Significance
The study of ecological succession remains at the core of ecology. Understanding the trajectories and mechanisms controlling ecological succession is crucial to predicting the responses of ecosystems to environmental change and projecting their future states. By definition, deterministic succession is expected under homogeneous abiotic and biotic starting conditions. This study, however, shows that the succession of groundwater microbial communities in response to nutrient amendment is primarily stochastic, but that the drivers controlling biodiversity and succession are dynamic rather than static. By identifying the mechanisms controlling microbial community assembly and succession, this study makes fundamental contribution to the mechanistic understanding essential for a predictive microbial ecology of many systems ranging from microbiomes of humans and plants to natural and managed ecosystems.
Keywords: community assembly, disturbances, metagenomics, GeoChip, remediation
Abstract
Unraveling the drivers of community structure and succession in response to environmental change is a central goal in ecology. Although the mechanisms shaping community structure have been intensively examined, those controlling ecological succession remain elusive. To understand the relative importance of stochastic and deterministic processes in mediating microbial community succession, a unique framework composed of four different cases was developed for fluidic and nonfluidic ecosystems. The framework was then tested for one fluidic ecosystem: a groundwater system perturbed by adding emulsified vegetable oil (EVO) for uranium immobilization. Our results revealed that groundwater microbial community diverged substantially away from the initial community after EVO amendment and eventually converged to a new community state, which was closely clustered with its initial state. However, their composition and structure were significantly different from each other. Null model analysis indicated that both deterministic and stochastic processes played important roles in controlling the assembly and succession of the groundwater microbial community, but their relative importance was time dependent. Additionally, consistent with the proposed conceptual framework but contradictory to conventional wisdom, the community succession responding to EVO amendment was primarily controlled by stochastic rather than deterministic processes. During the middle phase of the succession, the roles of stochastic processes in controlling community composition increased substantially, ranging from 81.3% to 92.0%. Finally, there are limited successional studies available to support different cases in the conceptual framework, but further well-replicated explicit time-series experiments are needed to understand the relative importance of deterministic and stochastic processes in controlling community succession.
Understanding how local communities assemble from a regional species pool is a central issue in community ecology (1–4). Two types of processes (deterministic vs. stochastic) influence the assembly of species into a local community. However, whether a local community structure is controlled by stochastic or deterministic processes is hotly debated (5–7). Traditional niche-based theory assumes that deterministic factors, including species traits, interspecies interactions (e.g., competition, predation, mutualisms, and tradeoffs), and environmental conditions, control local community compositions (8, 9). Consequently, the resulting local communities generally have little site-to-site variation in species composition (low β-diversity) when the environmental conditions are similar (10). In contrast, neutral theory (6) hypothesizes that all species are ecologically equivalent and ecological drift, i.e., stochastic processes of birth, death, colonization, extinction, and speciation (6, 11), govern the diversity and species composition of local communities independent of their traits and niches. When stochastic processes are coupled with priority effects, where early-arriving species influence the establishment and growth of later-arriving species (12), local communities with greater site-to-site variations (high β-diversity) in species compositions can emerge under similar, even identical, environmental conditions (10, 13, 14). The site-to-site variation in species composition due to stochastic processes (i.e., stochasticity) is unpredictable. To avoid confusion, we refer in this paper to such unpredictable variation in community composition as “compositional stochasticity.”
It is now more generally accepted that both deterministic and stochastic processes occur simultaneously in the assembly of local communities (5, 15, 16), but their relative importance remains difficult to resolve (5, 13). It is also known that several factors such as habitat connectivity and size (4), productivity (15), disturbance (13), predation (10), and resource availability (17) influence the relative importance of stochastic vs. deterministic processes in the assembly of local communities. However, it is not clear whether and how their relative importance varies with time. In addition, although the mechanisms shaping the structure of ecological communities have been intensively studied (5, 14–16, 18–21), the drivers controlling ecological succession in response to environmental perturbations are poorly understood.
The study of ecological succession remains at the core of ecology because knowledge of the temporal dynamics of ecological communities can help predict changes of biodiversity and ecosystem services in response to environmental change (22, 23). Ecological succession refers to more or less niche-based deterministic development of ecological community structure after perturbations (22, 24). Although, by definition, deterministic succession is expected under identical or rather similar environmental conditions, very few studies have examined the roles of stochastic processes controlling the succession of ecological communities (13, 15, 22). Neutral theory (6) predicts that chance, the stochasticity inherent in various probabilistic biological processes (such as dispersal, colonization, extinction, speciation, biotic interactions, and initial population heterogeneity) could lead to unpredictable variability in community composition (13, 22) (i.e., compositional stochasticity). However, assessing the degree of stochasticity and its role in ecological succession in field studies is challenging (18, 22) because the three components of ecological succession (stages, trajectories, and mechanisms) depend on the individual characteristics of community members, environment, and perturbations (23). Thus, manipulative experiments under similar, if not identical, initial conditions are valuable for disentangling the drivers controlling the succession of natural communities in response to environmental perturbations (18, 22).
The physical characteristics of an ecosystem have dramatic impacts on its succession in response to environmental perturbations (23). System fluidity (connectivity) is expected to be a major factor in natural systems ranging from fluidic ecosystems (e.g., groundwater, rivers, ocean, lakes, wetlands, wastewater treatment plants, bioreactors, intestinal tract) to low or nonfluidic ecosystems (e.g., soils, sediments, deep subsurface). One key feature associated with the stochasticity of a fluidic ecosystem, as represented by the planktonic groundwater community examined in this study, is that dispersal is not significantly restricted at local scales due to high hydraulic conductivity. In contrast, microbial dispersal would be much more limited in nonfluidic ecosystems. Thus, it is expected that the stages, trajectories, and mechanisms of succession following a perturbation will vary dramatically between fluidic and nonfluidic ecosystems.
In general, environmental perturbations have been classified into two categories (25): (i) increased nutrient input, especially with complex carbon (C) substrates (e.g., nutrient amendment, eutrophication, oil spill) and (ii) disturbances, which increase mortality and decrease biomass (e.g., drought, tillage, toxic chemicals, extreme temperature, salt and pH, predation). The type of environmental perturbation is also thought to influence the relative importance of stochasticity vs. determinism in community assembly (13, 22, 25). Nutrient input is believed to increase compositional stochasticity by enhancing ecological drift (e.g., stochastic processes of birth, death, colonization, and possibly extinction, and random change in species relative abundance) (5), and weakening niche selection by providing more resources (C, energy, nutrients). In contrast, it is generally believed that extreme disturbances such as drought often decrease compositional stochasticity by acting as selection factors (13), eliminating a large portion of the regional species pool. In addition, perturbations can also be classified as pulses or presses, depending on their duration (26). Whereas pulse perturbations are relatively discrete and short term, press perturbations are continuous and of long duration.
Microorganisms play integral and unique roles in various ecosystem processes and functions and are of enormous importance in global biogeochemistry, human health (27), energy (28), environmental remediation (29), engineering (30), and agriculture (31). However, in contrast to their known ecological primacy, the drivers controlling the succession of microbial communities in response to environmental perturbations are poorly understood. Thus, in this study, we developed a novel theoretical framework to conceptualize the relationships of succession to stochasticity. This framework is composed of four different models to predict the relative importance of stochastic processes in mediating microbial community succession with respect to ecosystem characteristics (fluidic vs. nonfluidic) and environmental perturbations (nutrient input vs. disturbance). We then tested one of these models for a specific fluidic system: a groundwater system perturbed by addition of emulsified vegetable oil (EVO) for uranium immobilization as a part of a long-term field-scale bioremediation experiment (32). The advantages of this system are that it is open and well connected with high hydraulic conductivity so that dispersal appeared to not be a major limiting factor in community assembly and that the study had sufficient replicate time-series data on many community variables (i.e., genes/functional populations) to allow null model analysis.
Our main objectives of this study were to answer the following questions: (i) How does a groundwater microbial community’s structure respond to the environmental perturbation of C amendment? (ii) What are the relative roles of deterministic and stochastic factors in determining community composition and succession? (iii) Does the relative importance of deterministic and stochastic factors change over time? Our results suggest that stochastic processes play predominant roles in controlling the succession of the groundwater microbial communities in response to the nutrient amendment but that the relative importance of stochastic and deterministic processes is time dependent. To the best of our knowledge, this is the first study to conceptualize the relationships between stochasticity and ecological succession and to demonstrate the importance and dynamic behavior of stochastic processes in controlling the succession of microbial communities.
Theoretical Framework
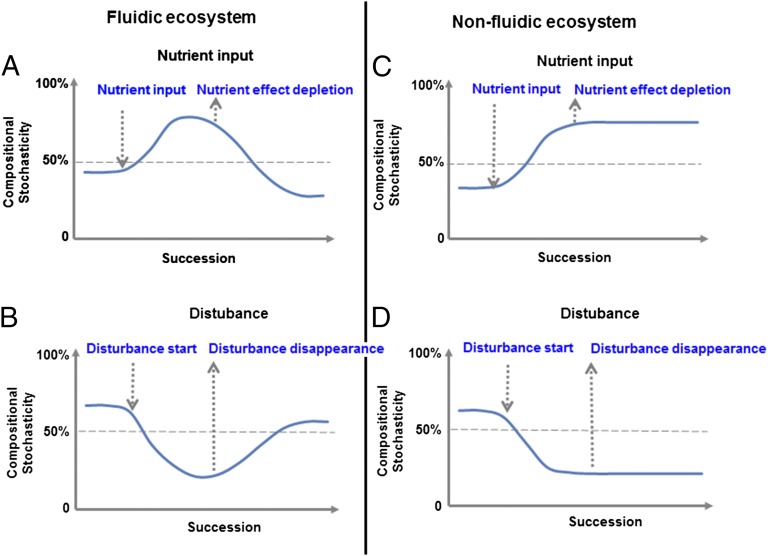
Below we conceptualize the relationships of succession to stochasticity, ecosystem characteristics, and environmental perturbations. Four basic scenarios are proposed and we tested one using the experimental results of groundwater (fluidic) ecosystem. Experimental support for the remaining scenarios is then discussed. The hypothesized relationships are diagrammed in Fig. 1. It should be noted that this study is primarily focused on community succession, i.e., the processes of community changes immediately following a perturbation, but less on community resilience, i.e., the outcome of community succession after perturbation(s). Here, resilience is referred to as the capacity of an ecosystem to recover from perturbation(s) without shifting to an alternative structure state (33). To determine the mechanisms controlling community succession in response to perturbations, replicate time-series data on many community variables are needed to perform null model simulations.
Fig. 1.
Basic conceptual frameworks for modeling the relationships of succession to stochasticity, ecosystem characteristics, and environmental perturbations. (A) Fluidic ecosystem with nutrient input. (B) Fluidic ecosystem with disturbance. (C) Nonfluidic ecosystem with nutrient input. (D) Nonfluidic ecosystem with disturbance. In all these cases, the degree of the compositional stochasticity before a perturbation is arbitrary, just for illustrative purposes in a relative scale. In the groundwater communities examined here, deterministic processes played a larger role than stochastic processes before the perturbation (e.g., EVO injection). Thus, in scenario A, we assume that the communities before perturbations are more under deterministic control. Also, the time for succession is at an ecological time scale and is more or less arbitrary. It could be days, months, years, and decades (63), depending on particular ecosystems and the nature and intensity of perturbations.
Scenario A: Fluidic Ecosystems with Nutrient Input.
In a fluidic ecosystem with nutrient input of complex C substrates, it is expected that compositional stochasticity increases after nutrient input (Fig. 1A) primarily due to three major processes. First, nutrients can be used by a variety of microorganisms as C and energy sources to stimulate growth, especially growth of less abundant microbial populations (e.g., the “rare biosphere”) that could physiologically respond differentially to nutrient addition. Such growth/stimulation could enhance stochastic processes of birth, death, colonization, extinction, and random changes in relative population abundance. Nutrient addition could also weaken niche selection by reducing competition for resources and by providing more diverse resources (C, energy, nutrients) to some species. In addition, nutrient addition could strengthen priority effects (13). Enhanced growth of early-arriving species could result in the exclusion or facilitation of later arriving species (17). All of the above could lead to unpredictable site-to-site variation in species compositions (i.e., compositional stochasticity) under similar environmental conditions.
After a certain time following depletion of added nutrients, deterministic processes gain in importance (Fig. 1A) for several reasons. First, the effects of nutrient amendment (i.e., enhancing stochastic processes, weakening niche selection, promoting priority effects) on the local communities become diminished, and hence the relative roles of deterministic processes become stronger. Second, relatively speaking, due to shorter fluid residence time and/or higher population dispersal rates, substantial portions of the populations, if not all, in local communities might be replaced by the populations from the common regional species pool. Third, the local environmental conditions could return to a state similar to the original levels in which deterministic factors play relatively bigger roles. All of these could decrease the relative importance of stochastic processes in controlling microbial community structure.
The outcome of the succession with this scenario is an unpredictable divergence from original community structure following nutrient input, followed by convergence toward the original structure. Ultimately, these communities would show a high degree of resilience. Consequently, a community state, which is highly similar to the initial state, is expected. However, because deterministic processes (e.g., changes in abiotic environmental conditions, species interactions) still play roles in regulating community structure, especially in the late phase of succession, the recovered communities could be the same, very similar to, or statistically significantly different from the original communities. In addition, because nutrient input could potentially stimulate growth of a variety of populations and weaken competitions among different species, compared with the original communities before nutrient input, the average α-diversity of the local communities is expected to be higher during succession and similar at the end of succession.
Scenario B: Fluidic Ecosystems with Disturbance.
In a fluidic ecosystem with a disturbance, a successional trajectory opposite to scenario A would be expected. Stochasticity decreases after a disturbance (Fig. 1B) for several reasons. First, by increasing mortality and decreasing biomass, disturbance acts as a selection factor. As a result, much of the regional species pool will be eliminated and the growth of many of the remaining species will be inhibited (13). Because of resulting smaller population size and lower growth rates, the probability of ecological drifts associated with birth and death, and fluctuations in relative population abundance becomes smaller. As a result, β-diversity among the local communities will decrease as the regional species pool decreases (1). Also, niche selection could be stronger after a disturbance because disturbance itself acts as a selection factor. In addition, priority effects could become less important because the growth of many populations could be slowed down or inhibited, and hence the chance for the dominance of early-arriving species and for excluding the coexistence of late arriving species could be lower. All of the above would lead to more predictable site-to-site variation in species compositions of the local communities (i.e., reduced stochasticity) after a disturbance.
Once the disturbance is removed, stochasticity would increase to near the original level (Fig. 1B). This is because, first, the disturbance effect (e.g., weakening stochastic processes, strengthening niche selection, weakening priority effects) on the local communities becomes weaker. Also, because of higher population dispersal rates in the fluidic ecosystem, ecological drift (e.g., stochastic processes of birth, death, and colonization) becomes stronger. In addition, the local environmental conditions could become similar to the original levels (more benign) under which stochastic factors are relatively more important. All of these factors could lead to greater relative importance of stochastic processes in shaping microbial community structure.
The outcome of the succession with this scenario is that the communities will diverge away from original communities following a disturbance and converge toward the original ones after the disturbance effect is gone. Similar to scenario A, the communities would also show a high degree of resilience. Due to strong niche selection imposed by the disturbance during the succession, the ultimately recovered communities could be the same, very similar to, or statistically significantly different from the original communities. In addition, compared with the original communities before the disturbance, the local communities will have lower average α-diversity during succession and have a similar α-diversity at the end of succession.
Scenario C: Nonfluidic Ecosystem with Nutrient Input.
Similar to scenario A, stochasticity will also increase in nonfluidic ecosystems after nutrient input (Fig. 1C). This is because nutrient addition would enhance stochastic processes, weaken niche selection, and promote priority effects as described in scenario A.
However, different from scenario A, after time following added nutrient depletion, stochasticity would remain at a similar high level, at least at short ecological time scales (Fig. 1C). This is primarily because dispersal is generally limited in nonfluidic ecosystems due to the lack of rapid fluid movement. Thus, the population turnover is expected to be very slow. Also, nutrient input will alter the local environmental conditions. Due to the lack of rapid fluid movement like those observed in the fluidic ecosystems, it will take much longer for the local environmental conditions to return to a state similar to the original. As a result, the unpredictable site-to-site variation in species compositions will stay relatively high even after a nutrient is gone.
The outcome of this succession scenario is that, similar to scenario A, the communities will diverge away from original communities with longer lag time after nutrient input. However, different from scenario A, the local communities would not converge toward the original ones over a relative short ecological time scale. Thus, after nutrient input, the local communities will be quite different from original communities and would show a low degree of resilience. In addition, due to availability of more diverse C substrates and growth stimulation, local communities with a higher α-diversity would be expected, compared with the original communities before nutrient input.
Scenario D: Nonfluidic Ecosystems with Disturbance.
In a nonfluidic ecosystem with a disturbance, it is expected that the relationships of succession to stochasticity would be opposite to scenario C (Fig. 1D). Similar to scenario B, once a disturbance is applied, stochasticity will decrease. This is because disturbance would weaken stochastic processes, enhance niche selection, and weaken priority effects as described in scenario B.
However, different from scenario B but similar to scenario C, after a certain time following the disturbance event, stochasticity would remain at a similarly low level, at least at short ecological time scales (Fig. 1D). This is also due to the lack of rapid fluid movement in the nonfluidic ecosystems, leading to dispersal limitation (low population turnover) and slower recovery as described in scenario C. Also, similar to scenario C, the communities will diverge away from the original communities with a longer lag time after a disturbance and would not converge toward original communities at relatively short ecological time scales as seen in scenarios A and B. Thus, after a disturbance, the local communities will be quite different from original communities and would show a low degree of resilience. Due to the selection effects from disturbance, the local communities would have, on average, lower α-diversity compared with the original communities before disturbance.
Results
Geochemical Conditions Before and After Nutrient Injection.
To understand how the groundwater microbial community responded to EVO amendment, groundwater samples were collected from seven down-gradient monitoring wells and one up-gradient well as a control (32) (Fig. S1) at seven different time points (days 0, 4, 17, 31, 80, 140, and 269) over a 9-mo period for combined physical, chemical, and biological analyses. Based on acetate appearance and disappearance (Fig. S2A), these samples can be roughly divided into groups belonging to three different phases: (i) early phase included the samples collected before EVO amendment (day 0); (ii) middle phase contained samples obtained after EVO injection and before EVO depletion (days 4, 17, 31, 80, and 140); and (iii) final phase comprised samples collected on day 269 at which time acetate was not detectable (Fig. S2A) (32). The geochemical conditions at both early and final phases represent the stress environment, which is toxic (low pH and high concentrations of nitrate, Al, and various other metals) and C resource limited.
Based on Mann–Whitney u test, the differences among the majority (∼80%) of geochemical variables between the control well and the monitoring wells before EVO injection were not significant. Similarly, all geochemical variables except acetate between the monitoring wells before injection and the control well across different time points were also not significantly different. Hence environmental conditions before injection were quite homogeneous, which was most likely due to high hydraulic conductivity [∼0.4 d for the residence time for this groundwater system (32)].
Enhanced Local and Regional Diversity by Nutrient Amendment.
A total of 55 samples were analyzed with the GeoChip array, which contains tens of thousands of functional genes involved in biogeochemical cycling of C, nitrogen, phosphorus, sulfur, metals, and degradation of various organic contaminants, and is able to provide species–strain level resolution (34, 35). An average of 1,762 genes/populations was detected before EVO injection (Fig. S2C). At the local scale (<10 m, Fig. S1), there were significant effects of the EVO amendment on the gene richness of microbial communities (ANOVA, F7,47 = 3.77, P < 0.003). In general, the numbers of functional genes detected increased by approximately threefold in the middle phase of the succession (Fig. S2C). At the final phase (day 269), the number of genes detected decreased to a level closer to that observed in the early phase (day 0) (Fig. S2C). In addition, a strong positive correlation was observed between gene richness and productivity, measured with DNA yields as a proxy of microbial biomass, by Spearman ranked correlation test (rho = 0.397, P < 0.01) (Fig. S2B), suggesting that the EVO amendment enhanced both gene richness and productivity. Interestingly, high gene richness was obtained during the intermediate time after EVO amendment (from 17 to 140 d). Such an α-diversity pattern of the local communities observed in this study is apparently consistent with the intermediate disturbance hypothesis (36–38), which predicts that the maximum species diversity will be observed at intermediate levels of disturbance as measured by intensity or frequency of disturbance or by the time since disturbance (39).
A total of 12,868 gene sequences/populations, which belong to 279 functional genes (e.g., nirS, nirK, amoA), were detected across all wells and all time points. The total number of genes detected across all wells at each time point (γ-diversity) varied by more than a factor of two during the experimental period (Fig. S2C). Also, only a subset of the total gene/population pool could persist in the early (30.2%) and final (41.6%) phases of the community succession in which added nutrient was depleted, indicating a strong environmental selection effect on community assembly at these phases. Alternatively, the majority of the regional functional genes/populations (97.1%) were detected, at least on one occasion, in the middle phase of the succession, suggesting that environmental selection effects could be much less important in this phase. In addition, most of the genes/populations observed at day 0 (71.4–84.5%) or day 269 (61.5–87.7%) were detected at the other time points (Fig. S3 A and B). Almost all genes detected at day 0 (98.5%) and day 269 (97.4%) were observed at least at one of these time points. These results indicated that the functional populations present at the early and final phases were almost entirely nested within the regional pool of the functional genes/populations that could potentially respond to EVO amendment. Finally, about 53.4% of the detected genes/populations were only detected in the middle phase of the succession (Fig. S3C), suggesting that most of the functional populations in the groundwater ecosystem were responsive to EVO amendment, and they became rare under the stress conditions.
Dramatic Shift of the Groundwater Microbial Community Structure by Nutrient Amendment.
To understand whether EVO amendment impacts microbial community structure, β-diversity as measured by Jaccard or Bray–Curtis dissimilarities was compared. Based on the GeoChip data, the overall functional community structures were significantly different (P < 0.05) among various time points except for the communities at days 31, 80, and 140 based on Bray–Curtis dissimilarity, indicating that EVO amendment had dramatic impacts on the microbial community composition and structure. Also, many important functional groups involved in C degradation, methanogenesis, sulfate reduction, denitrification, dissimilatory nitrate reduction, organic contaminant degradation, and metal resistance were greatly stimulated, especially at day 17 (Fig. S4). In addition, considerable portions of the detected genes/populations (1.9–29.2%) at day 0 were well specific (Table S1), suggesting significant initial population heterogeneity in the groundwater communities.
The average β-diversity of the microbial communities varied considerably over the experimental period. Generally speaking, the average β-diversities at the middle phase (Jaccard’s incidence-based dissimilarity: DJ = 0.489–0.603; Bray–Curtis’s abundance-based dissimilarity: DBC = 0.480–0.611) tended to be slightly higher than that at the early phase (DJ = 0.578; DBC = 0.518), but considerably higher than that at the final phase (DJ = 0.481; DBC = 0.313), as well as that from the control well (DJ = 0.419; DBC = 0.290). Interestingly, no significant relationship at P = 0.05 level was observed between stochasticity and productivity as measured by DNA yields (Spearman ranked correlation: r = 0.714, P = 0.058) as previously demonstrated (15). The distinct differences in β-diversity imply that the mechanisms underlying the community succession could be different among these three phases (5).
Convergence of Community Trajectories Toward the Original Communities After Nutrient Depletion.
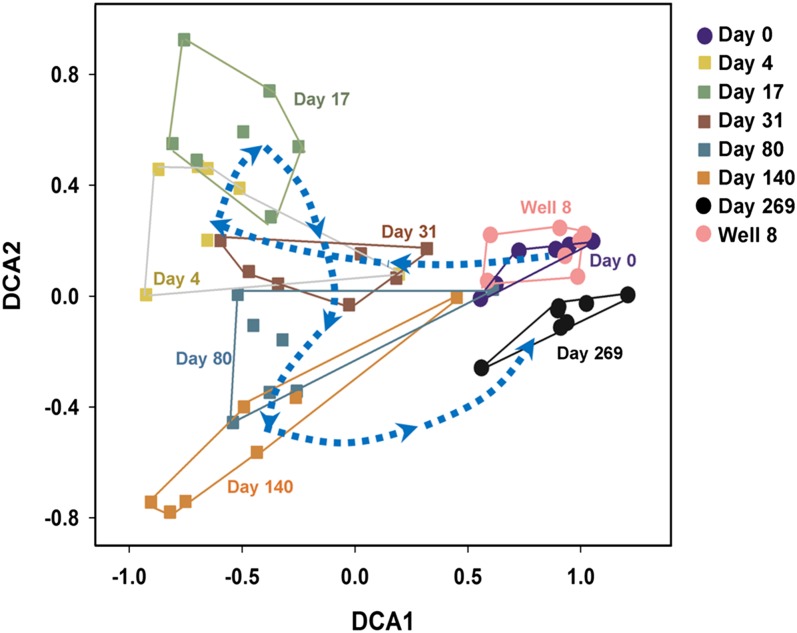
To portray the succession of microbial communities in response to EVO amendment, detrended correspondence analysis (DCA) was performed. After receiving EVO, the microbial communities started diverging away from the original communities, roughly following the trajectories of day 4, 17, 31, 80, and 140 (Fig. 2). Also, the microbial communities at the early phase (day 0) were tightly clustered with the control well communities from different time points (Fig. 2), which is consistent with the geochemistry-based classification (32). These results suggested that there were strong linkages between groundwater geochemistry and community structure. In addition, the microbial communities at the final phase in which EVO was depleted converged to be more similar to those at the early phase and the control well (Fig. 2), indicating the resilience of the microbial communities.
Fig. 2.
Detrended correspondence analysis (DCA) of all detected functional genes, showing successional trajectories of the groundwater microbial communities in the seven downgradient wells at day 0 (preinjection), 4, 17, 31, 80, 140, and 269 as well the upgradient control well. Colors of symbols represent different days for sampling.
Although the microbial communities were more closely grouped together between the early and final phases based on DCA ordination (Fig. 2), the community composition and structure between these phases were significantly different (P < 0.03) as indicated by the three complementary nonparametric tests [Adonis, analysis of similarity (ANOSIM), and multiresponse permutation procedure (MRPP)] at the levels of the whole community and individual functional gene categories (Table S2). Further analysis indicated that 51% of the individual functional genes detected were significantly different between these two phases. These significantly different genes are mostly involved in sulfate reduction, C degradation, nitrogen cycling, organic contaminant degradation, and metal resistance, and they were more abundant at the final phase than early phase (Table S3). Many of these genes would be those expected to be important for growth and survival of microorganisms under such harsh environmental conditions and could be important indicators for adaptation of microbial communities. Thus, it seems that successional processes over this time scale resulted in a new community state that was similar to, but distinct from, the initial microbial community. If the abundance of functional genes can be used as proxy for community functions, the new communities may have improved functional capability in terms of metal reduction, growth, and survival in this harsher environment.
Null Model Analysis.
β-Diversity can provide insights on community assembly mechanisms (5). Three distinct community assembly mechanisms can cause high β-diversity: purely deterministic, purely stochastic, or both (15). Low dissimilarity among communities that are otherwise identical in environmental conditions would imply a more important role for deterministic assembly, whereas high dissimilarity would suggest a large role of stochastic assembly (13, 15). However, because β-diversity covaries with α- and γ-diversity (40), the observed differences in β-diversity in different phases could be simply due to the differences in their α- and γ-diversity, which were considerably different in this study as shown above.
To disentangle the importance of deterministic mechanisms from stochastic mechanisms underlying community assembly, a null model analysis is needed. Null model analysis assumes that an assemblage (a community) is not structured by species interactions (41). If ecological drift (e.g., stochastic colonization and extinction) and possibly priority effects leading to multiple stable equilibria (1, 13) play predominant roles in determining community composition, the similarity observed across the communities at a certain time point will be statistically indistinguishable from the random null expectation. On the other hand, if community assembly is primarily shaped by deterministic processes, the actual similarity observed will be significantly higher than the random null expectation (13).
Two types of null model analyses were performed. The first null model analysis is based on the method proposed by Chase et al. (40) by holding gene richness at each time point (α-diversity) and across all time points (γ-diversity) constant. Here, the regional species pool is defined as the total number of all genes found in all of the samples surveyed over the experimental period. Interestingly, the permutational analysis of multivariate dispersions (PERMDISP) test revealed that the observed β-diversity was indistinguishable from the null expectation for all communities in the middle phase at P < 0.01 (Table 1), whereas the observed β-diversity was significantly different from the null random expectations for the groundwater communities at the early and final phases as well as the control well (Table 1). Also, standard effect size (SES), which is used to measure the influence of deterministic factors on community composition and abundance (19), was quite high for the communities at the early and final phase as well as the control well (Fig. S5). The SES was substantially decreased during the middle phase after EVO amendment (Fig. S5).
Table 1.
Significance test of the differences of centroids between the groundwater microbial communities and null model simulations across different time points
| Day | Centroid of actual communities | Centroid of the null model | F | P |
| 0 | 0.3724 | 0.5187 | 16.3428 | 0.0024 |
| 4 | 0.3926 | 0.4411 | 0.8498 | 0.3748 |
| 17 | 0.3196 | 0.3721 | 3.0699 | 0.1052 |
| 31 | 0.3616 | 0.4143 | 1.7391 | 0.2119 |
| 80 | 0.3722 | 0.4093 | 5.6219 | 0.0353 |
| 140 | 0.3962 | 0.4120 | 0.0663 | 0.8012 |
| 269 | 0.3115 | 0.4506 | 10.4535 | 0.0072 |
Permutational analysis of multivariate dispersions (PERMDISP) was used. P values <0.01 in bold.
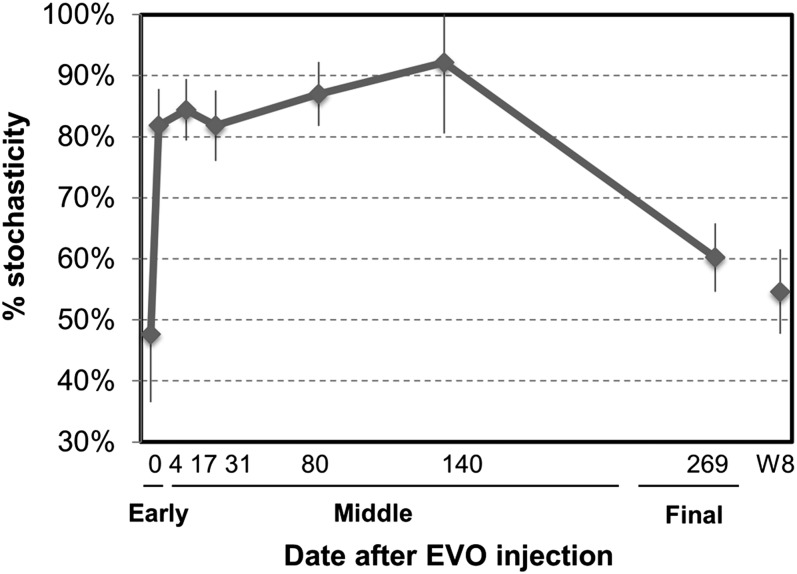
To further quantify the relative importance of deterministic vs. stochastic processes in shaping the groundwater community structure, similar to SES, the proportion of the difference between the observed similarity for each pairwise comparison and the null expected similarity divided by the observed similarity was calculated (Fig. 3). For convenience, such ratio is referred to as selection strength (SS) because it provides a quantitative estimation of the role of niche-based deterministic selection processes in shaping community composition. Its complement (1-SS) measures the relative importance of the compositional stochasticity in shaping biodiversity. As illustrated in Fig. 3, significant differences in the compositional stochasticity were observed for the communities across different time points (PERMANOVA: F7,154 = 98.03, P < 0.001). The stochastic processes contributed an average of 46.4% of the community variations at the early phase. However, the roles of stochastic processes in controlling community composition increased substantially during the middle phase, ranging from 81.3% to 92.0% (Fig. 3), further supporting that stochastic assembly dictated the succession of the groundwater communities in response to the EVO amendment. At the end of the experiment, the stochastic processes become less important (59.8%). In addition, the stochasticity for the majority (95%) of the detected individual functional genes showed significant correlations to that of the entire community, indicating that the mechanisms (stochastic vs. deterministic) shaping the structure of the individual functional guilds were very similar to that at the whole community level. Hence, all of the above results indicated that stochastic processes dictated the succession of the groundwater microbial communities in response to EVO amendment.
Fig. 3.
Dynamic changes of stochasticity during the succession of the groundwater microbial communities. The stochasticity is defined as the complement of the selection strength, a proportion of the differences between the observed total similarity and the null expected similarity divided by the total similarity.
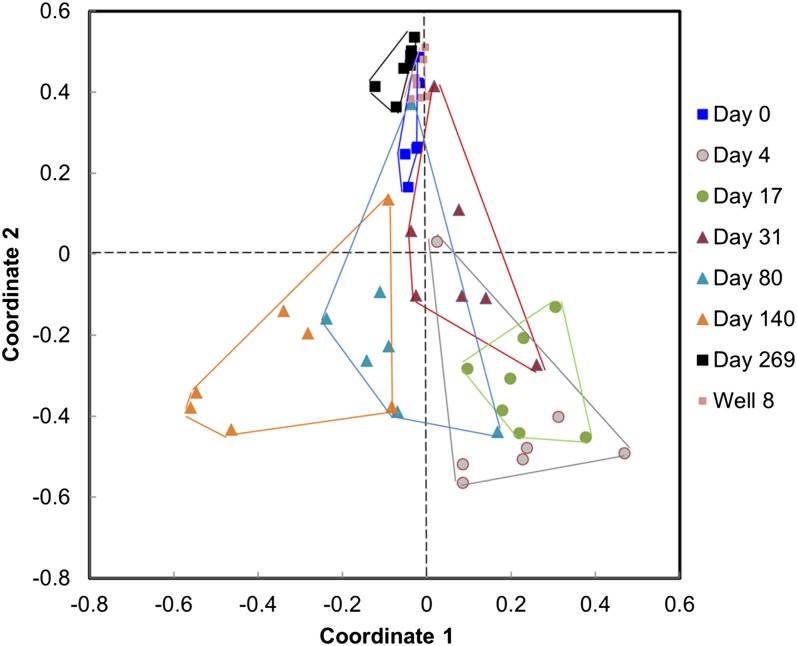
Because the results from null model analyses are very sensitive to the models, approaches, and algorithms used (42), a second more restricted null model analysis (15) was performed based on EcoSim (43), keeping the number of species per site and the number of sites occupied by each species constant. Then, a probability distribution of the deviations from the null-expected number of the shared species among pairwise community comparisons was obtained (15), which were overall significantly different among various time points (PERMDISP: F7,47 = 7.38, P < 0.001). Our results revealed that the groundwater microbial communities at the early phase were more similar (Fig. 4), indicating that they deviated from the null expectation (15). However, the communities at the different time points (days 4, 17, 31, 80, and 140) of the middle phase were farther apart and spread over the entire space (Fig. 4), demonstrating that they were less deviant from the null expectation. Similar to those at the early phase, the communities at the final phase as well as the control well were also more similar (Fig. 4). These results suggest that the relative importance of stochastic processes is time dependent, playing a greater role during the middle phases of community response to nutrient addition.
Fig. 4.
Ordination of community composition by nonmetric multidimensional scaling (NMDS) based on the modified Raup–Crick dissimilarity metric (15). The communities that are closer together are more deviant from the null expectation, whereas the communities that are farther apart are less deviant from the null expectations. The data suggested that the communities at days 4, 17, 31, 80, and 140 were less different from null model expectation, whereas the communities at day 0, 269, and the control well were far more different from null model expectation.
Discussion
Selection of the Groundwater Ecosystem for Testing the Theoretical Framework.
There are several reasons to suggest this groundwater ecosystem offers advantages for testing some of the proposed theoretical models. First, whereas a deterministic process involves nonrandom, niche-differentiation–based mechanisms, a stochastic process generates community patterns indistinguishable from random chance alone (5). Four distinct ecological processes/mechanisms can be visualized in controlling community compositions: selection, drift, speciation, and dispersal (33). Whereas selection is definitely deterministic and drift is stochastic, speciation and dispersal could contribute to both (5). One major challenge in examining community assembly is the difficulty in estimating real dispersal among sites (44). Because this groundwater ecosystem is open, well connected (fluidic) with rapid dispersion and population movement, dispersal is not a major limiting factor influencing community assembly at the local scale (<10 m). Because the experimental time (9 mo) is short for evolution, speciation can also be ignored. Thus, the microbial community changes we saw are most likely driven by selection (deterministic processes) and/or drift (stochastic processes). These points greatly simplify the analyses in disentangling the importance of niche-based (deterministic) processes from stochastic processes.
A second advantage of this groundwater ecosystem, compared with other fluidic ecosystems such as oceans and lakes, is that the high hydraulic conductivity (at the scale of <10 m) allows informative community monitoring at relative short time scales. Third, compared with terrestrial ecosystems such as soils, groundwater microbial communities are relatively less complex so that more comprehensive and cost-effective monitoring of microbial changes at the whole community scale can be achieved. In addition, groundwater ecosystems are inherently open systems like many other fluidic ecosystems such as oceans, lakes, rivers, wetlands, but they can provide for more defined spatial (hence temporal) resolution.
Consistency Between Experimental Results and Theoretical Predictions.
As predicted by the theoretical framework (Fig. 1A), our results show that both deterministic and stochastic processes are important in shaping the community’s assembly and succession in response to EVO amendment, but that their relative importance is time dependent. Whereas stochastic processes played predominant roles in controlling the middle phase of the succession, they were less important in the early and final phases of the succession. Such results are consistent with site geochemistry. Because the groundwater contained high levels of U, Al, NO3−, and other contaminants and had very low C (45), the groundwater communities were highly stressed before nutrient injection and after EVO depletion. Under such stress conditions, it is expected that deterministic processes would play significant roles due to the selection effects imposed by the harsh conditions. After nutrient amendment, the groundwater communities were less stressed due to high C and energy supply. Thus, they could respond to substrate amendment in a stochastic way, rather than in a predicable manner for the following reasons. First, EVO is a low viscosity liquid containing a combination of less bioavailable (vegetable oil, 60%) and more bioavailable (lactic acid, 4%) electron donors plus other nutrients and growth factors, which can stimulate growth and reproduction of a variety of microorganisms, especially less abundant and/or dormant microorganisms. The initial differences among microbial communities will thus be greatly amplified by substrate addition. Second, as mentioned above, because the groundwater turnover time is very short, about 0.4 d, the stochastic processes of birth, death, immigration, and emigration (13, 33) would be greatly enhanced by the amended complex substrates. In addition, once the communities were stimulated, competitive interactions could play important roles to further intensify the differences of community structure created by various stochastic processes.
Support for Alternative Models.
To test the proposed theoretical framework, time-series data with sufficient replicates on many community variables (i.e., operational taxonomic units, genes/functional populations) are needed to assess the changes of stochasticity over time based on null model analysis. Although ecological succession and its related topics (e.g., stability) have been intensively examined in ecological literature, many published studies could not be directly used for testing the proposed framework because they do not have sufficient replicates, lack time-series sampling, and/or have few community variables measured. In the following, we spotlight some examples that appear to lend indirect support to the theoretical framework based on the consistency of the succession outcomes (i.e., diversity changes and resilience) to the predictions of different models.
Whereas scenario A was tested with the groundwater ecosystem, results from the Deepwater Horizon oil spill in the Gulf of Mexico also support this model. Essentially, the oil spill represents nutrient input into a marine ecosystem. Various snap-shot samples from both plume and nonplume areas 2 mo after the spill were analyzed with omic technologies (46, 47). Those results show that the communities from the oil plume were less similar than those from nonplume based on PhyloChip (figure 2A in ref. 46), phospholipid fatty acid (figure 2B in ref. 46), and GeoChip analyses (figure 2 in ref. 47). All of these results suggest that stochasticity plays a larger role after the spill. Because no time-series samples were available for further analysis, it is not clear whether the original marine microbial communities were reestablished after the oil was gone. Nevertheless, these results are consistent with scenario A (Fig. 1A).
Several studies have also examined the resilience of lake microbial communities after disturbances (26, 48–51). The experimental results with lake epilimnion microbial communities (26, 48–51) also support scenario A. Generally, lake mixing increases nutrient input to the nutrient limited epilimnion (50), which should stimulate microbial diversity. The epilimnion microbial community diversity consistently increased after a whole-ecosystem perturbation (26). All of the epilimnion microbial communities recovered after perturbations by typhoons (49), nutrient addition or mixing (50), and whole-ecosystem mixing (26), which is consistent with the scenario A prediction. However, due to the lack of sufficient replicate data for null model analysis, it is not clear how compositional stochasticity changed with time in response to disturbances.
Although no experiments were designed to directly test the other three theoretical models (Fig. 1 B–D), literature data provide support for these scenarios. For example, the experimental results from aquatic ecosystems (artificial ponds) perturbed by drought (13) are more or less consistent with scenario B. In this 4-y experiment, 10 artificial ponds were dried in the middle phase of the experiment (2 y). The results showed that the β-diversity of the communities (animals and plants) in the drought ponds were significantly less than that in the permanent ponds, indicating that drought decreased the stochasticity in the dry ponds. For another example, the experimental results on the lake hypolimnion microbial communities from the lake mixing studies also appear to support scenario B (26, 49, 50). Lake mixing increases the oxygen concentration in the anoxic hypolimnion, which likely stresses the anaerobic hypolimnion communities, explaining the measured decrease in microbial diversity (26). Similarly, all of the hypolimnion microbial communities recovered following perturbation by typhoons (49), oxygen addition or mixing (50), and whole-ecosystem mixing (26). The high degree of resilience of the hypolimnion microbial communities is consistent with the prediction of scenario B. Finally, another field-scale aquifer experiment examined the changes of diversity patterns during the succession in a trichloroethylene (TCE) bioremediation experiment (52) and is supportive of scenario B. In this experiment phenol or toluene was injected into the aquifer as C source to stimulate cometabolism of TCE. During the second phase of this experiment, 1,1-dichloroethene (1,1-DCE), a potential intermediate, was also injected, whose products are toxic. Interestingly, the microbial community diversity was dramatically decreased by 1,1-DCE, and remained low during subsequent phenol-feeding phase, but increased to the original levels during the fourth phase of the experiment following toluene feeding (figure 2B in ref. 52). Six of the seven dominant microbial populations recovered after the severe disturbance imposed by 1,1-DCE, indicating that this aquifer microbial community was quite resilient. The decrease in diversity and high degree of resilience of the aquifer microbial communities are consistent with the predictions of scenario B. However, no replicate data are available to evaluate the changes of stochasticity during succession.
Scenario C has some support from experiments with fertilized grasslands (25). The plant communities in the fertilization plots were more dissimilar than those in the control plots (figures 4 and 5B in ref. 25), which is most likely because community dispersion increases with the increased productivity that typically results from fertilization (25). Also, our recent GeoChip-based analysis of a long-term grassland fertilization experiment at Rothamstead, England, revealed that the microbial communities from plots fertilized for ∼150 y displayed a greater spread between samples than those from the control plots without fertilization (Fig. S6A). These results indicate that stochasticity plays larger roles in the microbial communities with long-term fertilization than the control communities, which is consistent with the prediction from scenario C.
In addition, some evidence supports scenario D. For example, tillage is a disturbance to soils in many agricultural ecosystems. Tillage is expected to impose various stresses to soil microbial communities through destruction of soil structure, causing erosion, and leading to enormous losses in soil organic C and nutrients (53–55). In particular, soil aggregation, which is critical for key resources (air, water, and nutrients) and provides a relatively stable accommodation for microbes (56), is decreased by tillage. Thus, tillage is expected to decrease stochasticity in soil communities. Our recent GeoChip-based analysis with samples collected from tillage and nontillage plots with monocultures of annually rotating corn, soybean, and wheat at the Kellogg Biological Station Long-Term Ecological Research experimental site indicated that communities from the tillage treatment were much less disperse than those from the control plots (Fig. S6B), suggesting that tillage reduced stochasticity. As another example, forest harvesting leads to organic matter removal and soil compaction, which are key disturbances for forest soil microbial communities (57). The soil microbial communities were significantly different between the timber harvest sites and control sites even 15 y after harvesting (57). This suggests that the soil microbial communities have a low degree of resilience, which is consistent with the prediction of scenario D.
Challenges in Testing the Theoretical Models.
The relationships of succession to stochasticity, ecosystem characteristics, and environmental perturbations are likely more complicated than the four basic scenarios outlined above. First, we classify the ecosystems as fluidic and nonfluidic. but in nature there is a continuum from fluidic to nonfluidic, dependent on relative spatial and temporal scales as well as aggregated behaviors and/or lifestyles (e.g., biofilm, planktonic, flocs). For instance, the dynamics of the cells attached to the surface (i.e., biofilm community) in a fluidic ecosystem (e.g., groundwater, wastewater treatment plants) could be very different from the planktonic community. The turnover of the biofilm communities in groundwater will be considerably slower than the planktonic communities. However, relatively speaking (e.g., compared with soils), the biofilm community in a fluidic ecosystem is under faster dynamic exchanges and has intensive interactions with associated planktonic communities via attachment and detachment processes. Consequently, over relatively longer time periods and at smaller spatial scales, from the perspective of compositional stochasticity, the dynamic behavior of the biofilm community in a fluidic ecosystem should be similar to the planktonic community. Second, the two types of environmental perturbations could interact with each other to stimulate stochasticity as revealed by Houseman et al. (25) in grassland system. The species traits and their interactions with disturbance could also be critical to determining the effects of disturbance on stochasticity. Third, the shift in community composition and structure by nutrient input could be substrate dependent. A simple substrate, such as the ethanol used for U bioremediation (58, 59), could have less effect in driving communities from determinism to stochasticity. Fourth, under natural settings, an ecosystem is always under multiple and continuous perturbations. The successional trajectories of a particular ecosystem will definitely be dependent on the numbers of perturbations, their intensity, and time. For an example, if the communities in an fluidic ecosystem received a perturbation and/or several perturbations for multiple times, it is anticipated that the trajectories of scenarios A and B could be more like those in scenarios C and D, respectively. In addition, in some fluidic ecosystems, dispersal could also be limited or very limited, depending on the spatial and temporal scales examined. The differences in dispersal rates and regional species pool sizes will have considerable effects on the successional trajectories of microbial communities. Finally, in the four basic scenarios outlined above, like many community ecology studies (33), we assume that community succession occurs during a relatively short ecological time scale so that evolutionary dynamics such as speciation and extinction could be negligible. Over a longer time scale, speciation and extinction could play significant roles in determining the relative importance of deterministic vs. stochastic processes in shaping community structure. However, understanding whether and how evolutionary processes affect successional trajectories of microbial communities will be very challenging, given the extreme difficulty in tracking the evolutionary changes of individual microbial populations over time, especially under field settings.
Conclusions and Implications
Several theoretical models have been developed to conceptualize the relationships between stochasticity and ecological succession with respect to ecosystem characteristics and environmental perturbations, providing a general framework for testing the responses and succession of ecosystems to environmental perturbations. One of the models was tested with the groundwater ecosystem in response to nutrient addition. Contradictory to the expected, rule-based, deterministic succession, community succession responding to nutrient amendment was primarily controlled by stochastic processes. Whereas both deterministic and stochastic processes played roles in controlling community assembly, their relative importance was time dependent. This is the first demonstration that the drivers controlling biodiversity and community succession are dynamic rather than static in the fluidic ecosystems.
Our findings have important implications for biodiversity preservation, ecosystem restoration, and environmental management. Because both stochastic and deterministic processes play important roles in mediating ecological succession, to achieve a desired state, any ecosystem restoration programs must consider approaches to facilitate both processes. Also, in the fluidic ecosystems like the groundwater ecosystem examined here, the exact successional trajectories are unpredictable because they are stochastic. However, based on the model predictions (scenario A), the ultimate community states appear to be more predictable if deterministic processes play more roles at the end of succession. If the regional pool is large enough to contain the desired populations, a desired new community state, which is closely related to, but significantly different from, the initial state, could also result. This could be very important for many manipulation programs in fluidic ecosystems such as groundwater bioremediation (58). If ecosystems have high hydraulic conductivity and high dispersal rates, as does the groundwater ecosystem examined here, the theoretical models (scenarios A and B) and results presented in this study predict that manipulated fluidic ecosystems would eventually return to a state similar to their original states (i.e., high resilience). Thus, in this case, the impacts or risks in changing ecosystem structure and associated functions could be small. However, to generalize whether the phenomenon observed in this groundwater ecosystem is applicable to other fluidic ecosystems requires further testing.
Method Summary
Details for all methods are provided in SI Materials and Methods. Briefly, groundwater samples (2 L) were taken for geochemical and microbial analyses from the seven monitoring wells and the control well at different time points, −28, 4, 17, 31, 80, 140, and 269 d after EVO amendment (32). For convenience, the samples taken at the 28 d before EVO injection were treated as the samples of day 0. Groundwater was filtered in situ with 8-μm filters to remove large particles, followed by filtering with 0.2-μm filters for sample collection (32). Although groundwater and sediment microbial populations may differ, groundwater samples were focused in this study because sampling sediment was not possible over the temporal and spatial scales of the experiment.
The community DNA was extracted with the SDS-based chemical lysis method (60). GeoChip 3.0 was used for analyzing microbial community functional gene structure as described elsewhere (34, 35, 61). GeoChip 3.0 is a functional gene array containing ∼28,000 probes covering ∼57,000 gene variants from 292 functional gene families involved in C, nitrogen, phosphorus, and sulfur cycles, energy metabolism, antibiotic resistance, metal resistance, and organic contaminant degradation. GeoChip hybridization, imaging, and data preprocessing were described previously (34). All statistical analyses, including DCA, ANOSIM, Adonis, and MRPP, PERMANOVA, and PERMDISP, were carried out with the Vegan package in R (62). Null model analyses were performed as described elsewhere (15, 40, 41, 43).
Supplementary Material
Acknowledgments
We thank three reviewers for their invaluable suggestions on improving the presentation. This work conducted by Ecosystems and Networks Integrated with Genes and Molecular Assemblies was supported by the Office of Science, Office of Biological and Environmental Research, of the US Department of Energy under Contract No. DE-AC02-05CH11231, US Department of Energy Grant DE-FG02-07ER64398, the Office of Biological and Environmental Research Biological Systems Research on the Role of Microbial Communities in Carbon Cycling Program (DE-SC0004601), the US National Science Foundation (NSF) MacroSystems Biology program under Contract NSF EF-1065844, and the State Key Joint Laboratory of Environment Simulation and Pollution Control (11Z03ESPCT).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1324044111/-/DCSupplemental.
References
- 1.Chase JM. Community assembly: When should history matter? Oecologia. 2003;136(4):489–498. doi: 10.1007/s00442-003-1311-7. [DOI] [PubMed] [Google Scholar]
- 2.Dumbrell AJ, Nelson M, Helgason T, Dytham C, Fitter AH. Relative roles of niche and neutral processes in structuring a soil microbial community. ISME J. 2010;4(3):337–345. doi: 10.1038/ismej.2009.122. [DOI] [PubMed] [Google Scholar]
- 3.Holyoak M, Leibold MA, Holt RD. Metacommunities: Spatial Dynamics and Ecological Communities. Chicago: Univ of Chicago Press; 2005. [Google Scholar]
- 4.Orrock JL, Watling JI. Local community size mediates ecological drift and competition in metacommunities. Proc Biol Sci. 2010;277(1691):2185–2191. doi: 10.1098/rspb.2009.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chase JM, Myers JA. Disentangling the importance of ecological niches from stochastic processes across scales. Philos Trans R Soc Lond B Biol Sci. 2011;366(1576):2351–2363. doi: 10.1098/rstb.2011.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hubbell SP. The Unified Neutral Theory of Biodiversity and Biogeography. Princeton, NJ: Princeton Univ Press; 2001. [DOI] [PubMed] [Google Scholar]
- 7.Tilman D. Niche tradeoffs, neutrality, and community structure: A stochastic theory of resource competition, invasion, and community assembly. Proc Natl Acad Sci USA. 2004;101(30):10854–10861. doi: 10.1073/pnas.0403458101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chesson P. Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst. 2000;31:343. [Google Scholar]
- 9.Fargione J, Brown CS, Tilman D. Community assembly and invasion: An experimental test of neutral versus niche processes. Proc Natl Acad Sci USA. 2003;100(15):8916–8920. doi: 10.1073/pnas.1033107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chase JM, Biro EG, Ryberg WA, Smith KG. Predators temper the relative importance of stochastic processes in the assembly of prey metacommunities. Ecol Lett. 2009;12(11):1210–1218. doi: 10.1111/j.1461-0248.2009.01362.x. [DOI] [PubMed] [Google Scholar]
- 11.Chave J. Neutral theory and community ecology. Ecol Lett. 2004;7(3):241–253. [Google Scholar]
- 12.Fukami T, Bezemer TM, Mortimer SR, van der Putten WH. Species divergence and trait convergence in experimental plant community assembly. Ecol Lett. 2005;8(12):1283–1290. [Google Scholar]
- 13.Chase JM. Drought mediates the importance of stochastic community assembly. Proc Natl Acad Sci USA. 2007;104(44):17430–17434. doi: 10.1073/pnas.0704350104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou J, et al. 2013. Stochastic assembly leads to alternative communities with distinct functions in a bioreactor microbial community. MBio 4(2) pii: e00584–12.
- 15.Chase JM. Stochastic community assembly causes higher biodiversity in more productive environments. Science. 2010;328(5984):1388–1391. doi: 10.1126/science.1187820. [DOI] [PubMed] [Google Scholar]
- 16.Ofiteru ID, et al. Combined niche and neutral effects in a microbial wastewater treatment community. Proc Natl Acad Sci USA. 2010;107(35):15345–15350. doi: 10.1073/pnas.1000604107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kardol P, Souza L, Classen AT. Resource availability mediates the importance of priority effects in plant community assembly and ecosystem function. Oikos. 2013;122(1):84–94. [Google Scholar]
- 18.Ellwood MDF, Manica A, Foster WA. Stochastic and deterministic processes jointly structure tropical arthropod communities. Ecol Lett. 2009;12(4):277–284. doi: 10.1111/j.1461-0248.2009.01284.x. [DOI] [PubMed] [Google Scholar]
- 19.Kraft NJB, et al. Disentangling the drivers of β diversity along latitudinal and elevational gradients. Science. 2011;333(6050):1755–1758. doi: 10.1126/science.1208584. [DOI] [PubMed] [Google Scholar]
- 20.Caruso T, et al. Stochastic and deterministic processes interact in the assembly of desert microbial communities on a global scale. ISME J. 2011;5(9):1406–1413. doi: 10.1038/ismej.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stegen JC, Lin XJ, Konopka AE, Fredrickson JK. Stochastic and deterministic assembly processes in subsurface microbial communities. ISME J. 2012;6(9):1653–1664. doi: 10.1038/ismej.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kreyling J, Jentsch A, Beierkuhnlein C. Stochastic trajectories of succession initiated by extreme climatic events. Ecol Lett. 2011;14(8):758–764. doi: 10.1111/j.1461-0248.2011.01637.x. [DOI] [PubMed] [Google Scholar]
- 23.Prach K, Walker LR. Four opportunities for studies of ecological succession. Trends Ecol Evol. 2011;26(3):119–123. doi: 10.1016/j.tree.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Connell JH, Slatyer RO. Mechanisms of succession in natural communities and their role in community stability and organization. Am Nat. 1977;111(982):1119–1144. [Google Scholar]
- 25.Houseman GR, Mittelbach GG, Reynolds HL, Gross KL. Perturbations alter community convergence, divergence, and formation of multiple community states. Ecology. 2008;89(8):2172–2180. doi: 10.1890/07-1228.1. [DOI] [PubMed] [Google Scholar]
- 26.Shade A, et al. Fundamentals of microbial community resistance and resilience. Front Microbiol. 2012;3:417. doi: 10.3389/fmicb.2012.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 28.Werner JJ, et al. Bacterial community structures are unique and resilient in full-scale bioenergy systems. Proc Natl Acad Sci USA. 2011;108(10):4158–4163. doi: 10.1073/pnas.1015676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu MY, et al. Responses of microbial community functional structures to pilot-scale uranium in situ bioremediation. ISME J. 2010;4(8):1060–1070. doi: 10.1038/ismej.2010.31. [DOI] [PubMed] [Google Scholar]
- 30.Curtis TP, Head IM, Graham DW. Theoretical ecology for engineering biology. Environ Sci Technol. 2003;37(3):64A–70A. doi: 10.1021/es0323493. [DOI] [PubMed] [Google Scholar]
- 31.Kennedy AC, Smith KL. Soil microbial diversity and the sustainability of agricultural soils. Plant Soil. 1995;170(1):75–86. [Google Scholar]
- 32.Gihring TM, et al. A limited microbial consortium is responsible for extended bioreduction of uranium in a contaminated aquifer. Appl Environ Microbiol. 2011;77(17):5955–5965. doi: 10.1128/AEM.00220-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vellend M. Conceptual synthesis in community ecology. Q Rev Biol. 2010;85(2):183–206. doi: 10.1086/652373. [DOI] [PubMed] [Google Scholar]
- 34.He Z, et al. GeoChip 3.0 as a high-throughput tool for analyzing microbial community composition, structure and functional activity. ISME J. 2010;4(9):1167–1179. doi: 10.1038/ismej.2010.46. [DOI] [PubMed] [Google Scholar]
- 35.Zhou JZ, et al. Microbial mediation of carbon-cycle feedbacks to climate warming. Nat Clim Change. 2012;2(2):106–110. [Google Scholar]
- 36.Connell JH. Diversity in tropical rain forests and coral reefs. Science. 1978;199(4335):1302–1310. doi: 10.1126/science.199.4335.1302. [DOI] [PubMed] [Google Scholar]
- 37.Svensson JR, Lindegarth M, Jonsson PR, Pavia H. Disturbance-diversity models: What do they really predict and how are they tested? Philos Trans R Soc Lond B Biol Sci. 2012;279(1736):2163–2170. doi: 10.1098/rspb.2011.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller AD, Roxburgh SH, Shea K. How frequency and intensity shape diversity-disturbance relationships. Proc Natl Acad Sci USA. 2011;108(14):5643–5648. doi: 10.1073/pnas.1018594108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Loneragan WA, Duggin JA, Grant CD. Issues affecting the measurement of disturbance response patterns in herbaceous vegetation: A test of the intermediate disturbance hypothesis. Plant Ecol. 2004;172(1):11–26. [Google Scholar]
- 40.Chase JM, Kraft NJB, Smith KG, Vellend M, Inouye BD. Using null models to disentangle variation in community dissimilarity from variation in α-diversity. Ecosphere. 2011;2(2):art24. [Google Scholar]
- 41.Ulrich W, Gotelli NJ. Null model analysis of species associations using abundance data. Ecology. 2010;91(11):3384–3397. doi: 10.1890/09-2157.1. [DOI] [PubMed] [Google Scholar]
- 42.Gotelli NJ. Null model analysis of species co-occurrence patterns. Ecology. 2000;81(9):2606–2621. [Google Scholar]
- 43.Gotelli NJ, Entsminger GL. 2009. EcoSim: Null models software for ecology. Version 7.
- 44.Lindström ES, Langenheder S. Local and regional factors influencing bacterial community assembly. Environ Microbiol Rep. 2012;4(1):1–9. doi: 10.1111/j.1758-2229.2011.00257.x. [DOI] [PubMed] [Google Scholar]
- 45.Wu WM, et al. Pilot-scale in situ bioremediation of uranium in a highly contaminated aquifer. 1. Conditioning of a treatment zone. Environ Sci Technol. 2006;40(12):3978–3985. doi: 10.1021/es051954y. [DOI] [PubMed] [Google Scholar]
- 46.Hazen TC, et al. Deep-sea oil plume enriches indigenous oil-degrading bacteria. Science. 2010;330(6001):204–208. doi: 10.1126/science.1195979. [DOI] [PubMed] [Google Scholar]
- 47.Lu Z, et al. Microbial gene functions enriched in the Deepwater Horizon deep-sea oil plume. ISME J. 2012;6(2):451–460. doi: 10.1038/ismej.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gonzalez A, et al. Characterizing microbial communities through space and time. Curr Opin Biotechnol. 2012;23(3):431–436. doi: 10.1016/j.copbio.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones SE, et al. Typhoons initiate predictable change in aquatic bacterial communities. Limnol Oceanogr. 2008;53(4):1319–1326. [Google Scholar]
- 50.Shade A, et al. Resistance, resilience and recovery: Aquatic bacterial dynamics after water column disturbance. Environ Microbiol. 2011;13(10):2752–2767. doi: 10.1111/j.1462-2920.2011.02546.x. [DOI] [PubMed] [Google Scholar]
- 51.Shade A, et al. Lake microbial communities are resilient after a whole-ecosystem disturbance. ISME J. 2012;6(12):2153–2167. doi: 10.1038/ismej.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fries MR, Hopkins GD, McCarty PL, Forney LJ, Tiedje JM. Microbial succession during a field evaluation of phenol and toluene as the primary substrates for trichloroethene cometabolism. Appl Environ Microbiol. 1997;63(4):1515–1522. doi: 10.1128/aem.63.4.1515-1522.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blanco-Canqui H, Lal R. No-tillage and soil-profile carbon sequestration: An on-farm assessment. Soil Sci Soc Am J. 2008;72(3):693–701. [Google Scholar]
- 54.Lal R. Soil carbon sequestration impacts on global climate change and food security. Science. 2004;304(5677):1623–1627. doi: 10.1126/science.1097396. [DOI] [PubMed] [Google Scholar]
- 55.Martens DA. Nitrogen cycling under different soil management systems. Adv Agron. 2001;70:143–192. [Google Scholar]
- 56.Herrick JE, et al. Field soil aggregate stability kit for soil quality and rangeland health evaluations. Catena. 2001;44(1):27–35. [Google Scholar]
- 57.Hartmann M, et al. Significant and persistent impact of timber harvesting on soil microbial communities in Northern coniferous forests. ISME J. 2012;6(12):2199–2218. doi: 10.1038/ismej.2012.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu WM, et al. Pilot-scale in situ bioremedation of uranium in a highly contaminated aquifer. 2. Reduction of U(VI) and geochemical control of U(VI) bioavailability. Environ Sci Technol. 2006;40(12):3986–3995. doi: 10.1021/es051960u. [DOI] [PubMed] [Google Scholar]
- 59.Wu WM, et al. In situ bioreduction of uranium (VI) to submicromolar levels and reoxidation by dissolved oxygen. Environ Sci Technol. 2007;41(16):5716–5723. doi: 10.1021/es062657b. [DOI] [PubMed] [Google Scholar]
- 60.Zhou JZ, Bruns MA, Tiedje JM. DNA recovery from soils of diverse composition. Appl Environ Microbiol. 1996;62(2):316–322. doi: 10.1128/aem.62.2.316-322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.He Z, et al. GeoChip: A comprehensive microarray for investigating biogeochemical, ecological and environmental processes. ISME J. 2007;1(1):67–77. doi: 10.1038/ismej.2007.2. [DOI] [PubMed] [Google Scholar]
- 62.Dixon P. VEGAN, a package of R functions for community ecology. J Veg Sci. 2003;14(6):927–930. [Google Scholar]
- 63.Bardgett RD, Bowman WD, Kaufmann R, Schmidt SK. A temporal approach to linking aboveground and belowground ecology. Trends Ecol Evol. 2005;20(11):634–641. doi: 10.1016/j.tree.2005.08.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.