Significance
Many influential models in economics, finance, and neurobiology assume risk preferences are a stable trait. In this study we find they are not. We examine the effects of chronic stress on financial risk taking by raising cortisol levels in volunteers over an 8-d period using individually tailored hydrocortisone regimens. We find that they become more risk-averse and that the overweighting of small probabilities becomes more exaggerated among men relative to women. We designed our protocol to maintain ecological validity: The increase in cortisol among participants replicated levels we had previously observed in real traders when faced with uncertainty and market volatility. Physiology-induced shifts in risk preferences may thus be a cause of market instability that has been hitherto overlooked by economists, risk managers, and central bankers.
Abstract
Risk taking is central to human activity. Consequently, it lies at the focal point of behavioral sciences such as neuroscience, economics, and finance. Many influential models from these sciences assume that financial risk preferences form a stable trait. Is this assumption justified and, if not, what causes the appetite for risk to fluctuate? We have previously found that traders experience a sustained increase in the stress hormone cortisol when the amount of uncertainty, in the form of market volatility, increases. Here we ask whether these elevated cortisol levels shift risk preferences. Using a double-blind, placebo-controlled, cross-over protocol we raised cortisol levels in volunteers over 8 d to the same extent previously observed in traders. We then tested for the utility and probability weighting functions underlying their risk taking and found that participants became more risk-averse. We also observed that the weighting of probabilities became more distorted among men relative to women. These results suggest that risk preferences are highly dynamic. Specifically, the stress response calibrates risk taking to our circumstances, reducing it in times of prolonged uncertainty, such as a financial crisis. Physiology-induced shifts in risk preferences may thus be an underappreciated cause of market instability.
Risk is inescapable. We take risks whenever we play a sport, enter a personal relationship, or choose a career. In the financial sector, the appetite for—and skill at—risk taking among those working on the world’s trading floors affects the stability of the market, the growth of the economy, and, through these effects, the health and well-being of the wider population. A scientific understanding of risk-taking behavior is therefore of pressing concern for individual investors, managers of financial institutions, and policy makers alike.
To address this concern, decision sciences such as economics and finance have placed risk taking at the very heart of their research agendas. Historically, many of these sciences have built theories upon the assumption that people make consistent choices based on relatively stable preferences through time. Such an assumption leads to transitive (i.e., noncontradictory, and therefore rational) choices and permits the building of models that are tractable and elegant. This assumption of stable risk preferences has been widely influential in economics and finance (1–3), and its influence has extended into certain branches of biology, such as evolutionary game theory (4). However, is this assumption justified? Are our risk preferences indeed stable?
Since the financial crisis of 2007–2009, evidence has suggested they are not. For example, a small number of empirical studies have shown that financial risk preferences do fluctuate (5). In addition, anecdotal evidence suggests that traders and investors experience a greater willingness to take risks during a rising market and a reduced willingness during a falling one. If risk preferences do indeed move in tandem with the market cycle, they may exaggerate the peaks and troughs, thereby contributing to financial instability. What physiological mechanisms could cause risk preferences to fluctuate in this manner?
The hormone cortisol, a glucocorticoid produced by the adrenal glands and one of the main stress hormones, might play a particularly important role here, because circulating levels of this hormone increase in situations of novelty, uncertainty, and uncontrollability (6–8). When in a novel environment or a state of uncertainty we do not know what to expect, and rising levels of cortisol help us marshal a preparatory stress response. The financial markets present a unique venue for conducting controlled studies of uncertainty and stress because uncertainty in this setting can be quantified precisely: The greater the uncertainty, the greater the volatility, measured objectively by the variance in securities prices. In a previous study we examined the effects of market volatility on a group of traders in the City of London and found that as volatility increased over an 8-d period the traders experienced a 68% increase in their mean daily cortisol levels (9).
An important question emerged from this fieldwork: Does the increase in cortisol stemming from market uncertainty in itself affect risk preferences? If so, do the effects of hypercortisolism differ between an acute (short-lived, i.e., minutes to hours) and a chronic (sustained, i.e., days to weeks) exposure?
Acute and chronic exposures to cortisol can have very different, and in many cases opposite, effects. Acute cortisol elevation has been found to increase physical arousal (10), aid in the recall of important memories (11), and, by interacting with dopaminergic pathways in the brain, promote learning, motivated behavior, and sensation seeking (12–14). Taken together these observations might suggest that an acute exposure to cortisol could promote risk taking, although there is only a limited amount of literature to support this contention (13, 14). By contrast, longer-term exposure to raised cortisol levels, as one might experience during periods of sustained uncertainty, can impair many physiological responses. For example, it can contribute to metabolic dysfunction (15) and immunological impairment (16); in the brain, it can impair attentional control and behavioral flexibility (17–19), and it can promote anxiety (20), depression (21), and learned helplessness (22). These latter effects could be expected to discourage risk taking.
We therefore developed the hypothesis that whereas an acute exposure to raised cortisol levels would have either no significant effect or, at most, modest ones on promoting risk taking, a chronic exposure would promote risk aversion.
To test this hypothesis, we conducted a randomized double-blind, placebo-controlled, cross-over study in which hydrocortisone—the pharmaceutical form of cortisol—or placebo was administered to 36 healthy volunteers, 20 men and 16 women, aged 20–36 y, over an 8-d period. Subjects were randomly assigned to one of three treatment schedules: (i) active–washout–placebo, (ii) placebo–washout–active, or (iii) placebo–washout–placebo (this last schedule serving as a control to test for learning effects during the study). A series of computer tasks were used to measure participants’ risk preferences under control conditions (placebo-treated), under conditions of acutely elevated cortisol, and under chronically elevated cortisol. This protocol permitted us to assess whether risk preferences remained stable and, if they did not, whether the fluctuations in risk preference were physiologically driven, specifically by changes in the level of circulating cortisol.
Results
Individualized Hydrocortisone Dosing Replicates Levels Found in Traders.
We sought to replicate the changes in cortisol levels observed in the field study of traders (9). Accordingly, we aimed to raise cortisol levels ∼68% above normal daily requirements for a period of 8 d.
In brief, participants visited the study site on the first and last day of each treatment schedule, provided saliva and venous blood samples, were fitted with a heart rate monitor, and were then dosed with either hydrocortisone or placebo. Dosing regimens were individualized for each subject using a well-established weight-based algorithm (23). After a 90-min wait, to allow capsule absorption, they provided further saliva and blood samples immediately before playing the computer tasks. For the next 7 d participants took hydrocortisone or placebo capsules at home. To ensure a sustained elevation of cortisol levels with loss of the normal circadian rhythm, participants took hydrocortisone tablets three times a day (Methods). They also collected saliva samples every second day, and these later allowed us to monitor serial changes in cortisol and to confirm compliance with the dosing regimens.
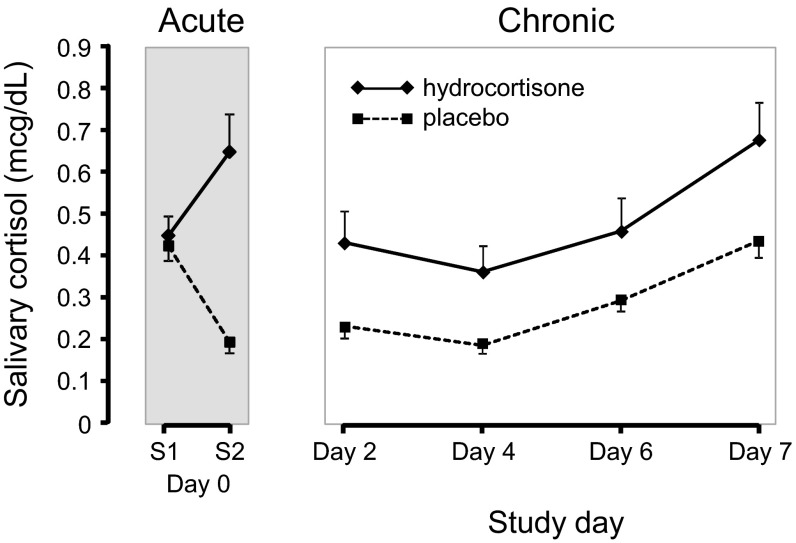
Compared with placebo, hydrocortisone treatment increased salivary cortisol levels acutely on the first day of the study by 235% (panel regression t test, t = 5.00, P < 0.0001, df = 35) and chronically over the next 8 d by 69% (t = 5.32, P < 0.0001, df = 35) (Fig. 1). Interestingly, in both the active and placebo arms of the study, cortisol levels on day 7 were higher than during the intervening days, possibly reflecting participants’ anticipation of the study tasks and the providing of blood samples. However, cortisol levels on day 7 in placebo-treated subjects were almost identical to those at baseline (day 0) (Fig. 1). Importantly, though, this upward trend from mid-week toward day 7 was observed with both active and placebo treatment curves, thus maintaining a relatively constant difference between them. Finally, we did not observe any significant difference between treatment schedules in men’s testosterone levels or women’s estradiol levels (Methods).
Fig. 1.
Cumulative exposure to cortisol over 8 d. The acute plot shows average salivary cortisol levels on the first day of the study, before (day 0, S1) and 90 min after (day 0, S2) the first dose of hydrocortisone or placebo. The decline in cortisol levels observed in the placebo arm of day 0 is consistent with the normal diurnal rhythm of cortisol secretion. The chronic plot shows average cortisol levels on every second day of the study (days 2, 4, and 6) and on day 7 during the hydrocortisone and placebo schedules. Results are shown as means ± SEMs.
Chronic Exposure to Raised Cortisol Is Not Associated with Changes in Autonomic Nervous System Function.
Cardiac monitoring allowed us to compare participants’ heart rate and heart rate variability between treatment schedules, and thereby to assess potential changes in autonomic nervous system function in response to raised cortisol levels. Heart rate variability (i.e., the acceleration and deceleration of the heart rate across the respiratory cycle) is an important indicator of the relative activation of the sympathetic to parasympathetic nervous systems: The greater the heart rate variability, the greater the parasympathetic control (via the vagus nerve) over the heart (24). In contrast, activation of the sympathetic nervous system, as occurs in stressful situations, reduces heart rate variability. During the study no significant changes in heart rate or heart rate variability were observed between treatment schedules (Methods).
The absence of any changes in these cardiac parameters or in sex steroid levels increases the likelihood that any differences we observed in risk preferences could be attributed to the elevated cortisol, and not to secondary effects stemming from changes in the autonomic nervous system or gonadal function.
Computerized Risk Tasks Show Greater Risk Aversion Under Chronic Hypercortisolism.
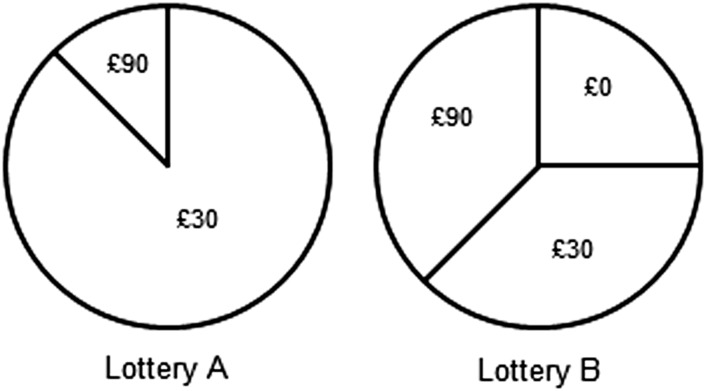
Participants played a computerized choice task that displayed lotteries offering real monetary payoffs (Fig. 2). We chose this specific task because it is a well-validated means of gauging changes in risk preferences (25, 26). More importantly, the lotteries in this task enabled us to separate the participants’ observed risk taking into two components: first, the way they valued the payouts of the lotteries (i.e., their utility functions) and, second, the way they weighted the probabilities of each payout when making their choices (i.e., their probability weighting functions) (26). We will consider each of these functions in turn.
Fig. 2.
A sample screen from the computerized choice task. Participants were presented with two lotteries at a time, from which they chose to play one. In this example, lottery A offers the player the certainty of some return: a high probability of receiving £30 and a low probability of £90. Lottery B offers a higher chance of receiving £90 but also some chance of receiving £0. The expected return of lottery B is high relative to lottery A, but so is its variance (i.e., the dispersion of possible payouts), which range from £90 to £0. Lottery B is thus the risky bet, and lottery A the safe bet.
To choose between two lotteries, a person could compare each lottery’s expected return, that is, its payout multiplied by its probability. A gamble that pays £100 with a 50% chance of winning has an expected return of £50, as does one with a £1,000 payout and a 5% chance of winning. Here the expected returns of the two bets are equal. One might assume people to be indifferent when choosing between these two bets. However, previous studies have found that people on average are not (27); they tend to choose the safer bet (i.e., the one offering the higher chance of winning, even if the actual payout is lower). They are, in short, risk-averse. The reason often proposed by psychologists and economists for this observation is that most people value the first £50 of winnings more than they do, say, the £50 that takes their winnings from £950 to £1,000. Stated in terms of what is called expected utility theory, most people derive a declining marginal utility from each increment in a bet’s payoff. Economists accordingly depict risk aversion graphically as a utility curve with a concave shape (Fig. 3A). The flatter this curve, the greater an individual’s risk aversion.
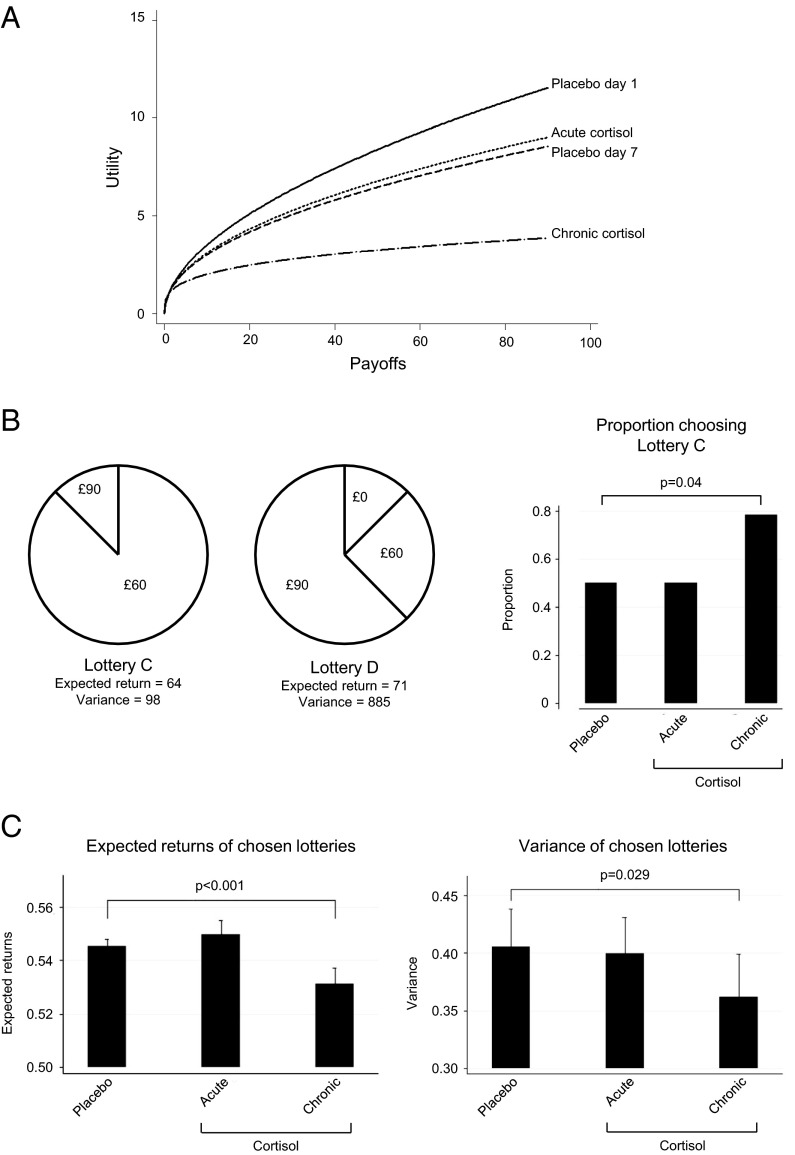
Fig. 3.
Effect of cortisol on risk aversion. (A) Changes in risk aversion as measured by the curvature of the utility function. Utility curves were averaged from all participants in the study. Utility can be thought of as the participants’ subjective valuation of the monetary outcomes. Most people derive a decreasing marginal utility from each increment in a bet’s payoff and are therefore risk-averse. The flatter their utility curve, the greater their risk aversion. (B) A sample lottery choice under placebo, acute, and chronically raised cortisol. In this sample choice, under chronically elevated cortisol, participants preferred the safer lottery C (P = 0.04). (C) Changes in the expected return and variance of the chosen lotteries. Under chronically raised cortisol participants chose safer lotteries (i.e., ones with lower payoffs and a lower variance of possible payoffs). Results are shown as means ± SEMs.
In the present study participants exhibited typical risk aversion, with the average utility curve under placebo conditions displaying a concave shape (Fig. 3A). Indeed, the estimated coefficient of risk aversion, which determines the curvature in the average utility function, was r = 0.50, a value consistent with estimates reported in previous empirical studies (28, 29) (Supporting Information).
We used a maximum likelihood estimation for all analyses of utility functions and Z-tests for tests of statistical significance. We found no evidence of learning effects on the computer tasks (Methods), nor did we find any significant difference in utility curves, and therefore risk aversion, between men and women (two-tailed Z-test, P = 0.572, n = 36). Previous work has suggested that women are more risk-averse than men (30). Although our results have the limitation of a relatively small sample size, they nonetheless provide no support for this conclusion.
Next, we compared utility curves, averaged from all participants, for each treatment schedule. We found no significant difference between the average utility curve when participants received an acute dose of cortisol and their curve when receiving placebo (two-tailed Z-test, P = 0.328, n = 36) (Fig. 3A). In marked contrast, when participants were exposed to a sustained elevation in cortisol over 8 d, their utility curves became more concave (i.e., they displayed a greater risk aversion) (two-tailed Z-test, P = 0.022, n = 36). This result is robust to other specifications of the utility function, such as the normalized constant risk aversion utility function (two-tailed Z-test, P < 0.001, n = 36) (Supporting Information).
An alternative way of analyzing the data is to look at the expected return and variance of the chosen lotteries, the variance being a measure of the dispersion of possible payouts. Under the influence of chronically elevated cortisol the participants preferred safer lotteries, in other words, ones with a lower expected return (linear regression; two-tailed t test, t = 4.54, P < 0.001, n = 36) and a lower variance of return (linear regression; two-tailed t test, t = 2.28, P = 0.029, n = 36) (Fig. 3 B and C) than they did under placebo.
The effect size of the observed change in risk aversion was large, with the coefficient of risk aversion dropping from 0.50 to 0.35, this 0.15 change representing one SD in the distribution of individual risk aversion observed in previous studies (28, 29). This effect size is perhaps more intuitively represented by translating it into changes in the “certainty equivalent” (i.e., the amount of cash a person would accept as a replacement for taking a gamble). For example, flipping a coin with a payoff of £100 for heads, £0 for tails, is a lottery (a gamble) with an expected return of £50. If a person would accept a guaranteed payment of, say, £30 or more instead of playing this lottery, then this amount is defined as his or her certainty equivalent. The difference between the £30 and the gamble’s £50 expected return is called the risk premium—the amount of extra return a person requires for taking risk—and is a measure of this person’s risk aversion. In our study, participants under placebo had a certainty equivalent of £25; under sustained cortisol exposure this number fell to £14, a decrease of 44%.
Moving from between-group results to within-subject measures, we looked at how variations in each participant’s cortisol levels predicted his or her risk aversion. We used within-subject variations in cortisol levels (relative to day 0) as a predictor in the model estimating risk aversion. We found that under chronic conditions higher levels of cortisol predicted greater risk aversion (two-tailed Z-test, P = 0.002, n = 28).
Chronic Hypercortisolism Distorts the Probability Weighting Function.
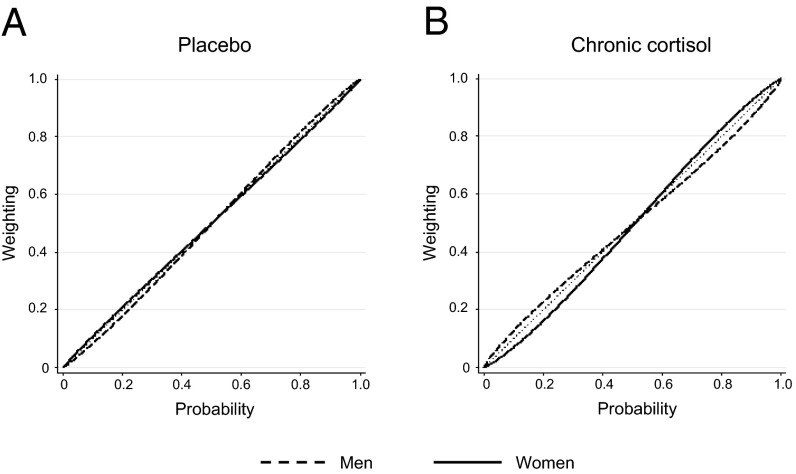
Next, we examined the effect of changing cortisol levels on each participant’s probability weighting function. This function shows how a person judges the significance of a probability when making choices. People tend to behave differently when presented with equal changes in probabilities. For example, when making decisions, people often treat an increase in probability from 5% to 10% as a more significant change than one from 40% to 45%. In general, there is a tendency to overweight small probabilities and underweight large probabilities. If we represent probabilities graphically, then a linear increase in objective probability from 0% to 100% can be plotted as a 45° line and the subjective weighting function as an S-shape, snaking around this 45° line (31, 32) (Fig. 4).
Fig. 4.
Probability weighting functions. The 45° line represents a linear increase in objective probability from 0% to 100%. The inverted S-shape curve centered on the 45° line represents the weighting of probabilities people display when making choices. When compared with placebo (A), under conditions of chronically elevated cortisol (B), men relative to women become more sensitive to small probabilities and less sensitive to large probabilities.
Examination of the participants’ probability weighting function revealed a significant sex interaction under conditions of chronically elevated cortisol, with the weighting of probabilities changing significantly in men (two-tailed Z-test, P = 0.028, n = 36), but not in women. Specifically, relative to women, male subjects exhibited greater sensitivity to small probabilities and less to large ones (two-tailed Z-test, P = 0.014) (Fig. 4) (see also refs. 14, 33, and 34).
The probability weighting function underlies an alternative model of risk aversion developed by behavioral economists, known as the rank-dependent expected utility function (32). It differs from the classical expected utility model because probability weights can adopt the S-shape depicted above and therefore have a nonlinear effect on the utility function (Supporting Information). We therefore repeated the analysis of risk aversion using the probability weighting function. We found that under chronically raised cortisol participants’ risk aversion, as measured by rank-dependent expected utility, increased significantly (two-tailed t test, P = 0.023, n = 36). When we reran the within-subjects analysis we found once again that participants’ cortisol levels predicted their rank-dependent risk aversion (two-tailed Z-test, P = 0.004, n = 28).
Discussion
We have found that an acute elevation of cortisol has no significant effect on financial risk taking whereas a sustained elevation leads to greater risk aversion (Fig. 3A), with study participants preferring lower expected return and lower-variance bets (Fig. 3 B and C).
In designing our protocol we have at all times tried to maintain ecological validity. We did so by combining field work with laboratory work. Specifically, we began, in a previous study, by observing a chronic elevation in cortisol levels among a group of traders in the City of London during a period of market volatility; we followed this, in the present study, by inducing among a group of volunteers the same cortisol increases, for the same period, as observed in the traders (9). The field work ensured that we were studying physiology and behavior that do in fact occur in the financial world; the present laboratory-based study permitted us to analyze in a more controlled manner the effects of these hormonal changes on risk preferences.
There are two other aspects of our dosing regimen that merit consideration. First, we have targeted changes in cortisol levels that fall within a normal physiological range, thus avoiding the pitfalls of extrapolating to normal subjects findings that may be relevant only to subjects exposed to supraphysiologic glucocorticoid concentrations. Second, our dosing protocol tailored the hydrocortisone dose to each individual, rather than using a “one-size-fits-all” approach. Dosing was individualized by weight, and the hydrocortisone was delivered three times a day to suppress cortisol’s normal diurnal rhythm. We adapted a protocol used with patients suffering adrenal failure, and it proved remarkably accurate: The traders followed in the previous study experienced a 68% increase in cortisol levels over an 8-d period, and in the current study we raised participants’ cortisol levels by 69% over the same period (Fig. 1).
The effects of chronic hypercortisolism on our participants were large, so we should briefly consider the likely neural mechanisms through which cortisol could have exerted its effects. Previous studies have shown that glucocorticoids have dramatic effects on the brain. In the hippocampus, chronically elevated glucocorticoids can reduce spine density, suppress neurogenesis, and reduce hippocampal volume (21, 35). In the amygdala, glucocorticoids can cause dendritic arborization (36) and promote corticotrophin-releasing hormone gene expression, with a resulting anxiety (20, 37). Together these effects are thought to underlie the observed tendency of chronically stressed individuals to develop a selective attention to negative precedents, to find threat where none exists, and even to experience depression and learned helplessness (20–22). Although full morphological changes in the brain occur over a long time period, many of the central effects of elevated cortisol, even over an 8-d period, could begin to promote an aversion to uncertainty and potential monetary loss. As an aside, our findings also suggest that risk aversion could well be an unwanted consequence of medically prescribed long-term synthetic glucocorticoid therapy (e.g., prednisolone or dexamethasone).
The prefrontal cortex (PFC) is also an important target for glucocorticoids. Chronically elevated glucocorticoids, acting on the PFC, can impair working memory, reduce attentional control, and limit behavioral flexibility (17–19). These effects on the PFC raise the possibility that chronic stress may shift a person’s decision making from goal-directed processes to more habitual ones (38, 39), and it may reduce their motivation and ability to consider novel actions (40). Risk taking requires that we search across a range of opportunities, but stress, by limiting attentional shift and behavioral flexibility, may constrain our choices to those that are familiar and require the least amount of search. In the financial markets, during times of crisis participants display a strong preference for familiar securities, such as government bonds and home markets.
Our findings have relevance for economics and finance. It is widely assumed in these sciences that risk preferences, or more precisely the utility functions underlying risk preferences, remain largely constant through time (1–3). We find, on the contrary, that financial risk preferences shift, and do so substantially. We further find that these shifts occur under the influence of a physiological mechanism, specifically the stress response. Economics, and the financial world more generally, could therefore benefit from considering the effects of physiology on risk taking. Existing research on risk taking proceeds largely within a paradigm of cognitive processing. However, when people take risk, including financial risk, they do more than just think about it—they prepare for it physically (41). Their endocrine, metabolic, and cardiovascular systems prime them for impending activity, and these changes then feedback on the brain (42, 43), calibrating their appetite for risk to current circumstances (34). Our findings suggest that when people are stressed by chronic uncertainty or uncontrollable threat their endocrine systems discourage them from taking risk.
It is worth pointing out that the rise in cortisol we observed in the trading floor study, and replicated among participants in the current study, was in line with that found in other studies of moderately stressed individuals (44). We should add, however, that such a cortisol increment is relatively modest compared with the increases that can be observed in individuals subjected to major physiological stressors such as trauma or anesthesia/surgery, where a 400–500% rise is not uncommon (45). It is difficult to say whether cortisol levels could rise this high in the financial world. However, we do know that during the credit crisis of 2007–2009 volatility in, for example, US equities spiked from 12% to over 70%. It seems reasonable to assume that such historically high levels of uncertainty would have caused stress hormones to rise substantially, and for a much longer period than we observed in our study. It is therefore possible that rising stress hormones contributed to the wide-spread risk aversion during the crisis that came to be known as “irrational pessimism.”
Indeed, our findings point to an alternative model of risk taking. In it risk preferences are not stable; rather, they are highly dynamic. Such a model might help explain why the risk premium on equities rises and falls with volatility (46), and why the appetite for risk among the financial community seems to expand during a rising market, and contract during a declining one. Critically, if cortisol responds powerfully to increases in uncertainty and volatility, and volatility rises most strongly during a financial crisis, then risk taking may decrease just when the economy needs it most: when markets are crashing and need traders and investors to buy distressed assets. Physiologically driven shifts in risk preferences may thus be a source of financial market instability that has been overlooked by economists, risk managers, and central bankers alike.
Methods
Hydrocortisone Dosing Algorithms.
Hydrocortisone dosages were calculated using a weight-based algorithm, which allows the prediction of total daily hydrocortisone requirements in subjects who have had both adrenal glands surgically removed or suffered total adrenal failure (23). In healthy subjects, cortisol levels rise just after waking and decline over the day, but in chronically stressed individuals levels remain high throughout the day. To mimic the diurnal cortisol pattern of a chronically stressed individual, we required subjects to take their hydrocortisone in three divided doses, at 7:00 AM, 1:00 PM, and 7:00 PM. Hydrocortisone (5 mg) and placebo capsules were manufactured to be indistinguishable from each other (Pharmacy Technical Services, St. George’s Hospital, NHS Trust). Participants kept a tablet diary and returned any unused tablets at the end of each study phase; together with monitoring of salivary cortisol levels, these were used to confirm compliance with the prescribed regimen.
Serum and Saliva Collection.
Whole blood (5mL) was taken into serum tubes following standard venipuncture. Samples collected on days 0 and 7 of each treatment schedule were centrifuged and separated within 30 min and stored at −80 °C. Saliva (2 mL) was collected by passive drool using Greiner cryovials according to the manufacturer’s instructions. Participants kept saliva samples collected on days 2, 4, and 6 of each treatment schedule in a refrigerator at 4 °C and returned these on day 7. Following centrifugation these samples were also stored at −80 °C.
All samples were batch-analyzed. Salivary cortisol was measured using a competitive immunoassay (Salimetrics) with each measurement performed in duplicate. Serum cortisol was measured in the Cambridge University Hospitals NHS Foundation Trust Clinical Pathology Accreditation laboratory using a competitive immunoassay (ADVIA Centaur; Siemens). The intraassay coefficient of variation in all assays was <4%.
Our results were robust to the use of salivary or serum measures. However, because we collected only salivary samples between participants’ visits to the test site, we have reported salivary measures in the main text.
Cardiac Monitoring of Heart Rate and Heart-Rate Variability.
Participants were fitted with a two-lead portable cardiac monitor (Actiwave Cardio; CamNTech Ltd) at the start of each visit to the study center. Heart rate (HR) and interbeat intervals were recorded before and during the four times the participants played the lottery tasks. Heart-rate variability (HRV) was assessed using the square root of the mean of the sum of the squares of differences between adjacent NN intervals. We compared HR and HRV data both by treatment (i.e., placebo or active) and by task using a random effects generalized least squares regression. We found no effects of treatment schedule (HR P = 0.3798, HRV P = 0.4971) or task (HR P = 0.4263, HRV P = 0.7153) or of treatment × task (HR P = 0.6183, HRV P = 0.8916).
Sampling of Sex Steroids.
We sampled sex steroids on days 0, 2, 4, 6, and 7. We did not specifically control for the phase of each female participant’s menstrual cycle, but we found that mean estradiol levels between treatment schedules were not significantly different (t = 1.59, P = 0.138, n = 15). Men’s testosterone levels also did not differ (t = 1.09 P = 0.296, n = 13).
Recruitment and Monetary Payments.
Participants were recruited from the general population by means of advertisements. Randomization into treatment schedules (discussed above) was performed separately for men and women to ensure an approximately equal number in each treatment schedule. Participants were paid £100 to take part in the study. They were also offered an additional payment, based on their risk choices during the tasks. This variable payment had a maximum value of £165. Participants could thus receive up to a total of £265. To determine the variable portion of the payment, a single lottery choice made by each participant was chosen at random. All subjects signed an informed consent form. The study was approved by the ethics committee of the School of Biological Sciences at the University of Cambridge.
Computer Tasks.
At each visit, participants were presented with 45 different lottery pairs and took on average 25–30 min to complete the full series. Because each participant played the task on four separate days, they made a total of 4 × 45 = 180 lottery choices. Results of the computerized risk tasks performed on the first day of the study were used to gauge the effects of an acute elevation of cortisol, and those on the seventh day the effects of a chronic elevation.
Statistical Analysis.
We did not observe any learning effects on risk preferences. A group of eight subjects were assigned to the placebo–washout–placebo arm and we did not observe any significant changes in their risk preferences over time (two-tailed Z-test, P = 0.787, n = 8). We also did not find any significant differences in risk preferences on days 0 and 7 of the placebo phase of each treatment schedule, whether this was on the placebo–washout–active schedule (two-tailed Z-test, P = 0.311, n = 14) or the active–washout–placebo schedule (two-tailed Z-test, P = 0.459, n = 14).
Coefficients’ SEs were computed using a clustered robust matrix of variance to control for the fact that participants were making several choices and that different choices from a given individual cannot be considered independent (25). In all statistical tests, however, the number N represented the number of participants (and therefore of clusters), not the number of choices. By default, we used between-subject analysis, including all our subjects, with n = 36. We complemented these analyses with some within-subject estimation, which involved dropping the eight participants who were assigned to the placebo–placebo arm, giving us n = 28.
All tests of significance for the parameters of the utility model are Z-tests derived from the maximum likelihood estimations of the model with n = 36 participants. Because these tests are asymptotic, we indicated the number of participants (i.e., cluster of observations) with which the tests are computed, to clearly indicate the relative sample size and give an indication of the power of the tests. We used a regression model to measure the statistical significance of the differences in expected return and variance of the chosen lotteries as observed in Fig. 3C. This statistical method also allowed us to use a clustered robust matrix of variance. All tests of significance for the parameters estimated by these linear regressions are Student t tests with n = 36 participants. We reported test statistics for the t tests and the number of participants (clusters of observations).
Supplementary Material
Acknowledgments
We thank Sheila Skidmore for technical and administrative support, Søren Brage for processing the heart-rate data, and Alexis Barr for critical readings of the manuscript. This research was supported by a Programme Grant from the Economic and Social Research Council. A.S.P. and M.G. are supported by the National Institute for Health Research Cambridge Biomedical Research Centre.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1317908111/-/DCSupplemental.
References
- 1.Luigino B, Sugden R. The road not taken: How psychology was removed from economics, and how it might be brought back. Econ J. 2007;117(516):146–173. [Google Scholar]
- 2.Gai P, Vause N. Measuring investors' risk appetite. Int J Cent Bank. 2006;2:167–188. [Google Scholar]
- 3. European Central Bank (2007) Measuring investors' risk appetite. Financial Stability Review, June: 166–171.
- 4.Samuelson L. Evolutionary Games and Equilibrium Selection. Vol 1. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- 5.Guiso L, Sapienza P, Zingales L. 2013. Time varying risk aversion. NBER Working Paper No. 19284 (National Bureau of Economic Research, Cambridge, MA)
- 6.Mason JW. A review of psychoendocrine research on the pituitary-adrenal cortical system. Psychosom Med. 1968;30(5):576–607. [PubMed] [Google Scholar]
- 7.Hennessy J, Levine S. Stress, arousal, and the pituitary-adrenal system: A psychoendocrine hypothesis. In: Sprague J, Epstein A, editors. Progress in Psychobiology and Physiological Psychology. 8th Ed. San Diego: Academic; 1979. pp. 133–178. [Google Scholar]
- 8.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 9.Coates JM, Herbert J. Endogenous steroids and financial risk taking on a London trading floor. Proc Natl Acad Sci USA. 2008;105(16):6167–6172. doi: 10.1073/pnas.0704025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dallman M, Bhatnagar S. Comprehensive Physiology. New York: Wiley; 2010. Chronic stress and energy balance: Role of the hypothalamo-pituitary-adrenal axis; pp. 179–210. [Google Scholar]
- 11.Lupien SJ, et al. The modulatory effects of corticosteroids on cognition: Studies in young human populations. Psychoneuroendocrinology. 2002;27(3):401–416. doi: 10.1016/s0306-4530(01)00061-0. [DOI] [PubMed] [Google Scholar]
- 12.Piazza PV, et al. Corticosterone in the range of stress-induced levels possesses reinforcing properties: Implications for sensation-seeking behaviors. Proc Natl Acad Sci USA. 1993;90(24):11738–11742. doi: 10.1073/pnas.90.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Putman P, Antypa N, Crysovergi P, van der Does WA. Exogenous cortisol acutely influences motivated decision making in healthy young men. Psychopharmacology (Berl) 2010;208(2):257–263. doi: 10.1007/s00213-009-1725-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Bos R, Harteveld M, Stoop H. Stress and decision-making in humans: Performance is related to cortisol reactivity, albeit differently in men and women. Psychoneuroendocrinology. 2009;34(10):1449–1458. doi: 10.1016/j.psyneuen.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. Clinical review: The pathogenetic role of cortisol in the metabolic syndrome: A hypothesis. J Clin Endocrinol Metab. 2009;94(8):2692–2701. doi: 10.1210/jc.2009-0370. [DOI] [PubMed] [Google Scholar]
- 16.McEwen BS, et al. The role of adrenocorticoids as modulators of immune function in health and disease: Neural, endocrine and immune interactions. Brain Res Brain Res Rev. 1997;23(1-2):79–133. doi: 10.1016/s0165-0173(96)00012-4. [DOI] [PubMed] [Google Scholar]
- 17.McEwen BS, Morrison JH. The brain on stress: Vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79(1):16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radley JJ, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125(1):1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci USA. 2009;106(3):912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korte SM. Corticosteroids in relation to fear, anxiety and psychopathology. Neurosci Biobehav Rev. 2001;25(2):117–142. doi: 10.1016/s0149-7634(01)00002-1. [DOI] [PubMed] [Google Scholar]
- 21.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57(10):925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 22.Kademian SM, Bignante AE, Lardone P, McEwen BS, Volosin M. Biphasic effects of adrenal steroids on learned helplessness behavior induced by inescapable shock. Neuropsychopharmacology. 2005;30(1):58–66. doi: 10.1038/sj.npp.1300577. [DOI] [PubMed] [Google Scholar]
- 23.Mah PM, et al. Weight-related dosing, timing and monitoring hydrocortisone replacement therapy in patients with adrenal insufficiency. Clin Endocrinol (Oxf) 2004;61(3):367–375. doi: 10.1111/j.1365-2265.2004.02106.x. [DOI] [PubMed] [Google Scholar]
- 24.Porges SW. The polyvagal perspective. Biol Psychol. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hey J, Orme C. Investigating generalizations of expected utility theory using experimental data. Econometrica. 1994;62(6):1291–1326. [Google Scholar]
- 26.Conte A, Hey J, Moffatt P. Mixture models of choice under risk. J Econom. 2011;162(1):79–88. [Google Scholar]
- 27.Bernoulli D. Exposition of a new theory on the measurement of risk. Econometrica. 1954;22(1):23–36. [Google Scholar]
- 28.Holt C, Laury S. Risk aversion and incentive effects. Am Econ Rev. 2002;92(5):1644–1655. [Google Scholar]
- 29.Harrison G, Lau M, Rutström E. Estimating risk attitudes in Denmark: A field experiment. Scand J Econ. 2007;109(2):341–368. [Google Scholar]
- 30.Eckel C, Grossman P. Men, women and risk aversion: Experimental evidence. In: Plott C, Smith V, editors. Handbook of Experimental Economics Results. Vol 1. Amsterdam: Elsevier; 2008. pp. 1061–1073. [Google Scholar]
- 31.Kahneman D, Tversky A. Prospect theory: An analysis of decision under risk. Econometrica. 1979;47(2):263–291. [Google Scholar]
- 32.Wakker P. Prospect Theory: For Risk and Ambiguity. Cambridge, UK: Cambridge Univ Press; 2010. [Google Scholar]
- 33.Lighthall NR, et al. Gender differences in reward-related decision processing under stress. Soc Cogn Affect Neurosci. 2012;7(4):476–484. doi: 10.1093/scan/nsr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coates JM, Gurnell M, Sarnyai Z. From molecule to market: Steroid hormones and financial risk-taking. Philos Trans R Soc Lond B Biol Sci. 2010;365(1538):331–343. doi: 10.1098/rstb.2009.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61(2):203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 36.Mitra R, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci USA. 2008;105(14):5573–5578. doi: 10.1073/pnas.0705615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corodimas KP, LeDoux JE, Gold PW, Schulkin J. Corticosterone potentiation of conditioned fear in rats. Ann N Y Acad Sci. 1994;746:392–393. doi: 10.1111/j.1749-6632.1994.tb39264.x. [DOI] [PubMed] [Google Scholar]
- 38.Dias-Ferreira E, et al. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325(5940):621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- 39.Schwabe L, Wolf OT. Stress prompts habit behavior in humans. J Neurosci. 2009;29(22):7191–7198. doi: 10.1523/JNEUROSCI.0979-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10(6):410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coates J. The Hour Between Dog and Wolf: How Risk Taking Transforms Us, Body and Mind. New York: Penguin-Random House; 2012. [Google Scholar]
- 42.Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 43.Bechara A, Damasio A. The somatic marker hypothesis: A neural theory of economic decision. Games Econ Behav. 2005;52:336–372. [Google Scholar]
- 44.Schulz P, Kirschbaum C, Prüßner J, Hellhammer D. Increased free cortisol secretion after awakening in chronically stressed individuals due to work overload. Stress Med. 1998;14(2):91–97. [Google Scholar]
- 45.Nicholson G, Burrin JM, Hall GM. Peri-operative steroid supplementation. Anaesthesia. 1998;53(11):1091–1104. doi: 10.1046/j.1365-2044.1998.00578.x. [DOI] [PubMed] [Google Scholar]
- 46.Graham J, Harvey C. 2009. The equity risk premium amid a global financial crisis. Available at http://ssrn.com/abstract=1405459.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.