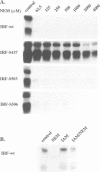
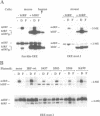
Abstract
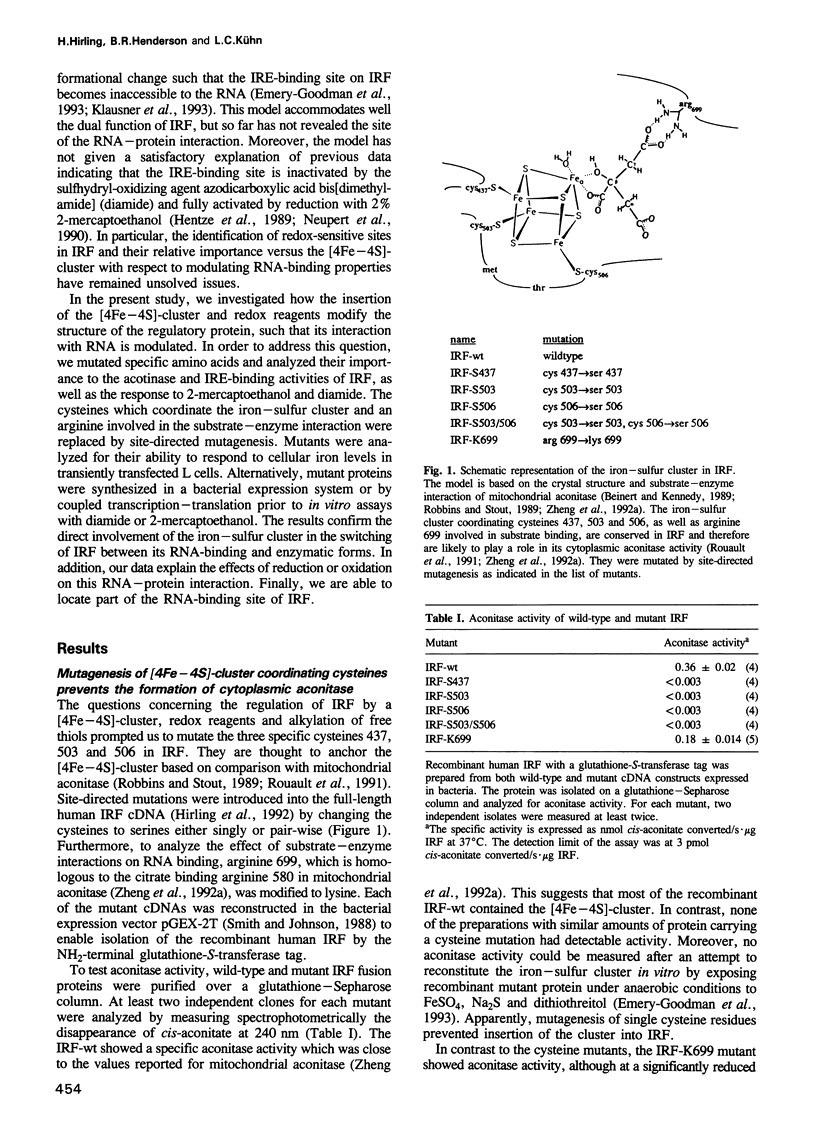
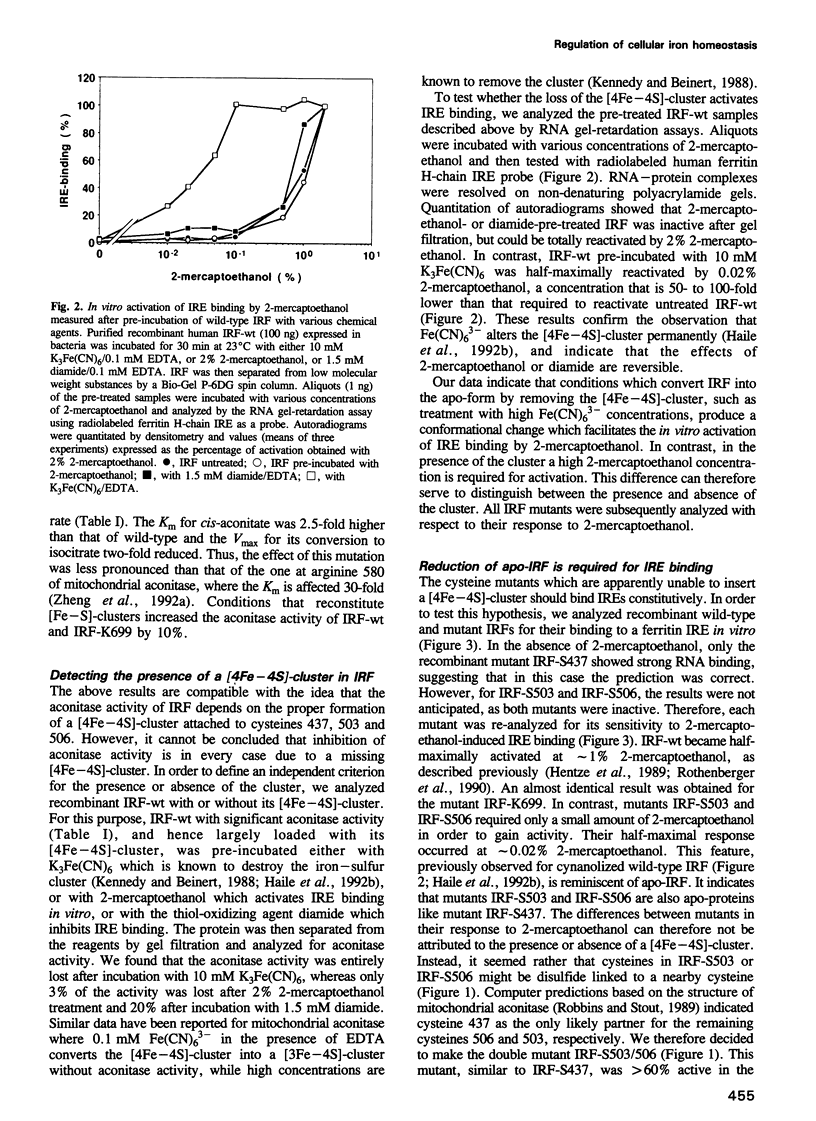
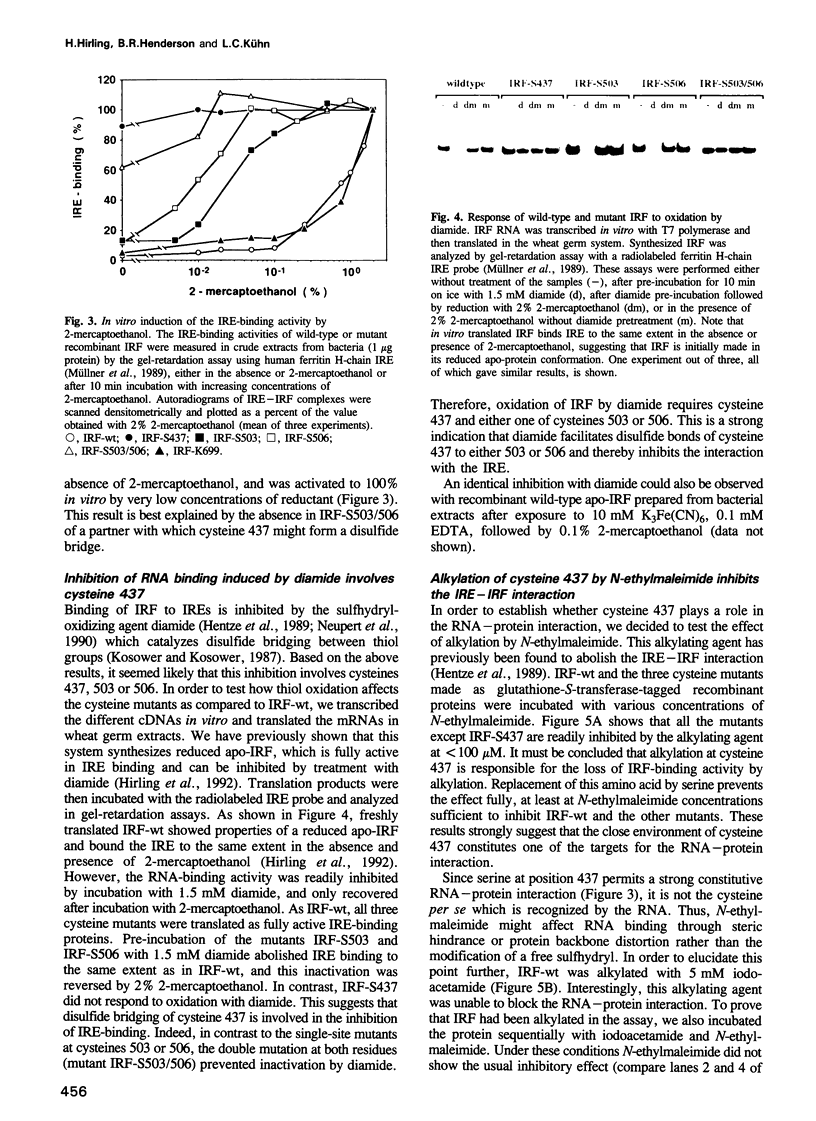
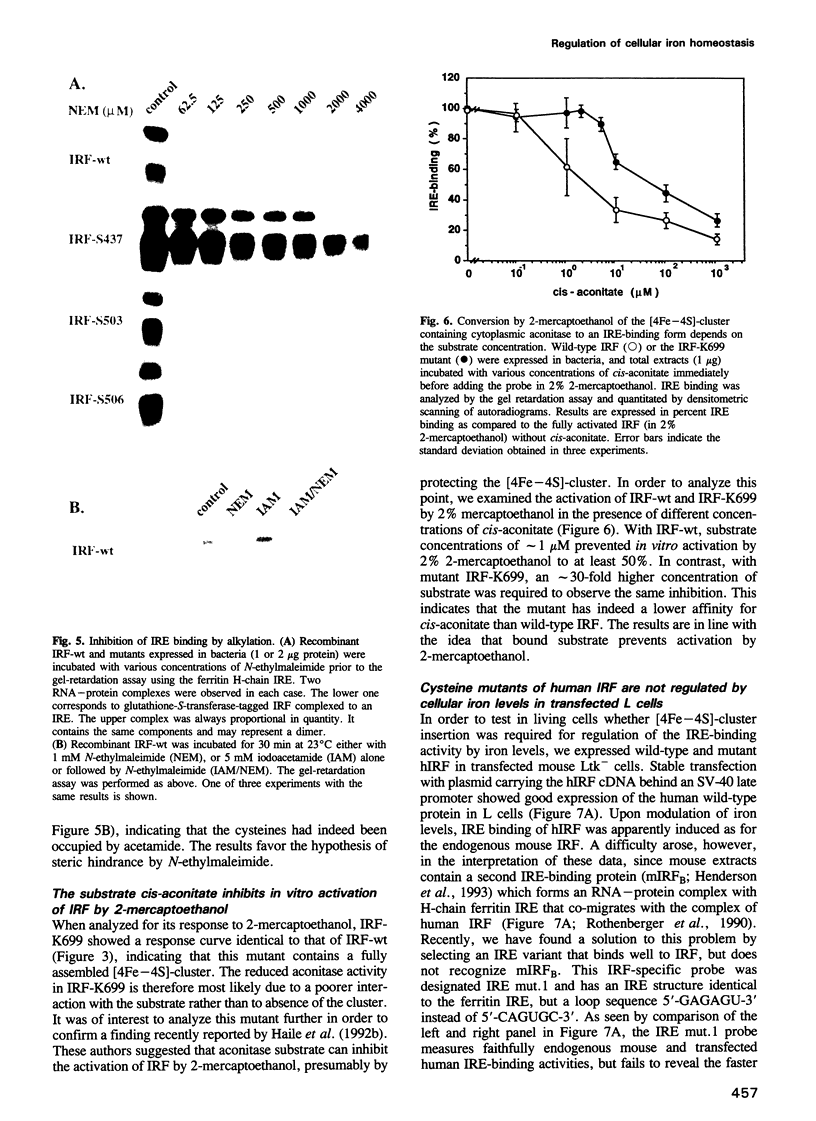
The control of cellular iron homeostasis involves the coordinate post-transcriptional regulation of ferritin mRNA translation and transferring receptor mRNA stability. These regulatory events are mediated by a soluble cytoplasmic protein, iron regulatory factor (IRF), which binds specifically to mRNA hairpin structures, termed iron-responsive elements (IREs), in the respective mRNAs. IRF is modulated by variations of cellular iron levels and exists as either an apo-protein or a [4Fe-4S]-cluster protein. The two conformations show distinct, mutually exclusive functions. High-affinity IRE binding is observed with the apo-form induced by iron deprivation, but is lost under high iron conditions when IRF is converted to the [4Fe-4S]-cluster form which shows cytoplasmic aconitase activity. Moreover, IRE binding is inactivated by the sulfhydryl-oxidizing agent diamide and fully activated in vitro by 2% 2-mercapto-ethanol, whereas alkylation of IRF inhibits IRE binding. In the present study, we analyzed each of the above features using site-directed mutants of recombinant human IRF. The results support the bifunctional nature of IRF. We conclude that cysteines 437, 503 and 506 anchor the [4Fe-4S]-cluster, and are essential to the aconitase activity. Mutagenesis changing any of the cysteines to serine leads to constitutive RNA binding in 0.02% 2-mercaptoethanol. Cysteine 437 is particularly critical to the RNA-protein interaction. The spontaneous or diamide-induced formation of disulfide bonds between cysteines 437 and 503 or 437 and 506, in apo-IRF, as well as its alkylation by N-ethylmaleimide, inhibit binding to the IRE.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aziz N., Munro H. N. Iron regulates ferritin mRNA translation through a segment of its 5' untranslated region. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8478–8482. doi: 10.1073/pnas.84.23.8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinert H., Kennedy M. C. 19th Sir Hans Krebs lecture. Engineering of protein bound iron-sulfur clusters. A tool for the study of protein and cluster chemistry and mechanism of iron-sulfur enzymes. Eur J Biochem. 1989 Dec 8;186(1-2):5–15. doi: 10.1111/j.1432-1033.1989.tb15170.x. [DOI] [PubMed] [Google Scholar]
- Bhasker C. R., Burgiel G., Neupert B., Emery-Goodman A., Kühn L. C., May B. K. The putative iron-responsive element in the human erythroid 5-aminolevulinate synthase mRNA mediates translational control. J Biol Chem. 1993 Jun 15;268(17):12699–12705. [PubMed] [Google Scholar]
- Casey J. L., Hentze M. W., Koeller D. M., Caughman S. W., Rouault T. A., Klausner R. D., Harford J. B. Iron-responsive elements: regulatory RNA sequences that control mRNA levels and translation. Science. 1988 May 13;240(4854):924–928. doi: 10.1126/science.2452485. [DOI] [PubMed] [Google Scholar]
- Constable A., Quick S., Gray N. K., Hentze M. W. Modulation of the RNA-binding activity of a regulatory protein by iron in vitro: switching between enzymatic and genetic function? Proc Natl Acad Sci U S A. 1992 May 15;89(10):4554–4558. doi: 10.1073/pnas.89.10.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox T. C., Bawden M. J., Martin A., May B. K. Human erythroid 5-aminolevulinate synthase: promoter analysis and identification of an iron-responsive element in the mRNA. EMBO J. 1991 Jul;10(7):1891–1902. doi: 10.1002/j.1460-2075.1991.tb07715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandekar T., Stripecke R., Gray N. K., Goossen B., Constable A., Johansson H. E., Hentze M. W. Identification of a novel iron-responsive element in murine and human erythroid delta-aminolevulinic acid synthase mRNA. EMBO J. 1991 Jul;10(7):1903–1909. doi: 10.1002/j.1460-2075.1991.tb07716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Murine cytotoxic activated macrophages inhibit aconitase in tumor cells. Inhibition involves the iron-sulfur prosthetic group and is reversible. J Clin Invest. 1986 Sep;78(3):790–797. doi: 10.1172/JCI112642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapier J. C., Hirling H., Wietzerbin J., Kaldy P., Kühn L. C. Biosynthesis of nitric oxide activates iron regulatory factor in macrophages. EMBO J. 1993 Sep;12(9):3643–3649. doi: 10.1002/j.1460-2075.1993.tb06038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery-Goodman A., Hirling H., Scarpellino L., Henderson B., Kühn L. C. Iron regulatory factor expressed from recombinant baculovirus: conversion between the RNA-binding apoprotein and Fe-S cluster containing aconitase. Nucleic Acids Res. 1993 Mar 25;21(6):1457–1461. doi: 10.1093/nar/21.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossen B., Caughman S. W., Harford J. B., Klausner R. D., Hentze M. W. Translational repression by a complex between the iron-responsive element of ferritin mRNA and its specific cytoplasmic binding protein is position-dependent in vivo. EMBO J. 1990 Dec;9(12):4127–4133. doi: 10.1002/j.1460-2075.1990.tb07635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Haile D. J., Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. Regulation of interaction of the iron-responsive element binding protein with iron-responsive RNA elements. Mol Cell Biol. 1989 Nov;9(11):5055–5061. doi: 10.1128/mcb.9.11.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile D. J., Rouault T. A., Harford J. B., Kennedy M. C., Blondin G. A., Beinert H., Klausner R. D. Cellular regulation of the iron-responsive element binding protein: disassembly of the cubane iron-sulfur cluster results in high-affinity RNA binding. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11735–11739. doi: 10.1073/pnas.89.24.11735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile D. J., Rouault T. A., Tang C. K., Chin J., Harford J. B., Klausner R. D. Reciprocal control of RNA-binding and aconitase activity in the regulation of the iron-responsive element binding protein: role of the iron-sulfur cluster. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7536–7540. doi: 10.1073/pnas.89.16.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze M. W., Argos P. Homology between IRE-BP, a regulatory RNA-binding protein, aconitase, and isopropylmalate isomerase. Nucleic Acids Res. 1991 Apr 25;19(8):1739–1740. doi: 10.1093/nar/19.8.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze M. W., Caughman S. W., Rouault T. A., Barriocanal J. G., Dancis A., Harford J. B., Klausner R. D. Identification of the iron-responsive element for the translational regulation of human ferritin mRNA. Science. 1987 Dec 11;238(4833):1570–1573. doi: 10.1126/science.3685996. [DOI] [PubMed] [Google Scholar]
- Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. Oxidation-reduction and the molecular mechanism of a regulatory RNA-protein interaction. Science. 1989 Apr 21;244(4902):357–359. doi: 10.1126/science.2711187. [DOI] [PubMed] [Google Scholar]
- Hirling H., Emery-Goodman A., Thompson N., Neupert B., Seiser C., Kühn L. C. Expression of active iron regulatory factor from a full-length human cDNA by in vitro transcription/translation. Nucleic Acids Res. 1992 Jan 11;20(1):33–39. doi: 10.1093/nar/20.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptain S., Downey W. E., Tang C., Philpott C., Haile D., Orloff D. G., Harford J. B., Rouault T. A., Klausner R. D. A regulated RNA binding protein also possesses aconitase activity. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10109–10113. doi: 10.1073/pnas.88.22.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. C., Beinert H. The state of cluster SH and S2- of aconitase during cluster interconversions and removal. A convenient preparation of apoenzyme. J Biol Chem. 1988 Jun 15;263(17):8194–8198. [PubMed] [Google Scholar]
- Kennedy M. C., Mende-Mueller L., Blondin G. A., Beinert H. Purification and characterization of cytosolic aconitase from beef liver and its relationship to the iron-responsive element binding protein. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11730–11734. doi: 10.1073/pnas.89.24.11730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Rouault T. A., Harford J. B. Regulating the fate of mRNA: the control of cellular iron metabolism. Cell. 1993 Jan 15;72(1):19–28. doi: 10.1016/0092-8674(93)90046-s. [DOI] [PubMed] [Google Scholar]
- Koeller D. M., Casey J. L., Hentze M. W., Gerhardt E. M., Chan L. N., Klausner R. D., Harford J. B. A cytosolic protein binds to structural elements within the iron regulatory region of the transferrin receptor mRNA. Proc Natl Acad Sci U S A. 1989 May;86(10):3574–3578. doi: 10.1073/pnas.86.10.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M. Formation of disulfides with diamide. Methods Enzymol. 1987;143:264–270. doi: 10.1016/0076-6879(87)43050-4. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Kühn L. C., Hentze M. W. Coordination of cellular iron metabolism by post-transcriptional gene regulation. J Inorg Biochem. 1992 Aug 15;47(3-4):183–195. doi: 10.1016/0162-0134(92)84064-t. [DOI] [PubMed] [Google Scholar]
- Leibold E. A., Guo B. Iron-dependent regulation of ferritin and transferrin receptor expression by the iron-responsive element binding protein. Annu Rev Nutr. 1992;12:345–368. doi: 10.1146/annurev.nu.12.070192.002021. [DOI] [PubMed] [Google Scholar]
- Leibold E. A., Munro H. N. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5' untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2171–2175. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May B. K., Bhasker C. R., Bawden M. J., Cox T. C. Molecular regulation of 5-aminolevulinate synthase. Diseases related to heme biosynthesis. Mol Biol Med. 1990 Oct;7(5):405–421. [PubMed] [Google Scholar]
- Melefors O., Goossen B., Johansson H. E., Stripecke R., Gray N. K., Hentze M. W. Translational control of 5-aminolevulinate synthase mRNA by iron-responsive elements in erythroid cells. J Biol Chem. 1993 Mar 15;268(8):5974–5978. [PubMed] [Google Scholar]
- Müllner E. W., Kühn L. C. A stem-loop in the 3' untranslated region mediates iron-dependent regulation of transferrin receptor mRNA stability in the cytoplasm. Cell. 1988 Jun 3;53(5):815–825. doi: 10.1016/0092-8674(88)90098-0. [DOI] [PubMed] [Google Scholar]
- Müllner E. W., Neupert B., Kühn L. C. A specific mRNA binding factor regulates the iron-dependent stability of cytoplasmic transferrin receptor mRNA. Cell. 1989 Jul 28;58(2):373–382. doi: 10.1016/0092-8674(89)90851-9. [DOI] [PubMed] [Google Scholar]
- Müllner E. W., Rothenberger S., Müller A. M., Kühn L. C. In vivo and in vitro modulation of the mRNA-binding activity of iron-regulatory factor. Tissue distribution and effects of cell proliferation, iron levels and redox state. Eur J Biochem. 1992 Sep 15;208(3):597–605. doi: 10.1111/j.1432-1033.1992.tb17224.x. [DOI] [PubMed] [Google Scholar]
- Neupert B., Thompson N. A., Meyer C., Kühn L. C. A high yield affinity purification method for specific RNA-binding proteins: isolation of the iron regulatory factor from human placenta. Nucleic Acids Res. 1990 Jan 11;18(1):51–55. doi: 10.1093/nar/18.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D., Kühn L. C. Noncoding 3' sequences of the transferrin receptor gene are required for mRNA regulation by iron. EMBO J. 1987 May;6(5):1287–1293. doi: 10.1002/j.1460-2075.1987.tb02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodromou C., Artymiuk P. J., Guest J. R. The aconitase of Escherichia coli. Nucleotide sequence of the aconitase gene and amino acid sequence similarity with mitochondrial aconitases, the iron-responsive-element-binding protein and isopropylmalate isomerases. Eur J Biochem. 1992 Mar 1;204(2):599–609. doi: 10.1111/j.1432-1033.1992.tb16673.x. [DOI] [PubMed] [Google Scholar]
- Robbins A. H., Stout C. D. Structure of activated aconitase: formation of the [4Fe-4S] cluster in the crystal. Proc Natl Acad Sci U S A. 1989 May;86(10):3639–3643. doi: 10.1073/pnas.86.10.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberger S., Müllner E. W., Kühn L. C. The mRNA-binding protein which controls ferritin and transferrin receptor expression is conserved during evolution. Nucleic Acids Res. 1990 Mar 11;18(5):1175–1179. doi: 10.1093/nar/18.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault T. A., Hentze M. W., Caughman S. W., Harford J. B., Klausner R. D. Binding of a cytosolic protein to the iron-responsive element of human ferritin messenger RNA. Science. 1988 Sep 2;241(4870):1207–1210. doi: 10.1126/science.3413484. [DOI] [PubMed] [Google Scholar]
- Rouault T. A., Stout C. D., Kaptain S., Harford J. B., Klausner R. D. Structural relationship between an iron-regulated RNA-binding protein (IRE-BP) and aconitase: functional implications. Cell. 1991 Mar 8;64(5):881–883. doi: 10.1016/0092-8674(91)90312-m. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Tang C. K., Chin J., Harford J. B., Klausner R. D., Rouault T. A. Iron regulates the activity of the iron-responsive element binding protein without changing its rate of synthesis or degradation. J Biol Chem. 1992 Dec 5;267(34):24466–24470. [PubMed] [Google Scholar]
- Walden W. E., Patino M. M., Gaffield L. Purification of a specific repressor of ferritin mRNA translation from rabbit liver. J Biol Chem. 1989 Aug 15;264(23):13765–13769. [PubMed] [Google Scholar]
- Weiss G., Goossen B., Doppler W., Fuchs D., Pantopoulos K., Werner-Felmayer G., Wachter H., Hentze M. W. Translational regulation via iron-responsive elements by the nitric oxide/NO-synthase pathway. EMBO J. 1993 Sep;12(9):3651–3657. doi: 10.1002/j.1460-2075.1993.tb06039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Andrews P. C., Hermodson M. A., Dixon J. E., Zalkin H. Cloning and structural characterization of porcine heart aconitase. J Biol Chem. 1990 Feb 15;265(5):2814–2821. [PubMed] [Google Scholar]
- Zheng L., Kennedy M. C., Beinert H., Zalkin H. Mutational analysis of active site residues in pig heart aconitase. J Biol Chem. 1992 Apr 15;267(11):7895–7903. [PubMed] [Google Scholar]
- Zheng L., Kennedy M. C., Blondin G. A., Beinert H., Zalkin H. Binding of cytosolic aconitase to the iron responsive element of porcine mitochondrial aconitase mRNA. Arch Biochem Biophys. 1992 Dec;299(2):356–360. doi: 10.1016/0003-9861(92)90287-7. [DOI] [PubMed] [Google Scholar]