Significance
Understanding how widespread human-induced global changes are affecting the movement and dispersal of organisms is critical for maintaining species diversity and making sound land management decisions. In contrast with animal-dispersed species, little is known about how wind-dispersed species are affected by conservation strategies such as corridors. We use a combination of mechanistic models and field data to show that habitat corridors alter wind dynamics in a way that promotes seed dispersal and appears to increase plant diversity. Wind direction also interacts with landscape orientation to determine when corridors can provide connectivity.
Keywords: diversity, plant community, habitat structure, reserve design, long-distance dispersal
Abstract
Determining how widespread human-induced changes such as habitat loss, landscape fragmentation, and climate instability affect populations, communities, and ecosystems is one of the most pressing environmental challenges. Critical to this challenge is understanding how these changes are affecting the movement abilities and dispersal trajectories of organisms and what role conservation planning can play in promoting movement among remaining fragments of suitable habitat. Whereas evidence is mounting for how conservation strategies such as corridors impact animal movement, virtually nothing is known for species dispersed by wind, which are often mistakenly assumed to not be limited by dispersal. Here, we combine mechanistic dispersal models, wind measurements, and seed releases in a large-scale experimental landscape to show that habitat corridors affect wind dynamics and seed dispersal by redirecting and bellowing airflow and by increasing the likelihood of seed uplift. Wind direction interacts with landscape orientation to determine when corridors provide connectivity. Our results predict positive impacts of connectivity and patch shape on species richness of wind-dispersed plants, which we empirically illustrate using 12 y of data from our experimental landscapes. We conclude that habitat fragmentation and corridors strongly impact the movement of wind-dispersed species, which has community-level consequences.
Habitat loss and fragmentation sever movement pathways, posing major risks to population persistence and community diversity (1). As a result, landscape connectivity––the degree to which landscapes facilitate movement––is receiving growing attention as a means to increase long-distance dispersal (LDD) of individuals and persistence of species under global change (2, 3). In particular, habitat corridors, or linear strips of habitat connecting otherwise isolated habitat patches, have become one of the most commonly applied conservation tools (4, 5). However, corridors (and connectivity more generally) are almost exclusively considered a conservation strategy for animals or animal-dispersed organisms, not for the great diversity of species that are passively transported by wind.
Wind is a frequent means of movement for many organisms, including plant seeds, pollen, spores, insects, and pathogens (6–8). The changes in habitat structure (e.g., edge creation) that accompany habitat fragmentation and connectivity may strongly influence the flow of air, particularly the amount of vertical uplifting––a critical factor known to drive LDD of seeds by wind (9–11). Over the past decade, our mechanistic understanding of wind-driven seed dispersal has substantially increased (12, 13) and models have begun to incorporate landscape heterogeneity (11, 14–18). Combining mechanistic insight from dispersal models with dispersal patterns from real landscapes is the next frontier in understanding dispersal in fragmented habitats (12, 19–21).
Gaining a mechanistic understanding of how habitat fragmentation and connectivity affect wind dispersal (and dispersal in general), however, has proven challenging: whereas short-distance dispersal events can be empirically quantified, LDD events––the unusually long movements accomplished by only a small fraction of individuals in a population (Materials and Methods and SI Materials and Methods)––are difficult to detect empirically. As a consequence, LDD events are typically predicted using mechanistic models, but these predictions are rarely empirically tested (19).
Here, we surmount these challenges by combining predictions for wind dynamics and seed dispersal patterns from an advanced fluid-dynamics model with empirical wind and seed dispersal data from a unique large-scale landscape fragmentation experiment that controls for patch area, shape, and connectivity. This comprehensive approach allows for evaluation of habitat fragmentation and connectivity impacts on both short- and long-distance dispersal of seeds by wind. Our work reveals several revealing mechanisms by which landscape structure impacts the movement of wind-dispersed plants, and demonstrates how landscape effects on wind can have consequences for plant community diversity.
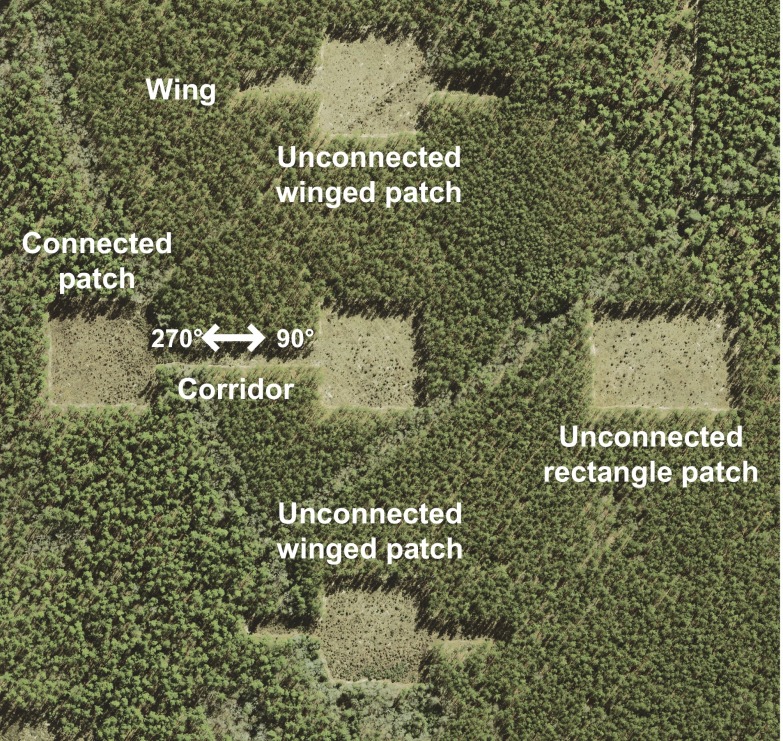
We developed and tested our model predictions within a large-scale experimental landscape (∼50 ha) at the Savannah River Site, SC (Fig. 1) comprising 1.375 ha connected and unconnected open-habitat longleaf pine savanna patches surrounded by mature pine plantation. “Connected” patches test for connectivity effects whereas unconnected “rectangular” and “winged” patches test for patch area and shape effects, respectively (Materials and Methods). Longleaf pine savanna supports some of the most diverse plant communities in the world (22, 23) and is typified in part by a large proportion of wind-dispersed plant species (22). At our study site, for example, wind-dispersed species constitute the most common plant dispersal mode.
Fig. 1.
Experimental landscape at Savannah River Site, SC. Patch types are connected (with a corridor), unconnected winged, or unconnected rectangular. The long axis of the corridor is aligned along 90° and 270° to correspond with Figs. 2 and 3.
To understand how connectivity and habitat fragmentation affect wind and seed dispersal dynamics, we applied and tested a mechanistic model of wind-driven dispersal in our experimental landscape. We used the Regional Atmospheric Modeling System-based Forest Large Eddy Simulation model (RAFLES; see Materials and Methods, ref. 24, and Fig. S1), a mechanistic model that explicitly incorporates 3D heterogeneous habitat structure at meter-scale resolution. We tested the model’s predictions by empirically measuring wind dynamics and LDD patterns of experimentally released artificial seeds in our highly controlled landscape. We also tested the predicted implications of these model results for plant community dynamics by evaluating changes in species richness of wind-dispersed plants among our experimental patch types across 12 y of community development.
Results
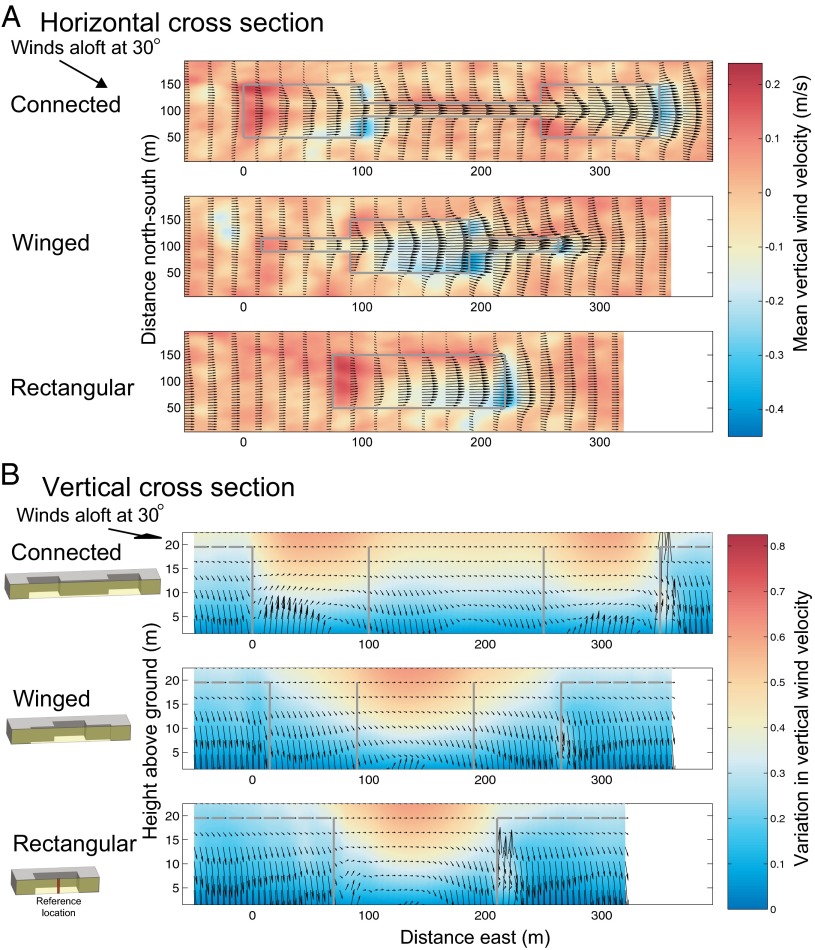
The model predicts that, in general, wind speeds accelerate in habitat openings relative to the surrounding closed forest, causing increased turbulence and uplift probabilities (Fig. 2; Figs. S2–S4). It also illuminates three distinct effects of habitat openings on wind dynamics (Fig. 2)––redirecting, bellowing, and ejection hotspots (these terms are described below). All of these effects are affected by corridors and the shapes of patches, altering seed dispersal patterns (Fig. 3 A and B).
Fig. 2.
Cross-sections of wind dynamics and seed ejection probabilities when overall wind forcing aloft was 30° relative to the long axis of each patch (see SI Materials and Methods for 0°, 60°, and 90° cases). An arrow at the top left of each panel marks the mean, above-canopy wind direction. Each figure is a visualization of the results from the full simulation domain (Fig. S1) and is an average of results from two identical patches. (A) The redirecting effect (shown by arrows pointing along the major axis of the landscape structures rather than the 30° forcing wind aloft) and the bellowing effect (shown by the high wind speeds at the right end of the corridor). The horizontal cross-section below the canopy top is at 13.5 m above ground. Solid gray lines are edges of patches. The color bar shows mean vertical wind speed (red = up, blue = down). Arrows correspond to horizontal wind speeds (longer arrows indicate faster wind speeds with maximal wind speed of 2.75 m/s) and directions. (B) Ejection hotspots (shown by arrows indicating uplift probability, on a log scale, relative to the uplift probability at the same height at a reference location at the center of the rectangular patch depicted by a red solid vertical bar). Vertical cross-sections are along patch centers (yellow translucent rectangles in icons to the left of each panel depict where the vertical section was taken). Solid gray lines are patch edges and dashed gray lines are the mean canopy height of 22 m. Color bar represents the SD of vertical wind speed, where strong variation in vertical wind speed (red) creates ejection hotspots. Upward-pointing arrows mark locations where the probability is higher than the reference area, downward-pointing arrows where it is lower. A slight forward tilt for upward and backward tilt to downward arrows was added to make the arrows more easily distinguishable from each other. Details in SI Materials and Methods.
Fig. 3.
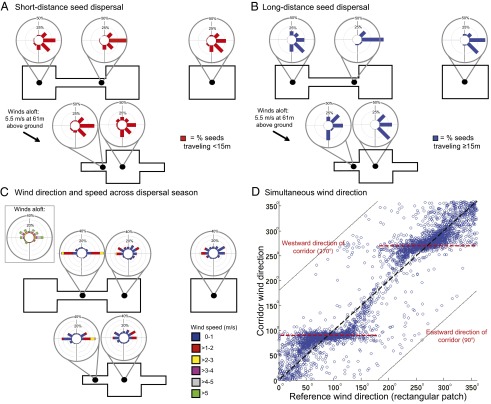
(A) Direction of short-distance seed movements from key locations (dots) within each patch. The mean directionality of seeds released in the corridor significantly differed from all other locations (Watson–Williams F tests, P ≤ 0.05). (B) Direction of long-distance seed movements from key locations (dots). Mean directionality was significantly different between the corridor and all other locations except the wing (P ≤ 0.05 and P = 0.43, respectively). (C) Wind directions and speeds over the entire dispersal season from October to December 2008 at key locations (dots) within each patch. (D) Simultaneous redirection of wind by corridors compared with reference wind conditions in the area (as measured in the center of the rectangular patch). Over a wide range of reference wind directions (40–130° and 210–300°), wind tends to orient through the corridor (90° or 270°, dashed red line). Each point represents a 30-min average of wind directions within long-term wind data collected over two dispersal seasons. The experimental landscape and data in this figure were rotated such that the long axis of the corridor is aligned along 90° and 270°, matching the simulation domain and facilitating visualization of the results (the long axis of the corridor in the field is actually aligned along 246° and 66°).
First, wind direction in all patches rotates toward the long axis of each patch, in line with the corridor or wings. This “redirecting” effect causes wind to converge into the corridor or wing when either is located on the downwind side of a patch [e.g., Fig. 2A: wind blows directly along the long axes of the patches (eastward) even though the above-canopy wind forcing was at 30° toward the southeast, an effect that can begin even in the forested matrix before the wind reaches the patches; Materials and Methods]. The spatial extent of this effect is longest in connected patches, intermediate in winged patches, and shortest in rectangular patches (Fig. 2A), suggesting that seeds are moved farthest among connected patches, intermediate distances in winged patches, and least in rectangular patches. The redirection effect is strongest close to the ground, affects the wind direction in the matrix near patches, and is weaker above the canopy.
Second, a “bellowing” effect occurs when winds accelerate inside a patch, leading to relatively strong winds at the downwind end of corridors and wings (e.g., arrows in Fig. 2A). The strongest enhancement of wind speed is at the center of wings and corridors because wind in the patches experiences less drag relative to wind at the same height in the surrounding forest matrix due to the lack of tree-canopy obstacles (25). Although the bellowing effect occurs in both winged and connected patches, it only promotes between-patch movement for connected patches (Fig. 2A).
Third, increased “ejection hotspots”––locations in which seeds have a relatively high probability of being uplifted (sensu ref. 11)––are more likely to occur in connected than unconnected patches (Fig. 2B: upward arrows around the centers of connected patches at ∼40 m and ∼300 m downwind are much stronger than those in the centers of winged and rectangular patches at ∼140 m downwind). Ejection hotspots occur when increased turbulence, and particularly increased variation of the vertical component of wind speed, results in increased updrafts and downdrafts such that the former leads to an increased probability of seeds being transported long distances (9–11). This effect, in combination with the redirection and bellowing effects, makes it more likely that seeds will move among connected than unconnected patches and that corridors will promote LDD of wind-dispersed seeds among open-habitat patches.
Empirically determined wind dynamics and seed dispersal patterns in our experimental landscapes (Materials and Methods) provide evidence consistent with these model predictions. Across a dispersal season, corridors increased wind speeds and rotated the wind direction to be in line with the long axis of the patch, in the same direction as the corridors and wings (Fig. 3C). Wind data collected simultaneously in a corridor and reference patch (rectangular patch) across two years (blue dots, Fig. 3D) show that wind directions consistently rotate to be in line with the corridor (Fig. 3D; red horizontal lines show corridor orientation shown in Fig. 1). Experimental releases of 5,400 artificial seeds (with a terminal velocity similar to native wind-dispersed species; Materials and Methods) corroborate these results. In five separate release events, 300 seeds were simultaneously released under the same overall meteorological conditions from different locations within our landscapes (Table S1). A greater proportion of seeds dispersed in the same direction as the corridor when released near the corridor entrance than when released elsewhere (Fig. 3 A and B). In the winged patch with high edge-to-area ratios, but no connection to other patches, similar increases in wind speeds and redirection were also observed but to a lesser degree than in the corridor of connected patches (Fig. 3C).
Overall, modeled and observed dispersal kernels from all release locations were significantly correlated (Materials and Methods, r2 = 0.50–0.94, the slopes of modeled vs. observed data were not significantly different from 1, Table S1 and Fig. S5), suggesting that the model captures the underlying wind and seed dispersal processes in our study landscape and provides reasonable predictions for seed dispersal patterns.
Our modeled and empirical results show that empirically determined LDD is indeed greatest among connected patches, moderately high in winged patches, and lowest in rectangular patches. Higher dispersal should yield greater species richness of wind-dispersed species in connected patches, especially when aligned with the predominant winds, by reducing extinction rates and increasing rescue effects (26–28). First, the redirection effect should promote colonization of seeds dispersing into both connected and, to a lesser extent, winged patches. Second, the bellowing effect should further increase colonization rates between connected patches. Third, the ejection hotspot effect, when considered together with the two above effects, predicts that seeds are more likely to be uplifted and therefore more likely to be redirected and bellowed among connected patches and, to a lesser degree, redirected into winged patches.
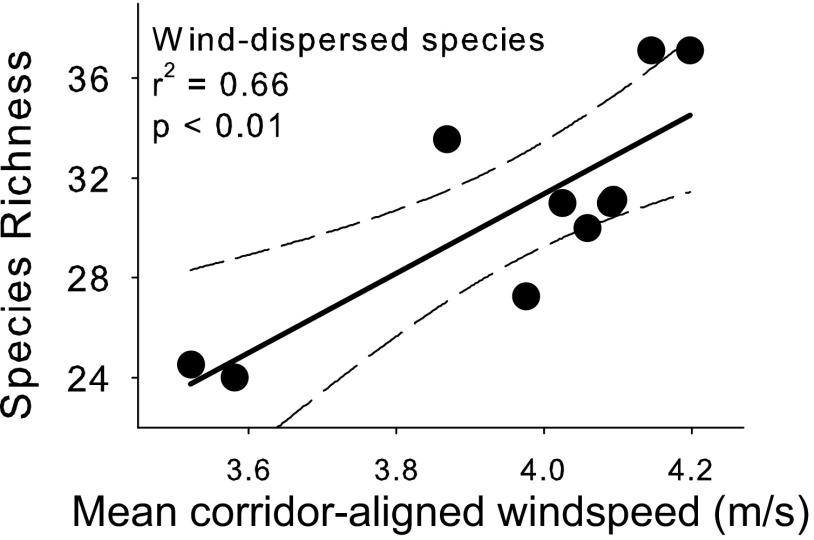
Plant community richness within our fragmentation experiment over the past 12 y matches the prediction of increased species richness in connected patches, moderately high species richness in winged patches, and lowest species richness in rectangular patches. Connected patches have gained 15% more wind-dispersed species than either unconnected winged or rectangular patches [Fig. S6; repeated-measures analysis of covariance (ANCOVA), F1,46.9 = 19.24, P = 0.002] and winged patches have gained 5% more wind-dispersed species than rectangular patches (Fig. S6; repeated-measures ANCOVA, F1,47.1 = 6.23, P = 0.016). Furthermore, when corridors are aligned with the predominant wind direction, their impact on wind-dispersed plant species richness increases (Fig. 4). Because our experimental landscapes control for edge effects associated with corridor creation (i.e., “patch shape;” connected and winged patches have nearly identical edge-to-area ratios), we have controlled for the dominant landscape factor affecting differences in microclimate, such that it is highly unlikely that microclimate differences are responsible for the shifts in community composition of wind-dispersed species that we observed. Once species colonize, we know from prior studies that corridors affect species interactions such as pollination (29) and seed predation (30), which may help to maintain elevated diversity in connected patches. Colonization sets an upper limit on the size of the local species pool, but it remains to be determined how much dispersal, relative to other factors, contributes to the long-term maintenance of elevated species richness.
Fig. 4.
Richness of wind-dispersed species is significantly related to the average velocity of wind during the seed dispersal season (September–January) that is aligned within 30° of the angle of the long axis of the corridor from a central patch to the connected patch. Each point is the mean species richness from 2001 to 2010 for the connected patch in each of 10 experimental blocks. Solid line represents the least-squares fit to the data; dashed lines are 95% confidence intervals. Details in SI Materials and Methods.
Discussion
By coupling mechanistic models, field experiments, and empirical wind measurements, in conjunction with large-scale experimental landscapes, we provide results that have substantial implications for understanding plant movement. Our findings show that both connectivity and patch shape increase the richness of wind-dispersed species, highlighting how corridors uniquely impact wind-dispersed species relative to animal-dispersed species, whose richness is impacted by corridors but not patch shape (31). Importantly, wind-driven dispersal is rarely considered in reserve design planning, yet our results suggest that patch shape and corridor configuration strongly impact the movement of wind-dispersed seeds.
Our results support the hypothesis that wind-driven dispersal depends on how wind is oriented relative to landscape features. Our mechanistic model suggests that corridors are most effective for promoting LDD of seeds when they are aligned with the wind (between 0° and 30° of the long axis of the corridor; Fig. 2; Figs. S2–S4). For example, when wind is close to perpendicular to the long axis of a patch (60° or 90°), the redirecting and bellowing effects are almost completely lost (Figs. S3A and S4A). In addition, the probability of uplift changes dramatically, either becoming much weaker when the wind rotates to 60° of the patch’s long axis (Fig. S3B), or stronger at 90° (Fig. S4B) but with no accompanying redirection or bellowing effects to transport uplifted seeds down the corridor (Fig. S4A). Empirical data support the predicted shifts in wind caused by corridors (Fig. 3 C and D), providing a mechanism for observed increases in wind-dispersed species richness in connected patches (Fig. 4). Because corridors in our experimental landscape were oriented without respect to prevailing wind direction, our results conservatively estimate the effect of corridors on wind-dispersed plant species richness and demonstrate that corridor-aligned winds can increase wind-dispersed plant species richness (Fig. 4). Other factors, such as topography (17), the heterogeneity of canopy height, the dimensions of corridor width relative to length, and species-specific seed traits (e.g., size, shape, and terminal velocity) may also play a role in effective wind-driven dispersal down corridors.
Many threatened and endangered plant species that are dependent on open habitats (e.g., smooth purple coneflower, Echinacea laevigata; Willamette daisy, Erigeron decumbens var. decumbens; spring goldenrod, Solidago verna) live in patchy metapopulations and may require dispersal through a forested matrix (32). Conservation planning for such plants (and likely other wind-dispersed organisms) should, therefore, consider the impacts of corridor alignment with predominant winds alongside other management goals and constraints. For example, connectivity planning under climate change should consider aligning corridors with predominant winds amid other goals such as promoting movement among suitable current and future climates (33, 34). Similarly, the restoration of habitat patches and corridors should also consider predominant winds.
Our findings are particularly important for conservation planning in open habitats (e.g., grasslands, rocky outcrops, savannas) where plant communities have larger proportions of wind-dispersed species than forested, closed-habitat systems (8, 35), can be highly diverse at small to intermediate scales (23), and are often surrounded by forested landscapes. Conservation planning for open-habitat species is becoming increasingly important as open habitats are threatened by woody encroachment due to altered historical disturbance regimes (e.g., decreased fire, 36) and global afforestation (e.g., plantation forests, 37). Our multifaceted approach can also be used to elucidate wind dynamics and dispersal in other ecosystems, including forested patches in an open matrix, and for other wind-dispersed organisms.
We show how habitat fragmentation and corridors alter wind dynamics in multiple ways that can be predicted from models, and can be used to determine LDD of seeds, with apparent consequences for the plant community. These findings provide revealing insights about fragmentation effects on wind dynamics, compelling evidence for previously undocumented impacts of habitat fragmentation and corridors on wind-dispersed organisms, and new evidence for how landscape structure may mediate patterns of species richness by altering dispersal.
Materials and Methods
Landscape Experiment.
Ten experimental landscapes at the Savannah River Site, SC (Fig. 1) contain a central 100 × 100-m patch surrounded by four peripheral patches that are either connected or unconnected. Connected patches are 100 × 100 m and have a 150 m long × 25 m wide corridor connecting to the central patch. Unconnected patches are either rectangular patches (100 × 137.5 m and equivalent in area to the connected patch plus its corridor) or winged (100 × 100 m with two 25 × 75-m “wings” on either side of the patch that also have the same ratio of edge-to-core habitat as the corridor, but do not connect habitat patches). Experimental patches consist of open savanna habitat patches within a surrounding matrix of dense mature loblolly (Pinus taeda) and longleaf (Pinus palustris) pine plantation forest (Fig. 1), which contains far fewer species than the experimental patches (38) and creates a sharp contrast in habitat suitability and structure between the patches and surrounding matrix. Additional details are in SI Materials and Methods and elsewhere (29, 39).
RAFLES Model.
The full simulation domain was 1.5 × 1.5 × 1.5 km3 at a mesh–grid spacing of 5 × 5 × 3 m3 (in the eastward, northward, and upward directions, respectively). The model surface included two replicates of each patch type separated by at least 150 m (Fig. S1). The model was run for 4.5 h to spin-up from initial conditions and then an additional 30 min were used to provide data for analyses. Data at each grid cell in the model were time averaged for these 30 min of simulations and further averaged between the corresponding grid cell location in the identically structured replicate patch in a different area of the simulation domain (e.g., patches A and B in Fig. S1). In RAFLES, atmosphere–vegetation interactions are resolved through several physical mechanisms: leaves affect flow and dissipate turbulence kinetic energy as a function of their drag coefficient, leaf density, and the wind speed; stems restrict flow and limit the free space within the canopy; and light is attenuated and reflected by this multilayered representation of the canopy. RAFLES includes an explicit, 3D, heterogeneous representation of leaf densities and tree-stem volumes at very high resolution, explicitly resolving the vertical distribution of leaf densities, and the horizontal differences between individual tree crowns. Detailed methods are in SI Materials and Methods.
Wind Dynamics.
From October to December 2008, over the course of an entire dispersal season and when most wind-dispersed plants in our study site and much of the eastern United States (25) release their seeds, we placed five meteorological towers at key locations within the experimental patches (Fig. 3C) of one experimental unit. Each tower had one 3D ultrasonic anemometer (RM Young 81000) mounted at 5 m above the ground and data were recorded at 10 Hz to data loggers (Campbell Scientific CR1000). From October 2009 to January 2010, we placed seven meteorological towers at key locations within the experimental patches and one within the forested matrix. These towers were mounted with 3D ultrasonic anemometers at 10 m above ground and two of them also had ultrasonic anemometers at 5 m above ground. Above-canopy wind data were collected from a nearby meteorological tower. See SI Materials and Methods for further details.
Seed Releases.
We simultaneously released 300 artificial seeds over 30-min period release events from seven different locations within our experimental landscape during three unique wind directions (Table S1) for a total of 5,400 released seeds. Artificial seeds were made out of synthetic fibers and fluorescent dye powder and were constructed to have a seed terminal falling velocity (Vt) well within the range of native wind-dispersed plants in our study system. Seed colors allowed identification of the release point of that seed. Artificial seeds were released from 4.5 m above ground. Five seeds were simultaneously released every 30 s for 30 min (n = 300) from each release location (Table S1) in our experimental landscape. This process was repeated five times under different conditions. Seeds were recovered and their locations were recorded. Detectability was very high and thus did not bias our results (see SI Materials and Methods). Watson–Williams F tests were used to compare the directionality of seeds dispersed (<15 m and ≥15 m separately for short- and long-distance dispersal, respectively) among release locations. See SI Materials and Methods for detailed methods and our definition of LDD.
Comparing Modeled and Empirical Dispersal Kernels.
To evaluate the relationship between model-predicted and empirical seed dispersal patterns we used linear regression and permutation tests to test significance of the fit. See SI Materials and Methods for further details.
Plant Communities.
Eight experimental habitat patches were annually censused for all plant species from May 15 to July 15 from 2001 through 2012, except 2004 when a prescribed fire took place. All patches are equal in area. Detailed methods are described in SI Materials and Methods and refs. 31, 39.
Supplementary Material
Acknowledgments
Thanks to J. Blake, E. Olson, J. Segar, C. Hobson, and the US Department of Agriculture (USDA) Forest Service-Savannah River for maintaining the experimental landscapes and to R. Kurzja and the Savannah River National Lab for providing access to meteorological data and towers. Simulations were conducted at the Ohio Supercomputer Center under Award PAS0409-2. Thanks to the Corridor Research Group and field technicians for data collection. Funding was provided by the National Science Foundation (DEB-0733746, DEB-0919074, DEB-0614333, DEB-0918869, and DEB-0911461); the Center for Programs and the International Center for Advanced Renewable Energy and Sustainability at Washington University; the USDA Forest Service-Savannah River (a National Environmental Research Park), under Interagency Agreement DE-AI09-00SR22188 with the Department of Energy, Aiken, SC; and the USDA National Institute for Food and Agriculture Air Quality Grant 2010-65112-20564. R.N.’s studies on seed dispersal by wind were supported by the Israel Science Foundation (ISF-474/02), the US National Science Foundation (IBN-9981620, DEB-0453665), the Adelina and Massimo Della Pergola Chair of Life Sciences, and the Minerva Center for Movement Ecology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1308968111/-/DCSupplemental.
References
- 1.Fahrig L. Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst. 2003;34(1):487–515. [Google Scholar]
- 2.Heller NE, Zavaleta ES. Biodiversity management in the face of climate change: A review of 22 years of recommendations. Biol Conserv. 2009;142(1):14–32. [Google Scholar]
- 3.Krosby M, Tewksbury J, Haddad NM, Hoekstra J. Ecological connectivity for a changing climate. Conserv Biol. 2010;24(6):1686–1689. doi: 10.1111/j.1523-1739.2010.01585.x. [DOI] [PubMed] [Google Scholar]
- 4.Hilty JA, Lidicker WZ, Jr, Merenlender A, Dobson AP. Corridor ecology: The science and practice of linking landscapes for biodiversity conservation. Washington, DC: Island Press; 2006. [Google Scholar]
- 5.Gilbert-Norton L, Wilson R, Stevens JR, Beard KH. A meta-analytic review of corridor effectiveness. Conserv Biol. 2010;24(3):660–668. doi: 10.1111/j.1523-1739.2010.01450.x. [DOI] [PubMed] [Google Scholar]
- 6.Foissner W. Biogeography and dispersal of micro-organisms: A review emphasizing protists. Acta Protozool. 2006;45(2):111–136. [Google Scholar]
- 7.Friedman J, Barrett SCH. Wind of change: New insights on the ecology and evolution of pollination and mating in wind-pollinated plants. Ann Bot (Lond) 2009;103(9):1515–1527. doi: 10.1093/aob/mcp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howe HF, Smallwood J. Ecology of seed dispersal. Annu Rev Ecol Evol Syst. 1982;13:201–228. [Google Scholar]
- 9.Nathan R, et al. Mechanisms of long-distance dispersal of seeds by wind. Nature. 2002;418(6896):409–413. doi: 10.1038/nature00844. [DOI] [PubMed] [Google Scholar]
- 10.Tackenberg O. Modeling long-distance dispersal of plant diaspores by wind. Ecol Monogr. 2003;73(2):173–189. [Google Scholar]
- 11.Bohrer G, Katul GG, Nathan R, Walko RL, Avissar R. Effects of canopy heterogeneity, seed abscission and inertia on wind-driven dispersal kernels of tree seeds. J Ecol. 2008;96(4):569–580. [Google Scholar]
- 12.Nathan R, Muller-Landau HC. Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends Ecol Evol. 2000;15(7):278–285. doi: 10.1016/s0169-5347(00)01874-7. [DOI] [PubMed] [Google Scholar]
- 13.Levin SA, Muller-Landau HC, Nathan R, Chave J. The ecology and evolution of seed dispersal: A theoretical perspective. Annu Rev Ecol Evol Syst. 2003;34:575–604. [Google Scholar]
- 14.Greene DF, Johnson EA. Wind dispersal of seeds from a forest into a clearing. Ecology. 1996;77(2):595–609. [Google Scholar]
- 15.Nuttle T, Haefner JW. Seed dispersal in heterogeneous environments: Bridging the gap between mechanistic dispersal and forest dynamics models. Am Nat. 2005;165(3):336–349. doi: 10.1086/428298. [DOI] [PubMed] [Google Scholar]
- 16.Schurr FM, Steinitz O, Nathan R. Plant fecundity and seed dispersal in spatially heterogeneous environments: Models, mechanisms and estimation. J Ecol. 2008;96(4):628–641. [Google Scholar]
- 17. Trakhtenbrot A (2011) Dispersal of plant seeds by wind in heterogeneous environments. PhD doctoral dissertation (The Hebrew Univ of Jerusalem, Jerusalem)
- 18.Nathan R, Horn HS, Chave J, Levin SA. Mechanistic models for tree seed dispersal by wind in dense forests and open landscapes. In: Levey DJ, Silva WR, Galetti M, editors. Seed Dispersal and Frugivory: Ecology, Evolution and Conservation. New York: CABI; 2002. [Google Scholar]
- 19.Bullock JM, Nathan R. Plant dispersal across multiple scales: Linking models and reality. J Ecol. 2008;96(4):567–568. [Google Scholar]
- 20.Trakhtenbrot A, Nathan R, Perry G, Richardson DM. The importance of long-distance dispersal in biodiversity conservation. Divers Distrib. 2005;11(2):173–181. [Google Scholar]
- 21.Nathan R. Long-distance dispersal of plants. Science. 2006;313(5788):786–788. doi: 10.1126/science.1124975. [DOI] [PubMed] [Google Scholar]
- 22.Jose S, Jokela EJ. In: The Longleaf Pine Ecosystem: Ecology, Silviculture, and Restoration. Miller DL, editor. New York: Springer; 2006. [Google Scholar]
- 23.Noss RF. Forgotten Grasslands of the South: Natural History and Conservation. Washington, DC: Island Press; 2013. [Google Scholar]
- 24.Bohrer G, Katul GG, Walko RL, Avissar R. Exploring the effects of microscale structural heterogeneity of forest canopies using large-eddy simulations. Boundary-Layer Meteorol. 2009;132:351–382. [Google Scholar]
- 25.Nathan R, Katul GG. Foliage shedding in deciduous forests lifts up long-distance seed dispersal by wind. Proc Natl Acad Sci USA. 2005;102(23):8251–8256. doi: 10.1073/pnas.0503048102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown JH, Kodric-Brown A. Turnover rates in insular biogeography: Effect of immigration on extinction. Ecology. 1977;58:445–449. [Google Scholar]
- 27.MacArthur RH, Wilson EO. The Theory of Island Biogeography. Princeton, NJ: Princeton Univ Press; 1967. [Google Scholar]
- 28.Levins R. Some demographic and genetic consequences of environmental heterogeneity for biological control. Bull Entomol Soc Am. 1969;15:237–240. [Google Scholar]
- 29.Tewksbury JJ, et al. Corridors affect plants, animals, and their interactions in fragmented landscapes. Proc Natl Acad Sci USA. 2002;99(20):12923–12926. doi: 10.1073/pnas.202242699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orrock JL, Danielson BJ, Burns MJ, Levey DJ. Spatial ecology of predator-prey interactions: Corridors and patch shape influence seed predation. Ecology. 2003;84(10):2589–2599. [Google Scholar]
- 31.Damschen EI, et al. The movement ecology and dynamics of plant communities in fragmented landscapes. Proc Natl Acad Sci USA. 2008;105(49):19078–19083. doi: 10.1073/pnas.0802037105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bohrer G, Nathan R, Volis S. Effects of long-distance dispersal for metapopulation survival and genetic structure at ecological time and spatial scales. J Ecol. 2005;93(5):1029–1040. [Google Scholar]
- 33.Nuñez TA, et al. Connectivity planning to address climate change. Conserv Biol. 2013;27(2):407–416. doi: 10.1111/cobi.12014. [DOI] [PubMed] [Google Scholar]
- 34.Beier P. Conceptualizing and designing corridors for climate change. Ecol Res. 2012;30(4):312–319. [Google Scholar]
- 35.Lorts CM, Briggeman T, Sang T. Evolution of fruit types and seed dispersal: A phylogenetic and ecological snapshot. J Syst Evol. 2008;46(3):396–404. [Google Scholar]
- 36.Bowman DM, et al. The human dimension of fire regimes on Earth. J Biogeogr. 2011;38(12):2223–2236. doi: 10.1111/j.1365-2699.2011.02595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bremer LL, Farley KA. Does plantation forestry restore biodiversity or create green deserts? A synthesis of the effects of land-use transitions on plant species richness. Biodivers Conserv. 2010;19(14):3893–3915. [Google Scholar]
- 38.Brudvig LA, Damschen EI, Tewksbury JJ, Haddad NM, Levey DJ. Corridors promote plant biodiversity spillover into non-target habitats. Proc Natl Acad Sci USA. 2009;106(23):9328–9332. doi: 10.1073/pnas.0809658106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Damschen EI, Haddad NM, Orrock JL, Tewksbury JJ, Levey DJ. Corridors increase plant species richness at large scales. Science. 2006;313(5791):1284–1286. doi: 10.1126/science.1130098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.