Abstract
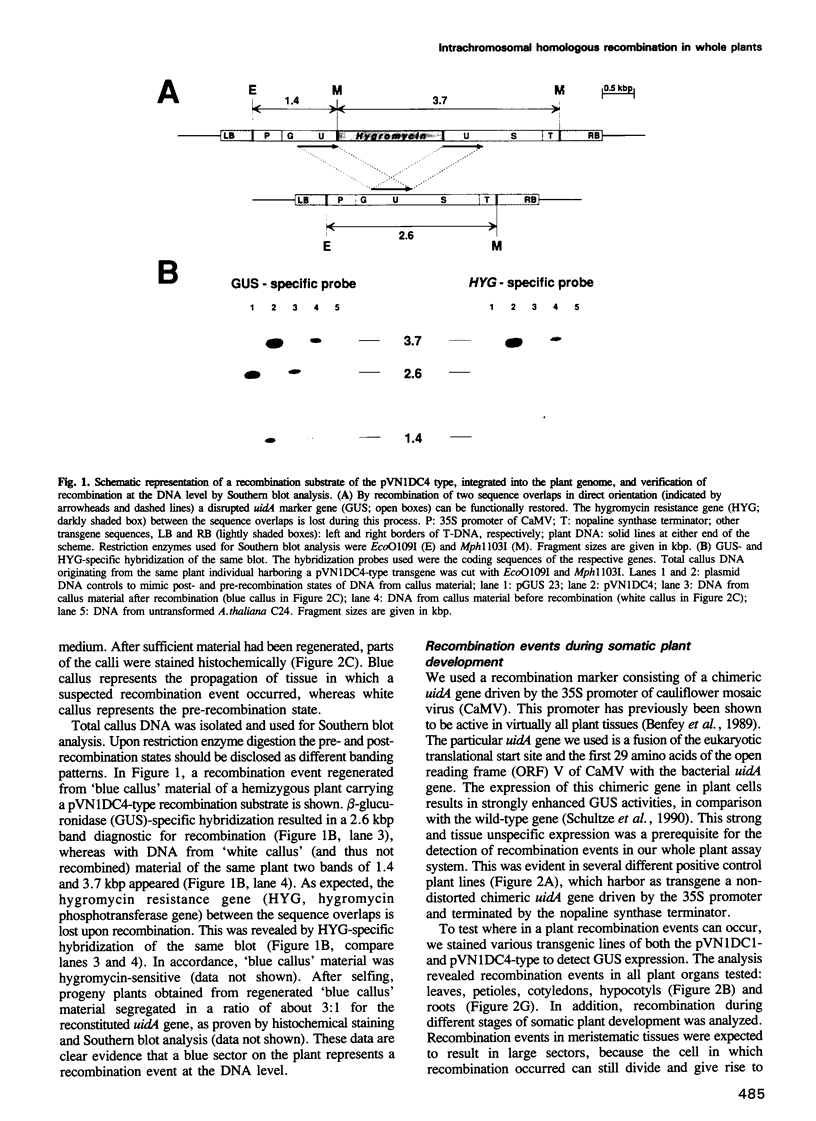
A system to assay intrachromosomal homologous recombination during the complete life-cycle of a whole higher eukaryote was set up. Arabidopsis thaliana plants were transformed with a recombination substrate carrying a non-selectable and quantitatively detectable marker gene. The recombination substrates contain two overlapping, non-functional deletion mutants of a chimeric beta-glucuronidase (uidA) gene. Upon recombination, as proven by Southern blot analysis, a functional gene is restored and its product can be detected by histochemical staining. Therefore, cells in which recombination events occurred, and their progeny, can be precisely localized in the whole plant. Recombination was observed in all plant organs examined, from the seed stage until the flowering stage of somatic plant development. Meristematic recombination events revealed cell lineage patterns. Overall recombination frequencies typically were in the range 10(-6)-10(-7) events/genome. Recombination frequencies were found to differ in different organs of particular transgenic lines.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assaad F. F., Signer E. R. Somatic and germinal recombination of a direct repeat in Arabidopsis. Genetics. 1992 Oct;132(2):553–566. doi: 10.1093/genetics/132.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker H. J. X-ray- and TEM-induced mitotic recombination in Drosophila melanogaster: unequal and sister-strand recombination. Mol Gen Genet. 1975;138(1):11–24. doi: 10.1007/BF00268823. [DOI] [PubMed] [Google Scholar]
- Benfey P. N., Ren L., Chua N. H. The CaMV 35S enhancer contains at least two domains which can confer different developmental and tissue-specific expression patterns. EMBO J. 1989 Aug;8(8):2195–2202. doi: 10.1002/j.1460-2075.1989.tb08342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. D., Smith J. B. Nuclear dna amounts in angiosperms. Philos Trans R Soc Lond B Biol Sci. 1976 May 27;274(933):227–274. doi: 10.1098/rstb.1976.0044. [DOI] [PubMed] [Google Scholar]
- Bollag R. J., Waldman A. S., Liskay R. M. Homologous recombination in mammalian cells. Annu Rev Genet. 1989;23:199–225. doi: 10.1146/annurev.ge.23.120189.001215. [DOI] [PubMed] [Google Scholar]
- Bowman J. T., Jr Spontaneous reversion of the white-ivory mutant of Drosophila melanogaster. Genetics. 1965 Nov;52(5):1069–1079. doi: 10.1093/genetics/52.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr B., Burr F. A. Controlling-element events at the shrunken locus in maize. Genetics. 1981 May;98(1):143–156. doi: 10.1093/genetics/98.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das O. P., Levi-Minzi S., Koury M., Benner M., Messing J. A somatic gene rearrangement contributing to genetic diversity in maize. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7809–7813. doi: 10.1073/pnas.87.20.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rocher E. J., Harkins K. R., Galbraith D. W., Bohnert H. J. Developmentally regulated systemic endopolyploid in succulents with small genomes. Science. 1990 Oct 5;250(4977):99–101. doi: 10.1126/science.250.4977.99. [DOI] [PubMed] [Google Scholar]
- Gal S., Pisan B., Hohn T., Grimsley N., Hohn B. Genomic homologous recombination in planta. EMBO J. 1991 Jun;10(6):1571–1578. doi: 10.1002/j.1460-2075.1991.tb07677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith D. W., Harkins K. R., Knapp S. Systemic Endopolyploidy in Arabidopsis thaliana. Plant Physiol. 1991 Jul;96(3):985–989. doi: 10.1104/pp.96.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondo Y., Gardner J. M., Nakatsu Y., Durham-Pierre D., Deveau S. A., Kuper C., Brilliant M. H. High-frequency genetic reversion mediated by a DNA duplication: the mouse pink-eyed unstable mutation. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):297–301. doi: 10.1073/pnas.90.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel E. G., Masson J., Bogucki A., Paszkowski J. Stress-induced intrachromosomal recombination in plant somatic cells. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):422–426. doi: 10.1073/pnas.90.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager D. L., Goodchild N. L. Homologous recombination between the LTRs of a human retrovirus-like element causes a 5-kb deletion in two siblings. Am J Hum Genet. 1989 Dec;45(6):848–854. [PMC free article] [PubMed] [Google Scholar]
- Mattanovich D., Rüker F., Machado A. C., Laimer M., Regner F., Steinkellner H., Himmler G., Katinger H. Efficient transformation of Agrobacterium spp. by electroporation. Nucleic Acids Res. 1989 Aug 25;17(16):6747–6747. doi: 10.1093/nar/17.16.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. G., Thompson W. F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panthier J. J., Condamine H. Mitotic recombination in mammals. Bioessays. 1991 Jul;13(7):351–356. doi: 10.1002/bies.950130709. [DOI] [PubMed] [Google Scholar]
- Peterhans A., Schlüpmann H., Basse C., Paszkowski J. Intrachromosomal recombination in plants. EMBO J. 1990 Nov;9(11):3437–3445. doi: 10.1002/j.1460-2075.1990.tb07551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchta H., Hohn B. A transient assay in plant cells reveals a positive correlation between extrachromosomal recombination rates and length of homologous overlap. Nucleic Acids Res. 1991 May 25;19(10):2693–2700. doi: 10.1093/nar/19.10.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchta H., Hohn B. The mechanism of extrachromosomal homologous DNA recombination in plant cells. Mol Gen Genet. 1991 Nov;230(1-2):1–7. doi: 10.1007/BF00290641. [DOI] [PubMed] [Google Scholar]
- Puchta H., Kocher S., Hohn B. Extrachromosomal homologous DNA recombination in plant cells is fast and is not affected by CpG methylation. Mol Cell Biol. 1992 Aug;12(8):3372–3379. doi: 10.1128/mcb.12.8.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze M., Hohn T., Jiricny J. The reverse transcriptase gene of cauliflower mosaic virus is translated separately from the capsid gene. EMBO J. 1990 Apr;9(4):1177–1185. doi: 10.1002/j.1460-2075.1990.tb08225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seperack P. K., Strobel M. C., Corrow D. J., Jenkins N. A., Copeland N. G. Somatic and germ-line reverse mutation rates of the retrovirus-induced dilute coat-color mutation of DBA mice. Proc Natl Acad Sci U S A. 1988 Jan;85(1):189–192. doi: 10.1073/pnas.85.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoye J. P., Fenner S., Greenoak G. E., Moran C., Coffin J. M. Role of endogenous retroviruses as mutagens: the hairless mutation of mice. Cell. 1988 Jul 29;54(3):383–391. doi: 10.1016/0092-8674(88)90201-2. [DOI] [PubMed] [Google Scholar]
- Swoboda P., Hohn B., Gal S. Somatic homologous recombination in planta: the recombination frequency is dependent on the allelic state of recombining sequences and may be influenced by genomic position effects. Mol Gen Genet. 1993 Feb;237(1-2):33–40. doi: 10.1007/BF00282781. [DOI] [PubMed] [Google Scholar]
- Tovar J., Lichtenstein C. Somatic and Meiotic Chromosomal Recombination between Inverted Duplications in Transgenic Tobacco Plants. Plant Cell. 1992 Mar;4(3):319–332. doi: 10.1105/tpc.4.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie T. M., Braun R. E., Ehrman W. J., Palmiter R. D., Hammer R. E. Germ-line intrachromosomal recombination restores fertility in transgenic MyK-103 male mice. Genes Dev. 1991 Jan;5(1):38–48. doi: 10.1101/gad.5.1.38. [DOI] [PubMed] [Google Scholar]
- Zijlstra C., Hohn T. Cauliflower Mosaic Virus Gene VI Controls Translation from Dicistronic Expression Units in Transgenic Arabidopsis Plants. Plant Cell. 1992 Dec;4(12):1471–1484. doi: 10.1105/tpc.4.12.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot M. J., Offringa R., Does M. P., Hooykaas P. J., van den Elzen P. J. Mechanisms of intermolecular homologous recombination in plants as studied with single- and double-stranded DNA molecules. Nucleic Acids Res. 1992 Jun 11;20(11):2785–2794. doi: 10.1093/nar/20.11.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]





