Significance
We have characterized the relative bioactivity of the physiological progestin, dihydroprogesterone (DHP), showing in vivo it maintains equine gestation in the absence of progesterone, generally believed the only steroid capable of supporting pregnancy. DHP activates the equine progesterone receptor (PR) as potently as progesterone itself, and the human PR potently enough to suggest support for human pregnancy. Thus, DHP joins this steroid class as the only other naturally synthesized progestin so defined. This settles five decades of speculation that DHP sustains equine pregnancies in the second half of gestation when circulating progesterone becomes undetectable. Expanding the progestin class in horses reminds us that the limited suite of physiologically relevant sex steroids currently recognized may be incomplete across taxa.
Keywords: evolution; steroid; 5α DHP; endometrium; 5α-pregnane-3,20-dione
Abstract
One of the most widely accepted axioms of mammalian reproductive biology is that pregnancy requires the (sole) support of progesterone, acting in large measure through nuclear progesterone receptors (PRs) in uterine and cervical tissues, without which pregnancy cannot be established or maintained. However, mares lack detectable progesterone in the latter half of pregnancy. Instead of progesterone, several (mainly 5α-reduced) pregnanes are elevated and have long been speculated to provide progestational support in lieu of progesterone itself. To the authors' knowledge, evidence for the bioactivity of a second potent endogenously synthesized pregnane able to support pregnancy in the absence of progesterone has never before been reported. The 5α-reduced progesterone metabolite dihydroprogesterone (DHP) was shown in vivo to stimulate endometrial growth and progesterone-dependent gene expression in the horse at subphysiological concentrations and to maintain equine pregnancy in the absence of luteal progesterone in the third and fourth weeks postbreeding. Results of in vitro studies indicate that DHP is an equally potent and efficacious endogenous progestin in the horse but that the PR evolved with increased agonistic potency for DHP at the expense of potency toward progesterone based on comparisons with human PR responses. Sequence analysis and available literature indicate that the enzyme responsible for DHP synthesis, 5α-reductase type 1, also adapted primarily to metabolize progesterone and thereby to serve diverse roles in the physiology of pregnancy in mammals. Our confirmation that endogenously synthesized DHP is a biopotent progestin in the horse ends decades of speculation, explaining how equine pregnancies survive without measurable circulating progesterone in the last 4 to 5 mo of gestation.
Since first crystallized almost eight decades ago, progesterone has remained the only endogenous member of the progestin class of steroids defined by its singular ability to maintain pregnancy (1), acting, in large measure, through nuclear progesterone receptors (PRs) in uterine and cervical tissues, without which pregnancy cannot be established or maintained (2). Birth is thought to be triggered by a decrease in systemic progesterone concentrations (withdrawal) (3), even though this is not evident in mares (4), women, or guinea pigs (5), a disparity that limits the utility of other animal models for preterm labor (6). The vast majority of studies have focused on measuring progesterone, with most using immunoassays that necessarily cross-react with multiple pregnanes (7), the bioactivity of which remain uncharacterized. This is reasonable because, in contrast to androgens, estrogens, and corticoids, for which multiple natural biopotent analogs are known, no other endogenous pregnane has ever been shown to substitute for progesterone in pregnancy in any mammal.
However, over five decades ago, Short (8) reported that circulating progesterone concentrations in pregnant mares were surprisingly low at <4 ng/mL, as did Holtan et al. (4) subsequently. Indeed, Holtan et al. (9) further confirmed by GC-MS that maternal progesterone concentrations in middle to late equine gestation were <0.5 ng/mL, and were low even in fetal circulation (10). Conversely, 5α-reduced metabolites like 5α-dihydroprogesterone (DHP) were very high in both pregnant mares and their fetuses (9, 10). Some human pregnancies that have extremely low concentrations of progesterone can survive to term also, as in patients who have congenital hypobetalipoproteinemia (11), and progesterone is low or undetectable in plasma of pregnant zebras (12), elephants (13), and the rock hyrax (14). Thus, alternative endogenous progestins have been postulated to exist in species other than horses, but definitive evidence of bioactivity is lacking. For instance, the results of studies investigating the activity of identified circulating pregnanes on equine (15) or human (16) myometrial contractility have not been consistent. Consequently, speculation about alternative progestins has been based mostly on binding assays in tissue extracts (17). However, binding assays alone do not reliably predict bioactivity (18, 19), and, to date, an alternative endogenous progestin capable of sustaining pregnancy in the absence of progesterone has yet to be identified in any mammal.
Here, we verify by a unique combination of in vivo and in vitro studies that endogenously synthesized pregnane DHP sustains pregnancy in the absence of detectable progesterone in mares by (i) inducing equine endometrial growth and stimulating expression of progesterone-responsive endometrial genes in vivo; (ii) maintaining equine pregnancy after progesterone withdrawal induced by luteal regression; (iii) activating the equine PR (ePR) in vitro with equal efficacy and potency to progesterone itself; and (iv) doing so at concentrations seen during the luteal phase and early pregnancy, as well as in the second half of equine gestation when progesterone itself is undetectable. Collectively, these data establish DHP as a biopotent progestin in the horse at concentrations seen during gestation. Evidence both in vivo and in vitro demonstrating that an endogenously synthesized pregnane is able to sustain pregnancy by activating the nuclear PR at physiological concentrations has not previously been reported for any species to our knowledge. Evolutionary implications relating to the synthetic enzymes involved, and the classical PR itself, were also explored and are discussed.
Results
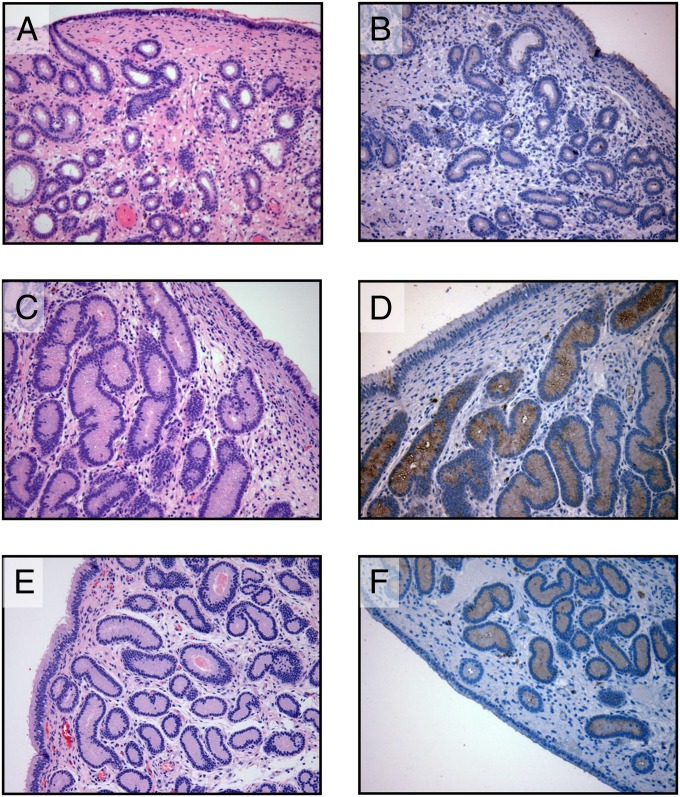
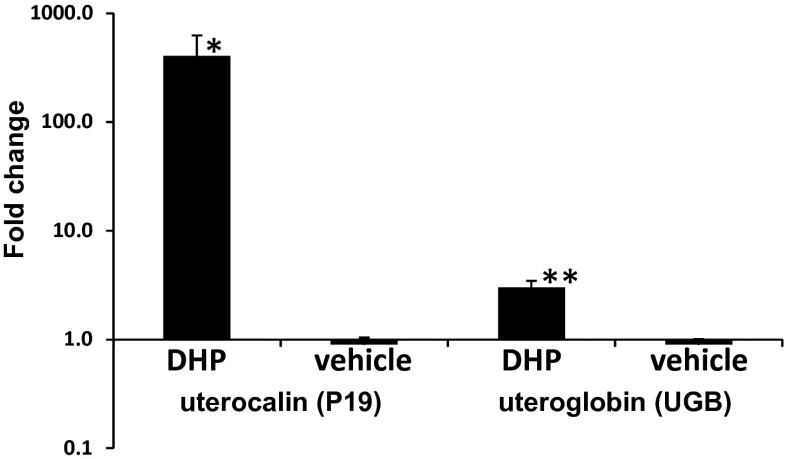
Studies to determine the bioactivity of DHP in vivo were initiated in ovariectomized mares treated daily for 10 d with 300 mg of DHP dissolved in vehicle (benzyl alcohol and benzyl benzoate in oil) or vehicle alone by i.m. injection using a cross-over design (n = 4). Mares were bled daily, and DHP concentrations were determined by liquid chromatography tandem mass spectrometry (LC-MS/MS). Histology performed on endometrial biopsies taken on day 10 of treatment demonstrated that glandular development was induced by DHP (Fig. 1 A vs. C) to a similar degree as that seen during a normal progestational luteal phase in cyclic mares (Fig. 1E). Systemic DHP in treated mares reached circulating concentrations of 3.8 ± 0.4 ng/mL by day 10, which was demonstrated to be below peaks seen both in the luteal phase (6.3 ± 1.0 ng/mL) and in pregnancy (see below). Vehicle induced no detectable response. Immunohistochemical staining of endometrial biopsies for uterocalin (P19), a progesterone-induced gene (20), verified the stimulatory response to DHP (Fig. 1 B vs. D) similar to that seen in the luteal phase (Fig. 1F). Furthermore, quantitative PCR for transcripts encoding P19 and another progesterone-responsive gene, uteroglobin (UGB), confirmed a significant induction of both by DHP (P19 fold over vehicle: 405.5 ± 220.1 vs. 0.9 ± 0.1, P < 0.05; UGB fold over vehicle: 3.0 ± 0.4 vs. 0.9 ± 0.1, P < 0.01) using equine β2-microglobulin as the reference gene (Fig. 2).
Fig. 1.
Endometrial biopsies from ovariectomized (A–D) and intact, luteal phase (E and F) mares. Sections were stained routinely with H&E (A, C, and E) or immunostained with antisera raised against equine P19 (B, D, and F). Biopsies were taken on day 0 (A and B) before treatment with DHP (300 mg/d) began, after 10 d of treatment (C and D), and from normal cyclic mares in the midluteal phase (E and F) of the estrus cycle. Note the presence of small, undeveloped endometrial glands on day 0 (A) with little or no evidence of P19 expression (B) and both glandular development (C) and intensely positive immunostaining for P19 (D) after 10 d of DHP treatment. This compares favorably with endometrial glandular development (E) and P19 expression (F) in the glandular epithelium of biopsies from luteal phase mares. (Magnification: 200×.)
Fig. 2.
Expression of P19 (NM_001082509.1) and UGB (NM_001081858.1) in endometrial biopsies taken from ovariectomized mares treated with DHP or vehicle for 10 d. P19 and UGB transcript levels were measured by quantitative PCR and expressed as fold change over the reference gene β2-microglobulin. Note that data are expressed on a log scale. Asterisks indicate significant differences between DHP and vehicle control treatments (*P < 0.05; **P < 0.01).
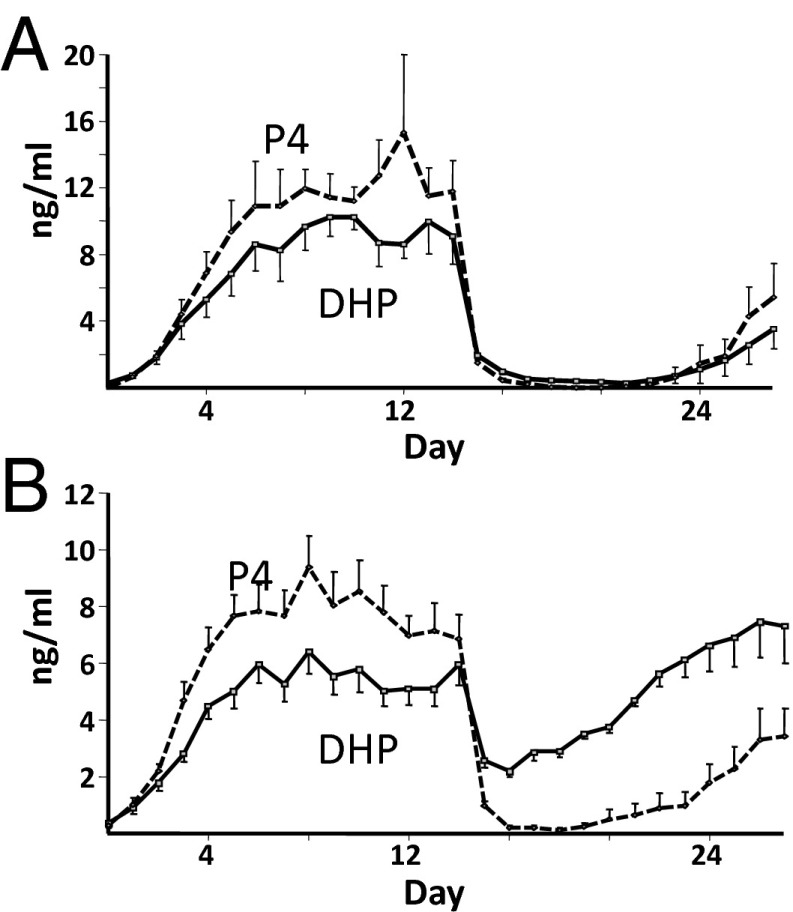
Experiments were also conducted to verify that DHP was sufficiently progestogenic to maintain pregnancy in the absence of luteal progesterone. Mares inseminated and diagnosed as pregnant by ultrasound on day 12 postovulation were given DHP (0.7 mg/kg, n = 9) or vehicle (n = 5) daily beginning on day 13 and were treated with prostaglandin F2α (PGF2α) on day 14 to induce luteolysis, eliminating luteal progesterone secretion. Luteolysis and disappearance of progesterone were confirmed in blood by LC-MS/MS (Fig. 3). Pregnancies were monitored daily by ultrasound through detection of a heartbeat and until day 27 of pregnancy, when conceptuses were recovered by uterine lavage. Seven of nine mares treated with DHP maintained pregnancy until day 27 with normal embryonic development; all five of five vehicle-treated mares lost their pregnancies (Fisher’s exact test, P < 0.05). DHP concentrations were maintained at >2 ng/mL (6 nM) in treated mares, and progesterone was <1 ng/ml by day 16 (2 d after PGF2α-induced luteal regression; Fig. 3).
Fig. 3.
Daily jugular venous concentrations of P4 and DHP in vehicle-treated (A; n = 5) and DHP-treated (B; 0.7 mg/kg, n = 9) mares administered a luteolytic dose of PGF2α on day 14 of pregnancy.
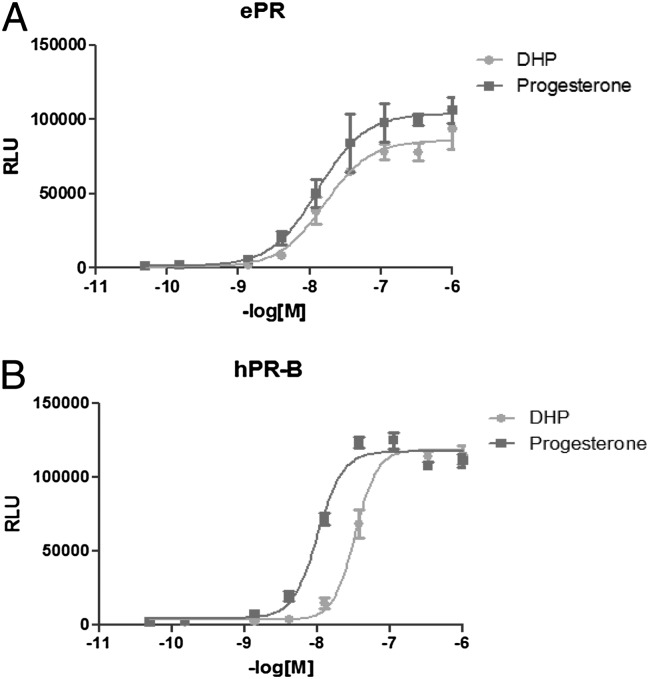
The relative biopotency of DHP compared with progesterone was investigated using an in vitro bioassay in cells expressing the ePR, and parallel experiments were conducted for comparison with human PR (hPR) responses. The ePR was cloned from an equine uterine cDNA library (21) and genomic DNA, sequenced, and subcloned into pcDNA3.1 (Life Technologies). Nucleotide identity of ePR with other mammalian PRs was extremely high overall: 96% in the ligand-binding domain as recently reported (22). Hepatocellular carcinoma G2 (HepG2) cells were cotransfected with the expression plasmids encoding either ePR-B or hPR-B (Fig. S1) with a luciferase reporter gene driven by the mouse mammary tumor virus promoter (MMTV)-luciferase and a β-gal reporter construct to correct for transfection efficiencies. DHP and progesterone (0.15 nM–1 μM) were added in DMSO and ethanol, respectively, and luciferase expression was measured 24–36 h later (Fig. 4). DHP exhibited a similar luciferase reporter activity induction profile as progesterone in cells transfected with the ePR construct, with similar half-maximal stimulating concentrations (EC50: 14.0 ± 1.1 nM and 13.7 ± 1.6 nM, respectively). By comparison, DHP was one-fifth as potent an agonist of the hPR as was progesterone (DHP and progesterone EC50: 23.1 ± 5.1 nM and 5.4 ± 2.4 nM, respectively) in these experiments, but both the ePR and hPR responses were saturated at 100 nM (Fig. 4).
Fig. 4.
Results of a representative experiment showing induction of MMTV-driven relative luciferase unit (RLU) activity in HepG2 cells also transiently expressing either ePR (A) or hPR-B (B) exposed to DHP or progesterone.
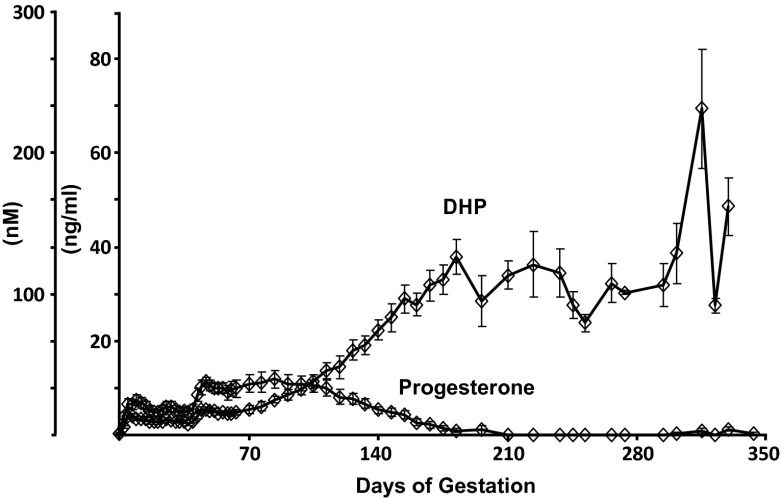
Concentrations of DHP and progesterone were measured by LC-MS/MS in plasma from pregnant mares collected throughout the course of gestation (Fig. 5). Progesterone concentrations peaked at 11.8 ± 1.8 ng/mL (37.5 nM) during the 12th week of pregnancy, by which time DHP concentrations were 7.5 ± 2.6 ng/mL (23.7 nM), both effectively twice the EC50 for the ePR measured in HepG2 cells. Thereafter, progesterone concentrations declined progressively as DHP continued to rise steadily. Concentrations of DHP exceeded those of progesterone by day 110 of gestation, and progesterone became undetectable by day 200. In contrast, DHP concentrations peaked at 69.5 ± 12.7 ng/mL (220 nM) from 310–320 d of gestation before declining slightly near the time of parturition (Fig. 5).
Fig. 5.
Jugular venous concentrations (nanograms per milliliter) of progesterone and DHP in serum from pregnant mares (n = 11) throughout pregnancy.
Quantitative PCR studies were conducted on tissues from pregnant mares (n = 5, 306–310 d of gestation) to identify the most likely isoform involved in DHP synthesis. Chorioallantois and endometrium exhibited a clear predominance of 5α-reductase type 1 (SRD5A1) over 5α-reductase type 2 (SRD5A2) expression (P < 0.01); five to six more cycles were required to generate equivalent amounts of SRD5A2 as SRD5A1 amplification product in both tissues. The phylogeny of a broad variety of vertebrate sequences supported the hypothesis that the duplication giving rise to the genes encoding SRD5A1 and SRD5A2 predates the most recent common ancestor of bony fish and tetrapods (Fig. S2). In addition, SRD5A1 has almost twice the rate of synonymous (0.6087 ± 0.0512 vs. 0.3050 ± 0.0282; P < 0.001) and nonsynonymous (0.1274 ± 0.0152 vs. 0.0762 ± 0.0106; P < 0.01) substitutions as SRD5A2.
Discussion
Collectively, the data presented here provide strong support for the conclusion that DHP exhibits progestogenic bioactivity in mares, stimulating sufficient endometrial growth and secretion to sustain pregnancy in the absence of luteal progesterone in this species. Progesterone concentrations in the second half of pregnancy in the mares studied were below the limits of detection (0.05 ng/mL), but peak concentrations of DHP were >10-fold higher than those shown to (i) induce endometrial growth and gene expression in ovariectomized mares, (ii) maintain early pregnancy after luteal ablation, and (iii) maximally stimulate the ePR in vitro. The reduction of progesterone to DHP in the placenta (23, 24) must be both rapid and virtually complete such that little progesterone escapes into the maternal or fetal circulation (10). To the authors’ knowledge, no prior studies in any species have provided evidence of the bioactivity of alternative endogenous progestins consistent with the capacity to maintain pregnancy in the absence of progesterone. Though tissue binding has been investigated, no previous report has demonstrated that DHP is a potent agonist of the ePR in vitro and in vivo, stimulating equine endometrial growth and sustaining pregnancy in the absence of progesterone. Horses are not likely to be unique among mammals in this regard. Elephants (13) and the rock hyrax (14) at least sustain pregnancy with low to undetectable concentrations of progesterone. Like the horse (17), endometrial extracts of elephant (17) and rock hyrax (14) bind DHP with comparable affinity to progesterone and, based on the current data, can also be assumed to stimulate a progestogenic response at concentrations consistent with the maintenance of pregnancy.
Collectively, these data suggest that DHP may be an important progestin in species from several mammalian orders and that the PR in at least some of these species appears to have evolved an increased affinity for DHP (17, 22) accordingly. It is of equal interest and significance that although not as potent an agonist for the hPR, DHP demonstrated similar efficacy at 10-fold higher concentrations (100 nM), consistent with estimates of relative binding to human uterine cytosol (25). Although perhaps not sufficiently high during the luteal phase of the menstrual cycle to be considered physiologically relevant, these concentrations of DHP are within the physiological range for women in their third trimester (26). The sometimes variable inhibitory effects of some 5α- and/or 5β-reduced pregnanes (so-called “neurosteroids”) on human myometrial contractility have been attributed, in part, to activity at GABAA receptors (27). Based on the results of the current studies, however, DHP must also be considered a relevant component of the progestogenic support of human pregnancies through PR activation, in addition to having other PR-independent effects (28).
Additionally, these data have significant physiological and potentially evolutionary implications for 5α-reductase, the enzyme system that catalyzes DHP synthesis from progesterone, because a physiological function for SRD5A1 has not been clear to date. Unlike SRD5A2 deficiency in human (29) and equine fetuses (30), which is associated with male pseudohermaphrodism, no known clinical case of SRD5A1 deficiency has ever been found in nature. Previous studies have shown that the endometrium and placenta are the major sites of 5α-reductase enzyme activity in equine tissues (23). The data presented here showing a predominance of SRD5A1 over SRD5A2 expression are consistent with results of studies on the human (31) and mouse (32) placenta, also suggesting that DHP synthesis is primarily dependent on SRD5A1. Women with SRD5A2 deficiency have normal concentrations of DHP (33), and pregnant SRD5A1 KO mice experience high rates of both fetal death (34) and impaired cervical ripening that prevents normal delivery (35), further supporting a predominant role for SRD5A1 in gestating females. The significance in terms of the support or termination of pregnancy would depend, importantly, on the bioactivity of DHP at the PR for each species, but the current data are consistent with those from KO mouse studies (36), which indicate a primary role for SRD5A1 in the physiology of gestation.
Insight within an evolutionary context was also sought by analysis of the SRD5A1, SRD5A2, and mammalian PR sequences (Fig. S2). The phylogeny constructed (Fig. S2) is consistent with that previously reported by Langlois et al. (37) but, based on amino acid substitution rates, suggests that SRD5A1 has undergone significantly more mutational change. SRD5A2 is far more conserved based on this analysis. This is consistent with the possibility that SRD5A1 serves a different physiological role than SRD5A2, and one that is far less conserved. The bioactivity of DHP, together with a role in the success of pregnancy, as discussed, provides a plausible explanation for why both type 1 and 2 isozymes metabolize progesterone more efficiently than testosterone in the mammals studied to date (38); progesterone is likely the more ancestral substrate. Collectively then, not only is DHP likely to be a potent progestin in multiple species, but 5α-reductase type 1 catalytic activity and expression may well have evolved primarily for metabolizing progesterone (dependent on the bioactivity of DHP at the PR in certain species), supporting a physiologically important role for this previously suspected but unverified progestin in pregnancy.
The phylogeny of mammalian PR remains unclear, but this lack of clarity may itself be significant. Wierer et al. (22) investigated DHP and progesterone binding to the human and elephant PR ligand-binding domains and, by mutating numerous residues, demonstrated that 722G promoted progesterone binding, whereas 722A promoted binding of DHP at the expense of progesterone. Neither the extensive sequence analysis by those authors nor our own provided any support for ancestry of the 722G/A residue in the ligand-binding domain of the mammalian PR. The present results provide evidence consistent with the data of Wierer et al. (22) that increases in DHP bioactivity subsequent to binding come at the expense of the biopotency of progesterone. Still, there is no clear evidence to support ancestry for either 722A (DHP binding) or 722G (progesterone binding) in mammals. Because this residue is a key determinant of the response to different pregnanes, this suggests the possibility that progesterone evolved to dominate in the role of progestational support of pregnancy among present-day mammals by a process whereby the response to other bioactive progestins, such as DHP, was lost. Further exploration of bioactivation of a larger number of mammalian PRs and a broader range of pregnanes is necessary to understand both receptor and agonist evolution better in this context.
The pioneering studies conducted by Short (8) and Holtan et al. (4) provided convincing evidence that progesterone concentrations were unexpectedly low in pregnant mares and that 5α-reduced pregnanes, including DHP, were surprisingly high (9). The combination of in vivo and in vitro data shown here defines the bioactivity of DHP physiologically and biochemically to a degree that resolves 50 y of speculation as to how pregnancy survives in mares without progesterone. It is, to date, the only identified physiologically active pregnane, other than progesterone itself, capable of sustaining pregnancy in a mammal. Multiple bioactive endogenously synthesized steroids are recognized within all other classes, estrogens, androgens, and corticoids, but progesterone has, until now, existed alone in the progestin class. The results reported here establish the existence of a biopotent, endogenous substitute for progesterone, and thereby provide impetus to reexamine the hormonal control of pregnancy and the events triggering normal birth as well as preterm labor. Consistent with this notion, previous studies with frequent sampling of mares around parturition indicate that DHP concentrations decline steadily over the last 2 d before foaling; progesterone did not drop until after birth (39). As Medawar famously said, “For ‘endocrine evolution’ is not an evolution of hormones but an evolution of uses to which they are put” (40). The evolution of steroid physiology depends on the enzymes that synthesize them and the receptors that they bioactivate. The equine nuclear PR appears to have adapted to become more responsive to DHP at the expense of the response to progesterone, and the SRD5A1 isozyme has evolved to promote DHP synthesis among mammals at least. However, simply recognizing the existence of a physiological progestin other than progesterone calls into question what is really known with certainty about endogenous, bioactive steroids of all classes where different species are concerned. This is especially true, given that so few species were used in the bioassays performed in the early decades of the past century, when steroid discovery experienced its golden age and the suite of steroids presumed to be the most bioactive was established in mammals.
Materials and Methods
In Vivo DHP Bioactivity in Ovariectomized Mares.
Animals used in all in vivo studies were housed at the Veterinary Medical Teaching Hospital, the Animal Science Horse Barn, or at the Center for Equine Health at the University of California, Davis, and were treated in accordance with approved University of California, Davis Institutional Animal Care and Use Committee protocols. Four mares (average age of 14 y, weighing 444–632 kg), ovariectomized for 8–19 mo before enrollment in the study, were given once-daily i.m. injections of 300 mg of DHP (total volume of 16 mL) or 16 mL of vehicle for 10 d in a cross-over design. Each of four mares received DHP and vehicle treatments with a washout period of at least 50 d between treatments. Two mares received DHP first, and two mares received vehicle first. A fifth ovariectomized mare was used preliminarily to verify that DHP was not detectable 25 d after the last injection. Blood samples were collected daily for steroid measurement, and endometrial biopsies were taken before treatment and again 24 h after the tenth and final treatment for histological evaluation, immunohistochemistry, and qualitative and quantitative RT-PCR. Day 0 was the day of pretreatment blood and biopsy sampling and the first injection, the final (10th) injection was given on day 9, and final blood and biopsies were taken on day 10. DHP (Steraloids) was dissolved in 30% (vol/vol) solvent (1:1 vol/vol benzyl alcohol/benzyl benzoate; Fisher Scientific) and 70% (vol/vol) cottonseed oil (Sigma–Aldrich). Solvents were measured, mixed, and heated in a 65 °C water bath with occasional vortexing until dissolved. Heated oil was filter-sterilized (0.45 μm) and added to the DHP-solvent solution [final concentration of 18.75 mg/mL DHP (16 mL for 300-mg dose)]. Vehicle was formulated similarly, excluding the addition of DHP. Peripheral blood samples were collected before daily DHP or vehicle injection. Collection was by jugular venipuncture into heparinized, evacuated glass tubes (Becton Dickinson), and samples were placed on ice until plasma was removed and stored at −20 °C for steroid analysis. Plasma concentrations of progesterone and DHP were measured by LC-MS/MS.
In Vivo DHP Bioactivity in Early Pregnant Mares.
Fertile mares were inseminated at estrus, and the day of ovulation (day 0) was determined ultrasonographically per rectum. Pregnancies were confirmed by ultrasonography on day 12, and mares were assigned to either a DHP treatment (n = 9) or vehicle control (n = 5) group. Mares received daily i.m. injections of DHP (0.7 mg/kg) or vehicle beginning on day 13. On the following day (day 14), PGF2α(10 mg i.m., Lutalyse; Pharmacia & Upjohn) was administered to induce luteolysis and eliminate endogenous progesterone production. Pregnancies were monitored daily by ultrasound (Pico; Universal Ultrasound) for maintenance, as indicated by cardiac motion and blood flow in the embryo proper until day 27, or, otherwise, for pregnancy loss. Jugular plasma was collected daily from insemination until day 27, and concentrations of progesterone and DHP were measured by LC-MS/MS. Differences in pregnancy maintenance between vehicle- and DHP-treated mares were tested by Fisher’s exact test.
Concentrations of Progesterone and DHP During Equine Pregnancy.
Jugular blood samples were taken from pregnant mares (n = 11) every second day for the first 2 mo of gestation, weekly to the sixth month, and twice each month until parturition. Plasma was collected, stored as before, and analyzed for progesterone and DHP concentration by LC-MS/MS.
LC-MS/MS Chemicals and Reagents.
Steroids for reference standards [DHP, progesterone (P4), and deuterium-labeled testosterone (d3-testosterone)] were obtained from Steraloids. Acetonitrile and water (HPLC grade) were obtained from Burdick and Jackson. Acetone, isopropanol, and ammonium hydroxide (Optima grade) were obtained from Thermo Fisher Scientific. Formic acid (American Chemical Society grade) was obtained from EMD Chemicals. Plasma samples (300 μL), including calibrator, quality control (QC), and test samples, were added to autosampler vials, followed by the addition of 150 μL of internal standard (50 ng/mL d3-testosterone solution in water). Samples were capped and vortex-mixed for 60 s, followed by centrifugation at ∼1,800 × g for 3 min. Calibrator, QC, and test samples were prepared concurrently for each sample run.
Two-Dimensional LC.
Online sample extraction and separation by 2D LC-MS/MS were accomplished as previously described (41). For chromatography, we used a Thermo Scientific Aria TLX-2 Turbulent Flow Chromatography system composed of two online degassers and four Shimadzu LC-10AD HPLC pumps, with two quaternary pumps and two binary pumps. A valve interface module with multiple switching valves allowed for multiplexing of the system. A CTC Analytics LEAP autosampler with a temperature-controlled compartment held at 7 °C was used to inject 50 μL of each sample into the system. The first dimension allowed the online extraction of DHP and P4 from plasma using a Thermo Scientific Cyclone extraction column (0.5 × 50 mm, 60-μm particle size). A quaternary pump composed of four solvents was used in the first dimension to extract the analytes, elute them to the second dimension, clean the column, and reequilibrate the system for the next injection. Following the transfer of analytes to the second dimension, compounds were separated over a linear gradient composed of water with 0.1% formic acid and acetonitrile with 0.1% formic acid on a Thermo Scientific Hypersil Gold column (2.1 × 50 mm, 3-μm particle size) at a flow rate of 300 µL/min. The column temperature was maintained at 40 °C by a Thermo Scientific Hot Pocket column heater.
MS/MS.
As reported previously (41), MS analysis was accomplished using a Thermo Scientific TSQ Vantage triple-stage quadrupole mass spectrometer with an atmospheric pressure chemical ionization (APCI) source operating in the positive mode. The APCI source was operated at 350 °C with sheath gas, and auxiliary gases were held at 45 and 25 arbitrary units of dry nitrogen, respectively. Spray voltage was set to 2,700 V, and the ion transfer tube temperature was set to 280 °C. Argon was used as a collision gas and set to 1.5 arbitrary units. The system was run at unit resolution (0.7 m/z) for both quadrupole 1 and quadrupole 3. Select reaction monitoring (SRM) was used to detect the analytes, with five SRM transitions monitored for both DHP and P4. A single SRM transition was used for quantitation of each analyte, whereas the remaining transitions were used for qualitative identification. Control equine plasma from castrated males was used to generate calibration curves and QC samples. Quantitation was accomplished by linear regression analysis using d3-testosterone as the internal standard. Linear calibration curves from 0.1–100 ng/mL and 0.25–100 ng/mL were obtained for progesterone and DHP, respectively. QC samples (n = 6 per level) were run at 0.3, 1, 10, and 50 ng/mL progesterone and DHP. Calibrators were run at the beginning and end of analysis, whereas QCs were interspersed throughout the sample analysis. The limits of quantitation were 0.25 ng/mL for DHP and 0.1 ng/mL for progesterone, and the limits of detection for DHP and progesterone were 0.1 ng/mL and 0.05 ng/mL, respectively. Intraassay precision (% coefficient of variation) for DHP ranged from 6.7–14.5%, and it ranged from 5.8–7.3% for progesterone; interassay precision ranged from 7.5–16.9% for DHP and from 5.8–13% for progesterone.
Tissue Collection, Preparation, and Immunohistochemistry.
Endometrial biopsy samples were collected from the corpus/cornual junction of the uterus immediately before the initial administration of DHP or vehicle (day 0) and 24 h after the 10th daily injection using a standard transcervical, rectally guided technique. Tissue samples were immediately quick-frozen on dry ice and then stored at −80 °C for RNA isolation or fixed in 4% (wt/vol) paraformaldehyde for histological evaluation after routine processing in paraffin and staining with H&E. Expression and localization of uterocalin (P19) used polyclonal antiserum to a recombinant-derived equine P19 raised in rabbit (courtesy of W. R. Allen, The Paul Mellon Laboratory of Equine Reproduction, Newmarket, Suffolk, United Kingdom) as reported previously (20). Sections were subjected to antigen retrieval [steam-heated for 5 min at 93 °C in antigen unmasking solution (Vector Laboratories)], incubated in primary anti-P19 antiserum (1:10,000) for 1 h at room temperature in a humidified chamber and in biotinylated secondary antiserum for 30 min, developed with a 3, 3′-diaminobenzidine peroxidase substrate kit (Vector Laboratories) for 5 min, and counterstained with hematoxylin. The primary antibody was omitted in negative control samples. In addition, tissues that included chorioallantois, endometrium, myometrium, and cervix were obtained from the reproductive tracts of five pregnant mares (306–310 d of gestation) for determination of the relative levels of expression of SRD5A1 and SRD5A2.
RT-PCR and Sequence Analysis.
Total RNA was isolated from frozen endometrial samples using TRIzol Reagent (Invitrogen) according to the manufacturer’s instructions. Total RNA was quantified by spectral analysis and reverse-transcribed (M-MLV reverse transcriptase; Promega) using 1 μg of RNA and oligo-dT primer. The RT product was phenol/chloroform-extracted and precipitated before full-length amplification by PCR using specific primers based on the known sequences for equine P19, UGB, β2-microglobulin, and GAPDH. Both Taq and Pfu DNA polymerases (Qiagen) were used in combination for high-fidelity PCR amplification. Cycling conditions consisted of an initial incubation at 94 °C for 1 min and then 30 repeated cycles at 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min, followed by 10 min at 72 °C before a final hold at 4 °C. PCR products were verified by gel electrophoresis and direct product sequencing (Biological Sciences DNA Sequencing Facility, University of California, Davis). The ePR was sequenced from clones isolated from an equine endometrial expression library (GenBank accession nos. KJ197859 and KJ197860), and sequence was confirmed by amplification (GenBank accession no. KJ197861) and sequencing of genomic DNA in regions of ambiguity. The full-length coding sequence was assembled in pcDNA3.1 for transient transfection and for the construction and maintenance of a CHO cell line stably expressing the ePR under geneticin selection. The hPR-B cDNA was expressed from a pcDNA3 plasmid as previously reported (42, 43).
Cell Culture and Transfection Assays.
Unless otherwise noted, all media and supplements were purchased from Invitrogen. HepG2 cells were purchased from the American Type Culture Collection and maintained in Eagle’s basal medium (Sigma–Aldrich) supplemented with 8% (vol/vol) FBS (HyClone Laboratories), 2 mM l-glutamine, 1 mM sodium pyruvate (NaPyr), and 0.1 mN nonessential amino acids (NEAA). Cells were grown in a 37 °C incubator with 5% (vol/vol) CO2. HepG2 cells were seeded at a density of 18,000 cells per well in 96-well culture plates in phenol red-free MEM supplemented with 8% (wt/vol) charcoal-stripped serum, 2 mM l-glutamine, 1 mM NaPyr, and 0.1 mM NEAA, and they were transfected with Lipofectin (Invitrogen) according to the manufacturer’s protocol. Briefly, each transfection was performed in triplicate using 550 ng of total DNA. Each transfection contained 500 ng of MMTV-luciferase reporter, 25 ng of ePR or hPR-B, and 25 ng of pCMV–β-Gal (Clontech) to normalize for transfection efficiency. Transfection into HepG2 cells was allowed to proceed for 24 h; progesterone and DHP (0.152, 1.37, 4.10, 12.3, 37.0, 111, 333, and 1,000 nM) were added for an additional 24- to 36-h period, after which luciferase activity was determined.
Quantitative RT-PCR.
Specific primer/probe sets for quantitative PCR were designed based on the known sequences of equine P19 and UGB as noted above, SRD5A1 and SRD5A2, and β2-microglobulin as a reference gene, using the Roche Universal Probe primer design Web site (www.roche-applied-science.com). Primers (100 μM) and probes (10 μM) were used in conjunction with the QuantiTect Probe PCR kit (Qiagen), containing HotStarTaq DNA polymerase and an optimized buffer containing dNTPs and ROX passive reference dye. Cycling conditions consisted of an initial incubation at 95 °C for 15 min, followed by 40 repeated cycles at 95 °C for 15 s and 60 °C for 60 s. Amplification of each gene was measured in duplicate using an ABI 7900 real-time PCR thermocycler (Applied Biosystems) at the University of California, Davis Lucy Whittier Molecular Core Facility. Relative quantities of mRNA were determined using an efficiency-calibrated model to calculate the fold change in expression after treatment. The threshold cycle (Ct; the average of duplicated wells) of the target gene after vehicle or DHP treatment was subtracted from the Ct of the target gene before treatment to determine the difference between Cts (∆Ct-target). The ∆Ct for the reference gene (β2-microglobulin) was determined in the same manner. The primer efficiency for each gene was then raised to the power of ∆Ct for that gene, and the ratio of the target to the reference was calculated to determine the fold change in expression after treatment. Differences between expression at day 0 and day 10 of DHP or vehicle treatment were analyzed by paired t tests for P19 and UGB corrected for β2-microglobulin expression. A similar quantitative PCR analysis for SRD5A1 and SRD5A2 expression was conducted using equine-specific probe sets on a transcript isolated from chorioallantois and endometrial tissue of pregnant mares (n = 5, 306–310 d of gestation). Relative levels of expression of SRD5A1 and SRD5A2 in these tissues were subjected to ANOVA using raw Ct values. Comparisons among tissues were precluded based on the variation in expression levels of β2-microglobulin.
Supplementary Material
Acknowledgments
We thank Prof. W. R. Allen for generously providing the P19 antisera, Lauren Matthewson for expert technical assistance, Jamie Deuel for skilled help with animal work, and Dr. Trish Berger for both statistical advice and assistance. We are equally grateful to several colleagues (Drs. Ray Rodgers, Larry Reynolds, and Paul Allen) who provided insightful, constructive, and valuable comments on numerous drafts of the manuscript. This project was supported, in part, by the Center for Equine Health with funds provided by the State of California pari-mutuel fund and contributions by private donors, the John P. Hughes Endowment, the Floyd and Mary Schwall Fellowship in Medical Research, the Albert G. Clay Endowment, National Institutes of Health Grant DK048807 (to D.P.M.), and Department of Defense Grant W81XWH-11-1-0063 (to S.K.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. KJ197859–KJ197861).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1318163111/-/DCSupplemental.
References
- 1.Conneely OM, Mulac-Jericevic B, DeMayo F, Lydon JP, O’Malley BW. Reproductive functions of progesterone receptors. Recent Prog Horm Res. 2002;57:339–355. doi: 10.1210/rp.57.1.339. [DOI] [PubMed] [Google Scholar]
- 2.Mendelson CR. Minireview: Fetal-maternal hormonal signaling in pregnancy and labor. Mol Endocrinol. 2009;23(7):947–954. doi: 10.1210/me.2009-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liggins GC, Thorburn GD. Initiation of parturition. In: Lamming GE, editor. Marshall’s Physiology of Reproduction. 4th Ed. Vol 3. London: Chapman & Hall; 1994. pp. 863–1002. [Google Scholar]
- 4.Holtan DW, Nett TM, Estergreen VL. Plasma progestins in pregnant, postpartum and cycling mares. J Anim Sci. 1975;40(2):251–260. doi: 10.2527/jas1975.402251x. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell BF, Taggart MJ. Are animal models relevant to key aspects of human parturition? Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R525–R545. doi: 10.1152/ajpregu.00153.2009. [DOI] [PubMed] [Google Scholar]
- 6.Challis JR, et al. Fetal signals and parturition. J Obstet Gynaecol Res. 2005;31(6):492–499. doi: 10.1111/j.1447-0756.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- 7.Behrman HR. The history of hormone assays. Prog Clin Biol Res. 1988;285:1–14. [PubMed] [Google Scholar]
- 8.Short RV. Progesterone in blood. IV. Progesterone in the blood of mares. J Endocrinol. 1959;19:207–210. doi: 10.1677/joe.0.0190207. [DOI] [PubMed] [Google Scholar]
- 9.Holtan DW, et al. Plasma progestagens in the mare, fetus and newborn foal. J Reprod Fertil Suppl. 1991;44:517–528. [PubMed] [Google Scholar]
- 10.Ousey JC, et al. Ontogeny of uteroplacental progestagen production in pregnant mares during the second half of gestation. Biol Reprod. 2003;69(2):540–548. doi: 10.1095/biolreprod.102.013292. [DOI] [PubMed] [Google Scholar]
- 11.Parker CR, Jr, Illingworth DR, Bissonnette J, Carr BR. Endocrine changes during pregnancy in a patient with homozygous familial hypobetalipoproteinemia. N Engl J Med. 1986;314(9):557–560. doi: 10.1056/NEJM198602273140906. [DOI] [PubMed] [Google Scholar]
- 12.Klima F, Rohleder M, Dehnhard M, Meyer HHD. Identification of progesterone and 5 alpha-dihydroprogesterone (5 alpha-DHP) in zebras by two-dimensional high-performance liquid chromatography and their dependence on the state of reproduction. Zoo Biol. 1999;18(4):325–333. [Google Scholar]
- 13.Hodges JK, Heistermann M, Beard A, van Aarde RJ. Concentrations of progesterone and the 5 alpha-reduced progestins, 5 alpha-pregnane-3,20-dione and 3 alpha-hydroxy-5 alpha-pregnan-20-one, in luteal tissue and circulating blood and their relationship to luteal function in the African elephant, Loxodonta africana. Biol Reprod. 1997;56(3):640–646. doi: 10.1095/biolreprod56.3.640. [DOI] [PubMed] [Google Scholar]
- 14.Kirkman S, Wallace ED, van Aarde RJ, Potgieter HC. Steroidogenic correlates of pregnancy in the rock hyrax (Procavia capensis) Life Sci. 2001;68(18):2061–2072. doi: 10.1016/s0024-3205(01)00999-7. [DOI] [PubMed] [Google Scholar]
- 15.Ousey JC, Freestone N, Fowden AL, Mason WT, Rossdale PD. The effects of oxytocin and progestagens on myometrial contractility in vitro during equine pregnancy. J Reprod Fertil Suppl. 2000;56(56):681–691. [PubMed] [Google Scholar]
- 16.Löfgren M, Holst J, Bäckström T. Effects in vitro of progesterone and two 5 alpha-reduced progestins, 5 alpha-pregnane-3,20-dione and 5 alpha-pregnane-3 alpha-ol-20-one, on contracting human myometrium at term. Acta Obstet Gynecol Scand. 1992;71(1):28–33. doi: 10.3109/00016349209007943. [DOI] [PubMed] [Google Scholar]
- 17.Jewgenow K, Meyer HH. Comparative binding affinity study of progestins to the cytosol progestin receptor of endometrium in different mammals. Gen Comp Endocrinol. 1998;110(2):118–124. doi: 10.1006/gcen.1997.7054. [DOI] [PubMed] [Google Scholar]
- 18.Glasser SR. A molecular bioassay for progesterone and related compounds. Methods Enzymol. 1975;36:456–465. doi: 10.1016/s0076-6879(75)36042-4. [DOI] [PubMed] [Google Scholar]
- 19.Giangrande PH, Kimbrel EA, Edwards DP, McDonnell DP. The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. Mol Cell Biol. 2000;20(9):3102–3115. doi: 10.1128/mcb.20.9.3102-3115.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart F, Gerstenberg C, Suire S, Allen WR. Immunolocalization of a novel protein (P19) in the endometrium of fertile and subfertile mares. J Reprod Fertil Suppl. 2000;56:593–599. [PubMed] [Google Scholar]
- 21.McDowell KJ, Adams MH, Adam CY, Simpson KS. Changes in equine endometrial oestrogen receptor alpha and progesterone receptor mRNAs during the oestrous cycle, early pregnancy and after treatment with exogenous steroids. J Reprod Fertil. 1999;117(1):135–142. doi: 10.1530/jrf.0.1170135. [DOI] [PubMed] [Google Scholar]
- 22.Wierer M, Schrey AK, Kühne R, Ulbrich SE, Meyer HH. A single glycine-alanine exchange directs ligand specificity of the elephant progestin receptor. PLoS ONE. 2012;7(11):e50350. doi: 10.1371/journal.pone.0050350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamon M, et al. Production of 5 alpha-dihydroprogesterone during late pregnancy in the mare. J Reprod Fertil Suppl. 1991;44:529–535. [PubMed] [Google Scholar]
- 24.Moss GE, Estergreen VL, Becker SR, Grant BD. The source of the 5-alpha-pregnanes that occur during gestation in mares. J Reprod Fertil Suppl. 1979;27(27):511–519. [PubMed] [Google Scholar]
- 25.Kontula K, et al. Progesterone-binding proteins: In vitro binding and biological activity of different steroidal ligands. Acta Endocrinol (Copenh) 1975;78(3):574–592. doi: 10.1530/acta.0.0780574. [DOI] [PubMed] [Google Scholar]
- 26.Milewich L, Gomez-Sanchez C, Madden JD, MacDonald PC. Isolation and characterization of 5alpha-pregnane-3,20-dione and progesterone in peripheral blood of pregnant women. Measurement throughout pregnancy. Gynecol Invest. 1975;6(5):291–306. [PubMed] [Google Scholar]
- 27.Putnam CD, Brann DW, Kolbeck RC, Mahesh VB. Inhibition of uterine contractility by progesterone and progesterone metabolites: Mediation by progesterone and gamma amino butyric acidA receptor systems. Biol Reprod. 1991;45(2):266–272. doi: 10.1095/biolreprod45.2.266. [DOI] [PubMed] [Google Scholar]
- 28.Wiebe JP, Zhang G, Welch I, Cadieux-Pitre HA. Progesterone metabolites regulate induction, growth, and suppression of estrogen- and progesterone receptor-negative human breast cell tumors. Breast Cancer Res. 2013;15(3):R38. doi: 10.1186/bcr3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson JD, Griffin JE, Russell DW. Steroid 5 alpha-reductase 2 deficiency. Endocr Rev. 1993;14(5):577–593. doi: 10.1210/edrv-14-5-577. [DOI] [PubMed] [Google Scholar]
- 30.Knobbe MG, Maenhoudt C, Turner RM, McDonnell SM. Physical, behavioral, endocrinologic, and cytogenetic evaluation of two Standardbred racehorses competing as mares with an intersex condition and high postrace serum testosterone concentrations. J Am Vet Med Assoc. 2011;238(6):751–754. doi: 10.2460/javma.238.6.751. [DOI] [PubMed] [Google Scholar]
- 31.Milewich L, Gant NF, Schwarz BE, Chen GT, MacDonald PC. 5 alpha-Reductase activity in human placenta. Am J Obstet Gynecol. 1979;133(6):611–617. doi: 10.1016/0002-9378(79)90006-1. [DOI] [PubMed] [Google Scholar]
- 32.Mahendroo MS, Cala KM, Russell DW. 5 alpha-reduced androgens play a key role in murine parturition. Mol Endocrinol. 1996;10(4):380–392. doi: 10.1210/mend.10.4.8721983. [DOI] [PubMed] [Google Scholar]
- 33.Milewich L, et al. Women with steroid 5 alpha-reductase 2 deficiency have normal concentrations of plasma 5 alpha-dihydroprogesterone during the luteal phase. J Clin Endocrinol Metab. 1995;80(11):3136–3139. doi: 10.1210/jcem.80.11.7593415. [DOI] [PubMed] [Google Scholar]
- 34.Mahendroo MS, Cala KM, Landrum DP, Russell DW. Fetal death in mice lacking 5alpha-reductase type 1 caused by estrogen excess. Mol Endocrinol. 1997;11(7):917–927. doi: 10.1210/mend.11.7.9933. [DOI] [PubMed] [Google Scholar]
- 35.Mahendroo MS, Porter A, Russell DW, Word RA. The parturition defect in steroid 5alpha-reductase type 1 knockout mice is due to impaired cervical ripening. Mol Endocrinol. 1999;13(6):981–992. doi: 10.1210/mend.13.6.0307. [DOI] [PubMed] [Google Scholar]
- 36.Mahendroo MS, Russell DW. Male and female isoenzymes of steroid 5alpha-reductase. Rev Reprod. 1999;4(3):179–183. doi: 10.1530/ror.0.0040179. [DOI] [PubMed] [Google Scholar]
- 37.Langlois VS, Zhang D, Cooke GM, Trudeau VL. Evolution of steroid-5alpha-reductases and comparison of their function with 5beta-reductase. Gen Comp Endocrinol. 2010;166(3):489–497. doi: 10.1016/j.ygcen.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Wilson JD. Metabolism of testicular androgens. In: Hamilton DW, Greep RO, editors. Endocrinology. Male Reproductive System. Vol V. Washington, DC: American Physiological Society; 1975. pp. 491–508. [Google Scholar]
- 39.Seamans KW, Harms PG, Atkins DT, Fleeger JL. Serum levels of progesterone, 5 alpha-dihydroprogesterone and hydroxy-5 alpha-pregnanones in the prepartum and postpartum equine. Steroids. 1979;33(1):55–63. doi: 10.1016/s0039-128x(79)80006-9. [DOI] [PubMed] [Google Scholar]
- 40.Medawar PB. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. In: Brown R, Danielli JF, editors. Evolution. Vol VII. Cambridge, UK: Cambridge Univ Press; 1953. pp. 320–338. [Google Scholar]
- 41.Moeller BC, Stanley SD. The development and validation of a turbulent flow chromatography-tandem mass spectrometry method for the endogenous steroid profiling of equine serum. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;905:1–9. doi: 10.1016/j.jchromb.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 42.Chang CY, Abdo J, Hartney T, McDonnell DP. Development of peptide antagonists for the androgen receptor using combinatorial peptide phage display. Mol Endocrinol. 2005;19(10):2478–2490. doi: 10.1210/me.2005-0072. [DOI] [PubMed] [Google Scholar]
- 43.Wade HE, et al. Multimodal regulation of E2F1 gene expression by progestins. Mol Cell Biol. 2010;30(8):1866–1877. doi: 10.1128/MCB.01060-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.