Significance
Dendritic cells (DCs) are important for the development of intestinal inflammation in humans with inflammatory bowel disease (IBD) and in mice with experimental colitis. We demonstrate that a subset of DCs, CD103− DCs, isolated from murine mesenteric lymph nodes, is highly pathogenic for murine intestinal inflammation and expresses high levels of the cytokine osteopontin (Opn). This Opn expression by CD103− DCs is crucial for their pathogenicity. We define a specific domain of Opn protein significant for inducing pathogenic properties in CD103− DCs. Blockade of the interaction of this Opn domain with integrin α9 expressed on CD103− DCs abrogated their harmful effects. These findings may open new paths in IBD treatment.
Abstract
Intestinal CD103− dendritic cells (DCs) are pathogenic for colitis. Unveiling molecular mechanisms that render these cells proinflammatory is important for the design of specific immunotherapies. In this report, we demonstrated that mesenteric lymph node CD103− DCs express, among other proinflammatory cytokines, high levels of osteopontin (Opn) during experimental colitis. Opn expression by CD103− DCs was crucial for their immune profile and pathogenicity, including induction of T helper (Th) 1 and Th17 cell responses. Adoptive transfer of Opn-deficient CD103− DCs resulted in attenuated colitis in comparison to transfer of WT CD103− DCs, whereas transgenic CD103− DCs that overexpress Opn were highly pathogenic in vivo. Neutralization of secreted Opn expressed exclusively by CD103− DCs restrained disease severity. Also, Opn deficiency resulted in milder disease, whereas systemic neutralization of secreted Opn was therapeutic. We determined a specific domain of the Opn protein responsible for its CD103− DC-mediated proinflammatory effect. We demonstrated that disrupting the interaction of this Opn domain with integrin α9, overexpressed on colitic CD103− DCs, suppressed the inflammatory potential of these cells in vitro and in vivo. These results add unique insight into the biology of CD103− DCs and their function during inflammatory bowel disease.
Inflammatory bowel diseases (IBDs), including Crohn disease (CD) and ulcerative colitis (UC), are caused by excessive inflammatory responses to commensal microflora and other antigens present in the intestinal lumen (1). Intestinal dendritic cells (DCs) contribute to these inflammatory responses during human IBD, as well as in murine colitis models (2). DCs that reside in draining mesenteric lymph nodes (MLNs) are also crucial mediators of colitis induction (3) and may be grouped based on their surface CD103 (integrin αE) expression as CD11chighCD103+ (CD103+ DCs) and CD11chighCD103− (CD103− DCs) (4–6). CD103+ DCs are considered important mediators of gut homeostasis in steady state (4, 5, 7–9), and their tolerogenic properties are conserved between mice and humans (5). However, their role during intestinal inflammation is not well defined. Instead, CD103− DC function has been described mostly during chronic experimental colitis (10–12). These cells secrete IL-23, IL-6, and IL-12 (10–12), contributing to the development of T helper (Th) 17 and Th1 cells, and are highly inflammatory during CD4+ T-cell transfer colitis (12) and during 2,4,6 trinitrobenzene sulfonic acid (TNBS)-induced chronic colitis (11). MLN CD103− DCs cultured in the presence of LPS, a Toll-like receptor (TLR) 4 agonist, or R848, a TLR7 agonist, express higher levels of TNF-α and IL-6 (7, 12). In fact, these cells secrete IL-23 and IL-12 even in the absence of TLR stimulation (10). Both MLN CD103− and CD103+ DC subsets are present in acute colitis (11, 13); however, their function, as well as their cytokine profile, during this phase of disease, reflecting colitis initiation, remains unknown.
Recent studies suggest a proinflammatory role for the cytokine osteopontin (Opn) in TNBS- and dextran sulfate sodium (DSS)-induced colitis (14, 15), which are the models for CD and UC, respectively. Opn is expressed by DCs and other immune cell types, such as lymphocytes, during autoimmune responses (16–22), and its expression by DCs during autoimmunity contributes to disease severity (17–19, 21, 23). In addition, Opn expression is highly up-regulated in intestinal immune and nonimmune cells and in the plasma of patients with CD and UC (24–29), as well as in the colon and plasma of mice with experimental colitis (14, 15, 27, 30). Increased plasma Opn levels are related to the severity of CD inflammation (29), and certain Opn gene (Spp1) haplotypes are modifiers of CD susceptibility (31), indicating that Opn could be used as an IBD biomarker (27). In general, Opn affects DC biology during several inflammatory conditions (17–21, 32–37) and could be a potential therapeutic target in IBD.
In this study, we initially asked whether Opn was expressed by MLN CD103− and CD103+ DCs during colitis. We found that CD103− DCs express excessive levels of Opn in addition to other proinflammatory cytokines. Conversely, CD103+ DCs express profoundly lower levels of Opn and are noninflammatory. Using adoptive transfer of purified specific DC subsets, we determined that MLN CD103− DCs are critical mediators of acute intestinal inflammation and that their Opn expression is essential for their proinflammatory properties in both acute and chronic colitis. Furthermore, Opn-deficient and Opn-neutralized mice developed significantly milder disease. In addition, we constructed transgenic (Tg) mice overexpressing Opn only in DCs. These mice developed exaggerated colitis, and adoptive transfer of their CD103− DCs into recipient mice dramatically exacerbated disease. Because Opn protein contains several domains interacting with various receptors, we defined a specific Opn domain significant for inducing proinflammatory properties in CD103− DCs. Blockade of the interaction of this Opn domain [containing functional Ser-Leu-Ala-Tyr-Gly-Leu-Arg (SLAYGLR) sequence] with integrin α9 expressed on CD103− DCs abrogated their proinflammatory profile and colitogenic effects in vivo.
Results
MLN CD103− DCs Isolated from Mice with Colitis Are Proinflammatory in Vivo and in Vitro.
MLNs are the main inductive site for IBD (3). During acute colitis, there was a significant increase in the numbers of 7-aminoactinomycin D− (7AAD−) CD3−MHCIIhighCD11chighCD103− DCs (CD103− DC subset) in colitic BALB/c mice in comparison to healthy controls (Fig. 1A; sequential gating strategy in Fig. 1B and Table S1). Most of these CD103− DCs (∼80% in colitis vs. 70% in healthy MLNs) expressed the CD11b+ marker (Fig. 1B and Table S1), denoting monocyte origin (38, 39). CD11b−CD103− DC numbers were not altered significantly between these two groups (Table S1). Approximately 20% of colitic vs. 8% of healthy CD11b+CD103− DCs expressed E-cadherin (Fig. 1B and Table S1), which characterizes a subset of monocyte-derived DCs (12). On the other hand, the lymphocyte antigen 6C+ (Ly6C+) CD11b+CD103− DC population was decreased during colitis (Table S1). Overall, acute colitis mainly enhanced total numbers of monocyte-derived CD103− DCs in MLNs. Accumulation of CD103− DCs in MLNs increased in accordance with disease severity and in a TNBS dose-increasing manner (Fig. 1A).
Fig. 1.
DC subpopulations in acute colitis. (A) Numbers and ratios of MLN CD103− and CD103+ DCs in healthy (PBS, −), mild colitic (1 mg of TNBS, 1) and severe colitic (2 mg of TNBS, 2) BALB/c mice. (B) Representative gating strategy for DC characterization used for all experiments. FSC-A, forward scatter area; SSC-H, side scatter height. (C) Relative cytokine mRNA expression (values normalized to hypoxanthine phosphoribosyltransferase) and IL-12p70 secretion by 2 × 105 MLN CD103− DCs. Values are expressed as mean ± SEM (n = 5–6 mice per group) from three separate experiments. Statistical significance was obtained by an unpaired Student t test (**P < 0.01; ***P < 0.0002).
In contrast, 7AAD− CD3−MHCIIhighCD11chighCD103+ DCs (CD103+ DC subset) numbers in MLNs of colitic mice were negatively correlated to increasing TNBS doses administered and the resulting degree of inflammation, and were significantly reduced overall (Fig. 1A). Healthy mice had higher numbers of CD103+ DCs vs. CD103− DCs in MLNs (Fig. 1A and Table S1). Accordingly, colitic mice had a significantly higher ratio of MLN CD103− DCs to CD103+ DCs (DC ratio hereafter) in comparison to healthy mice, as well as mice with mild colitis (Fig. 1A).
CD103− DCs from colitic mice exhibited higher Il23p19 and Il6 expression and higher IL-12p70 production compared with CD103− DCs isolated from healthy control mice, whereas there was no significant difference in Il10 expression (Fig. 1C). CD103− DCs from colitic mice also expressed significantly higher levels of Spp1 (Fig. 1C). The cytokine expression profile of MLN CD103− DCs suggested that they have proinflammatory potential. To address this, we adoptively transferred (i.p.) MLN CD103− DCs from colitic donors into healthy recipients 2 h before colitis induction. We analyzed recipient mice 5 d following colitis induction. As expected, the disease score was significantly higher in recipients of CD103− DCs compared with nontransferred mice (Fig. 2A). However, transfer of CD103+ DCs did not affect disease (Fig. 2A). In addition, coculture experiments of purified CD103− or CD103+ DCs from colitic mice with anti-CD3/28–stimulated CD4+ T cells showed that only CD103− DCs were effective at inducing CD4+ T-cell proliferation compared with CD103+ DCs (Fig. 2B). As expected, the two subsets promoted distinct T-cell responses, because CD103− DCs cocultured with CD4+ T cells predominantly induced differentiation toward Th17 and Th1 cells (Fig. 2C), whereas CD103+ DCs induced mostly forkhead box protein 3+ (Foxp3+) regulatory T cells (Tregs) (Fig. 2C). Therefore, MLN CD103− DCs from mice with acute colitis are proinflammatory and exacerbate disease upon transfer. Transferred MLN CD103− DCs were BrdU-labeled and were tracked in recipient MLNs after 72 and 120 h (Fig. S1A).
Fig. 2.
MLN CD103− DCs are proinflammatory in acute colitis. (A) Representative photomicrographs of H&E colonic sections and histological score from control colitic and mice transferred with 2 × 105 colitic MLN CD103− or CD103+ DCs. (Magnification: 20×.) (Scale bars: 100 μm.) (B) [3H] thymidine (TdR) incorporation of CD4+ T cells cocultured with colitic DCs (72 h). Results are shown as mean ± SEM of triplicate wells. (C) FACS analysis of cells from B for intracellular cytokine and Foxp3 detection. Numbers of IL-17A+, IFN-γ+, and Foxp3+ CD4+ T cells per 105 total cells are shown. Opn levels of MLN homogenates from healthy (h) and colitic (c) mice (D) and relative Spp1 mRNA expression in MLNCs (E) are shown. Values are expressed as mean ± SEM (n = 5–6 mice per group) from three separate experiments. (F) Relative Spp1 mRNA expression in colitic MLN CD103− and CD103+ DCs. Values are from one representative experiment (*P < 0.05; **P < 0.01; ***P < 0.0005).
Opn Expression Exacerbates Acute Colitis with Increased CD103− DC Recruitment.
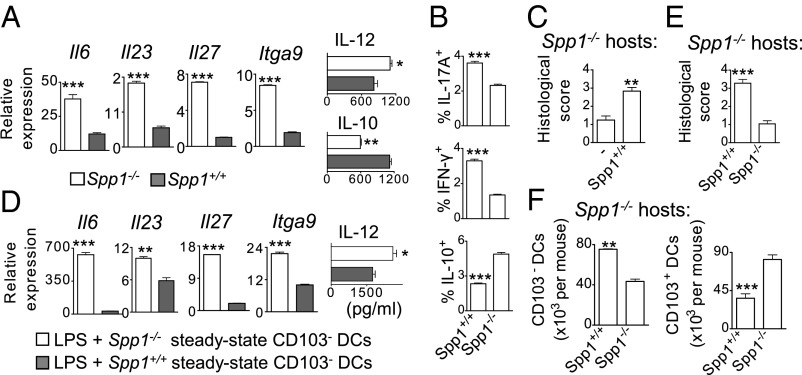
Opn expression is up-regulated in peripheral blood and intestinal mucosa of patients (24–29) and mice with IBD (14, 15, 27, 30). We found that Opn protein and mRNA (Spp1) levels were also significantly higher in MLN cells (MLNCs) from mice with acute colitis (∼fourfold and ∼46-fold, respectively) compared with healthy mice (Fig. 2 D and E). Specifically, MLN CD103− DCs from colitic mice expressed significantly higher Spp1 levels than CD103+ DCs (Fig. 2F).
Induction of colitis in Opn-deficient (Spp1−/−) mice resulted in attenuated disease with significantly reduced inflammatory cell infiltration in the colonic mucosa, submucosa, and muscularis propria (Fig. 3A). In comparison, WT mice had significantly enhanced inflammation, shortening of the colon, infiltration of inflammatory cells, and a high histological score (Fig. 3A). Epithelial hyperplasia, the cardinal feature of TNBS-induced colitis (40), was significantly decreased in the crypts of Spp1−/− mice, reminiscent of the crypts of noninflamed colonic tissue (Fig. 3A). Furthermore, Spp1−/− mice had lower mortality rates at the peak of disease (∼8.3%) compared with WT mice (∼20%).
Fig. 3.
BALB/c Spp1−/− mice are protected from acute colitis. (A) Representative colon photographs, photographs (H&E) of sections, length, histological scores, and differential cell counts of mice with TNBS colitis. HPF, high-power field; LMs, lymphomononuclear cells; Mϕs, macrophages; PMNs, polymorphonuclear leukocytes. (Magnification: 10×.) (Scale bars: 200 μm.) (B) Levels of cytokines in MLN homogenates. (C) Percentages of IL-17A+ and IFN-γ+ CD4+ T-cell MLNCs and numbers of IL-17A+, IFN-γ+, Foxp3+, and IL-10+ CD4+ T-cell MLNCs. Values are expressed as mean ± SEM (n = 6 mice per group) from five separate experiments. (D) Numbers of DC subpopulations gated as in Fig. 1B. Values are expressed as mean ± SEM (n = 4 mice per group) from two separate experiments (*P < 0.05; **P < 0.005; ***P < 0.001).
MLN homogenates from Spp1−/− mice contained decreased levels of IL-23 and IL-12p70 and increased IL-10 levels in comparison to homogenates from WT mice (Fig. 3B). Proliferation of CD4+ T cells isolated from MLNs from Spp1−/− colitic mice was significantly reduced (Fig. S1B). Also, their cytokine profile was characterized by significantly lower levels of IFN-γ and IL-17A, in comparison to CD4+ T cells from WT mice, whereas IL-10 secretion was significantly higher (Fig. S1C). Phenotypic analysis of CD4+ T-cell populations revealed that MLNs from colitic Spp1−/− mice had significantly reduced numbers of Th17 and Th1 cells (Fig. 3C). MLNs of colitic Spp1−/− mice contained significantly higher numbers of Foxp3+ and IL-10+ CD4+ T cells (Fig. 3C) with increased Foxp3 and Il10 expression (Fig. S1D). Spp1−/− mice also contained increased numbers of IL-10+ IL-17A+ CD4+ T cells, characterized as regulatory cells in another colitis model (41), in their MLNs (Fig. S1E).
Opn deficiency during acute colitis resulted in higher MLN numbers of 7AAD− CD3−MHCIIhighCD11chigh DCs in comparison to WT mice (Fig. 3D). Despite the overall increase in DC numbers, MLNs of Spp1−/− mice had significantly reduced numbers of the CD103− DC subset compared with WT mice (Fig. 3D). In addition, there were significantly higher numbers of CD11b+CD103− DCs and CD11b−CD103− DCs in Spp1+/+ vs. Spp1−/− colitic mice (Fig. 3D). The decrease of CD103− DCs in the Spp1−/− mice mainly reflected reduced numbers of the E-cadherin+ CD11b+ DC population (Fig. 3D). Decreased numbers of Ly6C-expressing CD11b+CD11chighCD103− DCs were also present in MLNs of Spp1−/− vs. Spp1+/+ colitic mice (Fig. 3D). In addition, CD103− DCs from Spp1−/− mice expressed significantly lower levels of C-C chemokine receptor type 7 (CCR7) (Fig. S1G), an important molecule for DC migration and homing to lymph node (LN) (42, 43). In vivo BrdU incorporation by CD103− DCs was similar between the two groups (Fig. S1F), suggesting that CD103− DC numbers were dampened in Spp1−/− mice as a result of decreased recruitment to MLNs, and not due to defective proliferation. There was no difference in proliferation of CD103+ DCs between Spp1+/+ and Spp1−/− colitic mice (Fig. S1F). Nevertheless, colitic Spp1−/− mice exhibited significantly increased numbers of CD103+ DCs (Fig. 3D) with higher CCR7 expression (Fig. S1G). Thus, the DC ratio was significantly lower in the MLNs of Spp1−/− colitic mice compared with WT mice (Fig. S1H). There were no differences in the numbers of MLN CD103− and CD103+ DCs between healthy Spp1−/− and WT mice (Fig. S1I).
Opn deficiency during colitis influenced lamina propria (LP) DC subset numbers as well. LP CD103− DC numbers were decreased in Spp1−/− mice similar to the MLNs. There was a significant decrease of CD103+ DC numbers in LP of Spp1−/− mice, which was in contrast to their representation in MLNs. In addition, there was no difference in numbers of total LP E-cadherin+ CD103− DCs between Spp1+/+ and Spp1−/− mice (Fig. S1J).
We found significantly decreased Il6, Il23p19, and Il27p28 expression; IL-12p70 secretion; and increased IL-10 secretion (Fig. 4A) by Spp1−/− MLN CD103− DCs in comparison to WT CD103− DCs. Also, Itga9 gene expression in DCs, encoding a known Opn receptor that favors IL-6 production in a rheumatoid arthritis (RA) model (19), was significantly lower in Spp1−/− CD103− DCs (Fig. 4A).
Fig. 4.
Opn-expressing CD103− DCs are proinflammatory. (A) Relative mRNA expression and IL-12p70 and IL-10 secretion by 2 × 105 LPS-stimulated MLN CD103− DCs from colitic mice. Values are expressed as mean ± SEM (n = 6 mice per group) from five separate experiments. (B) Percentages of IL-17A+, IFN-γ+, and IL-10+ CD4+ Spp1−/− T cells cocultured with Spp1−/− or Spp1+/+ colitic MLN CD103− DCs (72 h). Results are shown as mean ± SEM of triplicate wells from at least three separate experiments. (C) Histological scores of BALB/c Spp1−/− mice transferred with 2 × 105 colitic Spp1+/+ CD103− DCs and of control mice (−). (D) Relative mRNA expression and IL-12p70 secretion by 5 × 105 LPS-stimulated (24 h) CD103− DCs. Results from one representative experiment are shown as mean ± SEM of triplicate wells. (E) Histological scores of BALB/c Spp1−/− recipients of 2 × 105 LPS-treated CD103− DCs. (F) MLN CD103− and CD103+ DC numbers per Spp1−/− recipient. Values are expressed as mean ± SEM (n = 6 mice per group) from three separate experiments (*P < 0.05; **P < 0.005; ***P < 0.001).
We also examined the acute colitis susceptibility of Spp1−/− mice bred onto the Rag2−/− background, which lack both T and B lymphocytes. BALB/c Rag2−/−Spp1−/− mice were significantly protected from colitis, because they exhibited a lower histological disease score (Fig. S1K) and higher survival proportion (∼90%) than the Rag2−/−Spp1+/+ mice (∼50%). MLN homogenates from Rag2−/−Spp1−/− mice contained significantly lower amounts of IL-23, IL-12p70, and IL-10 compared with Rag2−/−Spp1+/+ mice (Fig. S1K). In addition, Rag2−/−Spp1−/− mice with acute colitis had significantly reduced numbers of both CD103− and CD103+ DC subsets in MLNs compared with the Rag2−/−Spp1+/+ mice (Fig. S1L). Isolated MLN CD103− DCs from colitic Rag2−/−Spp1+/+ mice expressed higher levels of Il6, Il23, and Itga9 compared with Rag2−/−Spp1−/− mice (Fig. S1M).
We also induced an additional acute colitis model, DSS colitis, in C57BL/6 Spp1+/+ and Spp1−/− mice. The effects of Opn were also proinflammatory in this model, because disease severity (Fig. S2A), as well as Th17 and Th1 cell numbers, was significantly enhanced in Spp1+/+ mice (Fig. S2B). Furthermore, CD103− DCs were highly accumulated in MLNs of Spp1+/+ mice, whereas CD103+ DCs were reduced in comparison to Spp1−/− mice (Fig. S3C). Thus, Opn had similar effects on DSS- and TNBS-induced acute colitis. Moreover, we observed that C57BL/6 Rag1−/−Spp1+/+ mice had increased severity of DSS-induced colitis in comparison to C57BL/6 Rag1−/−Spp1−/− mice (Fig. S2D).
We administered recombinant Opn protein (rOpn) in Spp1−/− mice during colitis. Opn-treated BALB/c Spp1−/− mice exhibited significantly elevated numbers of CD103− DCs in MLNs in comparison to untreated Spp1−/− mice, reaching the levels of DCs recruited in WT colitic mice (Fig. S3A). This was accompanied by significantly worsened disease phenotype compared with control Spp1−/− mice (Fig. S3A). In addition, rOpn administration resulted in significantly elevated numbers of effector Th17 cells, whereas Foxp3+ and IL-10+ CD4+ T cells were significantly decreased in MLNs (Fig. S3B). Overall, Opn expression increases numbers of CD103− DCs and exacerbates acute colitis in either the presence or absence of adaptive immunity.
Opn Expression by CD103− DCs Is Crucial for Their Inflammatory Pathogenicity.
We tested whether the observed high Opn expression by CD103− DCs is a component of their pathogenic capacity. For this, we performed coculture experiments of WT or Spp1−/− MLN CD103− DCs purified from colitic mice, together with anti-CD3/28–stimulated Spp1−/− CD4+ T cells. Colitic WT CD103− DCs induced naive Spp1−/− CD4+ T cells to become Th17 and Th1 cells, whereas colitic Spp1−/− CD103− DCs showed a significantly reduced ability to support Th17 and Th1 differentiation (Fig. 4B). WT CD103− DCs induced significantly lower numbers of IL-10+ CD4+ T cells in the cocultures in comparison to cultures with Spp1−/− CD103− DCs (Fig. 4B).
To assess the role of Opn expression by CD103− DCs further in terms of pathogenicity, we adoptively transferred Spp1−/− or WT CD103− DCs from colitic mice into BALB/c Rag2−/−Spp1−/− recipients reconstituted with naive WT CD4+ T cells, followed by colitis induction. As expected, recipient mice transferred with WT CD103− DCs had significantly higher disease scores than mice transferred with Spp1−/− CD103− DCs (Fig. S3C). Accordingly, numbers of Th17 and Th1 cells were significantly elevated in MLNs of WT CD103− DC recipients (Fig. S3D). Overall, Opn expression by CD103− DCs contributes significantly to their proinflammatory capacity in vivo.
Given the pathogenic potential of MLN CD103− DCs, we studied whether Opn production exclusively by these cells is efficient to exacerbate colitis in BALB/c Spp1−/− mice that are otherwise protected. For this, we transferred WT CD103− DCs obtained from MLNs of colitic mice into Spp1−/− recipients before mild colitis induction. Spp1−/− recipients of colitic WT CD103− DCs had a significantly enhanced colitis score (Fig. 4C). Additionally, transfer of WT CD103− DCs obtained from MLNs of C57BL/6 DSS-colitic mice into C57BL/6 Spp1−/− recipients enhanced colitis upon DSS administration (Fig. S2E). Transfer of Spp1−/− CD103− DCs did not exacerbate colitis in C57BL/6 Spp1−/− recipients (Fig. S2E).
MLN CD103− DCs exhibit high expression of TLR-4 (7, 12), whereas bacterial LPS is present in the intestinal lumen (44). Steady-state WT CD103− DCs stimulated with LPS had significantly higher expression of Il6, Il23p19, Il27p28, and Itga9, as well as a modest elevation in IL-12p70 secretion, compared with Spp1−/− CD103− DCs (Fig. 4D).
CD103− DCs isolated from colitic mice are preconditioned in an inflammatory environment, and this may alter their original function in addition to their Opn expression. Adoptive transfer of LPS-pretreated WT CD103− DCs from healthy mice into BALB/c Spp1−/− mice before colitis induction resulted in a significantly enhanced disease score compared with Spp1−/− CD103− DC recipients (Fig. 4E). Accordingly, the higher histological score was accompanied by significantly elevated total numbers of CD103− DCs in MLNs of WT CD103− DC recipients (Fig. 4F). Also, WT CD103− DC recipients had significantly decreased total numbers of MLN CD103+ DCs. Overall, these results demonstrate that steady-state Opn-expressing MLN CD103− DCs are predisposed to proinflammatory function. In addition, Opn derived exclusively from CD103− DCs worsens disease significantly.
Opn-Overexpressing CD103− DCs Are Highly Pathogenic.
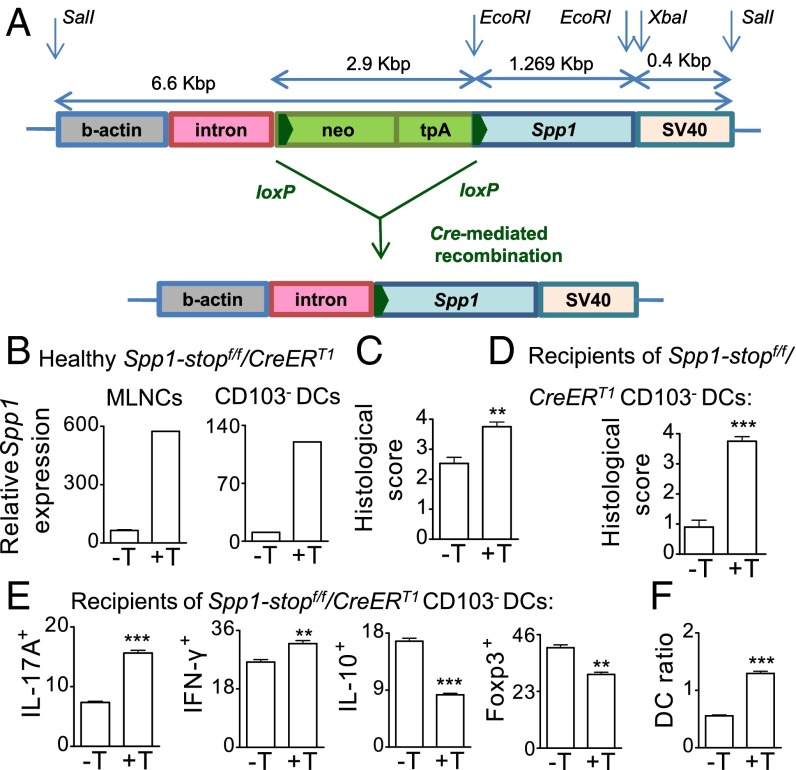
We have generated conditional C57BL/6 Tg mice that contain the Spp1 allele downstream of a stop cassette flanked by loxP sites (Spp1-stopf/f) (Fig. 5A). C57BL/6 Spp1-stopf/f mice overexpress the Spp1 allele when crossed to a mouse strain expressing Cre recombinase due to deletion of the stop cassette (Fig. 5A). We crossed the Spp1-stopf/f mice to the CreERT1 mice that express Cre and a mutated human estrogen receptor ligand-binding domain (ERT1) knocked into the ubiquitously expressed ROSA26 locus (R26Y). Treatment of the double-Tg (Spp1-stopf/f/CreERT1) littermates with tamoxifen induced Opn overexpression in MLNCs and in CD103− DCs (Fig. 5B). Inducible Opn overexpression did not have an effect in healthy mice but, upon colitis induction, enhanced disease score (Fig. 5C) and significantly boosted IL-17A, IL-6, and IFN-γ secretion by MLNCs stimulated ex vivo with LPS or anti-CD3/28 (Fig. S4A). There were higher numbers (2.2-fold) of activated CD4+ T cells with an effector memory phenotype (CD44highCD62L+) (Fig. S4B) and of CD103− DCs in MLNs of tamoxifen-treated double-Tg colitic mice compared with nontreated colitic littermates (Fig. S4C). We also isolated naive MLN CD103− DCs from treated or nontreated double-Tg Spp1-stopf/f/CreERT1 mice and transferred them into WT recipients. We induced colitis in recipient mice and observed that Spp1 overexpression exclusively by CD103− DCs was sufficient to exacerbate disease severity (Fig. 5D), accompanied by increased numbers of Th17 and Th1 cells, whereas it restrained Foxp3+ Tregs and IL-10+ CD4+ T cells in MLNs (Fig. 5E). The DC ratio was also significantly increased in recipients of Opn-overexpressing CD103− DCs (Fig. 5F), accompanied by elevated Spp1, Il17a, Il6, and ll23p19 expression and reduced Il10 and Foxp3 expression in MLNs compared with recipients of control CD103− DCs (Fig. S4D). A similar trend was noted for IL-17A, IL-6, IFN-γ, and IL-10 secretion by MLNCs (Fig. S4E). Conclusively, transfer of naive MLN CD103− DCs, which overexpress Opn, significantly boosts disease severity in WT recipient mice.
Fig. 5.
Opn-overexpressing CD103− DCs are pathogenic. (A) Scheme of the construct for the generation of Spp1-stopf/f Tg mice. C57BL/6 Spp1-stopf/f mice require crossing to Cre-expressing mice to overexpress Spp1. (B) Spp1 expression in MLNCs and CD103− DCs from C57BL/6 Spp1-stopf/fCreERT1 tamoxifen-untreated or -treated (−T/+T) mice. Histological scores of Spp1-stopf/fCreERT1 colitic mice (C) and of C57BL/6 Spp1+/+ mice transferred with CD103− DCs from healthy Spp1-stopf/fCreERT1 donors before colitis induction (D) are shown. Numbers of CD4+ T IL-17A+, IFN-γ+, IL-10+ (per 103 MLNCs), and Foxp3+ cells (per 106 MLNCs) (E) and DC ratios (F) are shown. Values are expressed as mean ± SEM (n = 6 mice per group) from three separate experiments (**P < 0.01; ***P < 0.001).
We also generated mice with DC-specific Opn overexpression by crossing the C57BL/6 Spp1-stopf/f mice to Itgaxcre mice. The double DC-Tg C57BL/6 (Spp1-stopf/f/Itgaxcre) mice overexpressed Opn only in DCs (Fig. S5A). Double DC-Tg Spp1-stopf/f/Itgaxcre mice had significantly enhanced disease upon colitis induction compared with control Spp1+/+/Itgaxcre littermates (Fig. 6A). Disease exacerbation was accompanied by significantly increased proliferation of isolated MLN CD3+ T cells (Fig. S5B). Stimulation of colitic Spp1-stopf/f/Itgaxcre MLNCs resulted in significantly increased Opn, together with IFN-γ, IL-17A, and IL-6 secretion (Fig. 6B). Percentages of MLN CD103− DCs in double DC-Tg Spp1-stopf/f/Itgaxcre colitic mice were enhanced compared with the percentages in Spp1+/+/Itgaxcre colitic littermates (Fig. S5C).
Fig. 6.
Opn overexpression by CD103− DCs exacerbates acute and chronic colitis. (A) Representative photographs of colonic sections from C57BL/6 Spp1+/+/Itgaxcre and Spp1-stopf/f/Itgaxcre colitic mice and histological scores. (Magnification: 10×.) (Scale bars: 200 μm.) (B) Levels of cytokine secretion by 106 MLNCs (120 h). (C) Histological scores of C57BL/6 Spp1−/− mice transferred with Spp1+/+/Itgaxcre (c) or Spp1-stopf/f/Itgaxcre (Tg) CD103− DCs before colitis induction. (D) Chronic colitis histological scores of C57BL/6 Rag1−/−Spp1−/− mice reconstituted with Spp1−/− CD45RBhighCD4+ T cells and naive Spp1+/+/Itgaxcre (c) or Spp1-stopf/f/Itgaxcre (Tg) CD103− DCs. (E) Cytokine secretion by 106 MLNCs (120 h) from recipients with chronic colitis. Values are expressed as mean ± SEM (n = 5–6 mice per group) from three separate experiments (*P < 0.05; **P < 0.01; ***P < 0.005).
In the above experimental approach, all DC subsets overexpress Opn. We thus transferred naive Opn-overexpressing MLN CD103− DCs from Spp1-stopf/f/Itgaxcre mice or control naive MLN CD103− DCs from Spp1+/+/Itgaxcre mice into Spp1−/− recipients. Induction of colitis in recipient mice resulted in significant disease exacerbation (Fig. 6C) and enhancement of Th17 and Th1 cellularity (Fig. S5D). Numbers of Foxp3+ Tregs were also decreased, whereas there was no significant difference in IL-10+ CD4+ T cells (Fig. S5D). Consistently, MLNCs derived from mice transferred with Opn-overexpressing CD103− DCs secreted higher levels of IFN-γ, Opn, IL-17A, and IL-6 compared with mice transferred with control CD103− DCs, whereas there was no significant difference in IL-10 levels (Fig. S5E). Therefore, Opn overexpression in CD103− DCs was efficient to exacerbate acute colitis in Spp1−/− recipient mice.
We also induced CD4+ T-cell transfer colitis (chronic model) by transferring naive Spp1−/− CD4+CD25−CD44−CD62L+CD45RBhigh T cells in C57BL/6 Rag1−/−Spp1−/− recipient mice. Recipients were transferred with CD103− DCs from MLNs of healthy Spp1-stopf/f/Itgaxcre mice or their Spp1+/+/Itgaxcre littermates. Mice that received Spp1-stopf/f/Itgaxcre CD103− DCs exhibited significantly exacerbated disease (Fig. 6D), and their MLNCs secreted enhanced levels of IFN-γ and IL-6 upon LPS stimulation and enhanced levels of IFN-γ, IL-17A, and IL-6 upon anti-CD3/28 stimulation compared with Spp1+/+/Itgaxcre CD103− DC recipients (Fig. 6E). MLN CD103− DC percentages in Spp1-stopf/f/Itgaxcre CD103− DC recipients were also increased compared with Spp1+/+/Itgaxcre CD103− DC recipients (Fig. S5F). Thus, Opn overexpressed just in CD103− DCs was sufficient to exacerbate chronic colitis significantly in Spp1−/− recipient mice.
Opn Secreted by CD103− DCs Mediates Colitis Exacerbation.
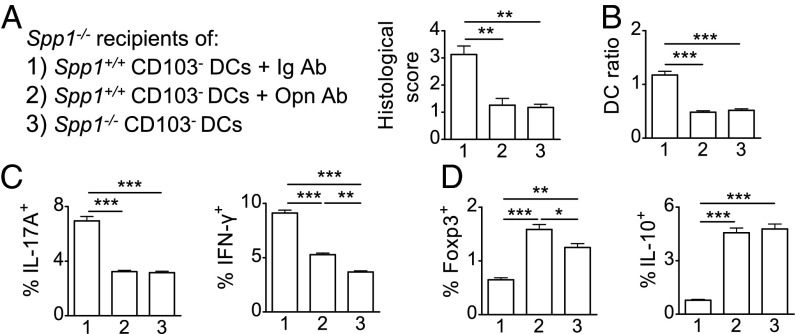
DCs express both the secreted Opn (Opn-s) (18, 19, 21, 35) and intracellular Opn (Opn-i) (17, 33, 34) forms. We preincubated isolated steady-state MLN CD103− DCs with LPS and then transferred these cells into Spp1−/− recipient mice. Subsequently, we induced colitis and in vivo neutralized Opn-s (produced only by the transferred DCs) with anti-Opn. Control recipient mice received Ig-isotype. A third group of Spp1−/− mice received Spp1−/− CD103− DCs. Disease histological scores were significantly enhanced in WT CD103− DC recipients treated with Ig-isotype compared with recipients treated with anti-Opn, which had similar scores as Spp1−/− CD103− DC recipients (Fig. 7A). This result was accompanied by significantly elevated DC ratios in MLNs (Fig. 7B). Th17 and Th1 responses were significantly increased in Ig-treated compared with anti-Opn–treated recipients of WT CD103− DCs and also compared with Spp1−/− CD103− DC recipients (Fig. 7C). As expected, MLNs from Ig-treated recipients produced significantly more IL-17A, IL-6, and IFN-γ ex vivo (Fig. S6A) and expressed higher levels of Il17a, Il6, Il23p19, and Itga9 compared with the two other groups (Fig. S6B). In contrast, anti-Opn–treated recipients of WT CD103− DCs and recipients of Spp1−/− CD103− DCs had similarly significantly increased numbers of Foxp3+ Tregs and IL-10+ CD4+ T cells in MLNs compared with Ig-treated WT CD103− DC recipients (Fig. 7D). Colitis protection conferred from neutralization of CD103− DC-derived Opn-s demonstrates the importance of this Opn isoform in this setting.
Fig. 7.
Opn secreted by CD103− DCs mediates colitis exacerbation. Histological scores of BALB/c Spp1−/− mice transferred with Spp1+/+ CD103− DCs and injected with Ig control (1), injected with Opn Ab (2), or transferred with Spp1−/− CD103− DCs (3) (A); MLN DC ratios (B); and percentages of IL-17A+, IFN-γ+ (C) and Foxp3+, IL-10+ (D) CD4+ T cells among MLNCs of recipients are shown. Values are expressed as mean ± SEM (n = 5 mice per group) from three separate experiments (*P < 0.05; **P < 0.01; ***P < 0.001).
Neutralization of Opn-s as a Colitis Treatment.
We also addressed the effects of systemic Opn neutralization in WT mice. Mice treated with anti-Opn were less susceptible to acute colitis, with significant differences in colon length (Fig. S7A) and histological inflammation scores (Fig. S7B) compared with Ig-treated controls. In addition, anti-Opn–treated mice exhibited significantly reduced numbers of macrophages, neutrophils, and lymphomononuclear cells (Fig. S7 C and D) in colonic mucosa. Supernatants of CD4+ T-cell cultures from the MLNs of anti-Opn–treated mice stimulated with anti-CD3 ex vivo had significantly decreased secretion of IL-17A and IFN-γ compared with CD4+ T-cell cultures from Ig-treated controls (Fig. S7E). Importantly, anti-Opn administration resulted in significantly impaired recruitment of CD103− DCs to MLNs; increased numbers of CD103+ DCs; and, consequently, decreased DC ratio (Fig. S7F). Effector Th17 and Th1 cell numbers were significantly decreased by anti-Opn administration, whereas the Foxp3+ Treg numbers were substantially elevated in MLNs (Fig. S7G). Our results demonstrate that in vivo neutralization of Opn-s ameliorates disease, suggesting a possible therapeutic approach.
SLAYGLR Motif of Opn-s Is Responsible for Its CD103− DC-Mediated Proinflammatory Action.
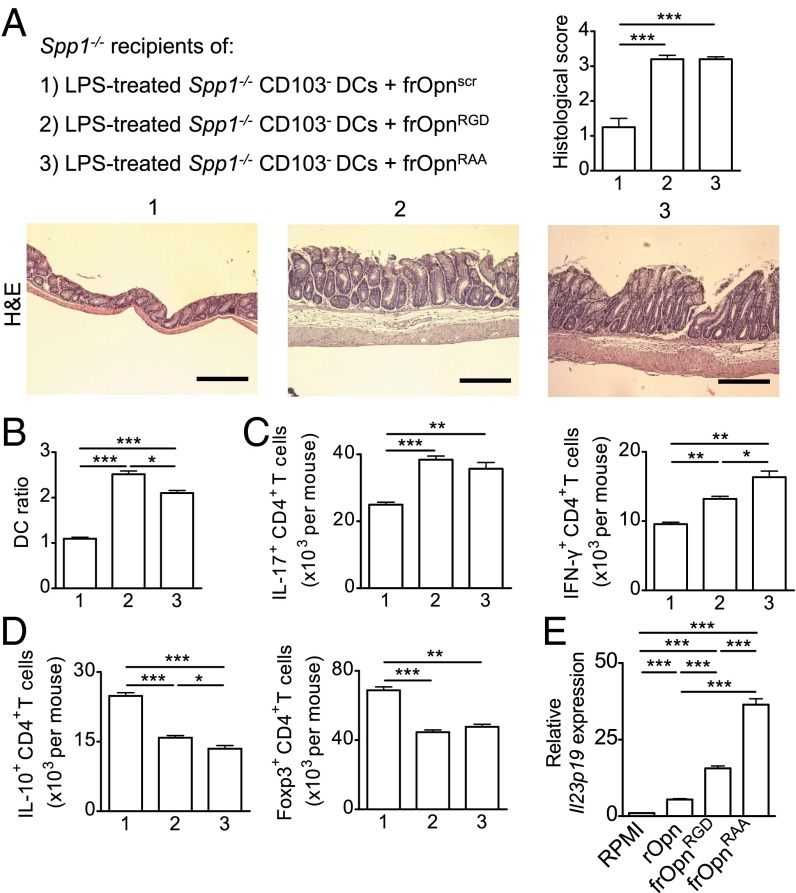
Opn-s has certain domains that interact with integrins expressed in DCs (45). We tested the effects of two synthetic Opn-s fragments (Opn134–153), RGD (Arg-Gly-Asp)-SLAYGLR (frOpnRGD) and RAA (Arg-Ala-Ala)-SLAYGLR (frOpnRAA), during colitis (Fig. S6C). The frOpnRGD includes the RGD domain that interacts with αvβ1, αvβ3, αvβ5, αvβ6, α5β1, and α8β1 (46–48), as well as the SLAYGLR motif that interacts with integrins α9β1, α4β1, and α4β7 (49–51) (Fig. S6C). The frOpnRAA contains the inactive RAA domain in the place of the RGD and the intact SLAYGLR motif (Fig. S6C). We chose a setting in which CD103− DCs could not secrete Opn (Spp1−/−) so that the observed effects would depend only on the exogenously provided Opn fragments. We transferred LPS-stimulated Spp1−/− CD103− DCs treated with frOpnRGD, frOpnRAA, or control scrambled frOpn into Spp1−/− recipient mice. Transfer of either frOpnRGD- or frOpnRAA-treated Spp1−/− CD103− DCs resulted in significant disease exacerbation (Fig. 8A), a higher DC ratio (Fig. 8B), and elevated numbers of Th17 and Th1 cells in MLNs (Fig. 8C) compared with control. Disease exacerbation was accompanied by decreases in Foxp3+ and IL-10+ CD4+ Treg populations (Fig. 8D). Therefore, the effects of Opn on the pathogenicity of CD103− DCs could be reproduced by Opn fragments that contain integrin-binding domains. Active SLAYGLR domain alone can reproduce the herein observed Opn effects on pathogenicity of CD103− DCs, because both frOpnRGD- and frOpnRAA-treated Spp1−/− CD103− DCs had similar colitogenic properties.
Fig. 8.
SLAYGLR Opn fragment is proinflammatory. (A) Representative photographs of colonic sections and histological scores of BALB/c Spp1−/− recipients of Spp1−/− CD103− DCs pretreated with scrambled frOpn (frOpnscr) (1), frOpnRGD (2), and frOpnRAA (3). (Magnification: 10×.) (Scale bars: 200 μm.) MLN DC ratios (B) and numbers of IL-17A+, IFN-γ+ (C) and IL-10+, Foxp3+ (D) MLN CD4+ T cells per mouse are shown. (E) Il23p19 mRNA expression by Spp1−/− CD103− DCs treated with RPMI or Opn fragments. Values are expressed as mean ± SEM (n = 6 mice per group) from three separate experiments (*P < 0.05; **P < 0.01; ***P < 0.001).
In support, LPS-stimulated Spp1−/− CD103− DCs treated with frOpnRAA expressed dramatically higher levels of Il23p19 compared with DCs treated with frOpnRGD, rOpn, or RPMI (Fig. 8E).
CD103− DC Surface Integrin α9 Mediates the Proinflammatory Effects of the Opn-s SLAYGLR Motif.
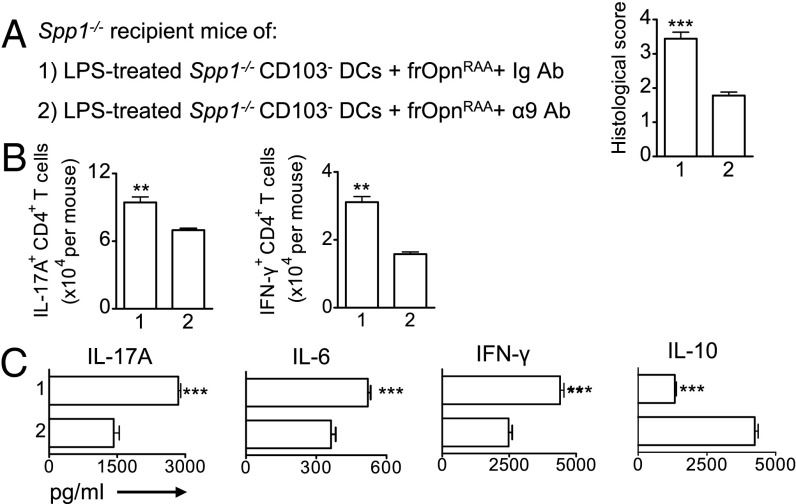
We asked which integrin expressed on the CD103− DC surface could account for the Opn-s SLAYGLR-mediated proinflammatory effects. The SLAYGLR domain is known to bind integrins α9 and α4. Integrin α9 was a highly likely candidate, because colitic WT CD103− DCs overexpress Itga9 (Fig. 4A) and Itga9 expressed on conventional DCs (cDCs) has a role in Th17 induction in an RA model (19). Therefore, we blocked integrin α9 expressed on Spp1−/− CD103− DCs during frOpnRAA treatment, and subsequently transferred them into Spp1−/− recipients. Transfer of the anti-α9/frOpnRAA–treated Spp1−/− CD103− DCs resulted in a significantly decreased disease score (Fig. 9A) and lower numbers of proinflammatory Th17 and Th1 cells in MLNs compared with the transfer of control Ig/frOpnRAA-treated Spp1−/− CD103− DCs (Fig. 9B). Accordingly, there was significantly decreased IL-17A, IL-6, and IFN-γ production by MLNCs from recipients transferred with anti-α9/frOpnRAA–treated CD103− DCs compared with Ig/frOpnRAA-treated CD103− DC recipients (Fig. 9C). Also, transfer of anti-α9/frOpnRAA–treated Spp1−/− CD103− DCs increased IL-10 levels in MLNCs compared with transfer of Ig/frOpnRAA-treated CD103− DCs (Fig. 9C). Interestingly, frOpnRGD or frOpnRAA treatment of LPS-stimulated CD103− DCs enhanced their Itga9 expression and not their Itga4 expression (Fig. S6D).
Fig. 9.
Disruption of interaction of frOpnRAA with integrin α9 on CD103− DCs suppresses their proinflammatory effects. (A) Histological scores of BALB/c Spp1−/− colitic mice that received Spp1−/− CD103− DCs pretreated with either Ig Ab/frOpnRAA (1) or with integrin α9 Ab/frOpnRAA (2). Numbers of MLN IL-17A+ and IFN-γ+ CD4+ T cells per mouse (B) and cytokine secretion levels by anti-CD3/28–stimulated (96 h) colitic MLNCs (C) are shown. Values are expressed as mean ± SEM (n = 5 mice per group) from two separate experiments (**P < 0.01; ***P < 0.001).
Discussion
Intestinal CD103− DCs have been generally characterized as proinflammatory for colitis (12). In this report, we demonstrate that MLN CD103− DCs are indeed pathogenic and express, among other proinflammatory cytokines, high levels of Opn. Although there is up-regulated Opn expression in plasma and intestinal tissue from patients and mice with IBD (14, 15, 24–30, 52), its specific role had not been determined. We found that Opn expression by CD103− DCs is crucial for their pathogenicity. Overall, our results elucidate a previously unappreciated role for Opn in colitis and its relation to CD103− DC-mediated responses.
The pathogenicity of CD103− DCs has been studied using only bone marrow-derived CD103− DCs resulting from in vitro treatment with GM-CSF (11, 12). Here, we show that CD103− DCs directly isolated from MLNs express IL-6, IL-12, IL-23, and Opn and are pathogenic for colitis. Acute colitis caused an elevation in MLN CD103− DC numbers, and this significant rise was mainly due to the development of CD11b+ DCs. Expression of CD11b by CD103− DCs denotes their origin from blood monocytes, and these monocyte-derived DCs (moDCs) present in MLNs have proinflammatory properties upon colitis (9, 12, 39, 53). Consequently, adoptive transfer of total MLN CD103− DCs (containing both the CD11b− and the CD11b+ subpopulations) into recipients predisposes them to severe colitis, and this potential is linked to their levels of Opn expression. Adoptive transfers of CD103− DCs in Opn-deficient Rag1−/− mice with chronic CD4+ T-cell transfer colitis resulted in disease augmentation, and inflammation was further elevated when transferred CD103− DCs overexpressed Opn (obtained from Spp1-stopf/f/Itgaxcre or tamoxifen-treated Spp1-stopf/f/CreERT1 mice). Opn expression in CD103− DCs isolated from healthy mice is essential for their colitogenic activity, which is also revealed upon adoptive transfer of these cells. Thus, recipients of Opn-deficient CD103− DCs showed dramatic attenuation of disease compared with recipients of WT CD103− DCs. Recent studies demonstrate that Opn derived from a broad DC subset, known as cDC subset, induces Th17 cells (17, 18) and has been linked to increased IL-6 production by cDCs (19). Past studies described the role of Opn in whole cDCs, whereas we addressed here the effects of Opn produced by CD103− DCs, a subpopulation of cDCs important for colitis (12). Our results showed Opn-mediated Th17 induction which was readily explained by the dramatic increase in IL-23 and IL-6 expression by Opn-expressing CD103− DCs. Interestingly, although Th1 responses were significantly enhanced, we found only modest elevations in IL-12 secretion by Opn-expressing CD103− DCs. It is possible that IL-23 and IL-6, apart from Th17 response induction, may also reinforce Th1 responses (54, 55). We also observed that CD103− DC-derived Opn enhances their IL-27 expression upon colitis. This is in contrast to a study describing that cDC-derived Opn induces Th17 responses mainly through the inhibition of IL-27 in the experimental autoimmune encephalomyelitis (EAE) model (17). However, a recent report demonstrates that IL-27 is detrimental for colitis because it mediates survival of activated CD4+ T cells, resulting in enhanced disease severity (56). In addition, IL-27 inhibits Foxp3 induction in CD4+ T cells in vitro (57). Thus, certain differences in the observed Opn effects on IL-27 production between our study and previous studies (17) may be attributed to special features of gut-associated CD103− DCs and require further investigation.
Colitis, in comparison to steady state, resulted in ∼20% elevation in the numbers of E-cadherin+ CD103−CD11b+ MLN DCs, a subset similar to the previously described highly proinflammatory moDC subset (12). However, the Ly6C+ subpopulation of CD11b+CD103− DCs was decreased upon colitis induction. Ly6C is a specific marker that also reliably identifies moDCs in the CD11b+ DC compartment (58). Opn expression in colitis, in comparison to Opn deficiency, resulted in enhanced MLN accumulation of the CD103− DC subset, reflecting equal elevations in both of their CD11b+ and CD11b− fractions but preferentially increased Ly6C+ (∼13-fold) vs. E-cadherin+ (∼fourfold) among the CD11b+CD103− DC subset. Thus, Opn expression during colitis has a dramatic effect on the accumulation of moDCs in MLNs. However, although Opn expression during colitis also enhanced the accumulation of CD11b+CD103− DCs in LP, similar to MLN, there were no significant increases in either E-cadherin+ or Ly6C+ moDC populations in that lymphoid compartment. It appears that Opn may be influencing influx of moDCs to MLN mainly. This could be due to direct and/or indirect effects of Opn on these cells. For example, we found that Opn expression during colitis enhances CCR7 expression on CD103− DCs, which is crucial for DC mobilization to LNs (42, 43).
Limited studies describe the role of CD103+ DCs during colitis (13, 59). In MLNs, 40–60% of DCs express CD103 (CD103+ DCs) and migrate to MLNs from LP in steady state (8) and in chronic colitis (6). Our experiments demonstrate that MLN CD103+ DCs were nonpathogenic in acute colitis and their Opn expression was significantly lower compared with CD103− DCs. Actually, CD103+ DCs appeared to exert a protective function, because they enhanced Foxp3+ Tregs and decreased Th1 and Th17 cell numbers. Our results are in agreement with the role of CD103+ DCs in the absence of inflammatory processes as mediators of gut homeostasis and oral tolerance as well as inducers of Foxp3+ Tregs (4–9).
A previous study demonstrated that Opn-deficient C57BL/6 mice had decreased severity of very early acute TNBS colitis (3 d) (14). However, studies addressing the role of Opn during DSS-induced colitis, another chemically induced model, have been contradictory (14, 15, 60–63). Opn deficiency in Black Swiss mice was protective (15), whereas Opn deficiency in C57BL/6 mice resulted in increased intestinal tissue destruction (60) and exacerbated disease phenotype (61). Our results demonstrate that Opn is proinflammatory in both the TNBS model and the DSS model, because WT mice have significantly increased severity and pathogenic Th17 and Th1 cell numbers in comparison to Opn-deficient mice. Contradictory results on the DSS-induced colitis can be attributed to differences in the methods used. For example, Da Silva et al. (60) administered a significantly higher dose of DSS in mice (5% DSS, double the dose used in standard protocols and in our protocol). Heilmann et al. (61) found that Opn was anti-inflammatory during acute DSS colitis but proinflammatory during chronic DSS colitis. We have not tested chronic DSS colitis; however, our studies on T-cell transfer chronic colitis showed that Opn expression by CD103− DCs exacerbated disease.
Here, we also demonstrate that TNBS and DSS colitis can develop in Rag−/− mice. TNBS-driven colitis is initiated by intrarectal instillation of a solution of ethanol, which breaks the mucosal barrier, with TNBS, which haptenizes autologous colonic or microflora proteins, leading to Th1 and Th17 responses (64). Despite the fact that the TNBS colitis model was traditionally considered T cell-dependent (65), studies have demonstrated that it can develop in the absence of adaptive immunity (66). Chemical haptens are sensitizers with a high ability to elicit DC migration from the sites of sensitization to draining LNs to present antigens and activate immune responses (67). Thus, initiation of the TNBS-induced colitis relies a lot on DCs and other innate cells (67). A previous study demonstrates that TNBS-driven colitis can develop in Rag−/− mice and can be suppressed with anti–α1-integrin treatment, which prevents mononuclear LP cells from proliferating and producing IFN-γ (66). In addition, DSS, another chemical that destroys the integrity of the mucosa, can cause colitis in the absence of adaptive immunity (68). In our experiments, Rag−/−Spp1+/+ mice are more susceptible to TNBS or DSS colitis compared with Rag−/−Spp1−/− mice. Isolated MLN CD103− DCs from colitic BALB/c Rag2−/−Spp1+/+ mice express higher levels of Il6 and Il23 compared with CD103− DCs from colitic Rag2−/−Spp1−/− mice. Also, Opn treatment of LPS-stimulated DCs induces IL-23 expression. IL-23 and IL-6 expressed by several subsets of innate immune cells are critical cytokines for IBD development (69, 70) and IL-23 drives T cell-independent colitis in Rag−/− mice (69, 71). In LNs and colonic LP of Rag−/− mice, only DCs, macrophages, and innate lymphoid cells from the innate compartment express IL-23R (72). In response to IL-23, DCs produce great amounts of IL-6, which activates other innate cells that may mediate tissue destruction (72). In addition, IL-23 stimulates innate lymphoid cells (ILCs) to secrete IL-17, IL-22, and IFN-γ in human colitis (73) and mouse colitis (71). Thus, Opn may contribute to innate colitis via induction of IL-23 in DCs and/or other innate cells. Also, Opn may have a yet unknown effect on ILCs. Because targeting of IL-17A in patients has not been efficient (74), the need for other cytokine targets for therapy that act upstream of IL-17, such as IL-23 and Opn, and the need for clarification of their pathways of action emerge.
DC Opn is expressed in two isoforms: Opn-s and Opn-i (34), generated by alternative translation of the Spp1 mRNA (17, 33). Neutralization of the CD103− DC-derived Opn in colitic Opn-deficient mice transferred with WT CD103− DCs resulted in ameliorated disease and revealed that it was the Opn-s isoform mainly responsible for the proinflammatory phenotype of the CD103− DCs. Opn-s, from CD103− DCs or other cellular sources, probably acts on CD103− DCs to potentiate their colitogenic function. Indeed, pretreatment of Opn-deficient CD103− DCs with rOpn or certain Opn fragments resulted in their enhanced pathogenicity upon transfer into recipients. Although we find that Opn-s drives the colitogenic activity of CD103− DCs, we do not exclude possible contributions by Opn-i in these cells during colitis. Elegant studies show that Opn-i expression by DCs promotes inflammation in antiviral (34) and autoimmune responses (17). Specifically, Opn-i mediates TLR-9–induced expression of IFN-α in plasmacytoid DCs (pDCs) via interactions with MyD88 (34), and Opn-i inhibition in IFN-α–activated cDCs dampens Th17 cell commitment through IL-27 production in EAE (17). Because MLN CD103− DCs express high levels of TLR-2, TLR-4, and TLR-9 (7, 12), they could possibly be subjected to Opn-i–mediated TLR regulation during intestinal inflammation. Specifically, because TLR-9 engagement and subsequent type I IFN production are protective in acute colitis (75), Opn-i from CD103− DCs and/or from other DC subsets, such as pDCs, could influence disease.
Through its RGD (46–48) and SLAYGLR domains (49, 50), Opn interacts with integrins expressed on several cell types, such as fibroblasts, endothelial cells, and epithelial cells, as well as on a variety of immune cells (45, 76). The SLAYGLR Opn domain contributes to RA development (77, 78) and interacts with integrins α9 and α4 (49–51). Upon inflammatory conditions, thrombin cleaves Opn-s right downstream of the SLAYGLR sequence, exposing an extra integrin-binding site (79). Integrin α4 can interact with SLAYGLR in full-length Opn, whereas integrin α9 interacts only with the thrombin-exposed SLAYGLR motif (79–81). Here, we demonstrate that the proinflammatory effect of Opn on CD103− DCs was mainly mediated by the Opn SLAYGLR domain, because DC pretreatment with a synthetic Opn fragment (frOpnRAA) containing exposed SLAYGLR and mutated RGD domain enhanced their pathogenicity. Blockade of integrin α9 during Opn SLAYGLR pretreatment of Opn-deficient CD103− DCs resulted in suppression of their proinflammatory profile and dramatically decreased their pathogenicity upon transfer into recipient mice. This indicated that integrin α9 mediates the observed Opn effects on CD103− DCs. In collagen-induced arthritis, blockade of the interaction of tenascin-C (TN-C) with integrin α9, expressed on cDCs, down-regulated their IL-6 expression (19). Our results demonstrated that colitis induction or LPS stimulation caused significant up-regulation of both Opn and Itga9 expression in WT CD103− DCs. Interestingly, frOpn treatment of LPS-stimulated CD103− DCs enhanced their Itga9 expression. Thus, it appears that inflammatory stimuli propagate circles of interactions of Opn and integrin α9. It has to be emphasized that there are other candidate ligands of integrin α9, such as TN-C, vascular cell adhesion molecule-1, and VEGF-A (19, 82–84), some of which are overexpressed in the colitic microenvironment (85, 86). It is unknown whether interactions of these molecules with integrin α9 on CD103− DCs enhance or restrain the described proinflammatory function of Opn.
As mentioned, CD103− DCs are a source of Opn, which can act on them in an autocrine manner. However, Opn secreted by other cells, such as epithelial and LP mononuclear cells in the colitic microenvironment (25, 26, 63), could affect CD103− DCs in a paracrine manner. Here, we show that Opn expressed by CD103− DCs is crucial for the colitogenic effect of these cells. One of the mechanisms mediating this potentiation could be autocrine or paracrine Opn-s (and especially its SLAYGLR domain) acting on CD103− DCs, enhancing their IL-23 expression. Nevertheless, receptors for Opn (several integrins and CD44 isoforms) are expressed on cell types like fibroblasts, endothelial cells, and epithelial cells, as well as on a variety of immune cells like T cells and macrophages (45, 76). This implies that cells other than DCs are subjected to changes through Opn signals in the inflammatory environment of colitis, which is Opn-rich (25).
We have demonstrated that Opn expression during allergen sensitization differentially influences recruitment of DC subsets by enhancing recruitment of proinflammatory cDCs while restraining recruitment of tolerogenic pDCs (35). In addition, Opn-deficient mice have a defect in trinitrochlorobenzene-induced DC migration in draining LNs (37), whereas Opn treatment may dampen DC migration during bacterial infection (87). In the current study, WT colitic mice and Opn-deficient colitic recipients of transferred WT CD103− DCs exhibited higher total MLN CD103− DC numbers in comparison to Opn-deficient mice and recipients of Opn-deficient CD103− DCs, respectively. Increases in CD103− DC numbers were accompanied by concomitant reductions in CD103+ DC numbers, and thus a higher MLN DC ratio (CD103−/CD103+ DCs), reflecting exacerbated disease. We showed that this Opn effect on MLN DC ratio was not due to enhanced proliferation of CD103− DCs and/or reduced proliferation of CD103+ DCs but to differential influence on the CD103+ and CD103− DC accumulation in MLNs. However, whether this is a direct effect of Opn on DC subset recruitment is unknown. Opn itself can act as a chemotaxis molecule for DCs (20, 37, 88). Whether CD103− DCs preferentially bear certain Opn receptors that eventually influence their MLN recruitment remains unknown. Our study suggests that the DC subset ratio, the numbers of CD103− DCs, and their Opn and possibly surface integrin α9 expression can be useful disease biomarkers.
Opn is up-regulated in IBD (24–29) and several other inflammatory conditions (16–21, 89). However, whether this up-regulation can be attributed to specific cellular sources remains elusive. It is possible that patients with CD have enhanced Opn or integrin α9 expression in CD103− DCs, predisposing them to disease. The Spp1-stopf/f/Itgaxcre Tg mice we constructed were overexpressing Opn only in their DCs, and this rendered them susceptible to severe colitis. Targeting of cytokines is extensively used in the therapy of immune disorders, including IBD (90). In addition to cytokines, several integrins like integrin α1β1 and integrin α4β7 are known to be essential for colitis pathology (66) and have been proposed as potential target molecules for therapy (66, 91, 92). However, their systemic blockade can cause generalized immunosuppression (92), similar to the broadly used anti–TNF-α (90). Thus, a more focused anti-inflammatory therapy for colitis is necessary. We showed that disrupting the interaction of the Opn SLAYGLR domain with integrin α9 overexpressed on the colitic CD103− DCs suppressed the proinflammatory function of these cells, opening scientific questions and suggesting future therapeutic approaches for IBD.
Materials and Methods
Colitis studies were performed in 8- to 10-wk-old male mice. For acute colitis induction, mice were administered 1–3 mg of TNBS (Sigma–Aldrich) in ethanol intrarectally as previously described (64). On day 5, mice were euthanized. Mice received 20 μg of a neutralizing Ab to mouse Opn (AF-808; R&D Systems) i.p. or 20 μg of isotype control 1 d before (d − 1) and 1 d following (d + 1) administration of TNBS. rOpn (2.5 μg; R&D Systems) was administered i.p. daily. Procedures have been described previously (93) for the acute DSS colitis model. Mice were analyzed on day 7. Purified DCs were transferred 2 h before colitis induction. For the CD4+ T-cell transfer model of chronic colitis, naive CD4+CD25−CD44−CD62L+CD45RBhigh T cells were sorted from C57BL/6 mice after enrichment with a CD4+ T-Cell Isolation Kit II (Miltenyi Biotec). The following anti-mouse mAbs were used for FACS sorting (FACSAria III; Becton Dickinson): CD3 (clone 145-2C11), CD4 (GK1.5), CD62L (MEL-14), CD25 (PC61.5), CD44 (IM7), and CD45RB (C363.16A). Sex-matched C57BL/6 Rag1−/− recipients were injected i.p. with 5 × 105 cells. Mice were euthanized at day 30 after cell transfer, and tissues were collected for cell populations or histological analysis.
Histology, mice, in vivo protocols, cell cultures, cytokine analysis, proliferation assays, flow cytometric analysis, adoptive transfers, and quantitative PCR assays are described in more detail in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank E. Anastasiadou, S. Georgopoulos, and L. Katsouri for assistance in the Tg mouse generation and E. Papaioannou, K. Petsiou, V. Fatourou, and G. Michas for technical assistance. This work was supported by the European Research Council (ERC) under the European Union’s Seventh Framework Program (FP/2007-2013)/ERC Grant Agreement 243322 (to V.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. K.M.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1316447111/-/DCSupplemental.
References
- 1.Baumgart DC, Carding SR. Inflammatory bowel disease: Cause and immunobiology. Lancet. 2007;369(9573):1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 2.Rescigno M, Di Sabatino A. Dendritic cells in intestinal homeostasis and disease. J Clin Invest. 2009;119(9):2441–2450. doi: 10.1172/JCI39134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakuraba A, et al. Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn’s disease. Gastroenterology. 2009;137(5):1736–1745. doi: 10.1053/j.gastro.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 4.Johansson-Lindbom B, et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202(8):1063–1073. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaensson E, et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205(9):2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Annacker O, et al. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202(8):1051–1061. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204(8):1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulz O, et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206(13):3101–3114. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivollier A, He J, Kole A, Valatas V, Kelsall BL. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J Exp Med. 2012;209(1):139–155. doi: 10.1084/jem.20101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerovic V, et al. Intestinal CD103(-) dendritic cells migrate in lymph and prime effector T cells. Mucosal Immunol. 2013;6(1):104–113. doi: 10.1038/mi.2012.53. [DOI] [PubMed] [Google Scholar]
- 11.Fortin G, et al. A role for CD47 in the development of experimental colitis mediated by SIRPalpha+CD103- dendritic cells. J Exp Med. 2009;206(9):1995–2011. doi: 10.1084/jem.20082805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siddiqui KR, Laffont S, Powrie F. E-cadherin marks a subset of inflammatory dendritic cells that promote T cell-mediated colitis. Immunity. 2010;32(4):557–567. doi: 10.1016/j.immuni.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spadoni I, Iliev ID, Rossi G, Rescigno M. Dendritic cells produce TSLP that limits the differentiation of Th17 cells, fosters Treg development, and protects against colitis. Mucosal Immunol. 2012;5(2):184–193. doi: 10.1038/mi.2011.64. [DOI] [PubMed] [Google Scholar]
- 14.Oz HS, Zhong J, de Villiers WJ. Osteopontin ablation attenuates progression of colitis in TNBS model. Dig Dis Sci. 2012;57(6):1554–1561. doi: 10.1007/s10620-011-2009-z. [DOI] [PubMed] [Google Scholar]
- 15.Zhong J, Eckhardt ER, Oz HS, Bruemmer D, de Villiers WJ. Osteopontin deficiency protects mice from Dextran sodium sulfate-induced colitis. Inflamm Bowel Dis. 2006;12(8):790–796. doi: 10.1097/00054725-200608000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Shinohara ML, et al. T-bet-dependent expression of osteopontin contributes to T cell polarization. Proc Natl Acad Sci USA. 2005;102(47):17101–17106. doi: 10.1073/pnas.0508666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shinohara ML, Kim JH, Garcia VA, Cantor H. Engagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: role of intracellular osteopontin. Immunity. 2008;29(1):68–78. doi: 10.1016/j.immuni.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murugaiyan G, Mittal A, Weiner HL. Increased osteopontin expression in dendritic cells amplifies IL-17 production by CD4+ T cells in experimental autoimmune encephalomyelitis and in multiple sclerosis. J Immunol. 2008;181(11):7480–7488. doi: 10.4049/jimmunol.181.11.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanayama M, et al. α9β1 integrin-mediated signaling serves as an intrinsic regulator of pathogenic Th17 cell generation. J Immunol. 2011;187(11):5851–5864. doi: 10.4049/jimmunol.1101524. [DOI] [PubMed] [Google Scholar]
- 20.Inoue M, Williams KL, Gunn MD, Shinohara ML. NLRP3 inflammasome induces chemotactic immune cell migration to the CNS in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2012;109(26):10480–10485. doi: 10.1073/pnas.1201836109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murugaiyan G, Mittal A, Weiner HL. Identification of an IL-27/osteopontin axis in dendritic cells and its modulation by IFN-gamma limits IL-17-mediated autoimmune inflammation. Proc Natl Acad Sci USA. 2010;107(25):11495–11500. doi: 10.1073/pnas.1002099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jansson M, Panoutsakopoulou V, Baker J, Klein L, Cantor H. Cutting edge: Attenuated experimental autoimmune encephalomyelitis in eta-1/osteopontin-deficient mice. J Immunol. 2002;168(5):2096–2099. doi: 10.4049/jimmunol.168.5.2096. [DOI] [PubMed] [Google Scholar]
- 23.Cantor H, Shinohara ML. Regulation of T-helper-cell lineage development by osteopontin: The inside story. Nat Rev Immunol. 2009;9(2):137–141. doi: 10.1038/nri2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masuda H, Takahashi Y, Asai S, Takayama T. Distinct gene expression of osteopontin in patients with ulcerative colitis. J Surg Res. 2003;111(1):85–90. doi: 10.1016/s0022-4804(03)00046-5. [DOI] [PubMed] [Google Scholar]
- 25.Sato T, et al. Osteopontin/Eta-1 upregulated in Crohn’s disease regulates the Th1 immune response. Gut. 2005;54(9):1254–1262. doi: 10.1136/gut.2004.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masuda H, Takahashi Y, Asai S, Hemmi A, Takayama T. Osteopontin expression in ulcerative colitis is distinctly different from that in Crohn’s disease and diverticulitis. J Gastroenterol. 2005;40(4):409–413. doi: 10.1007/s00535-005-1567-2. [DOI] [PubMed] [Google Scholar]
- 27.Neuman MG. Osteopontin biomarker in inflammatory bowel disease, animal models and target for drug discovery. Dig Dis Sci. 2012;57(6):1430–1431. doi: 10.1007/s10620-012-2120-9. [DOI] [PubMed] [Google Scholar]
- 28.Gassler N, et al. Expression of osteopontin (Eta-1) in Crohn disease of the terminal ileum. Scand J Gastroenterol. 2002;37(11):1286–1295. doi: 10.1080/003655202761020560. [DOI] [PubMed] [Google Scholar]
- 29.Mishima R, et al. High plasma osteopontin levels in patients with inflammatory bowel disease. J Clin Gastroenterol. 2007;41(2):167–172. doi: 10.1097/MCG.0b013e31802d6268. [DOI] [PubMed] [Google Scholar]
- 30.Oz HS, Chen TS, Nagasawa H. Comparative efficacies of 2 cysteine prodrugs and a glutathione delivery agent in a colitis model. Transl Res. 2007;150(2):122–129. doi: 10.1016/j.trsl.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glas J, et al. The role of osteopontin (OPN/SPP1) haplotypes in the susceptibility to Crohn’s disease. PLoS ONE. 2011;6(12):e29309. doi: 10.1371/journal.pone.0029309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Renkl AC, et al. Osteopontin functionally activates dendritic cells and induces their differentiation toward a Th1-polarizing phenotype. Blood. 2005;106(3):946–955. doi: 10.1182/blood-2004-08-3228. [DOI] [PubMed] [Google Scholar]
- 33.Shinohara ML, Kim HJ, Kim JH, Garcia VA, Cantor H. Alternative translation of osteopontin generates intracellular and secreted isoforms that mediate distinct biological activities in dendritic cells. Proc Natl Acad Sci USA. 2008;105(20):7235–7239. doi: 10.1073/pnas.0802301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shinohara ML, et al. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat Immunol. 2006;7(5):498–506. doi: 10.1038/ni1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xanthou G, et al. Osteopontin has a crucial role in allergic airway disease through regulation of dendritic cell subsets. Nat Med. 2007;13(5):570–578. doi: 10.1038/nm1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawamura K, et al. Differentiation, maturation, and survival of dendritic cells by osteopontin regulation. Clin Diagn Lab Immunol. 2005;12(1):206–212. doi: 10.1128/CDLI.12.1.206-212.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss JM, et al. Osteopontin is involved in the initiation of cutaneous contact hypersensitivity by inducing Langerhans and dendritic cell migration to lymph nodes. J Exp Med. 2001;194(9):1219–1229. doi: 10.1084/jem.194.9.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11(6):753–761. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- 39.Varol C, et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31(3):502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 40.Neurath MF, Fuss I, Kelsall BL, Stüber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182(5):1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huber S, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3− and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34(4):554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin-Fontecha A, et al. Regulation of dendritic cell migration to the draining lymph node: Impact on T lymphocyte traffic and priming. J Exp Med. 2003;198(4):615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Platt AM, Randolph GJ. Dendritic cell migration through the lymphatic vasculature to lymph nodes. Adv Immunol. 2013;120:51–68. doi: 10.1016/B978-0-12-417028-5.00002-8. [DOI] [PubMed] [Google Scholar]
- 44.Mazmanian SK, Kasper DL. The love-hate relationship between bacterial polysaccharides and the host immune system. Nat Rev Immunol. 2006;6(11):849–858. doi: 10.1038/nri1956. [DOI] [PubMed] [Google Scholar]
- 45.Uede T. Osteopontin, intrinsic tissue regulator of intractable inflammatory diseases. Pathol Int. 2011;61(5):265–280. doi: 10.1111/j.1440-1827.2011.02649.x. [DOI] [PubMed] [Google Scholar]
- 46.Liaw L, et al. The adhesive and migratory effects of osteopontin are mediated via distinct cell surface integrins. Role of alpha v beta 3 in smooth muscle cell migration to osteopontin in vitro. J Clin Invest. 1995;95(2):713–724. doi: 10.1172/JCI117718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu DD, Lin EC, Kovach NL, Hoyer JR, Smith JW. A biochemical characterization of the binding of osteopontin to integrins alpha v beta 1 and alpha v beta 5. J Biol Chem. 1995;270(44):26232–26238. doi: 10.1074/jbc.270.44.26232. [DOI] [PubMed] [Google Scholar]
- 48.Yokosaki Y, Tanaka K, Higashikawa F, Yamashita K, Eboshida A. Distinct structural requirements for binding of the integrins alphavbeta6, alphavbeta3, alphavbeta5, alpha5beta1 and alpha9beta1 to osteopontin. Matrix Biol. 2005;24(6):418–427. doi: 10.1016/j.matbio.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 49.Smith LL, et al. Osteopontin N-terminal domain contains a cryptic adhesive sequence recognized by alpha9beta1 integrin. J Biol Chem. 1996;271(45):28485–28491. [PubMed] [Google Scholar]
- 50.Green PM, Ludbrook SB, Miller DD, Horgan CM, Barry ST. Structural elements of the osteopontin SVVYGLR motif important for the interaction with alpha(4) integrins. FEBS Lett. 2001;503(1):75–79. doi: 10.1016/s0014-5793(01)02690-4. [DOI] [PubMed] [Google Scholar]
- 51.Lund SA, et al. Osteopontin mediates macrophage chemotaxis via α4 and α9 integrins and survival via the α4 integrin. J Cell Biochem. 2013;114(5):1194–1202. doi: 10.1002/jcb.24462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aziz MM, et al. MFG-E8 attenuates intestinal inflammation in murine experimental colitis by modulating osteopontin-dependent alphavbeta3 integrin signaling. J Immunol. 2009;182(11):7222–7232. doi: 10.4049/jimmunol.0803711. [DOI] [PubMed] [Google Scholar]
- 53.Zigmond E, et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 2012;37(6):1076–1090. doi: 10.1016/j.immuni.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 54.Lee YK, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30(1):92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamamoto M, Yoshizaki K, Kishimoto T, Ito H. IL-6 is required for the development of Th1 cell-mediated murine colitis. J Immunol. 2000;164(9):4878–4882. doi: 10.4049/jimmunol.164.9.4878. [DOI] [PubMed] [Google Scholar]
- 56.Kim G, Shinnakasu R, Saris CJ, Cheroutre H, Kronenberg M. A novel role for IL-27 in mediating the survival of activated mouse CD4 T lymphocytes. J Immunol. 2013;190(4):1510–1518. doi: 10.4049/jimmunol.1201017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neufert C, et al. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur J Immunol. 2007;37(7):1809–1816. doi: 10.1002/eji.200636896. [DOI] [PubMed] [Google Scholar]
- 58.Plantinga M, et al. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity. 2013;38(2):322–335. doi: 10.1016/j.immuni.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 59.Laffont S, Siddiqui KR, Powrie F. Intestinal inflammation abrogates the tolerogenic properties of MLN CD103+ dendritic cells. Eur J Immunol. 2010;40(7):1877–1883. doi: 10.1002/eji.200939957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Da Silva AP, et al. Exacerbated tissue destruction in DSS-induced acute colitis of OPN-null mice is associated with downregulation of TNF-alpha expression and non-programmed cell death. J Cell Physiol. 2006;208(3):629–639. doi: 10.1002/jcp.20701. [DOI] [PubMed] [Google Scholar]
- 61.Heilmann K, et al. Osteopontin as two-sided mediator of intestinal inflammation. J Cell Mol Med. 2009;13(6):1162–1174. doi: 10.1111/j.1582-4934.2008.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.da Silva AP, et al. Osteopontin attenuation of dextran sulfate sodium-induced colitis in mice. Lab Invest. 2009;89(10):1169–1181. doi: 10.1038/labinvest.2009.80. [DOI] [PubMed] [Google Scholar]
- 63.Chen F, et al. Osteopontin: Participation in inflammation or mucosal protection in inflammatory bowel diseases? Dig Dis Sci. 2013;58(6):1569–1580. doi: 10.1007/s10620-012-2556-y. [DOI] [PubMed] [Google Scholar]
- 64.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2(3):541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 65.Neurath MF, et al. Experimental granulomatous colitis in mice is abrogated by induction of TGF-beta-mediated oral tolerance. J Exp Med. 1996;183(6):2605–2616. doi: 10.1084/jem.183.6.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fiorucci S, et al. Importance of innate immunity and collagen binding integrin alpha1beta1 in TNBS-induced colitis. Immunity. 2002;17(6):769–780. doi: 10.1016/s1074-7613(02)00476-4. [DOI] [PubMed] [Google Scholar]
- 67.Kripke ML, Munn CG, Jeevan A, Tang JM, Bucana C. Evidence that cutaneous antigen-presenting cells migrate to regional lymph nodes during contact sensitization. J Immunol. 1990;145(9):2833–2838. [PubMed] [Google Scholar]
- 68.Dieleman LA, et al. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107(6):1643–1652. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- 69.Hue S, et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203(11):2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Uhlig HH, et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25(2):309–318. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 71.Buonocore S, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464(7293):1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Awasthi A, et al. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J Immunol. 2009;182(10):5904–5908. doi: 10.4049/jimmunol.0900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geremia A, et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med. 2011;208(6):1127–1133. doi: 10.1084/jem.20101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baeten DL, Kuchroo VK. How Cytokine networks fuel inflammation: Interleukin-17 and a tale of two autoimmune diseases. Nat Med. 2013;19(7):824–825. doi: 10.1038/nm.3268. [DOI] [PubMed] [Google Scholar]
- 75.Katakura K, et al. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J Clin Invest. 2005;115(3):695–702. doi: 10.1172/JCI22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Denhardt DT, Noda M, O’Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: Regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107(9):1055–1061. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamamoto N, et al. Essential role of the cryptic epitope SLAYGLR within osteopontin in a murine model of rheumatoid arthritis. J Clin Invest. 2003;112(2):181–188. doi: 10.1172/JCI17778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gravallese EM. Osteopontin: A bridge between bone and the immune system. J Clin Invest. 2003;112(2):147–149. doi: 10.1172/JCI19190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yokosaki Y, et al. The integrin alpha(9)beta(1) binds to a novel recognition sequence (SVVYGLR) in the thrombin-cleaved amino-terminal fragment of osteopontin. J Biol Chem. 1999;274(51):36328–36334. doi: 10.1074/jbc.274.51.36328. [DOI] [PubMed] [Google Scholar]
- 80.Bayless KJ, Davis GE. Identification of dual alpha 4beta1 integrin binding sites within a 38 amino acid domain in the N-terminal thrombin fragment of human osteopontin. J Biol Chem. 2001;276(16):13483–13489. doi: 10.1074/jbc.M011392200. [DOI] [PubMed] [Google Scholar]
- 81.Ito K, et al. The differential amino acid requirement within osteopontin in alpha4 and alpha9 integrin-mediated cell binding and migration. Matrix Biol. 2009;28(1):11–19. doi: 10.1016/j.matbio.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 82.Kanayama M, et al. Alpha9 integrin and its ligands constitute critical joint microenvironments for development of autoimmune arthritis. J Immunol. 2009;182(12):8015–8025. doi: 10.4049/jimmunol.0900725. [DOI] [PubMed] [Google Scholar]
- 83.Taooka Y, Chen J, Yednock T, Sheppard D. The integrin alpha9beta1 mediates adhesion to activated endothelial cells and transendothelial neutrophil migration through interaction with vascular cell adhesion molecule-1. J Cell Biol. 1999;145(2):413–420. doi: 10.1083/jcb.145.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yokosaki Y, et al. The integrin alpha 9 beta 1 mediates cell attachment to a non-RGD site in the third fibronectin type III repeat of tenascin. J Biol Chem. 1994;269(43):26691–26696. [PubMed] [Google Scholar]
- 85.Burns RC, et al. Antibody blockade of ICAM-1 and VCAM-1 ameliorates inflammation in the SAMP-1/Yit adoptive transfer model of Crohn’s disease in mice. Gastroenterology. 2001;121(6):1428–1436. doi: 10.1053/gast.2001.29568. [DOI] [PubMed] [Google Scholar]
- 86.Scaldaferri F, et al. VEGF-A links angiogenesis and inflammation in inflammatory bowel disease pathogenesis. Gastroenterology. 2009;136(2):585– e5. doi: 10.1053/j.gastro.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 87.Begum MD, et al. Suppression of the bacterial antigen-specific T cell response and the dendritic cell migration to the lymph nodes by osteopontin. Microbiol Immunol. 2007;51(1):135–147. doi: 10.1111/j.1348-0421.2007.tb03884.x. [DOI] [PubMed] [Google Scholar]
- 88.Tanaka K, et al. Effect of osteopontin alleles on beta-glucan-induced granuloma formation in the mouse liver. Am J Pathol. 2004;164(2):567–575. doi: 10.1016/s0002-9440(10)63146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ashkar S, et al. Eta-1 (osteopontin): An early component of type-1 (cell-mediated) immunity. Science. 2000;287(5454):860–864. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 90.Baumgart DC, Sandborn WJ. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet. 2007;369(9573):1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 91.Podolsky DK, et al. Attenuation of colitis in the cotton-top tamarin by anti-alpha 4 integrin monoclonal antibody. J Clin Invest. 1993;92(1):372–380. doi: 10.1172/JCI116575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lanzarotto F, Carpani M, Chaudhary R, Ghosh S. Novel treatment options for inflammatory bowel disease: Targeting alpha 4 integrin. Drugs. 2006;66(9):1179–1189. doi: 10.2165/00003495-200666090-00002. [DOI] [PubMed] [Google Scholar]
- 93.Okayasu I, et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98(3):694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.