Abstract
Rationale
Emergence of elevated liver function tests (LFTs) during a clinical trial may be due to underlying disease factors of the participants, thus cofounding safety assessments of therapies. Limited data exist addressing the frequency of elevated LFTs in the chronic disease setting of cystic fibrosis (CF). The objectives of this study were to characterize emergence rates of elevated LFTs in CF trials and their association with clinical outcomes.
Methods
The cohort was comprised of participants of three completed multicenter CF trials. LFTs were collected as safety endpoints, and hospitalization rates and changes in pulmonary function and weight were used to assess the association between elevated LFTs and clinical outcome.
Results
93/376 (25%) participants had at ≥1 emergent elevated LFT exceeding the normal reference range over an average 8.3 month follow-up, and only 12/93 (13%) had a value determined by the physician as clinically significant. Emergence of an elevated LFT was not significantly associated with a greater rate of decline in pulmonary function or weight as compared to participants with normal LFTs. Emergence of an elevated LFT value was however associated with a higher hospitalization risk (Relative risk:1.67, 95% confidence interval:1.11, 2.53).
Conclusions
Elevated LFTs are common among in CF trials, although in most cases are not deemed clinically significant. These elevated LFTs are associated with more frequent hospitalization, but additional studies are needed to determine the causality of this association. Therapeutic trials in CF must define a priori criteria for clinical significance of elevated LFTs to enable unbiased safety assessments.
Keywords: Cystic fibrosis, safety, liver function, randomized trial
Introduction
Liver disease in cystic fibrosis (CF) is considered a secondary consequence of the presence of the abnormal CF transmembrane regulator protein (CFTR) within the hepatobiliary tract or ducts (1). There is currently no gold standard for diagnosis of liver disease in CF (2). Nonetheless, the prevalence of liver disease among individuals with CF ranges from 2% to 37% (3), dependent on the definition used. While liver function tests (LFTs) and other serum markers have been found to have poor sensitivity and specificity predicting liver disease in patients with CF(4–7), these markers, in particular alanine transaminase (ALT), aspartate transaminase (AST), and gamma-glutamyl transpeptidase (GGT), are still the key safety laboratory parameters used in clinical trials to assess the emergence of drug related toxicities.
Limited data natural history data exist in CF addressing the extent and frequency of elevated LFTs that exceed an established upper limit of normal CF. Lindblad et.al. reported that over a 15 year follow-up period, 25% of individuals with CF older than 4 years of age had elevated LFTs (8). Consistent with that finding, Goss et. al. used data from a multicenter, randomized controlled trial in 504 children and adults with CF and reported that 16.1% and 11.8% of the subjects had higher than normal baseline ALT and AST, respectively(9). In addition, during the 24 week duration of the trial, significant fluctuation of LFTs occurred among the participants randomized to the placebo group. The significance of elevated LFTs remains unclear however. In a prospective observational study, Colombo et. al. reported that among those with CF without liver disease (defined clinically, sonographically, or biochemically), 41% experience at least one instance of elevated LFTs over a median 14 year follow-up(10), and Patriquin et. al found 21% of individuals with CF with normal liver sonograms had elevated LFTs in their cross-sectional study(11). These data suggest that there is a high prevalence and fluctuation of elevated LFTs of unclear etiology in CF. The presence of these potential LFT abnormalities among participants enrolled in therapeutic trials therefore presents a potential cofounder for the assessment of safety.
The data from three recently completed CF randomized, placebo-controlled clinical trials representing a diverse cohort of children and adults with CF provides a unique opportunity to better define the emergence rates of elevated LFTs that can be expected in the context of a CF clinical trial. Further, the standardized laboratory, adverse event, medication usage, and medical history data collected in these trials not only enables an assessment of the potential underlying etiology of elevated LFTs, but also characterization of the clinical significance of these events as determined by the treating physician. The key objectives of this study are therefore to (1) characterize expected emergence rates of elevated LFTs across age groups within a CF clinical trial, (2) determine the proportion of elevated LFTs that coincide with complications or medications indicative of hepatobiliary disease, and (3) determine the association between emergent elevated LFTs and clinical outcomes including pulmonary function and weight changes, hospitalization rates, and adverse event rates.
Materials and Methods
Study Cohort
The cohort was comprised of participants enrolled in three recently completed multicenter CF clinical trials. These trials included: (1) a 6-month randomized, placebo controlled trial of azithromycin in children and adults ages 6 and older with CF chronically infected with Pseudomonas aeruginosa conducted from December 2000 – May 2002 (AZ0001) (12), (2) a 6-month randomized, placebo controlled trial and subsequent 6-month open label follow-up study of azithromycin in children ages 6–18 uninfected with P. aeruginosa conducted from February 2007 – July 2009 (AZ0004) (13, 14) and (3) an 18-month randomized trial comparing anti-pseudomonal therapy regimens for treatment of new onset P. aeruginosa in children with CF ages 1–12 conducted from December 2004 – June 2009 (EPIC) (15). De-identified data included in the CF Therapeutics Development Network Data Archive were utilized for this study which was approved by the Institutional Review Board at Seattle Children’s Hospital, Seattle, Washington.
Eligibility
Eligibility criteria for the individual trials, in particular with relation to the exclusion of participants with abnormal liver function tests at screening, can be found in Table A.1 (Appendix A). Individuals with LFTs (ALT, AST, GGT) >2 times the upper limit of normal were excluded in these trials per the eligibility criteria. All randomized participants with LFTs within the normal reference range at baseline in the two azithromycin clinical trials were included in the current analysis. The decision to include both treatment arms in the azithromycin trials was due to the absence of treatment-related emergence of elevated LFTs in the original clinical trial (e.g. comparing azithromycin treated participants to placebo (12, 13)). Additionally, the generalizability of the current study results is strengthened by including individuals on study treatments that have now been integrated into standard of care. In particular, it is not uncommon that a subgroup of participants in CF clinical trials chronically uses azithromycin throughout the trial. Similarly, all randomized participants in the EPIC trial ages 6 months through 12 years of age with LFTs within the normal range at baseline who received intervention with inhaled tobramycin monotherapy were included. The group of randomized EPIC trial participants who received oral ciprofloxacin in combination with inhaled tobramycin were excluded from the current study as this combined treatment strategy has not been adopted into standard of care for chronic P. aeruginosa eradication therapy, in place of inhaled tobramycin monotherapy, due to lack of efficacy (16).
Data Collection
Liver function test results including ALT, AST, and GGT were collected prospectively as safety endpoints in each clinical trial according to the study visit schedule outlined in Table A.1. Laboratory parameters were processed at individual site laboratories and results recorded onto study-specific case report forms (CRFs). Site-specific normal reference ranges were not recorded in the CRFs, however elevated values greater than the upper limit of normal (ULN) were indicated in these CRFs according to these ranges as well as the physician’s determination of clinical significance as dictated by the Code of Federal Regulator, Title 21 Part 201(17). This document instructs the sponsor to identify adverse events (abnormal or elevated laboratory values in this case) that have significant clinical implications (e.g., those that are most commonly occurring, that result in discontinuation or dose modification, or that require monitoring). Study data utilized to assess the presence of conditions indicative of hepatobiliary disease included medical and medication use history, concomitant medication usage, and adverse events. All medications at study entry and new medications added during the study were reviewed among participants with elevated LFTs (blinded review in regards to the degree of the LFT elevation). This review was also performed for a random subset of participants without elevated LFTs, matched by study to the participants who experienced an elevated LFT. First, medications were classified related to whether they are employed to treat CF related liver disease; the primary medication used in CF is ursodeoxycholic acid (US registered trade names: Actigall, Urso, Urso 250, Urso Forte). In addition, medications with known hepatotoxicity were identified using MICROMEDEX® 2.0 by assessing each medication for reported common and serious adverse events that are associated with transaminitis, liver failure, liver dysfunction or liver disease. Clinical outcome and safety data including hospitalization rates, adverse event rates, change in pulmonary function as measured by forced expiratory volume in one second as a percentage of predicted (FEV1 % predicted), and change in weight in kilograms were used to assess clinical impact of elevated LFTs. Data were monitored at site visits during the clinical trial and CRF data was compared to source documentation from the laboratories and medical records.
Statistical Analysis
Demographic and baseline characteristics were descriptively summarized by study and overall. The emergence rates of elevated LFTs (AST, ALT, and GGT), defined as greater than the upper limit of normal, were estimated as rate per 100 months of follow up by age group (<6 years, 6–12 years, and >12 years) and gender, with corresponding 95% confidence intervals (CIs). Average LFT values observed throughout the study by whether they were elevated or normal and were summarized across participants and study visits using repeated measures regression utilizing generalized estimating equations with robust variance estimation and an independence working model, with 95% confidence intervals (CI) derived from the model (18).
Pulmonary function (forced expiratory volume in one second as a percentage of predicted [FEV1 % predicted]), weight, hospitalization and adverse event rates were compared between those with emergent elevated LFTs and those with normal LFTs throughout follow up to determine the association between the emergence of these events and clinical outcome over the duration of the clinical trials. Presence of an emergent elevated LFT was treated as a time independent covariate in these models due to imprecise timing of LFT values around clinical outcome data, and thus the model results are focused on overall associations rather than temporal causation. Rates of changes in FEV1 % predicted and weight were compared with repeated measures regression modeling utilizing generalized estimating equations (GEE) and robust variance estimation (18), adjusting for baseline age group, gender, and genotype which has been noted as a potential predictor of liver disease in CF (19). Risk of hospitalization was compared using Poisson regression adjusting for differential follow up time and baseline age group, gender, and genotype. Lastly, overall adverse event rates were compared using Poisson regression adjusting for baseline age group, gender, genotype, and follow up time and including an offset term for follow-up time. Analyses were performed using the statistical software SAS, version 9.1.3 (SAS Institute Inc., Cary, NC), R statistical package version 2.9.1 (R Foundation for Statistical Computing, Vienna, Austria), and Stata version 10.1 (StataCorp LP, College Station, TX).
Results
Cohort Characteristics
A total of 376 trial participants were included in the study with a mean age of 14 years (standard deviation [SD] = 7.40) and mean FEV1 % predicted of 87% (SD = 23.2). The majority (51%) of the cohort was less than 12 years of age as compared to 32% adolescents and approximately 17% adults ≥18 years of age. Table 1 displays details of the demographic and baseline characteristics of the cohort by participating trial and overall. Due to differences in eligibility criteria and study timeframes, study participants were not enrolled in more than one trial.
Table 1.
Baseline characteristics by study cohort
| AZ0001 N=139 | AZ0004 N=191 | EPIC N=46 | Total N=376 | |
|---|---|---|---|---|
|
| ||||
| Male | 68 (48.9%) | 109 (57.1%) | 18 (39.1%) | 195 (51.9%) |
|
| ||||
| Age (years) | ||||
| 6–12 | 18 (12.9%) | 130 (68.1%) | 44 (95.7%) | 192 (51.1%) |
| >12–18 | 59 (42.4%) | 61 (31.9%) | 2 (4.3%) | 122 (32.4%) |
| >18 | 62 (44.6%) | 0 (0.0%) | 0 (0.0%) | 62 (16.5%) |
| mean (SD) | 20.24 (8.379) | 10.76 (3.246) | 8.85 (2.087) | 14.03 (7.398) |
|
| ||||
| Genotype | ||||
| Delta F508 Homozygous | 60 (43.2%) | 87 (45.5%) | 25 (54.3%) | 172 (45.7%) |
| Delta F508 Heterozygous | 42 (30.2%) | 60 (31.4%) | 14 (30.4%) | 116 (30.9%) |
| Other | 6 (4.3%) | 17 (8.9%) | 4 (8.7%) | 27 (7.2%) |
|
| ||||
| Not Done | 31 (22.3%) | 27 (14.1%) | 3 (6.5%) | 61 (16.2%) |
|
| ||||
| Weight (kg) | ||||
| mean (SD) | 53.69 (13.135) | 37.96 (14.435) | 29.93 (8.478) | 42.82 (15.935) |
|
| ||||
| Weight (%)a | ||||
| mean (SD) | 34.08 (27.974) | 43.95 (26.806) | 41.27 (27.429) | 41.07 (27.427) |
|
| ||||
| BMI (kg/m2) | ||||
| mean (SD) | 20.12 (3.105) | 17.87 (2.947) | 17.16 (2.123) | 18.58 (3.135) |
|
| ||||
| BMI (%)a | ||||
| mean (SD) | 39.29 (28.207) | 49.59 (26.231) | 53.43 (25.889) | 47.53 (27.074) |
|
| ||||
| FEV 1 % predicted | ||||
| mean (SD) | 68.23 (20.997) | 98.54 (15.907) | 97.30 (16.628) | 87.10 (23.156) |
Weight and BMI percentiles calculated for those subjects ≤ 20 years of age using Center for Disease Control standard equations.
Emergence Rates of Elevated LFTs
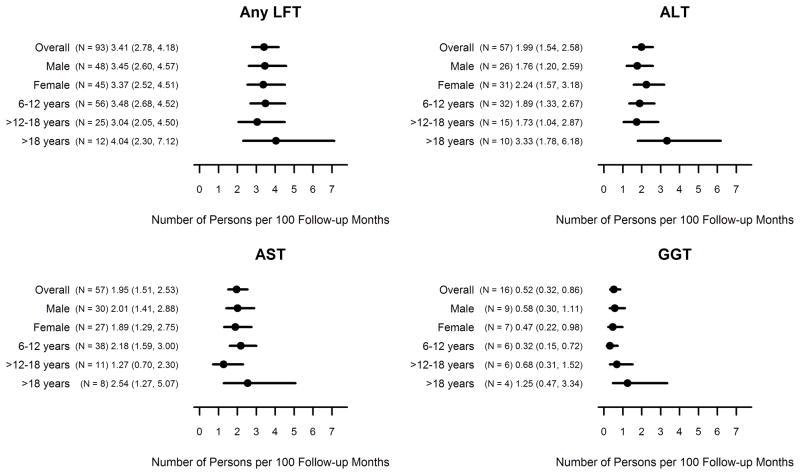
A total of 93/376 participants (25%) had at least one emergent elevated LFT exceeding the normal reference range in ALT, AST, or GGT throughout their follow-up period. Participants had an average of 3.4 observations over an average of 8.3 months of follow-up. Few of the participants (25/93, 27%) experienced more than one elevated LFT throughout the follow up. Figure 1 displays the emergence rates across studies by age group and gender. Detailed study-specific emergence rates by age group and gender for each LFT parameter can be found in Table A.2 (Appendix A). The overall rate of emergence of any elevated LFT (AST, ALT, or GGT) was 3.4 persons per 100 follow-up months (95% CI 2.8, 4.2). For a 6 month clinical trial with 100 participants, based on these estimates one would expect approximately 20.4 participants would have at least one emergent elevated LFT during the course of the study (95% CI: 16.8, 25.2). Emergence of an elevated LFT occurred equally across gender but slightly more frequently in those >18 years of age (4.0 persons per 100 follow up months, 95% CI: 2.3, 7.1) and least frequently in adolescents (3.0 persons per 100 follow up months, 95% CI: 2.1, 4.5). No significant associations between genotype or baseline FEV1 % predicted and the emergence of an elevated LFT were identified (data not shown). The individual emergence rates for elevated ALT and AST values were 2.0 persons per 100 follow-up months (95% CIs: 1.5, 2.6 and 1.5, 2.5 respectively). Emergence of an elevated GGT value was less common (0.5 persons per 100 follow-up months, 95% CI: 0.32, 0.86).
Figure 1.
Emergence rates (95% confidence intervals) of elevated LFTs by age group, gender, and overall across studies.
Overall, ALT values indicated as elevated were an average 32.3 U/L higher (95% CI: 26.8, 37.8) than ALT values within the normal range (average ALT among those with elevated values = 57.0 U/L, SD = 2.79 vs. 24.7 U/L among those with normal ALT values, SD=0.41). Similarly, elevated AST values were an average 21.3 U/L higher (95% CI: 16.8, 25.9) than AST values within the normal range (49.8 U/L, SD = 2.32 vs. 28.5 U/L, SD = 0.37) and elevated GGT values an average 34.8 U/L (95% CI: 16.8, 52.7) U/L higher (51.2, SD = 9.17 vs. 16.5, SD = 0.34).
Clinical Significance of the Emergence of Elevated LFTs
While 93/376 (25%) of trial participants experienced an emergent elevated LFT over their follow up, only 12/376 (3%) of all participants or 12/93 (13%) of those with emergent elevated LFTs had values deemed by the treating physician as clinically significant (Table A.2). The rate of emergence of clinically significant elevated LFTs was 0.4 per 100 follow up months (95% CI: 0.2, 0.7). Thus for a 6 month study of 100 participants, one would expect approximately 2.4 persons with a clinically significant emergent elevated LFT (95% CI: 1.2, 4.2).
Table 2 summarizes the potential indicators of hepatobiliary disease identified after medical and medication history, concurrent medication use, and adverse event review among the 93 participants with emergent elevated LFTs. As seen, only 11/93 (11.8%) of participants with an emergent elevated LFT had a potential indicator of hepatobiliary disease as identified by the medical review. In comparison, among the subset with normal LFTs throughout follow up, 7/93 (7.5%) also had a potential indicator of hepatobiliary disease. Only 3/93 (3.2%) of the participants with elevated LFTs had a coinciding emergent adverse event indicative of a hepatobiliary disorder, and 3/93 (3.2%) of participants were newly prescribed ursodeoxycholic acid. Further review of the medications of those with emergent elevated LFTs indicates that 89/93 (96%) of participants had documented use of at least one medication either historically or concurrently during the study that could be considered as potentially hepatotoxic as noted by both common and serious adverse effects noted in MICROMEDEX® 2.0.
Table 2.
Medical and medication history, initiation of new medications, and incidence of adverse events indicative of hepatobiliary disease among those subjects with emergent elevated LFTs and a random sample of subjects with normal LFTs throughout follow-up.
| Any Emergent Elevated LFT (N = 93) n (%) |
Subjects with Normal LFTsa (N = 93) n (%) |
|
|---|---|---|
|
| ||
| Overall | ||
|
| ||
| Number of subjects with any medical or medication history, initiation of new medication, or adverse event indicative of hepatobiliary disease | 11 (11.8%) | 7 (7.5%) |
|
| ||
| Medical History | ||
|
| ||
| Historical medical conditions indicative of hepatobiliary diseaseb, c | ||
|
| ||
| Elevated LFTs | 4 (4.3%) | 3 (3.2%) |
| Liver Disease | 5 (5.4%) | 1 (1.1%) |
| Gall Stones | 0 (0.0%) | 1 (1.1%) |
| Hepatomegally | 0 (0.0%) | 1 (1.1%) |
|
| ||
| Number of subjects with at least one historical medical condition indicative of hepatobiliary disease | 8 (8.6%) | 5 (5.4%) |
|
| ||
| Adverse Events | ||
|
| ||
| Adverse events indicative of hepatobiliary diseaseb | ||
|
| ||
| Adverse Drug Reaction | 1 (1.1%) | 1 (1.1%) |
| Hepatic atrophy | 1 (1.1%) | 0 (0.0%) |
| Liver Disorder | 1 (1.1%) | 0 (0.0%) |
|
| ||
| Number of subjects with at least one adverse event indicative of hepatobiliary disease | 3 (3.2%) | 1 (1.1%) |
|
| ||
| Medication Usage | ||
|
| ||
| Number of subjects with a history of ursodeoxycholic acid usage | 8 (8.6%) | 3 (3.2%) |
|
| ||
| Number of subjects initiating usage of ursodeoxycholic acid | 3 (3.2%) | 0 (0.0%) |
Random sample of study-matched subjects with normal LFTs throughout follow-up.
Not mutually exclusive. Subjects can have more than one medical condition.
Of the 6 subjects with liver disease among the 186 subjects evaluated, only 2 subjects (1 in each group) also had elevated LFTs historically. There were no other overlapping historical medical conditions among the subjects.
Association between Emergence of an Elevated LFT and Clinical Outcome
Table 3 summarizes the observed associations between the emergence of an elevated LFT and clinical outcomes including rate of change in FEV1 % predicted and weight, hospitalization rate, and adverse event rate. Emergence of an elevated LFT was not significantly associated with a difference in the rate of change in FEV1 % predicted or weight as compared to participants with normal LFTs throughout the follow up. Emergence of an elevated LFT value was however associated with a higher risk of hospitalization (Relative Risk [RR] 1.67, 95% CI: 1.11, 2.53), but not associated with a higher risk of adverse events (RR 0.91, 95% CI: 0.85, 0.97) as compared to those with normal LFTs.
Table 3.
Comparison of clinical outcomes between those with an emergent elevated LFT (≥1 emergent elevated LFT over the course of the trial) and those with normal LFTs (normal LFTs throughout follow-up).
| Normal LFTs throughout N = 283 |
Emergent Elevated LFT N = 93 |
|
|---|---|---|
| Mean rate of change (95% CI) per month of follow-up in FEV1 % predicteda | 0.24 (−0.02, 0.49) | −0.03 (−0.43, 0.37) |
| Avg. difference in rate of change (95% CI) as compared to those with Normal LFTsa | - | −0.26 (−0.75, 0.22) |
| Mean rate of change (95% CI) per month of follow-up in Weight (kg)a | 0.37 (0.23, 0.51) | 0.13 (−0.11, 0.37) |
| Avg. difference in rate of change (95% CI) as compared to those with Normal LFTa | - | −0.24 (−0.52, 0.04) |
| Number of Hospitalizations | 67 | 36 |
| Rate of Hospitalization (95% CI)b | 0.03 (0.02, 0.03) | 0.04 (0.03, 0.06) |
| Relative Risk (95% CI)c | - | 1.67 (1.11, 2.53) |
| Number of Adverse Events | 3534 | 1111 |
| Rate of Adverse Events (95% CI)b | 1.42 (1.38, 1.47) | 1.25 (1.18, 1.32) |
| Relative Risk (95% CI)c | - | 0.91 (0.85, 0.97) |
CI= confidence interval
Averages and confidence intervals are computed using generalized estimating equations with robust variance estimation to account for repeated measures, and adjusted for age group at baseline (<=12, >12–18, >18), sex and genotype (ΔF508 Homozygous, ΔF508 Heterozygous, Other/Unknown).
Rates and associated confidence intervals are calculated using Poisson regression with an offset for follow-up time.
Relative risks and associated confidence intervals are calculated using Poisson regression adjusting for age group at baseline (<=12, >12–18, >18), sex and genotype (ΔF508 Homozygous, ΔF508 Heterozygous, Other/Unknown).
Discussion
Clinical trials within the context of a chronic disease setting are complicated by underlying disease-related complications that are not related to treatment-induced safety endpoints. A pipeline of novel therapeutic interventions are currently under evaluation in CF clinical trials and these therapies are not only directed towards secondary consequences of the disease such as infection and inflammation, but also directed towards correcting the basic defect in CFTR (20). As CF is considered an orphan disease affecting only 30,000 children and adults in the United States and 70,000 worldwide, it is not always feasible to conduct placebo-controlled randomized trials either due to the lack of availability of patients or ethical concerns due to withholding treatment in order to administer a placebo (21). It is therefore critical to have a comprehensive characterization of expected rates of key safety endpoints, in particular those meant to directly capture drug toxicity such as LFTs, in order to benchmark the rates observed in future clinical trials of new interventions.
We conducted a retrospective cohort study utilizing data from completed CF clinical trials for which liver function tests were consistently evaluated across study participants. Emergence of elevated LFTs was surprisingly common in this cohort despite criteria which excluded participants abnormally high LFTs (> 2 times the upper limit of normal) at screening, and nearly 25% of participants with normal LFTs at baseline experienced an emergent elevated LFT greater than the upper limit of normal in at least one LFT over an average 8 month follow-up period. The data also suggest that older participants (>18 years of age) are more likely to experience emergence of an elevated LFT. The majority of elevated LFTs had unknown etiology and very few were associated with hepatobiliary related adverse events. Importantly, participants in CF trials are commonly on medications as part of their standard of care which can be considered as potentially hepatotoxic, and 96% of all participants with an elevated LFT had taken at least one medication considered as potentially hepatotoxic.
The majority of elevated LFTs were not felt to be clinically significant according to the treating physician. In fact, only 13% of the 93 participants with an elevated LFT had values determined by the physician to be clinically significant. Clinical significance is however a subjective assessment by the physician and is a determination that is not guided by formal Common Toxicity Criteria (CTC) typically used only for adverse event grading (22). Thus, it is unclear whether there is a bias in under-reporting clinical significance due to non-standardized criteria and variability in physician interpretation of individual results in relation to the disease and medical history prior to entering the trial. On the other hand, emergence of elevated LFTs was associated with higher risk of hospitalization. Unfortunately, this retrospective study was limited in our lack of ability to precisely model temporal associations between LFTs and hospitalization events. The association of elevated LFTs with hospitalization could merely reflect temporally synchronous abnormalities associated with the hospitalization, intercurrent illness, or medications employed during the hospitalization or illness including intravenous antibiotics. The abnormalities could have also preceded the hospitalization and/or followed the hospitalization. Thus, inferring any causality would be inappropriate within the context of this study.
The current study is limited by the usage of site specific laboratories rather than a centralized laboratory, and this limits comparisons of individual LFT values across trial participants. However, many CF studies utilize site-specific rather than centralized laboratories due to funding constraints and additionally, the rates of elevated LFTs based on site laboratories play an important role in standard of care assessments throughout the course of any clinical trial and elevated or “abnormal” results will often be recorded as adverse events in the trial database. Recent data has also suggested that individual lab normal values for AST and ALT may be inaccurate given the rising prevalence of obesity and non-alcoholic fatty liver disease (23). A further limitation is that this study included a relatively young and healthy cohort with mild lung disease, but the results among the subset of older participants with greater disease severity are comparable to those observed in older sicker populations as studied previously by Goss et. al (9). Comparisons of these two studies suggest that LFT elevations remain a relatively common occurrence in CF clinical trials even in a more contemporary CF population with improved standard of care. Another key limitation is that in the studies included in this analysis, no unified diagnostic criteria for CF related liver disease was employed. Subjects were excluded if they had elevated LFT’s at screening, but this criteria may not exclude subjects with significant CF related liver disease, a population that can have normal LFT’s. The diagnostic criteria for CF related liver disease remains a challenge, and it is hoped that ongoing prospective cohort studies will further elucidate this clinical entity.
In summary, the emergence of elevated LFTs should be expected in CF clinical trials and in many cases may be the result of underlying disease complications, as well as potentially the use of concurrent medications that have been noted to cause some degree of hepatoxicity. Although elevated LFTs were rarely deemed clinically significant during the short term follow-up of a clinical trial, a priori criteria for determining clinical significance should be developed and standardized across physicians in order to enable the unbiased evaluation of the safety of new therapies, in particular for trials lacking a placebo-control. Further studies would be needed to determine the longer term causality and impact, if any, of elevated LFTs on clinical outcomes of morbidity and mortality.
Supplementary Material
Acknowledgments
Funding Support
Support for this study was provided by Vertex Pharmaceuticals and NIH/NIDDK (P30 DK089507-01). The azithromycin trials were supported by Cystic Fibrosis Foundation Therapeutics, Inc. (CFFT) and the EPIC trial supported with collaboration between the CFFT and National Heart Lung and Blood Institute (NHLBI) and National Institute for Digestive Disorders and Kidney (NIDDK) grant number U01-HL080310.
Special thanks to the individuals with CF who participated in the participating clinical trials and whose dedication to research made this study possible.
Bibliography
- 1.Diwakar V, Pearson L, Beath S. Liver disease in children with cystic fibrosis. Paediatr Respir Rev. 2001;2(4):340–9. doi: 10.1053/prrv.2001.0170. [DOI] [PubMed] [Google Scholar]
- 2.Narkewicz MR. Markers of cystic fibrosis-associated liver disease. J Pediatr Gastroenterol Nutr. 2001;32(4):421–2. doi: 10.1097/00005176-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Nash KL, Allison ME, McKeon D, Lomas DJ, Haworth CS, Bilton D, Alexander GJ. A single centre experience of liver disease in adults with cystic fibrosis 1995–2006. J Cyst Fibros. 2008;7(3):252–7. doi: 10.1016/j.jcf.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Sidlova K, Skalicka V, Kotaska K, Pechova M, Chada M, Bartosova J, Hribal Z, Nevoral J, Vavrova V, Prusa R. Serum alpha-gluthathione S-transferase as a sensitive marker of hepatocellular damage in patients with cystic fibrosis. Physiology Research. 2003;52:361–5. [PubMed] [Google Scholar]
- 5.Bettinardi N, Felicetta I, Tomasi PA, Colombo C. Carbohydrate 19–9 antigen is not a marker of liver disease in patients with cystic fibrosis. Clin Chem Lab Med. 2003;41(3):311–6. doi: 10.1515/CCLM.2003.050. [DOI] [PubMed] [Google Scholar]
- 6.Wyatt HA, Dhawan A, Cheeseman P, Mieli-Vergani G, Price JF. Serum hyaluronic acid concentrations are increased in cystic fibrosis patients with liver disease. Arch Dis Child. 2002;86(3):190–3. doi: 10.1136/adc.86.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabolde M, Hubert D, Guilloud-Bataille M, Lenaerts C, Feingold J, Besmond C. The mannose binding lectin gene influences the severity of chronic liver disease in cystic fibrosis. J Med Genet. 2001;38(5):310–1. doi: 10.1136/jmg.38.5.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindblad A, Glaumann H, Strandvik B. Natural history of liver disease in cystic fibrosis. Hepatology. 1999;30(5):1151–8. doi: 10.1002/hep.510300527. [DOI] [PubMed] [Google Scholar]
- 9.Goss CH, Mayer-Hamblett N, Kronmal RA, Williams J, Ramsey BW. Laboratory parameter profiles among patients with cystic fibrosis. J Cyst Fibros. 2007;6(2):117–23. doi: 10.1016/j.jcf.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Colombo C, Battezzati PM, Crosignani A, Morabito A, Costantini D, Padoan R, Giunta A. Liver disease in cystic fibrosis: A prospective study on incidence, risk factors, and outcome. Hepatology. 2002;36(6):1374–82. doi: 10.1053/jhep.2002.37136. [DOI] [PubMed] [Google Scholar]
- 11.Patriquin H, Lenaerts C, Smith L, Perreault G, Grignon A, Filiatrault D, Boisvert J, Roy CC, Rasquin-Weber A. Liver disease in children with cystic fibrosis: US-biochemical comparison in 195 patients. Radiology. 1999;211(1):229–32. doi: 10.1148/radiology.211.1.r99ap13229. [DOI] [PubMed] [Google Scholar]
- 12.Saiman L, Marshall BC, Mayer-Hamblett N, Burns JL, Quittner AL, Cibene DA, Coquillette S, Fieberg AY, Accurso FJ, Campbell PW., 3rd Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2003;290(13):1749–56. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 13.Saiman L, Anstead M, Mayer-Hamblett N, Lands LC, Kloster M, Hocevar-Trnka J, Goss CH, Rose LM, Burns JL, Marshall BC, Ratjen F. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2010;303(17):1707–15. doi: 10.1001/jama.2010.563. [DOI] [PubMed] [Google Scholar]
- 14.Saiman L, Mayer-Hamblett N, Anstead M, Lands LC, Kloster M, Goss CH, Rose LM, Burns JL, Marshall BC, Ratjen F Team atAMS. Open-label, follow-on study of azithromycin in pediatric patients with CF uninfected with Pseudomonas aeruginosa. Pedatric Pulmonology. 2012 doi: 10.1002/ppul.21601. in press. [DOI] [PubMed] [Google Scholar]
- 15.Treggiari MM, Retsch-Bogart G, Mayer-Hamblett N, Khan U, Kulich M, Kronmal R, Williams J, Ramsey BW Investigators ftE. Comparative efficacy and safety of four randomized regimens to treat early Pseudomonas aeruginosa infection in children with cystic fibrosis. Arch Pediatr Adolesc Med. 2011;165(9):847–56. doi: 10.1001/archpediatrics.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saiman L, Cohen MB. What Have We Learned About Early Treatment of Pseudomonas aeruginosa Infection in Infants and Children With Cystic Fibrosis? Arch Pediatr Adolesc Med. 2011;165(9):867–8. doi: 10.1001/archpediatrics.2011.133. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Department of Health and Human Services FaDA, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER) Guidance for Industry: Adverse Reactions Section of Labeling for Human Prescription Drug and Biological Products -- Content and Format. 2006 http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm075057.pdf.
- 18.Diggle P, Heagerty P, Liang KY, Zeger S. Analysis of longitudinal data. Oxford, U.K: Oxford University Press; 2002. [Google Scholar]
- 19.Rowland M, Gallagher CG, O'Laoide R, Canny G, Broderick A, Hayes R, Greally P, Slattery D, Daly L, Durie P, Bourke B. Outcome in cystic fibrosis liver disease. Am J Gastroenterol. 2011;106(1):104–9. doi: 10.1038/ajg.2010.316. [DOI] [PubMed] [Google Scholar]
- 20.Ashlock MA, Beall RJ, Hamblett NM, Konstan MW, Penland CM, Ramsey BW, Van Dalfsen JM, Wetmore DR, Campbell PW., 3rd A pipeline of therapies for cystic fibrosis. Semin Respir Crit Care Med. 2009;30(5):611–26. doi: 10.1055/s-0029-1238919. [DOI] [PubMed] [Google Scholar]
- 21.Treggiari MM, Rosenfeld M, Mayer-Hamblett N, Retsch-Bogart G, Gibson RL, Williams J, Emerson J, Kronmal RA, Ramsey BW. Early anti-pseudomonal acquisition in young patients with cystic fibrosis: rationale and design of the EPIC clinical trial and observational study'. Contemp Clin Trials. 2009;30(3):256–68. doi: 10.1016/j.cct.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.U.S. National Institutes of Health NCI. Cancer Therapy Evaluation Program. http://ctepcancergov/protocolDevelopment/electronic_applications/ctchtm.
- 23.Schwimmer JB, Dunn W, Norman GJ, Pardee PE, Middleton MS, Kerkar N, Sirlin CB. SAFETY study: alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease. Gastroenterology. 2010;138(4):1357–64. 64 e1–2. doi: 10.1053/j.gastro.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.