Abstract
We previously showed that palmitic methyl ester (PAME) and stearic acid methyl ester (SAME) are simultaneously released from the sympathetic ganglion and PAME possesses potent vasodilatory properties which may be important in cerebral ischemia. Since PAME is a potent vasodilator simultaneously released with SAME, our hypothesis was that PAME/SAME confers neuroprotection in rat models of focal/global cerebral ischemia. We also examined the neuroprotective properties of Solutol HS15, a clinically approved excipient, because it possesses similar fatty acid compositions as PAME/SAME. Asphyxial cardiac arrest (ACA, 6min) was performed 30mins after PAME/SAME treatment (0.02mg/kg, IV). Solutol HS15 (2 ml/kg, IP) was injected chronically for 14 days (once daily). Histopathology of hippocampal CA1 neurons was assessed 7 days after ACA. For focal ischemia experiments, PAME, SAME, or Solutol HS15 was administered following reperfusion after 2 hrs of middle cerebral artery occlusion (MCAO). 2,3,5-triphenyltetrazolium staining of the brain was performed 24hrs after MCAO and the infarct volume was quantified. Following ACA, the number of surviving hippocampal neurons was enhanced by PAME (68%), SAME (69%), and Solutol HS15 (68%)-treated rats as compared to ACA only-treated groups. Infarct volume was decreased by PAME (83%), SAME (68%), and Solutol HS15 (78%) as compared to saline (vehicle) in MCAO-treated animals. PAME, SAME, and Solutol HS15 provide robust neuroprotection in both paradigms of ischemia. This may prove therapeutically beneficial since Solutol HS15 is already administered as a solublizing agent to patients. With proper timing and dosage, administration of Solutol HS15 and PAME/SAME can be an effective therapy against cerebral ischemia.
Keywords: asphyxial cardiac arrest, middle cerebral artery occlusion, palmitic acid methyl ester, stearic acid methyl ester, neuroprotection
Introduction
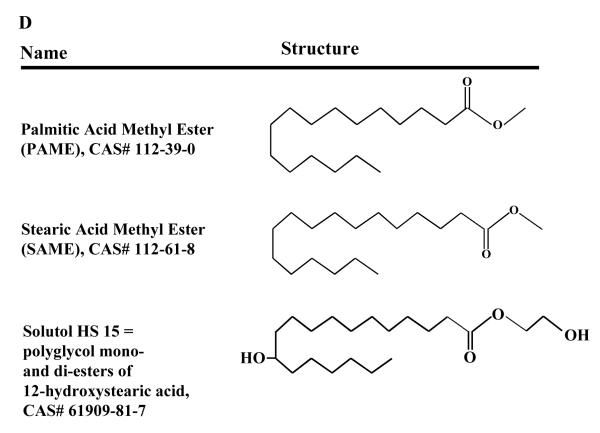
We previously showed that palmitic acid methyl ester (PAME) and stearic acid methyl ester (SAME) are simultaneously released upon chemical and electrical depolarization from the superior cervical ganglion (SCG) of the rat. Exogenous and endogenous application of PAME but not SAME induced potent rabbit aortic vasodilation (EC50 = 0.19 nM) and is 3000 times more potent than some nitric oxide (NO) donors [1]. Since the vast majority of vasodilators [i.e. NO, calcitonin gene-related peptide (CGRP), vasoactive intestinal peptide (VIP), and carbon monoxide (CO)] can exert intrinsic neuroprotection [2-7], we sought to investigate the possible neuroprotective effects of exogenous PAME and SAME in focal (middle cerebral artery occlusion with 2 mins of reperfusion, MCAO) and global (6 mins of asphyxial cardiac arrest, ACA) cerebral ischemia models. These two models of ischemia were selected to determine 1) delayed (7 days after ACA) neuronal cell death in the hippocampus as it is well-documented in the CA1 region of the hippocampus after ACA [8] and 2) observe brain infarct volume acutely 24 hrs in a stroke model (MCAO-focal cerebral ischemia) [9, 10]. Since SAME is a 18 carbon fatty acid, we also investigated the effects of Solutol HS 15 (polyoxyethylene glycol of 12-hydroxystearic acid), a highly soluble/stable in aqueous solution with low toxicity non-ionic solubilizer and emulsifying agent, that is clinically used to solubilize drugs such as: various vitamins, propanidid, miconazole, alfadolone, alfaxalone, nifedipine, and piroxicam; none of which are currently approved for the treatment of stroke or cerebral ischemia. Currently in the U.S., Solutol HS 15 is approved for oral consumption but has been marketed as an injectable drug product in Canada and Argentina [11].
Previous reports have suggested that fatty acids may provide robust neuroprotection after MCAO in rats [12-15]. We show that PAME, a potent vasodilator and SAME can provide neuroprotection against focal and global cerebral ischemia. Since SAME provides neuroprotection, we also tested Solutol HS 15 a structurally similar fatty acid as a possible neuroprotective agent. Our hypothesis was that similar to PAME and SAME, Solutol HS 15 can also confer neuroprotection in the rat in vivo. We show that pre and post-treatment of Solutol HS 15 confers neuroprotection in models of focal and global cerebral ischemia. Given the low toxicity of this drug, as attested by numerous clinical trials and possible clinical efficacy of Solutol HS 15 as a neuroprotective agent against cerebral ischemia, Solutol HS 15 could reach clinical trials for stroke in the near future.
Methods
Chemicals
Solutol HS 15 (BASF Corp, Florham Park, NJ, USA) and dimethyl sulfoxide (DMSO) were dissolved in sterile saline (0.9% NaCl). 600 μL of Solutol HS 15 was heated to 37°C and dissolved in 1.4 mL of sterile saline to achieve 2 ml/kg [16] injected (IV bolus or IP, daily for 14 days) into the rat used in MCAO and ACA models of ischemia. PAME and SAME were dissolved in 100% ethanol and diluted with sterile saline injected bolus IV (0.005% final ethanol concentration of PAME and SAME) for ACA model of cerebral ischemia. 0.005% ethanol was used as a vehicle (control). For MCAO studies, PAME, SAME, and 12-hydroxystearic acid (used as a negative control for MCAO studies, 12-HS) (Sigma-Aldrich, St. Louis, MO) was dissolved in 100% DMSO and diluted with sterile saline to achieve a concentration of 0.02 mg/kg. DMSO alone was used as vehicle control. Overall, 0.01% DMSO was injected into all groups with the exception of Solutol HS 15. Saline groups were injected with 0.9% NaCl with a final volume of 2 mL.
We used 0.02 mg/kg of PAME, SAME, and 12-HS in all of our studies. This was based on the fact that PAME produces maximum rabbit aortic vasorelaxation at 1 μM ex vivo [1]. Therefore, we hypothesized that utilizing 1 μM (This translates to 0.02 mg/kg in vivo in the 300g rat.) of PAME, SAME, or 12-HS can evoke neuroprotection in both ischemia animal models. Additionally, the dosages for PAME and SAME were derived from our previous in vivo two photon laser scanning microscopy (TPLSM) pial circulation studies (Figure 2E) suggesting that PAME (0.02 mg/kg) can enhance cerebral blood flow (CBF) 24 hrs after ACA. This was a starting point since we are the first to administer PAME or SAME in vivo. We administered the 18 carbon fatty acid, 12-HS as a negative control for PAME/SAME and Solutol HS 15. Therefore, the same concentration was used for 12-HS as with PAME/SAME. Dosage for Solutol HS 15 was determined according to manufacturer’s instructions as a novel excipient. Derived from previous studies [16], Solutol HS 15 was administered at 30 (Solutol HS 15): 70 (saline) (v:v) injected in 2 ml/kg (or 600 μl of solution containing Solutol HS 15 and saline, 30:70).
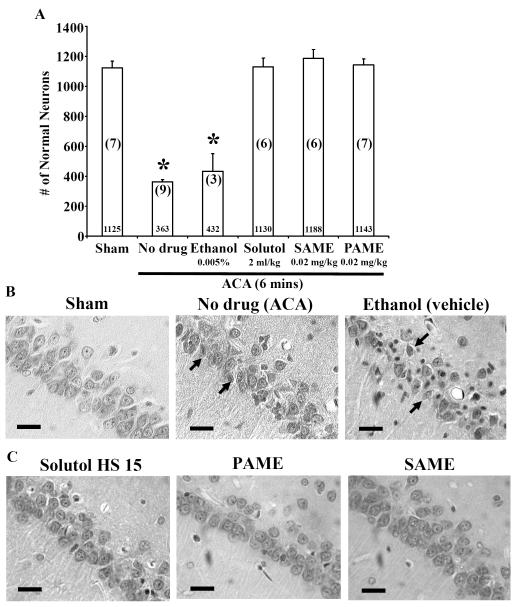
Figure 2.
Rats pretreated with PAME (0.02 mg/kg), SAME (0.02 mg/kg), and Solutol HS 15 (2 ml/kg) provide neuroprotection in the CA1 region of the rat hippocampus 7 days after ACA. Rats were pretreated with PAME/SAME (IV bolus 30 mins before ACA), and Solutol HS 15 (IP, once daily for 14 days before 6 mins of ACA). Sham (no ischemia), no drug (ACA only), and ethanol (vehicle control) groups were performed as internal controls. Neurons from the CA1 region of the hippocampus were counted and expressed in the bar graph shown in A. Numerical values in the bar graph represent the number of normal neurons counted. Numbers in parentheses indicate the number of animals used per group. Representative images of the CA1 region of the hippocampus are shown in B-C. Arrows represent typical neuronal cell death in the CA1 region of the hippocampus. Short horizontal solid bars represent 30 μm in length in the field of view of each representative image (n = 3-9, * p ≤ 0.05). (D) Schematic diagram of the experimental design of in vivo imaging of cortical microvessels via TPLSM in the anesthetized rat. (E) Linescans of the cerebral vessel were performed to determine CBF in cortical microvessels. Each animal serves as its own control (baseline measurement of CBF before ACA and/or PAME/SAME. The data is expressed as percent change in flow from baseline (control) (n = 3-6, * p ≤ 0.05).
Animal Preparation
All procedures were approved by the Institutional Animal Care and Use Committee (University of Miami, Miller School of Medicine). Adult male Sprague-Dawley rats (250-350g) were fasted overnight before surgery. Rats were anesthetized with 4% isoflurane and 30:70 mixture of O2 and N2O, followed by endotracheal intubation. Isoflurane was lowered to 1.5-2% for endovascular access. The femoral vein and artery were cannulated using a single-lumen (PE-50) catheter for blood pressure monitoring, blood gas analysis, and intravenous (IV) injection of pharmacological agents. Head and body temperatures were maintained at 37°C using heating blankets or lamps during the entire procedure in the presence or absence of ischemia. For physiological parameters for both global and focal ischemia models, please refer to Supplementary Tables 1-3.
Asphyxial Cardiac Arrest (ACA)
To induce ACA, apnea was induced by disconnecting the ventilator from the endotracheal tube. 6 mins after asphyxia, resuscitation was initiated by administering a bolus injection of epinephrine (0.005 mg/kg, IV) and sodium bicarbonate (1 meq/kg, IV) followed by mechanical ventilation with 100 % O2 to achieve return to spontaneous circulation (ROSC) [8]. Arterial blood gases were measured before and after ACA. Control animals (sham) were subjected to surgical procedures similar to ACA animals except without induction of ACA. Resuscitation drugs were not used; however, sham animals were treated with isoflurane similar to experimental animals. The rats were immobilized with vecuronium bromide (2.0 mg/kg, IV, administered every 10 mins) and maintained immobilized throughout the procedure [8].
Two-photon Laser Scanning Microscopy (TPLSM)
For detail methods of the thinned-skull method and the detection of CBF via TPLSM, please see Lin HW et al., 2010 and 2012 [8, 17]. Briefly, the animal was anasthetized and cannulated (femoral artery and vein). An incision was made at the midline of the scalp. Utilizing a high-speed micro-drill, a thin circular area of the skull (approximately ~ 2 mm in diameter) was made 1 mm from bregma. The skull is thin enough for visualization of blood vessels via 2-PM when it is approximately half the thickness of the skull or approximately 0.5 mm [18]. After thinning the skull, the rat was placed on a TPLSM (Lasersharp2000, BioRad, Hercules, CA) with a 20 X water immersion objective (Olympus XLUMPlanFl) lowered to the thinned-skull window. Fluorescent images were captured at an excitation wavelength of 910 nm with the intravenous introduction of fluorescein-dextran (average MW, 2,000,000, Sigma Aldrich, St. Louis, MO, USA) (0.2 mg/kg). Cortical cerebral blood vessel images were captured at 20 X, and 200 X with Z-series (20 X) and linescan (200 X) images obtained 30 mins before and 24 hrs after ACA (Figure 2D). PAME or SAME was introduced IV at 0.2 mg/kg 30 min before ACA. Linescan images were used to calculate CBF. For more details, please see Lin HW et al., 2010 and 2012 [8, 17].
Histopathology
7 days after ACA, rats were re-anesthetized with 4% isoflurane and perfused from the ascending aorta with physiologic saline (2 mins), following a mixture of 40% formaldehyde, glacial acetic acid, and methanol (FAM) for 18 mins and the head immersed in FAM at 4°C (24 hrs). Rat brains were removed from the skull, embedded in paraffin, and coronal sequential sections (10 μm thickness) from the brain were made and stained with hematoxylin and eosin. CA1 hippocampal sections were visualized at 40X magnification. Ischemic neurons were manually counted by a blinded operator at 18 fields/section along the medial to lateral extent of the CA1 region of the hippocampus 3.8 mm posterior to the bregma. The ischemic neuronal changes consist of severe cellular shrinkage, cytoplasmic eosinophilia, pyknotic triangular-shaped nucleus with dark basophilic staining, and eosinophilic staining nucleolus [19].
Middle Cerebral Artery Occlusion (MCAO)
Animals were prepared as described above (see “Animal Preparation” section). Animals were intubated and anesthesia was applied. Rats were immobilized with pancuronium bromide (0.75mg/kg, IV). Focal ischemia was induced in rats using an occluding intraluminal suture. A coated (with poly-D-lysine) 30 mm long segment of 3–0 nylon monofilament suture with the tip rounded by a flame was inserted into the stump of the external carotid artery and advanced into the internal carotid artery ~19–20 mm from the carotid bifurcation to occlude the ostium of the MCA for 2 hrs with 2 mins of reperfusion. Then, Solutol HS 15, PAME, SAME, or 12-HS were introduced IV. The intraluminal suture was left in place for 2 hrs and the sites of incisions (neck and femoral region for arterial and vein access) were sutured after 2 mins and reperfusion of drugs allowing the animal to recover (removal of intubation tube). 2 hrs later, the rats were re-anesthetized via mask with 1.5-2% isoflurane in the absence of pancuronium bromide. The intraluminal suture was removed and the animals were allowed to recover for 24 hrs. Rats were sacrificed with 5% isoflurane and 100% nitrous oxide after 24 hrs of reperfusion. The brain was quickly removed and sliced (2 mm thick) in preparation for the 2,3,5-triphenyltetrazolium chloride (TTC) staining [9, 10].
2,3,5-Triphenyltetrazolium chloride (TTC) staining
Rats were sacrificed 24 hrs after reperfusion. Brains were removed, placed in a brain matrix, and sliced into 2 mm coronal sections, resulting in six brain slices stained with 2% TTC. The brain slices were scanned on a flat-bed scanner for infarct assessment shown in white. Relative stroke area (ratio of infarct size relative to the ipsilateral hemisphere, corrected for edema via comparison of the contralateral hemisphere) was measured (via Image J software) [20] to assess infarct size from the six slices derived from each brain [9, 10].
Exclusion Criteria
MCAO model of focal ischemia
Excluded from the MCAO studies were rats that experience convulsions or prolonged disturbances of consciousness. Additionally, subarachnoid hemorrhage due to suture-induced arterial rupture was also excluded. In the present study, 4 animals were excluded from these studies.
ACA model of global ischemia
No animals were excluded with no mortality due to the fact that 6 min of ACA is a mild to moderate model of global ischemia. Occasionally, on average, 1 out of 10 animals will be excluded due to the fact that the animal did not survive the ACA upon cardiopulmonary resuscitation. Cause of death after cardiopulmonary resuscitation is almost always due to pulmonary edema by too much chest compression pressure by the operator.
Statistical Analysis
Results were expressed as means ± S.E.M. Statistical analysis was evaluated by one-way ANOVA followed by Tukey’s post hoc test as appropriate with SPSS statistical software (Chicago, IL). The p ≤ 0.05 level of probability was accepted as significant.
Results
PAME, SAME, and Solutol HS 15 after global cerebral ischemia
Our first hypothesis is that PAME, SAME, and Solutol HS 15 can confer neuroprotection after global (6 mins ACA) cerebral ischemia (Figures 1A and 1B). PAME (1143 ± 39.3 normal neurons) or SAME (1188 ± 57.8 normal neurons) pretreatment 30 min before ACA (6 min) conferred neuroprotection in the CA1 region of the hippocampus 7 days after ACA as compared to no drug treatment (ACA only) or vehicle (ethanol 0.005%) (Figure 2). Since SAME is an 18 carbon fatty acid, we investigated the possible neuroprotective effects of Solutol HS 15, which is a polyoxyethylene glycol (PEG) of 12-HS similar in structure to SAME with the exception of an additional methoxy group at C1 and hydroxyl group at C12.
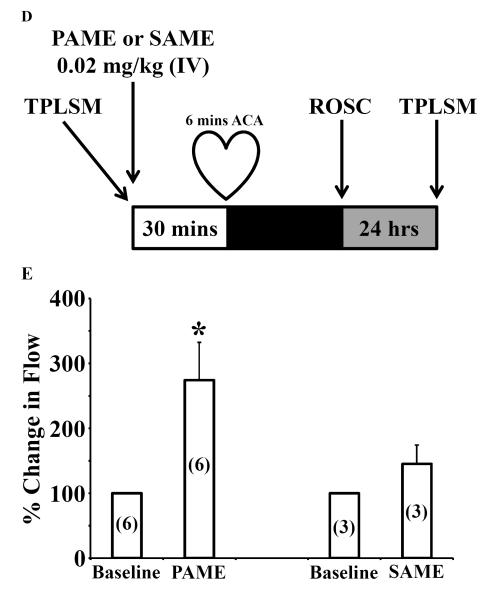
Figure 1.
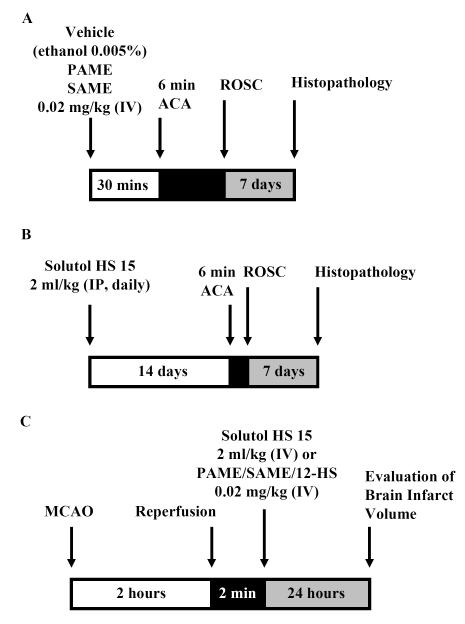
Schematic diagram of the experimental design. (A) PAME, SAME, or 12-HS (0.02 mg/kg, bolus, IV) was administered 30 mins before or (B) solutol HS 15 (2 ml/kg, IP) was administered once daily for 14 days before 6 mins of ACA. 7 days after ACA, whole brain sections were stained with hemotoxylin and eosin. Normal neurons of the CA1 region of the rat hippocampus were manually counted on both hemispheres of the brain. (C) MCAO (2 hrs) was performed on the rat. 2 mins after reperfusion, Solutol HS 15 (2 ml/kg, IV), PAME, SAME, or 12-HS (0.02 mg/kg, IV) were administered. After 24 hrs of recovery, the rats are sacrificed, brain slices stained with TTC and brain infarct volumes measured. (D) Two-dimensional structure and the Chemical Abstracts Service (CAS) numbers of PAME, SAME, and Solutol HS 15.
Since Solutol HS 15 is an excipient that is often administered chronically (i.e. nifedipine and miconazole), we surmised that chronic pretreatment would afford neuroprotection against ACA. We thus, pretreated rats with Solutol HS 15 (2 ml/kg, IP) for 14 days before ACA. This treatment provided robust neuroprotection in the CA1 region of the rat hippocampus 7 days after ACA. Rats pretreated with Solutol HS 15 in the presence of ACA presented with 1130 ± 60 normal neurons, similar to the sham-operated group (1125 ± 41.5 normal neurons) (Figures 2A-C). In contrast, rats without drug treatment (ACA only) presented with only 363 ± 15 normal neurons, indicating that 6 mins of ACA produced significant neuronal damage. Additionally, rats pretreated with PAME but not SAME (Figures 2D and E) enhanced CBF 24 hrs (almost 3 times more as compared to control) after ACA measuring cortical vessel red blood cells via TPLSM. Physiological parameters such as body weight, pH, PCO2, PO2 MABP, and blood glucose were measured (Supplementary Tables 1 and 2) to ensure consistency of the animals within and between groups. For a two-dimensional structure of the fatty acids, please see Figure 1D.
PAME, SAME, and Solutol HS 15 after focal cerebral ischemia
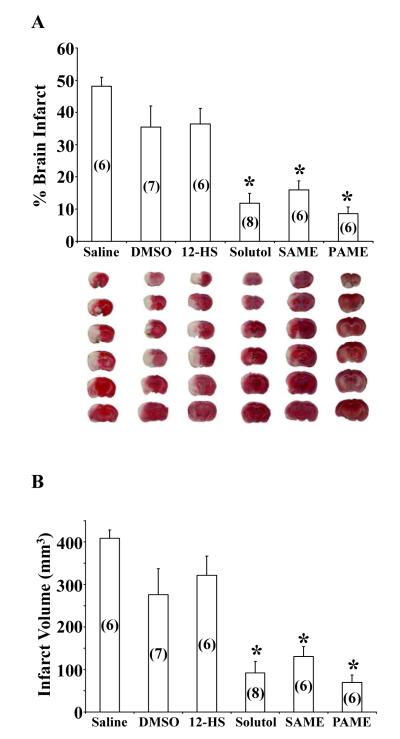
Although not all types of cerebral ischemia are the same, since PAME, SAME, and Solutol HS 15 afforded neuroprotection against ACA in the CA1 region of the hippocampus, we investigated the effects of these fatty acid-mediated neuroprotection (post-treatment, after MCAO reperfusion) using a stroke model of focal cerebral ischemia followed by TTC treatment for total brain infarct analyses (Figure 1C).
Saline (vehicle) (48.08 ± 2.80%), DMSO (vehicle) (35.43 ± 6.64%), and 12-HS (36.43 ± 4.82%)-treated groups presented with no reduction in percentage of infarct volume contrary to Solutol (11.79 ± 3.12%), SAME (15.97 ± 2.76%), and PAME (8.63 ± 2.03%)-treated groups (Figure 3A). Rats post-treated with saline or DMSO produced an increase in infarct volume while 12-HS (negative control), the main fatty acid component in Solutol HS 15, produced no reduction in infarct volume as compared to saline or DMSO vehicles. Typical TTC-treated rat brains are shown below the bar graph with corresponding treatments. Saline (408.6 ± 19.93 mm3), DMSO (276.1 ± 61.05 mm3), 12-HS (321.9 ± 45.0 mm3), Solutol (92.01 ± 27.07 mm3), SAME (130.6 ± 22.85 mm3), and PAME (69.24 ± 17.48 mm3)-treated groups were also measured for total brain infarct volume (Figure 3B). Physiological parameters such as body weight, pH, PCO2, PO2 MABP, and blood glucose were measured (Supplementary Table 3) to ensure consistency of the animals within and between groups.
Figure 3.
Post-treatment with PAME, SAME, or Solutol HS 15 after 2 hrs of MCAO decreases total rat brain infarct volume. Post-treatment with PAME (0.02 mg/kg, IV), SAME (0.02 mg/kg, IV), or Solutol HS 15 (2 ml/kg, IV) decreased ipsilateral hemisphere volume as compared to saline (vehicle for Solutol HS 15), DMSO [partial vehicle for (12-HS), PAME, or SAME], and 12-HS (negative control). TTC-treated brain slices were scanned and analyzed (Image J software) at the ipsilateral hemisphere. The percent of brain infarct volume is depicted in white and tabulated in panel (A) and the total brain infarct volume (expressed in mm3) is presented in (B). Representative images are shown below each bar graph corresponding to their relative drug treatment (A) (n = 6-8, * p ≤ 0.05).
Discussion
PAME, SAME, and Solutol HS 15 all can provide neuroprotection in rat models of global and focal cerebral ischemia, with a reduction in neuronal cell death in the CA1 region of the hippocampus [8] (7 days after ACA – delayed cell death) and this led to the utilization of the MCAO method to detect brain infarct volume acutely 24 hrs after MCAO (focal ischemia) [9, 10] as a model for stroke resulting in overall neuroprotection of the brain as observed using TTC staining. Additionally, pretreatment with PAME but not SAME can enhance CBF 24 hrs after ACA.
Stearic acid can provide neuroprotection against oxidative stress and oxygen/glucose deprivation in rat cortical/hippocampal slices [21, 22]. However, the esterified form of stearic acid (stearic acid methyl ester) has not been well-explored. Since SAME provides neuroprotection in both models of ischemia (global and focal), this led to further investigations into Solutol HS 15, a clinically used excipient that consists of PEG of 12-HS, similar in structure to SAME [23]. As a negative control, we administered 12-HS in a similar fashion as other drugs/vehicles tested (Figure 3). Neuronal damage was similar to that of DMSO/saline suggesting no protective effects of 12-HS alone, but the PEG of 12-HS may be crucial in providing neuroprotection. The mechanism of action of Solutol HS 15-induced neuroprotection is unknown, but PEG alone can stabilize and mechanically repair cell membranes in traumatic brain injury models [24, 25]. Interestingly, PEG is also thought to enter the cytosol and restructure reactive oxygen species/free radical-induced damaged mitochondria plasmalemma [26] leading to neuroprotection [27]. Unlike PEG, 12-hydroxy stearic acid alone did not elicit neuroprotection (present data). It is conceivable that this combined structure moiety is the key to eliciting neuroprotection due to neuronal cell membrane stabilization. Additionally, it is also important to note that PEG was administered at 1 ml IV injection of sterile saline with 30% by volume of PEG to achieve PEG-saline solution similar to our studies. However, the PEG used had a MW of 2000 unlike Solutol HS15 (MW = 1000). This discrepancy in molecular weight and structure could explain the possible differences in our results [24-26, 28].
There are examples of excipients (solvents) that may have therapeutic effects such as DMSO, which has been shown to be neuroprotective (> 0.1% DMSO) against cerebral ischemia [29]. However, these studies incorporate only 0.01% DMSO, which have been shown to be ineffective in providing neuroprotection. In both ischemia models, using low quantities of either ethanol or DMSO resulted in no measurable effects of neuroprotection.
Since PAME (hexadecanoic acid methyl ester, a 16 carbon chain fatty acid) is a potent vasodilator, it is highly likely that PAME’s neuroprotective properties are derived from the revival of CBF in otherwise ischemia-induced depression of CBF exacerbating neuronal damage. Like other vasodilators such as CGRP and NO, the depression of CBF can cause neuronal damage possibly reversed with PAME or other vasodilators (i.e. NO or CGRP). Cortical CBF was not measured in the MCAO model due to the technical complexity with the focal ischemia technique coupled with intravital TPLSM. Consistent with our previous findings [1], our results suggest that PAME, but not SAME (18 carbon fatty acid) can cause vasodilation to enhance CBF after global ischemia (asphyxial cardiac arrest) (Figures 2D and E). Since SAME, an 18 carbon fatty acid, did not cause vasodilation/constriction nor changes in CBF, we did not pursue this study further with similar 18 carbon fatty acids, Solutol HS15 or 12-HS. Our results suggest that PAME, but not SAME has vasodilatory actions (from previous studies) in vivo and in vitro resulting in enhanced brain perfusion after ischemia (Figures 2D and E). Besides enhanced perfusion (i.e. vasodilatory actions of PAME), the neuroprotective actions of PAME can be attributed to its’ inherent anti-inflammatory properties during cellular stress [30, 31]. However, the role of SAME-induced neuroprotection (not a vasodilator) [1] remains unknown. Interestingly, SAME is released at twice the concentration as compared to PAME upon depolarization of the SCG [1]. The exact mechanism of SAME-induced neuroprotection needs further investigation but may be similar to Solutol HS 15 by stabilizing cellular membranes under conditions of stress [24-28].
The concentration of PAME and SAME used in this study were entirely based on previous studies of PAME-induced aortic vasodilation of 1 μM in vitro [1]. This was extrapolated to the in vivo models of global (PAME/SAME pretreatment) and focal (PAME/SAME post treatment) ischemia. Acute/chronic pre- and post-treatments of PAME and SAME in both models of ischemia need to be further investigated. Similar experimental paradigms can be implemented to further define the role of Solutol HS 15. With well-defined fatty acid concentrations and time-points of administration, this strategy may be able to define fatty acids as a therapeutically viable option in the treatment against stroke and/or brain ischemia.
Global (ACA) cerebral ischemia was used as a model to study delayed-neuronal cell death (7 days after ischemia) of hippocampal CA1 neurons as they are highly susceptible to cell death under global ischemic conditions as previously shown [10, 17]. Aside from the fact that the ACA model of global ischemia is more clinically relevant than other global ischemia paradigms [8] (i.e. 2 or 4 vessel occlusion), global ischemia can also affect the parenchyma [32] known to affect cell survival in other regions of the brain including hippocampal CA1 neurons [33]. Therefore, we used the MCAO model (focal ischemia) of stroke coupled with TTC staining of the rat brain 24 hrs after ischemia to determine total infarct volume of the brain. The use of both methods further validates the effectiveness of PAME, SAME, and Solutol HS 15-induced neuroprotection under global and focal ischemic conditions.
We show that PAME, SAME, and Solutol HS 15 can provide neuroprotection in both models of cerebral ischemia. PAME, SAME and Solutol HS 15 can provide hippocampal CA1 neuroprotection and reduce brain infarction volume (regional neuroprotection). Mechanistic insight of PAME, SAME, and Solutol HS 15 may prove valuable in the near future to counter the detrimental effects of cerebral ischemia and/or stroke. Furthermore, neuroprotection studies involving pharmacological agents that utilize Solutol HS 15 as an excipient should be used with caution as this may exert inaccurate measures of cell survival.
Table 1. Asphyxial cardiac arrest physiological parameters.
Physiological parameters such as body weight, pH, PCO2, PO2, MABP, and blood glucose were measured to ensure consistency of the animals within and between groups. Values are mean ± S.E.M. ACA, asphyxial cardiac arrest; MABP, mean arterial blood pressure.
| Groups | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Sham (n= 7) |
No drug + ACA (n = 9) |
Ethanol + ACA (0.005%) (n = 3) |
Solutol + ACA (2 ml/kg) (n = 6) |
SAME + ACA (0.02 mg/kg) (n = 6) |
PAME + ACA (0.02 mg/kg) (n = 7) |
|
| Body wt (g) | 298.1 ± 16.2 | 317.8 ± 15.0 | 315.0 ± 10.1 | 327.0 ± 6.7 | 315.6 ± 10.1 | 305.2 ± 10.5 |
| pH | 7.46 ± 0.01 | 7.45 ± 0.01 | 7.46 ± 0.04 | 7.45 ± 0.02 | 7.49 ± 0.02 | 7.47 ± 0.01 |
| PCO2 (mm Hg) | 37.2 ± 1.0 | 35.8 ± 1.2 | 36.3 ± 1.5 | 38.3 ± 1.2 | 36.5 ± 2.2 | 34.6 ± 1.1 |
| PO2 (mm Hg) | 144.1 ± 7.4 | 129.6 ± 8.3 | 128.9 ± 10.6 | 126.8 ± 6.4 | 131.3 ± 5.1 | 141.9 ± 11.5 |
| MABP (mm Hg) | 118.6 ± 3.2 | 112.1 ± 6.5 | 113.7 ± 1.2 | 126.8 ± 2.0 | 109.0 ± 6.5 | 104.0 ± 1.5 |
| Glucose (mg/dL) | 142 ± 13 | 150 ± 20 | 137 ± 19 | 105.7 ± 12.1 | 131.0 ± 10.2 | 117 ± 17.9 |
Table 2. Asphyxial cardiac arrest physiological parameters.
Physiological parameters such as body weight, pH, PCO2, PO2, MABP, and blood glucose were measured to ensure consistency of the animals within and between groups.
| Groups | Variable | Before ACA | 30 mins after ACA | 24 hrs after ACA |
|---|---|---|---|---|
| PAME (0.02 mg/kg, IV) (n = 6) |
Body wt (g) | 333.5 ± 9.61 | – | – |
| pH | 7.46 ± 0.02 | 7.47 ± 0.04 | 7.40 ± 0.03 | |
| PCO2 (mm Hg) | 34.4 ± 1.3 | 33.1 ± 2.1 | 39.9 ± 2.4 | |
| PO2 (mm Hg) | 112.8 ± 15.7 | 224.7 ± 31.9* | 113.7 ± 6.3 | |
| MABP (mm Hg) | 102.7 ± 4.8 | 91.3 ± 3.05 | 90.5 ± 1.2 | |
| Glucose (mg/dL) | 118 ± 10 | |||
| SAME (0.02 mg/kg, IV) (n = 3) |
Body wt (g) | 313.3 ± 9.61 | – | – |
| pH | 7.48 ± 0.03 | 7.51 ± 0.04 | 7.48 ± 0.01 | |
| PCO2 (mm Hg) | 33.8 ± 2.2 | 31.2 ± 3.4 | 33.4 ± 0.7 | |
| PO2 (mm Hg) | 108.2 ± 12.5 | 380 ± 67* | 125 ± 7 | |
| MABP (mm Hg) | 103.3 ± 5.2 | 99.3 ± 6.44 | 93.3 ± 1.9 | |
| Glucose (mg/dL) | 103 ± 7 |
denotes significant difference from before and 24 hrs after ACA due to the delivery of 100 % O2 upon resuscitation to achieve ROSC. Values are mean ± S.E.M. ACA, asphyxial cardiac arrest; MABP, mean arterial blood pressure.
Table 3. Middle cerebral artery occlusion physiological parameters.
Physiological parameters such as body weight, pH, PCO2, PO2, MABP, and blood glucose were measured to ensure consistency of the animals within and between groups. Values are mean ± S.E.M. MCAO, middle cerebral artery occlusion; MABP, mean arterial blood pressure.
| Groups | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Saline (0.01 %) (n= 6) |
DMSO (0.01 %) (n = 7) |
12-HS (0.02 mg/kg) (n = 6) |
Solutol (2 ml/kg) (n = 8) |
SAME (0.02 mg/kg) (n = 6) |
PAME (0.02 mg/kg) (n = 6) |
|
| Body wt (g) | 312.8 ± 8.0 | 300.1 ± 8.3 | 303.3 ± 5.6 | 306.7 ± 3.9 | 300.8 ± 5.1 | 306.2 ± 2.8 |
| pH | 7.36 ± 0.01 | 7.40 ± 0.02 | 7.42 ± 0.04 | 7.34 ± 0.001 | 7.43 ± 0.01 | 7.43 ± 0.02 |
| PCO2 (mm Hg) | 37.6 ± 1.6 | 37.1 ± 1.3 | 37.3 ± 1.0 | 39.5 ± 1.6 | 38.4 ± 1.48 | 40.5 ± 0.69 |
| PO2 (mm Hg) | 108.1 ± 6.6 | 122.9 ± 5.8 | 126.4 ± 13.3 | 118.8 ± 8.1 | 137.4 ± 4.4 | 120.4 ± 5.8 |
| MABP (mm Hg) | 123.5 ± 6.4 | 123.6 ± 7.7 | 123.0 ± 10.9 | 112.6 ± 6.7 | 139.0 ± 5.1 | 142.0 ± 1.2 |
| Glucose (mg/dL) | 93 ± 6 | 111 ± 7 | 104 ± 7 | 101 ± 8 | 112 ± 2 | 132 ± 19 |
Acknowledgments
This work was supported by National Institutes of Health grants NS45676-01, NS054147-01, NS34773, NS073779, American Heart Association-Philips grant 10POST4340011, and AHA-13SDG13950014.
Footnotes
Disclosure/Conflict of Interest: The authors have no conflict of interest in this manuscript.
Compliance with Ethics Requirements: Hung Wen Lin, PhD; Isabel Saul, BS; Victoria L. Gresia, BS; Jake T. Neumann, PhD; Kunjan R. Dave, PhD; Miguel A. Perez-Pinzon, PhD: all declare no conflict of interest in this manuscript.
Human Subjects: N/A
Animal Subjects: All institutional and national guidelines for the care and use of laboratory animals were followed.
Clinical Trials: N/A
References
- 1.Lin HW, Liu CZ, Cao D, Chen PY, Chen MF, Lin SZ, et al. Endogenous methyl palmitate modulates nicotinic receptor-mediated transmission in the superior cervical ganglion. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(49):19526–31. doi: 10.1073/pnas.0810262105. doi:10.1073/pnas.0810262105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nature cell biology. 2001;3(2):193–7. doi: 10.1038/35055104. doi:10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 3.Choi YB, Tenneti L, Le DA, Ortiz J, Bai G, Chen HS, et al. Molecular basis of NMDA receptor-coupled ion channel modulation by S-nitrosylation. Nature neuroscience. 2000;3(1):15–21. doi: 10.1038/71090. doi:10.1038/71090. [DOI] [PubMed] [Google Scholar]
- 4.Liu L, Stamler JS. NO: an inhibitor of cell death. Cell death and differentiation. 1999;6(10):937–42. doi: 10.1038/sj.cdd.4400578. doi:10.1038/sj.cdd.4400578. [DOI] [PubMed] [Google Scholar]
- 5.Bell BA. The neuroprotective effect of calcitonin gene-related peptide following subarachnoid hemorrhage. European CGRP in Subarachnoid Haemorrhage Study Group. Annals of the New York Academy of Sciences. 1995;765:299–300. doi: 10.1111/j.1749-6632.1995.tb16588.x. [DOI] [PubMed] [Google Scholar]
- 6.Brenneman DE, Eiden LE. Vasoactive intestinal peptide and electrical activity influence neuronal survival. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(4):1159–62. doi: 10.1073/pnas.83.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang B, Cao W, Biswal S, Dore S. Carbon monoxide-activated Nrf2 pathway leads to protection against permanent focal cerebral ischemia. Stroke; a journal of cerebral circulation. 2011;42(9):2605–10. doi: 10.1161/STROKEAHA.110.607101. doi:10.1161/STROKEAHA.110.607101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin HW, Defazio RA, Della-Morte D, Thompson JW, Narayanan SV, Raval AP, et al. Derangements of post-ischemic cerebral blood flow by protein kinase C delta. Neuroscience. 2010;171(2):566–76. doi: 10.1016/j.neuroscience.2010.08.058. doi:10.1016/j.neuroscience.2010.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sick TJ, Xu G, Perez-Pinzon MA. Mild hypothermia improves recovery of cortical extracellular potassium ion activity and excitability after middle cerebral artery occlusion in the rat. Stroke; a journal of cerebral circulation. 1999;30(11):2416–21. doi: 10.1161/01.str.30.11.2416. discussion 22. [DOI] [PubMed] [Google Scholar]
- 10.Bright R, Raval AP, Dembner JM, Perez-Pinzon MA, Steinberg GK, Yenari MA, et al. Protein kinase C delta mediates cerebral reperfusion injury in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(31):6880–8. doi: 10.1523/JNEUROSCI.4474-03.2004. doi:10.1523/JNEUROSCI.4474-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ku S, Velagaleti R. Solutol HS15 as a Novel Excipient: Identification of the Need for and Implementation of a US Regulatory Strategy. Pharmaceutical Technology. 2010;34(11):108–10. [Google Scholar]
- 12.Belayev L, Khoutorova L, Atkins KD, Bazan NG. Robust docosahexaenoic acid-mediated neuroprotection in a rat model of transient, focal cerebral ischemia. Stroke; a journal of cerebral circulation. 2009;40(9):3121–6. doi: 10.1161/STROKEAHA.109.555979. doi:10.1161/STROKEAHA.109.555979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bazan NG, Molina MF, Gordon WC. Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases. Annual review of nutrition. 2011;31:321–51. doi: 10.1146/annurev.nutr.012809.104635. doi:10.1146/annurev.nutr.012809.104635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W, Li P, Hu X, Zhang F, Chen J, Gao Y. Omega-3 polyunsaturated fatty acids in the brain: metabolism and neuroprotection. Frontiers in bioscience : a journal and virtual library. 2012;17:2653–70. doi: 10.2741/3878. [DOI] [PubMed] [Google Scholar]
- 15.Belayev L, Khoutorova L, Atkins KD, Eady TN, Hong S, Lu Y, et al. Docosahexaenoic Acid Therapy of Experimental Ischemic Stroke. Translational stroke research. 2011;2(1):33–41. doi: 10.1007/s12975-010-0046-0. doi:10.1007/s12975-010-0046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ley JJ, Vigdorchik A, Belayev L, Zhao W, Busto R, Khoutorova L, et al. Stilbazulenyl nitrone, a second-generation azulenyl nitrone antioxidant, confers enduring neuroprotection in experimental focal cerebral ischemia in the rat: neurobehavior, histopathology, and pharmacokinetics. The Journal of pharmacology and experimental therapeutics. 2005;313(3):1090–100. doi: 10.1124/jpet.105.083386. doi:10.1124/jpet.105.083386. [DOI] [PubMed] [Google Scholar]
- 17.Lin HW, Della-Morte D, Thompson JW, Gresia VL, Narayanan SV, Defazio RA, et al. Differential effects of delta and epsilon protein kinase C in modulation of postischemic cerebral blood flow. Advances in experimental medicine and biology. 2012;737:63–9. doi: 10.1007/978-1-4614-1566-4_10. doi:10.1007/978-1-4614-1566-4_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu HT, Pan F, Yang G, Gan WB. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nature neuroscience. 2007;10(5):549–51. doi: 10.1038/nn1883. doi:10.1038/nn1883. [DOI] [PubMed] [Google Scholar]
- 19.Raval AP, Dave KR, Prado R, Katz LM, Busto R, Sick TJ, et al. Protein kinase C delta cleavage initiates an aberrant signal transduction pathway after cardiac arrest and oxygen glucose deprivation. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2005;25(6):730–41. doi: 10.1038/sj.jcbfm.9600071. doi:10.1038/sj.jcbfm.9600071. [DOI] [PubMed] [Google Scholar]
- 20.Stevens SL, Ciesielski TM, Marsh BJ, Yang T, Homen DS, Boule JL, et al. Toll-like receptor 9: a new target of ischemic preconditioning in the brain. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2008;28(5):1040–7. doi: 10.1038/sj.jcbfm.9600606. doi:10.1038/sj.jcbfm.9600606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang ZJ, Li GM, Tang WL, Yin M. Neuroprotective effects of stearic acid against toxicity of oxygen/glucose deprivation or glutamate on rat cortical or hippocampal slices. Acta pharmacologica Sinica. 2006;27(2):145–50. doi: 10.1111/j.1745-7254.2006.00259.x. doi:10.1111/j.1745-7254.2006.00259.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang ZJ, Liang CL, Li GM, Yu CY, Yin M. Stearic acid protects primary cultured cortical neurons against oxidative stress. Acta pharmacologica Sinica. 2007;28(3):315–26. doi: 10.1111/j.1745-7254.2007.00512.x. doi:10.1111/j.1745-7254.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- 23.Coon JS, Knudson W, Clodfelter K, Lu B, Weinstein RS. Solutol HS 15, nontoxic polyoxyethylene esters of 12-hydroxystearic acid, reverses multidrug resistance. Cancer research. 1991;51(3):897–902. [PubMed] [Google Scholar]
- 24.Koob AO, Colby JM, Borgens RB. Behavioral recovery from traumatic brain injury after membrane reconstruction using polyethylene glycol. Journal of biological engineering. 2008;2:9. doi: 10.1186/1754-1611-2-9. doi:10.1186/1754-1611-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koob AO, Duerstock BS, Babbs CF, Sun Y, Borgens RB. Intravenous polyethylene glycol inhibits the loss of cerebral cells after brain injury. Journal of neurotrauma. 2005;22(10):1092–111. doi: 10.1089/neu.2005.22.1092. doi:10.1089/neu.2005.22.1092. [DOI] [PubMed] [Google Scholar]
- 26.Liu-Snyder P, Logan MP, Shi R, Smith DT, Borgens RB. Neuroprotection from secondary injury by polyethylene glycol requires its internalization. The Journal of experimental biology. 2007;210(Pt 8):1455–62. doi: 10.1242/jeb.02756. doi:10.1242/jeb.02756. [DOI] [PubMed] [Google Scholar]
- 27.Lin HW, Thompson JW, Morris KC, Perez-Pinzon MA. Signal transducers and activators of transcription: STATs-mediated mitochondrial neuroprotection. Antioxidants & redox signaling. 2011;14(10):1853–61. doi: 10.1089/ars.2010.3467. doi:10.1089/ars.2010.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koob AO, Borgens RB. Polyethylene glycol treatment after traumatic brain injury reduces beta-amyloid precursor protein accumulation in degenerating axons. Journal of neuroscience research. 2006;83(8):1558–63. doi: 10.1002/jnr.20837. doi:10.1002/jnr.20837. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu S, Simon RP, Graham SH. Dimethylsulfoxide (DMSO) treatment reduces infarction volume after permanent focal cerebral ischemia in rats. Neuroscience letters. 1997;239(2-3):125–7. doi: 10.1016/s0304-3940(97)00915-4. [DOI] [PubMed] [Google Scholar]
- 30.Mantawy EM, Tadros MG, Awad AS, Hassan DA, El-Demerdash E. Insights antifibrotic mechanism of methyl palmitate: impact on nuclear factor kappa B and proinflammatory cytokines. Toxicology and applied pharmacology. 2012;258(1):134–44. doi: 10.1016/j.taap.2011.10.016. doi:10.1016/j.taap.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 31.El-Demerdash E. Anti-inflammatory and antifibrotic effects of methyl palmitate. Toxicology and applied pharmacology. 2011;254(3):238–44. doi: 10.1016/j.taap.2011.04.016. doi:10.1016/j.taap.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 32.Hickey RW, Ferimer H, Alexander HL, Garman RH, Callaway CW, Hicks S, et al. Delayed, spontaneous hypothermia reduces neuronal damage after asphyxial cardiac arrest in rats. Critical care medicine. 2000;28(10):3511–6. doi: 10.1097/00003246-200010000-00027. [DOI] [PubMed] [Google Scholar]
- 33.Dave KR, Saul I, Prado R, Busto R, Perez-Pinzon MA. Remote organ ischemic preconditioning protect brain from ischemic damage following asphyxial cardiac arrest. Neuroscience letters. 2006;404(1-2):170–5. doi: 10.1016/j.neulet.2006.05.037. doi:10.1016/j.neulet.2006.05.037. [DOI] [PubMed] [Google Scholar]