Abstract
Brain-derived neurotrophic factor (BDNF) promotes the survival and growth of neurons during brain development and mediates activity-dependent synaptic plasticity and associated learning and memory in the adult. BDNF levels are reduced in brain regions affected in Alzheimer’s, Parkinson’s, and Huntington’s diseases, and elevation of BDNF levels can ameliorate neuronal dysfunction and degeneration in experimental models of these diseases. Because neurons accumulate oxidative lesions in their DNA during normal activity and in neurodegenerative disorders, we determined whether and how BDNF affects the ability of neurons to cope with oxidative DNA damage. We found that BDNF protects cerebral cortical neurons against oxidative DNA damage-induced death by a mechanism involving enhanced DNA repair. BDNF stimulates DNA repair by activating cyclic AMP response element-binding protein (CREB), which, in turn, induces the expression of apurinic/apyrimidinic endonuclease 1 (APE1), a key enzyme in the base excision DNA repair pathway. Suppression of either APE1 or TrkB by RNA interference abolishes the ability of BDNF to protect neurons against oxidized DNA damage-induced death. The ability of BDNF to activate CREB and upregulate APE1 expression is abolished by shRNA of TrkB as well as inhibitors of TrkB, PI3 kinase, and Akt kinase. Voluntary running wheel exercise significantly increases levels of BDNF, activates CREB, and upregulates APE1 in the cerebral cortex and hippocampus of mice, suggesting a novel mechanism whereby exercise may protect neurons from oxidative DNA damage. Our findings reveal a previously unknown ability of BDNF to enhance DNA repair by inducing the expression of the DNA repair enzyme APE1.
Keywords: Running, Base excision repair, Exercise, Alzheimer, Oxidative stress, Akt kinase, Synaptic plasticity, Comet assay
Introduction
Neurons experience repeated bouts of membrane depolarization and ion fluxes that require large amounts of energy to sustain. Such electrochemical activity in neuronal circuits results in the generation of reactive oxygen species (ROS) by the mitochondrial electron transport chain and by various Ca2+-responsive enzymes (Nakamura and Lipton 2010; Mattson et al. 2008). ROS cause oxidative modification of DNA bases, which, if left unrepaired, can result in DNA damage and strand breaks that trigger apoptotic cell death (Martin et al. 2009). Oxidative damage to the DNA of neurons is elevated in acute brain injury and neurode-generative disorders (Lu et al. 2004; Yang et al. 2008). In addition, DNA damage may trigger the apoptosis that occurs in newly generated neurons during the development of the nervous system (Cheng et al. 2007; Gilmore et al. 2000).
Because life evolved under oxidizing conditions, elaborate mechanisms exist by which ROS are rapidly detoxified or oxidative damage to macromolecules is repaired. Enzymes involved in ROS detoxification include superoxide dismutases, glutathione peroxidases, and catalase. The primary mechanism by which cells remove oxidatively damaged DNA bases is base excision repair (BER); the BER pathway also repairs bases that are alkylated or deaminated (for review see Wilson and Bohr 2007). The primary enzymes of BER include a DNA glycosylase (OGG1) that recognizes and removes the damaged base, thus forming an apurinic/apyrimidinic (AP) site; AP endonuclease 1 (APE1) that cleaves at the AP site resulting in a single-strand break in the DNA; and a DNA polymerase (Pol-β) that inserts an undamaged base to complete the repair process. Studies of nonneural cells have demonstrated that APE1 can also promote the DNA-binding activity of several transcription factors including p53, NF-κB, AP-1, and HIF-1α (Evans et al. 2000). While much of the molecular machinery involved in DNA repair has been established, little is known regarding whether and how cells may increase their DNA repair capability. However, recent studies have shown that APE1 expression can be induced in cancer cells by the transcription factor MiTF (Liu et al. 2009) and BRCA1 (Saha et al. 2010), and in embryonic hippocampal neurons in response to pituitary adenylate cyclase-activating polypeptide (Stetler et al.2010) and glutamate (Yang et al. 2010).
Brain-derived neurotropic factor (BDNF) is produced by neurons in the mammalian brain in an activity-dependent manner and plays important roles in synaptic and behavioral plasticity, and cell survival (Mattson et al. 2004). BDNF expression is also induced by oxidative and metabolic stress and by environmental factors such as exercise, energy restriction, and environmental enrichment. BDNF can protect neurons in experimental models of Parkinson’s and Huntington’s diseases (Zuccato and Cattaneo 2009) and stroke (Schabitz et al. 1997). The neuroprotective effects of BDNF are mediated by the high-affinity cell surface receptor TrkB, which engages intracellular signaling pathways involving PI3, Akt, and MAP kinases, and the transcription factor cyclic AMP response element-binding protein (CREB) (Mattson et al. 2004). Here, we show that BDNF and exercise enhance DNA repair in cerebral cortical and hippocampal neurons by a mechanism involving CREB-mediated expression of APE1.
Materials and Methods
Cerebral Cortical Cell Cultures and Experimental Treatments
Cultures of primary cortical neurons were prepared from 17-day-old Sprague–Dawley rat embryos. Cells were plated in 100-mm-diameter plastic dishes on a polyethyleneimine substrate in minimum essential medium with Earle’s salts supplemented with 10 % heat-inactivated fetal bovine serum and containing 1 mM l-glutamine, 1 mM pyruvate, 20 mM KCl, and 26 mM sodium bicarbonate (pH 7.2). Following cell attachment (3–4 h after plating), the medium was replaced with Neurobasal medium containing B-27 supplements (Life Technologies, Inc.), 1 mM HEPES, 2 mM l-glutamine, and 0.001 % gentamycin sulfate. All experiments were performed in 7- to 9-day-old cultures in which >95 % of the cells were neurons. Cortical neurons were treated with 20 μg/μl BDNF in 50 % conditioned Neurobasal medium (50 % fresh Neurobasal plus B-27 supplement and 50 % conditioned Neruobasal medium) at 37 °C. Treated neurons were collected at 30 min, 1, 3, 6, and 24 h for immunoblot analysis. For inhibitor treatments, the cultured neurons were treated with the PI3 kinase inhibitor wortmannin (1 μM; Calbiochem), the Akt inhibitor PKI-AKT-005 (0.5 μM; Signagen), TrkB inhibitor ANA-12 (30 μM; Sigma-Aldrich), or vehicle (0.1 % dimethylsulfoxide in saline) for 1.5 h. BDNF (or saline vehicle) was then added to the culture medium (final concentration 10 ng/ml), and the cultures were incubated for 30 min, 1, 3, 6, or 24 h, at which time the cells were analyzed.
Measurement of Cell Viability
Rat cortical neurons were plated and maintained in 35-mm gridded glass-bottom petri dishes as described above. One group of cells was pretreated with 10 ng/ml BDNF overnight (~14 h) before menadione treatment. Menadione (2-methyl-1,4-naphthoquinone, Sigma) was freshly prepared in Neurobasal medium. Neurons were treated with 40 μM menadione for 10 min at 37 °C. Cells were washed with warm Neurobasal medium after treatment, and BDNF (10 ng/ml) was included in the cultures that had been pretreated with BDNF. In the method of direct neuron counting, images of designated microscope fields (20× objective) were acquired at 0, 6, 12, 24, 36, 48, and 72 h. Neuronal survival at postmenadione treatment time points was expressed as a percentage of the number of viable neurons present at the 0-h time point For lactate dehydrogenase (LDH) method, cell death was determined by measuring lactate dehydrogenase (LDH) levels in the culture medium using a kit (Cytotoxicity Detection Kit; Roche).
Alkali Single-Cell Gel Electrophoresis Analysis of DNA Damage
Alkali single-cell electrophoresis (comet assay) is a method for the detection and quantification of nuclear DNA damage. Treatments of cultured neurons with BDNF and menadione were as described above, except that the concentration of menadione was decreased to 20 μM to avoid cell death. Neurons were collected for comet assay at 6 and 24 h after treatment. Cells were washed with cold PBS to remove dead cells and debris and were then harvested in 1 ml PBS and gently triturated. A 10-μl aliquot of the cells (approximately 2,000 cells) was mixed with 75 μl of prewarmed 0.5 % low melt point agarose, and the cells were spread onto an agarose-coated glass slide. A cover slip was added to the slide, and the slide was placed in an ice-chilled aluminum tray for 5 min. The cover slip was removed, another 75 μl of low melt point agarose was added, a cover slip was added, and the slide was placed in a chilled aluminum tray for 5 min. The cover slip was removed, and the slide incubated in lysis buffer (2.5 M NaCl, 100 mM EDTA, 10 mM Tris, and 1 % Triton X-100) for at least 4 h. The slides were then washed (three 10-min washes) with neutralization buffer (0.4 M Tris, pH 7.4), followed by a 10-min incubation with REC buffer (10 mM HEPES–KOH, 100 mM KCl, 10 mM EDTA, 0.1 mg/ml BSA; pH 7.4). Each slide was then incubated with 8 units (in 100 μl volume) of formamido-pyrimidine DNA glycosylase (Fpg, New England Biolabs) at 37 °C for 1 h. The Fpg-treated slides were rinsed with Alkali buffer (300 mM NaOH and 1 mM EDTA; pH 12.1) for 30 min to denature DNA. Electrophoresis was then performed at 25 volts for 15 min, and the slides were dehydrated in 100 % ethanol for 5 min and were then stained with ethidium bromide (10 ng/ml). The images of nuclear DNA were acquired using a Zeiss Axiovert 200 M epifluorescence microscope system and were analyzed using Komet 5.5 software (Kinetic Imagine).
Lentiviral shRNA Knockdown of TrkB, CREB, and APE1
The packaging plasmid (psPAX2), envelope plasmid (pMD2.G), and scrambled control shRNA (5′-CCTAAGGTTAAGTCGCCCTCGCTCGAGCGAGGGCGACTTAACCTTAGG-3′) were purchased from Addgene. The shRNA plasmids of Trkb/Nrt2 (5′-TTTCCTGTACATGATGCTCTC-3′), CREB (5′- TTCCCTGTTCTTCATTAGACG -3′), and Apex1 (5′- AAATTCAGCCACAATCACCCG-3′) were purchased from Thermo Scientific Open Biosystems. All shRNAs were incorporated into the pLKO.1 vector. HEK 293T cells were transfected with shRNA, packaging, and envelope plasmids using FuGene 6 (Roche) simultaneously to produce lentiviral particles. Cultured cortical neurons (4 days after plating) were infected with lentivirus using procedures and conditions optimized for neurons according to the Addgene plasmid 10878 protocol (http://www.addgene.org/pgvec1?f=c&cmd=showcol&colid=170&page=2).
Immunoblot Analysis
Cultured neurons were extracted in RIPA buffer (150 mM NaCl, 0.1 % SDS, 0.5 % sodium deoxycholate, 1× protease inhibitor cocktail (Roche), phosphatase inhibitor cocktail (Pierce), and 50 mM Tris; pH 8.0), and the total protein concentration of cell extracts was determined using a BCA™ protein assay kit (Pierce). Thirty micrograms of total protein from each sample was loaded into precast 10 % SDS polyacrylamide gels (NuPage, Invitrogen) and electrophoresed to separate proteins; the proteins were then electrophoretically transferred to a PVDF membrane (Invitrogen). The membrane was then washed with 0.1 % Tween 20 in Tris-buffered saline (20 mM Tris and 150 mM NaCl; pH 7.4), and the blocking buffer (5 % skim milk in washing buffer) was added. The dilution factors for the primary antibodies were the following: OGG1 (1:200; Santa Cruz); polβ (1:500, Abcam); APE1 (1:500, Santa Cruz); pAkt (1:10,000, Abcam); pCREB (1:500, Santa Cruz); and actin (1:5,000; Sigma-Aldrich). The secondary antibodies (Vector Laboratories) were diluted 1:5,000. Immunolabeled proteins were visualized using an enhanced chemiluminescence kit (Amersham); reprobing of the same blot with additional antibodies was accomplished by removing the original antibodies using Restore Western Blot Stripping buffer (Pierce).
APE1 Enzyme Incisional Activity Assays
The cultured neurons were rinsed with PBS, scraped into 1 ml PBS, and pelleted by centrifugation. Cells were then either extracted immediately or stored at −80 °C for future use. Cells were extracted by resuspending in buffer I (10 mM Tris–HCl, 200 mM KCl, pH 7.8) and adding an equal volume of buffer II (10 mM Tris–HCl, 600 mM KCl, 2 mM EDTA, 40 % (v/v) glycerol, 0.2 % (v/v) nonidet P-40, 2 mM dithiothreitol (DTT), 0.5 mM phenylmethyl-sulfonyl fluoride (PMSF), and protease inhibitor cocktail (Roche; pH 7.8)). The lysate was briefly sonicated to completely disrupt cell and nuclear membranes. A 16,000g centrifugation at 4 °C for 10 min was performed to remove cellular debris and DNA. The cell extract was dialyzed overnight with buffer III (25 mM HEPES–KOH, 100 mM KCl, 12 mM MgCl2, 1 mM EDTA 17 % glycerol, 1 mM DTT, pH 8.0) at 4 °C. A brief centrifugation was employed to remove precipitation after dialysis. Five nanograms of total protein was used for APE1 incision activity assays. The procedures for incision assays were described previously (Weissman et al. 2007, 2009).
In Vivo Experiments
Male C57BL/6 mice (3–4 months of age) were randomly assigned to either an exercise group or a sedentary group (10 mice per group) and were housed 1 mouse per cage. The mice were provided food and water ad libitum, and were maintained on a 12-h light/12-h dark cycle. Mice in the exercise group were provided with a running wheel in their cage, while the sedentary group did not have the opportunity to run. The rotations of the running wheel and corresponding daily running distances were recorded and calculated using Clocklab (Coulbourn Instruments) and MATLAB software (Mathworks, Inc). After 10 days, mice in each group were euthanized, their brains were removed, and the hippocampi and cerebral cortex were removed, flash-frozen, and stored at −80 °C. All procedures were approved by the National Institute on Aging Animal Care and Use Committee and complied with NIH guidelines.
Statistics
Statistical comparisons were made using two-tail ANOVA or Student’s t test for pairwise comparisons (*P < 0.05, **P < 0.01, ***P < 0.001). All values shown in graphs are the mean and standard deviation (SD).
Results
BDNF Enhances DNA Repair, Protects Neurons Against Oxidative DNA Damage, and Selectively Increases APE1 Protein Levels
Menadione is a synthetic chemical that has been shown to induce oxidative modification of DNA bases and DNA strand breaks that can trigger apoptosis in a range of cell types including neurons (Kulkarni et al. 2008; Woods et al.1997). We first treated cultured cortical neurons with a concentration of menadione (20 μM) that we found, in preliminary studies, caused oxidative DNA damage without killing the neurons during the first 24 h of exposure. Cultures were pretreated overnight with 10 ng/ml BDNF or vehicle, and were then exposed to menadione for 10 min, followed by harvesting of the cells either immediately, or 6 or 24 h after exposure to menadione, for comet assay analysis. For the comet assay, cell nuclei were treated with Fpg, a glycosylase that specifically incises a number of oxidative DNA lesions producing various sizes of DNA fragments. Neurons in cultures treated with menadione alone exhibited a large (more than tenfold) increase in the amount of DNA damage within 10 min of exposure to menadione (Fig. 1a, b). During the ensuing 24 h, the amount of oxidative DNA damage progressively decreased, consistent with ongoing repair of the damage (Fig. 1b). Whereas menadione caused an initial amount of DNA damage in BDNF-pretreated neurons that was similar to that of neurons pretreated with vehicle, the BDNF-pretreated neurons exhibited a significantly greater reduction in DNA damage during the ensuing 24 h (Fig. 1b), suggesting that BDNF signaling enhances the ability of the neurons to repair oxidative DNA lesions.
Fig. 1.
BDNF protects cerebral cortical neurons against oxidative DNA damage by a mechanism involving upregulation of apurinic/apyrimidinic endonuclease 1 (APE1). a Representative images of neuronal nuclear DNA as visualized in comet assay gels. The upper two images show nuclei from cortical neurons in an untreated control culture and a culture treated with 10 ng/ml BDNF for 24 h. The images in the middle row show nuclear DNA from cortical neurons that had been treated with 20 μM menadione for the indicated time periods. The lower row of images show nuclear DNA from cortical neurons that had been pretreated for 14 h with 10 ng/ml BDNF and then exposed to 20 μM menadione for the indicated time periods. b Results of comet assay analysis of oxidative DNA damage and repair. Values are the mean and SD (n = 4–6 separate cultures). *P < 0.05, **P < 0.01, ***P < 0.001. c Immunoblots showing relative levels of the indicated proteins in cerebral cortical neurons that had been treated with vehicle or 10 ng/ml BDNF for the indicated time periods. The graph shows the results of densitometric analysis of immunoblots showing levels of the indicated DNA repair proteins in cortical neurons treated with BDNF for 6 or 24 h relative to the level of the proteins in control cultures. Values are the mean and SD of determinations made on samples from 4 to 6 separate cultures. **P < 0.01. d Cortical neurons were infected with lentivirus expressing shRNA directed against the APE1 mRNA (APE1 KD), which resulted in reduced levels of APE1 protein in the neurons as demonstrated by the immunoblot (inset at lower left). Neurons were pretreated overnight with 10 ng/ml BDNF or vehicle and were then were exposed to 40 μM menadione. Neuronal viability was quantified at the indicated time points. Parallel control cultures were exposed to the vehicle in which menadione was dissolved or to a scrambled control shRNA for 72 h. Values are the mean and SD (n = 4–6 separate cultures). *P < 0.05, **P < 0.01, ***P < 0.001 compared to the corresponding value for the menadione alone group
To elucidate how BDNF signaling enhances repair of oxidative DNA damage, we determined whether treatment of cultured cortical neurons with BDNF affected the levels of the major enzymes involved in BER (OGG1, APE1, DNA polymerase β, and DNA ligase III). Cultures were treated with 10 ng/ml BDNF, and neurons were harvested either immediately or 6 or 24 h later. Immunoblot analysis of cell lysates revealed that BDNF treatment resulted in significant increases in the amount of APE1 protein at both the 6- and 24-h time points (Fig. 1c). In contrast, levels of OGG1, polymerase b, and DNA ligase III were unchanged in BDNF-treated neurons (Fig. 1c).
We next determined whether BDNF affects neuronal vulnerability to oxidative DNA damage by pretreating cultured rat cortical neurons with 10 ng/ml BDNF or vehicle control, and then exposing them to 40 μM menadione. Cell viability was then quantified at designated time points (6, 12, 24, 36, 48 and 72 h). The concentration of BDNF was chosen based on previous studies, showing that 10 ng/ml BDNF promotes the survival of cultured hippocampal neurons (Kharebava et al. 2008), and the concentration of menadione was chosen based upon preliminary experiments, showing that 40 μM menadione causes a 50 % reduction in neuronal survival during a 24-h exposure period. We observed a progressive reduction in the number of surviving neurons in cultures exposed to menadione such that approximately 50 % of the neurons were viable at 24 h and 34 % remained alive at 72 h (Fig. 1d). In contrast, significantly more neurons survived exposure to menadione in cultures pretreated with BDNF, such that more than 70 % of the neurons remained viable during the 72-h time period (Fig. 1d). To determine whether APE1 played a critical role in the neuroprotective action of BDNF, we infected cortical neurons with a lentivirus expressing APE1 shRNA or a control shRNA with a scrambled sequence. Immunoblot analysis demonstrated that levels of APE1 protein were greatly reduced in neurons infected with the APE1 shRNA lentivirus compared to those infected with the control lentivirus (Fig. 1d, inset). Neurons with reduced APE1 levels were more sensitive to menadione toxicity and were not protected by BDNF (Fig. 1d). These results suggested that upregulation of APE1 plays a key role in the mechanism by which BDNF protects neurons against oxidative DNA damage.
BDNF Increases Levels of APE1 Protein and Enzyme Activity by a Mechanism Involving the Activation of Akt and CREB
Activation of TrkB by BDNF is known to promote neuronal survival by engaging the PI3 kinase–Akt signaling pathway, the extracellular signal-regulated kinases (ERKs), and the transcription factor CREB in neurons (Finkbeiner et al. 1997; Han and Holtzman 2000; Almeida et al. 2005). To determine whether BDNF activates Akt and CREB in the cultured cortical neurons, we treated cultures with BDNF and then harvested the cells immediately or 30 min, 1, 3, 6, or 24 h later. Immunoblot analysis of cell lysates using antibodies that selectively recognize phosphorylated (activated) Akt (pAkt) or CREB (pCREB) revealed that pAkt levels increased markedly within 30 min of exposure of neurons to BDNF, remained elevated at the 3-h time point, and thereafter decreased toward basal levels at the 24-h time point (Fig. 2a). Levels of pCREB were also elevated within 30 min of exposure to BDNF and remained elevated at the 24-h time point. APE1 levels were significantly elevated at the 3, 6, and 24-h time points, with the highest levels (greater than twofold elevation) occurring at the 6-h time point (Fig. 2a).
Fig. 2.
BDNF upregulates APE1 expression and enzymatic activity via a mechanism involving PI3 kinase, AKT, and CREB. a–d Cultured cerebral cortical neurons were pretreated for 1.5 h with vehicle (a), 1 μM wortmannin (b), 10 μM LY294002 (c) or 0.5 μM PKI-AKT-005 (d). Cultures were then treated with 10 ng/ml BDNF for the indicated time periods, and relative levels of p-AKT, p-CREB, and APE1 were assessed by immunoblot analysis. The graphs show the results of densitometric analysis of APE1 protein levels. Values are the mean and SD of determinations made in 4 separate cultures. BDNF treatment resulted in a rapid increase in levels of p-Akt and p-CREB followed by an elevation of APE1 levels. The ability of BDNF to activate CREB and increase APE1 levels was blocked by both wortmannin and PKI-AKT-005. e Biochemical results demon-strated incision activities of APE1 were increased in cortical neurons treated with BDNF for 6 or 24 h relative to the activity of the vehicle-treated control cultures (empty circle 32P labeling; filled circle tetrahydrofuran lesion site). **P < 0.01
We next determined whether activation of the PI3 kinase–Akt pathway was required for the activation of CREB and upregulation of APE1 in cortical neurons in response to BDNF. Cultures were pretreated for 1.5 h with either the PI3 kinase inhibitors wortmannin (1 μM) or LY294002 (10 μM), the Akt inhibitor PKI-AKT-005 (0.5 μM) or vehicle (0.1 % dimethylsulfoxide in PBS). BDNF (10 ng/ml) was then added to the culture medium, and cells were harvested either immediately (time 0) or 30 min, 1, 3, 6, or 24 h later. Wortmannin completely prevented BDNF-induced activation of Akt and CREB, and also blocked the ability of BDNF to increase levels of APE1 (Fig. 2b). The Akt inhibitor PKI-AKT-005 blocks ATP binding to Akt (Barnett et al. 2005; Zhao et al. 2005). We found that PKI-AKT-005 blocked the ability of BDNF to induce sustained activation of Akt and completely prevented CREB activation (Fig. 2c). BDNF did induce a rapid transient increase in the level of p-Akt at 30 min, presumably the result of the mechanism of action of PKI-AKT-005. The enzymatic activity of APE1 was also tested using a synthetic tetrahydrofuran oligonucleotide substrate, a DNA lesion recognized by APE1, to determine APE1 incision activity. BDNF treatment resulted in elevated APE1 incision activity with levels rising 1.5- and 1.2-fold after 6 and 24 h, respectively, compared to vehicle-treated control neurons (Fig. 2d). Collectively, these findings suggest that BDNF activates the PI3 kinase–Akt pathway in cortical neurons, resulting in CREB activation and upregulation of APE1 expression and activity.
To confirm that the Akt–CREB signaling pathway is initiated by TrkB activation, we used both highly specific antagonists and shRNA of TrkB to suppress activation of TrkB. ANA-12 is a relatively new and a highly selective antagonist of TrkB (Cazorla et al. 2011), which is commonly applied to block activation of TrkB from BDNF. The cortical neurons were treated with 30 μM of ANA-12 for 1.5 h before administration of 10 ng/ml BDNF. The results of Western blotting demonstrated that ANA-12 was able to suppress BDNF-induced APE1 expression (Fig. 3a, d). Levels of phosphorylated Akt and CREB were not significantly changed following BDNF treatment in the presence of ANA-12 (data not shown). Because BDNF can engage either TrkB or p75 receptors, we next employed RNA interference technology to selectively reduce levels of TrkB in cultured cortical neurons using a lentivirus engineered to produce TrkB shRNA. As a control, a lentivirus with a scrambled shRNA sequence was used. As shown in Fig. 3b, c and e, BDNF did not increase APE1 levels in neurons in which TrkB was knocked down. As shown in Fig. 3f, the ability of BDNF to protect neurons from being killed by menadione was abolished when TrkB was knocked down. These findings suggest that the effects of BDNF on APE1 expression and protection against oxidative DNA damage-induced neuronal death are mediated by TrkB.
Fig. 3.
Suppression of TrkB activation using either a selective antagonist (ANA-12) or lentiviral vector-mediated expression of TrkB shRNA blocks the ability of BDNF to protect cortical neurons against oxidative DNA damage. a Cultured cortical neurons were pretreated for 1.5 h with 30 μM of ANA-12, and 10 ng/ml BDNF was then added to the culture medium. Cells were then harvested at the indicated time points for immunoblot analysis using APE1 antibody. Control cultures were treated with vehicle for 24 h. b and c Cultured cortical neurons were infected with TrkB shRNA lentiviral vector, and 3 days later, 10 ng/ml BDNF was then added to the culture medium. Cells were then harvested at the indicated time points for immunoblot analysis using TrkB (b) or APE1 (c) antibodies. d Results of densitometric analysis of relative amounts of APE1 (normalized to actin and expressed as fold change versus the value for Control cultures) in cortical neurons from the experiments described for panel a (treatments with ANA-12 and BDNF). e Results of densitometric analysis of relative amounts of APE1 (normalized to actin and expressed as fold change versus the value for Control cultures) in cortical neurons from the experiments described for panels b and c (treatments with TrkB shRNA and BDNF). f Cultured cortical neurons were infected with TrkB shRNA lentiviral vector (TrkB KD), and 3 days later, 10 ng/ml BDNF (or no vehicle) was then added to the culture medium. Fourteen hours later, cultures were then were exposed to 40 μM menadione for 10 min. Neuronal viability was quantified by LDH assay at the indicated time points. Control cultures were infected with a scrambled control shRNA and were not treated with menadione. Values are the mean and SD (n = 4–6 separate cultures). *P < 0.05, **P < 0.01, compared to the BDNF + menadione value at the corresponding time point
CREB and APE1 Mediate BDNF-Induced Enhancement of DNA Repair
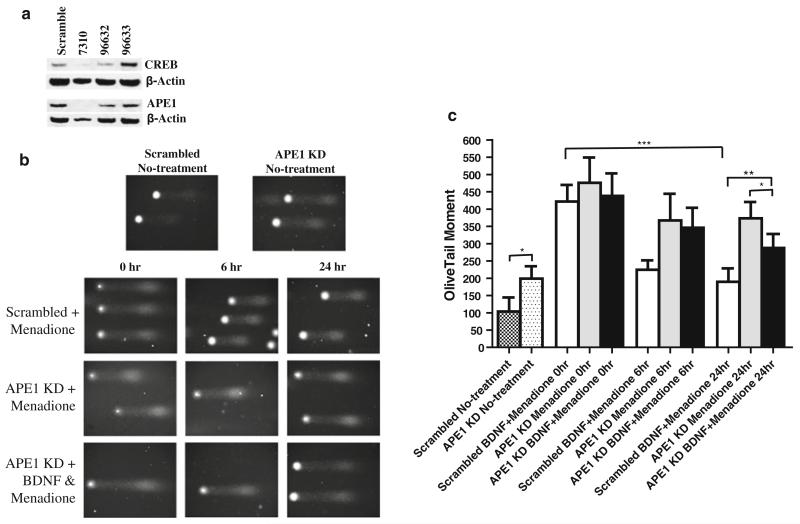
To determine whether CREB activation and increased APE1 expression are pivotal for BDNF-mediated protection of neurons against oxidative DNA damage, we employed RNA interference technology to selectively reduce levels of CREB and APE1 in cultured cortical neurons. We first tested three different lentiviral constructs encoding shRNAs directed against CREB mRNA for their abilities to reduce levels of CREB and APE1 in cultured cortical neurons. Neurons were infected with lentivirus and treated with BDNF on culture day 4, cells were harvested 3 days later, and relative levels of CREB were determined by immunoblot analysis of cell lysates. CREB shRNA sequence 7310 was highly effective in reducing CREB protein levels, whereas sequence 96632 was less effective and sequence 96633 did not decrease CREB levels (Fig. 4a). Neurons expressing the shRNA sequence 7310 exhibited a major reduction in APE1 protein levels (Fig. 4a), demonstrating that CREB is required for APE1 expression.
Fig. 4.
Posttranscriptional suppression of APE1 production blocks the ability of BDNF to enhance repair of oxidative DNA damage in cultured cerebral cortical neurons. a Cultured cortical neurons were infected with a lentiviral vector that expresses an shRNA directed against the CREB mRNA, and 3 days later, cells were harvested for immunoblot analysis. The upper band is CREB and that the numbers, 7310, 96632, and 96633, are the three different shRNAs directed against the CREB mRNA. Neurons infected with the CREB shRNA exhibited greatly reduced levels of APE1 compared to parallel cultures infected with a lentivirus to express a shRNA with a scrambled sequence. Similar results were obtained in 3 separate experiments. b Representative images of neuronal nuclear DNA as visualized in comet assay gels. The upper two images show nuclei from cortical neurons in cultures infected with lentiviruses to express either scrambled shRNA or APE1 shRNA. The next three rows of images show nuclear DNA from cortical neurons that had been treated with 20 μM menadione for the indicated time periods. Prior to exposure to menadione, the neurons were infected with lentiviruses to express scrambled shRNA or APE1 shRNA, in the absence or presence of 10 ng/ml BDNF. c Results of quantification of comet assay results. Values are the mean and SD (n = 4–6 separate cultures). *P < 0.05, **P < 0.01, ***P < 0.001
Lentivirus-mediated expression of a CREB-targeted shRNA resulted in a marked reduction in APE1 protein levels in cortical neurons (Fig. 4a). Cortical neurons in which APE1 protein production was selectively suppressed with shRNA exhibited a significant elevation of the basal level of oxidative DNA damage and a significant impairment in their ability to repair menadione-induced DNA damage at both the 6- and 24-h time points (Fig. 4b, c). Moreover, whereas BDNF significantly enhanced the repair of oxidatively damaged DNA in neurons infected with lentivirus to express a scrambled control shRNA sequence, the ability of BDNF to enhance DNA repair was significantly attenuated in neurons expressing APE1-specific shRNA (Fig. 4c).
Running Wheel Exercise Increases Levels of Activated CREB, BDNF, and APE1 in the Cerebral Cortex and Hippocampus
Previous studies have shown that voluntary running wheel exercise increases the expression of BDNF in cerebral cortical and hippocampal neurons (Neeper et al. 1996; Stranahan et al. 2009; Sartori et al. 2009). In addition, voluntary running results in a prolonged activation of CREB in hippocampal neurons of mice (Shen et al. 2001). We therefore divided mice into two groups (10 mice per group): a sedentary control group and a voluntary running group (see Methods), and after 10 days euthanized the mice and removed hippocampal and cortical tissue for immunoblot analysis to measure relative levels of pCREB, BDNF, and APE1 in these two brain regions. Levels of pCREB, BDNF, and APE1 were significantly greater in the cerebral cortex (Fig. 5a, b) and hippocampus (Fig. 5c, d) of mice that had run during the 10-day period compared to the sedentary control mice. These findings reveal a previously unknown stimulatory effect of exercise on APE1 and are consistent with the conclusion that BDNF signaling and CREB activation enhance the ability of neurons to repair oxidatively damaged DNA.
Fig. 5.
Voluntary running wheel exercise results in elevated levels of BDNF, p-CREB, and APE1 in the cerebral cortex and hippocampus. Adult male mice were maintained in cages lacking or containing running wheels for 10 days and cerebral cortical (a, b) and hippocampal (c, d) tissue samples were analyzed by immunoblot analysis using antibodies against BDNF, p-CREB, and APE1. Graphs show the results of densitometric analysis of the blots. Values are the mean and SD (n = 10 mice in each group). *P < 0.05, **P < 0.01, ***P < 0.001 compared to the value for the control (no running wheel exercise) group
Discussion
Our findings reveal a previously unknown biological action of BDNF; it enhances BER in neurons resulting in heightened resistance of the neurons to oxidative DNA damage. Pharmacological and genetic dissection of the underlying signaling pathway demonstrated pivotal roles for TrkB, PI3 kinase, Akt, and CREB leading to the upregulation of the BER enzyme APE1 (Fig. 6). Previous studies have shown that BER is a major pathway for the repair of oxidatively modified DNA bases in neurons (Wilson and McNeill 2007). Thus, OGG1 deficiency increases the vulnerability of cerebral cortical neurons to focal ischemic stroke, which was associated with increased levels of oxidative DNA modifications including 8-oxoG, FapyAde, and FapyGua (Liu et al. 2011). Overexpression of APE1 protects neurons from death induced by the mitochondrial toxin MPP+, demonstrating a role for unrepaired oxidative DNA damage in an experimental model of Parkinson’s disease (Huang et al. 2010). In addition, the death of neurons subjected to combined oxygen and glucose deprivation can be prevented by direct repletion of NAD+ by a mechanism involving restoration of nuclear BER; when APE1 expression was selectively inhibited, the cell survival-promoting effect of NAD+ was attenuated (Wang et al. 2008).
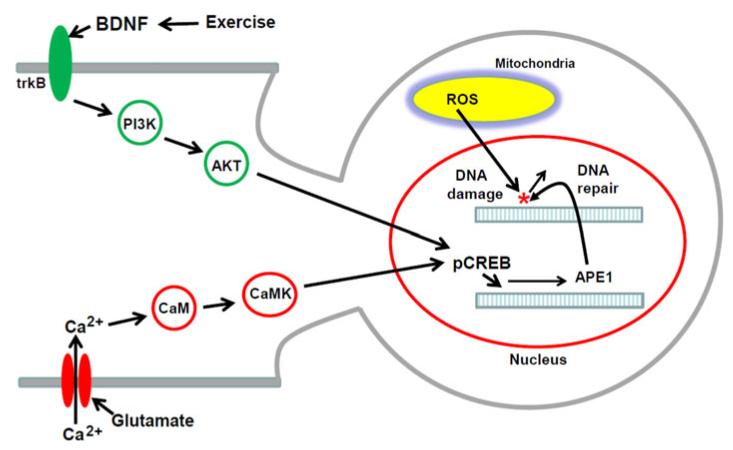
Fig. 6.
Model for the mechanism by which BDNF enhances repair of oxidative DNA damage in neurons. Neurons generate considerable amounts of reactive oxygen species (ROS) in mitochondria (superoxide and hydrogen peroxide) which can cause oxidative damage to DNA bases. Under usual conditions, neurons express sufficient amounts of the base excision repair (BER) enzymes that repair oxidative DNA lesions (OGG1, APE1, DNA ligase and DNA polymerase β). However, when levels of excitatory synaptic activity are elevated, or during injury, aging, and neurodegenerative disease, basal levels of repair are insufficient to prevent excessive accumulation of DNA lesions. Activation of BDNF receptors (TrkB) results in activation of PI3 kinase (PI3K) which phosphorylates AKT. AKT, in turn, phosphorylates the transcription factor CREB (pCREB), which then binds to the promoter of the APE1 gene to induce the expression of APE1 and enhance BER. In addition, Ca2+ influx through ionotropic glutamate receptor channels can upregulate APE1 expression via a pathway involving calmodulin (CaM) and calmodulin-dependent protein kinase (CaMK) which phosphorylates and activates CREB (13). By upregulating APE1 and BER, environmental factors that induce BDNF expression, such as regular exercise, may increase the resistance of neurons to oxidative DNA damage
TrkB is a receptor tyrosine kinase that is widely expressed in neurons, wherein its activation by BDNF plays roles in regulating neurite outgrowth, synaptic plasticity, and learning and memory processes (Lu et al. 2008). Binding of BDNF to TrkB results in receptor dimerization and trans-autophosphorylation, which, in turn, engages multiple kinase signaling cascades including those involving PI3 kinase, Akt, ERKs, and protein kinase C (Reichardt 2006). BDNF also binds to p75, the low affinity neurotrophin receptor p75 that plays roles in development, cell survival, and apoptosis (Khursigara et al. 2001; Reichardt 2006). We found that the effects of BDNF on APE1 levels and protection of cortical neurons against oxidative DNA damage (menadione) were completely abolished when TrkB levels were selectively reduced using TrkB shRNA. These findings suggest that TrkB, and not p75, mediates upregulation of BER in response to BDNF.
Transcription factors activated by BDNF signaling include CREB and NF-κB. Interestingly, Bdnf gene expression is induced by CREB in an activity-dependent manner, and the BDNF produced may then act on neigh-boring neurons or in a self-amplifying autocrine loop (Marini et al. 2008). We found that pharmacological inhibition of PI3 kinase with wortmannin and Akt with PKI-AKT-005 blocked the abilities of BDNF to activate CREB and to upregulate expression of APE1. The Ape1 gene promoter DNA sequence includes a CREB-binding site (Grosch and Kaina 1999), and it was previously reported that CREB upregulates APE1 mRNA levels in cultured neurons (Yang et al. 2010). CREB is the major transcription factor responsible for BDNF-induced APE1 expression because selective suppression of CREB production using RNA interference technology prevented induction of APE1 by BDNF. Because CREB also induces BDNF expression, it is possible that that reduced BDNF expression mediates, in part, the reduction in APE1 expression in response to CREB knockdown. Collectively, the data suggest a working model in which binding of BDNF to TrkB in cortical neurons results in the sequential activation of PI3 kinase, Akt, and CREB. CREB binds to the Ape1 gene promoter resulting in increased production of APE1 and enhanced BER capacity in the neurons (Fig. 6). Consistent with this model, we found that running wheel exercise increased BDNF, p-CREB, and APE1 levels in the hippocampus and cerebral cortex of adult mice.
BDNF is produced and released from neurons in response to excitatory synaptic activity that occurs during physiological processes including learning and memory and exercise (Gomez-Pinilla 2008). Such activity-dependent production of BDNF plays a critical role in synaptic plasticity/learning and memory, and also promotes the survival of neurons. Activation of glutamate receptors appears to be a major mechanism by which neuronal network activity stimulates BDNF production; this occurs by a signaling pathway involving calcium influx and activation of calcium/calmodulin-dependent protein kinase II (CaM-KII) and CREB or NF-κB (Marini et al. 2007). Interestingly, transient activation of glutamate receptors can enhance the ability of neurons to repair oxidative DNA damage by a mechanism involving CaMKII and CREB (Yang et al. 2010). The present findings suggest a potential role for BDNF-mediated upregulation of APE1 in activity-dependent enhancement of BER in neurons.
Ischemic preconditioning in adult rats was reported to enhance BER in cortical brain cells by a mechanism involving increased expression of DNA polymerase β and DNA ligase III (Li et al. 2007); APE1 expression status was not determined in this study. Because ischemic preconditioning results in increased expression of BDNF (Marini et al. 2007) and activation of CREB (Rybnikova et al. 2008; Terasaki et al. 2010), it is possible that BDNF plays a role in upregulation of BER in response to moderate energetic/oxidative stress. However, we found that BDNF signaling did not upregulate the expression of DNA polymerase β or DNA ligase III in cultured cerebral cortical neurons. Moreover, the ability of BDNF to protect neurons against oxidative damage-induced death was abolished when APE1 levels were selectively reduced by infection with a lentiviral vector that expressed a shRNA directed against the APE1 mRNA. Thus, APE1 plays a critical role as a mediator of BDNF’s ability to maintain oxidative DNA lesions below a level that triggers cell death when neurons are under stressful conditions.
Accumulating evidence from studies of human subjects and animal models suggest roles for perturbed neuronal network activity and reduced neurotrophic signaling in Alzheimer’s, Parkinson’s, and Huntington’s diseases (Roze et al. 2008; McIntyre and Hahn 2010; Kapogiannis and Mattson 2011; Gleichmann et al. 2011). Oxidative DNA damage in neurons is a common theme in studies of these neurodegenerative disorders, but no mechanism has been established that could link perturbed network activity to neuronal DNA damage. Our findings suggest that impaired synaptic activity and associated reductions in BDNF signaling may compromise BER resulting in the accumulation of oxidative DNA damage. By enhancing DNA repair capability in neurons, interventions that elevate BDNF levels, such as exercise or antidepressants, may protect neurons against neurodegenerative disease processes.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health, Chang Gung Medical Foundation Grant No. CLRPG871342, CMRPG8A0781, CRPG8B0051, and research grants NSC 101-2320-B-182A-007- and NSC 102-2320-B-182A-012- from National Science Council Taiwan.
Footnotes
Conflict of interest The authors declare that they have no conflicts of interest to report.
Contributor Information
Jenq-Lin Yang, Laboratory of Neurosciences, National Institute on Aging, Intramural Research Program, 251 Bayview Boulevard, Baltimore, MD 21224, USA; Laboratory of Molecular Gerontology, National Institute on Aging Intramural Research Program, 251 Bayview Boulevard, Baltimore, MD 21224, USA; Center for Translation Research in Biomedical Sciences, Kaohsiung Chang Gung Memorial Hospital, 123 Ta Pei Road, Kaohsiung 83301, Taiwan.
Yu-Ting Lin, Center for Translation Research in Biomedical Sciences, Kaohsiung Chang Gung Memorial Hospital, 123 Ta Pei Road, Kaohsiung 83301, Taiwan.
Pei-Chin Chuang, Department of Medical Research, Kaohsiung Chang Gung, Memorial Hospital, 123 Ta Pei Road, Kaohsiung 83301, Taiwan.
Vilhelm A. Bohr, Laboratory of Molecular Gerontology, National Institute on Aging Intramural Research Program, 251 Bayview Boulevard, Baltimore, MD 21224, USA
Mark P. Mattson, Laboratory of Neurosciences, National Institute on Aging, Intramural Research Program, 251 Bayview Boulevard, Baltimore, MD 21224, USA
References
- Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Graos MM, et al. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death and Differentiation. 2005;12:1329–1343. doi: 10.1038/sj.cdd.4401662. [DOI] [PubMed] [Google Scholar]
- Barnett SF, feo-Jones D, Fu S, Hancock PJ, Haskell KM, Jones RE, et al. Identification and characterization of pleckstrin-homology-domain-dependent and isoenzyme-specific Akt inhibitors. Biochemical Journal. 2005;385:399–408. doi: 10.1042/BJ20041140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazorla M, Premont J, Mann A, Girard N, Kellendonk C, Rognan D. Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. The Journal of Clinical Investigation. 2011;121:1846–1857. doi: 10.1172/JCI43992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Shin-ya K, Wan R, Tang SC, Miura T, Tang H, et al. Telomere protection mechanisms change during neurogenesis and neuronal maturation: Newly generated neurons are hypersensitive to telomere and DNA damage. Journal of Neuroscience. 2007;27:3722–3733. doi: 10.1523/JNEUROSCI.0590-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AR, Limp-Foster M, Kelley MR. Going APE over ref-1. Mutation Research. 2000;461:83–108. doi: 10.1016/s0921-8777(00)00046-x. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. CREB: A major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–1047. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- Gilmore EC, Nowakowski RS, Caviness VS, Jr, Herrup K. Cell birth, cell death, cell diversity and DNA breaks: How do they all fit together? Trends in Neurosciences. 2000;23:100–105. doi: 10.1016/s0166-2236(99)01503-9. [DOI] [PubMed] [Google Scholar]
- Gleichmann M, Chow VW, Mattson MP. Homeostatic disinhibition in the aging brain and Alzheimer’s disease. Journal of Alzheimer’s disease. 2011;24:15–24. doi: 10.3233/JAD-2010-101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla F. The influences of diet and exercise on mental health through hormesis. Ageing Research Reviews. 2008;7:49–62. doi: 10.1016/j.arr.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosch S, Kaina B. Transcriptional activation of apurinic/apyrimidinic endonuclease (Ape, Ref-1) by oxidative stress requires CREB. Biochemical and Biophysical Research Communications. 1999;261:859–863. doi: 10.1006/bbrc.1999.1125. [DOI] [PubMed] [Google Scholar]
- Han BH, Holtzman DM. BDNF protects the neonatal brain from hypoxic-ischemic injury in vivo via the ERK pathway. Journal of Neuroscience. 2000;20:5775–5781. doi: 10.1523/JNEUROSCI.20-15-05775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E, Qu D, Zhang Y, Venderova K, Haque ME, Rousseaux MW, et al. The role of Cdk5-mediated apurinic/apyrimidinic endonuclease 1 phosphorylation in neuronal death. Nature Cell Biology. 2010;12:563–571. doi: 10.1038/ncb2058. [DOI] [PubMed] [Google Scholar]
- Kapogiannis D, Mattson MP. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurology. 2011;10:187–198. doi: 10.1016/S1474-4422(10)70277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharebava G, Makonchuk D, Kalita KB, Zheng JJ, Hetman M. Requirement of 3-phosphoinositide-dependent protein kinase-1 for BDNF-mediated neuronal survival. Journal of Neuroscience. 2008;28:11409–11420. doi: 10.1523/JNEUROSCI.2135-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khursigara G, Bertin J, Yano H, Moffett H, DiStefano PS, Chao MV. A prosurvival function for the p75 receptor death domain mediated via the caspase recruitment domain receptor-interacting protein 2. Journal of Neuroscience. 2001;21:5854–5863. doi: 10.1523/JNEUROSCI.21-16-05854.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni A, McNeill DR, Gleichmann M, Mattson MP, Wilson DM., III XRCC1 protects against the lethality of induced oxidative DNA damage in nondividing neural cells. Nucleic Acids Research. 2008;36:5111–5121. doi: 10.1093/nar/gkn480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Wu H, Yang S, Chen D. Ischemic preconditioning induces XRCC1, DNA polymerase-beta, and DNA ligase III and correlates with enhanced base excision repair. DNA Repair (Amst) 2007;6:1297–1306. doi: 10.1016/j.dnarep.2007.02.027. [DOI] [PubMed] [Google Scholar]
- Liu D, Croteau DL, Souza-Pinto N, Pitta M, Tian J, Wu C, et al. Evidence that OGG1 glycosylase protects neurons against oxidative DNA damage and cell death under ischemic conditions. Journal of Cerebral Blood Flow and Metabolism. 2011;31:680–692. doi: 10.1038/jcbfm.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Fu Y, Meyskens FL., Jr MiTF regulates cellular response to reactive oxygen species through transcriptional regulation of APE-1/Ref-1. The Journal of Investigative Dermatology. 2009;129:422–431. doi: 10.1038/jid.2008.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Christian K, Lu B. BDNF: A key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiology of Learning and Memory. 2008;89:312–323. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, et al. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- Marini AM, Jiang H, Pan H, Wu X, Lipsky RH. Hormesis: A promising strategy to sustain endogenous neuronal survival pathways against neurodegenerative disorders. Ageing Research Reviews. 2008;7:21–33. doi: 10.1016/j.arr.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Marini AM, Jiang X, Wu X, Pan H, Guo Z, Mattson MP, et al. Preconditioning and neurotrophins: A model for brain adaptation to seizures, ischemia and other stressful stimuli. Amino Acids. 2007;32:299–304. doi: 10.1007/s00726-006-0414-y. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Liu Z, Pipino J, Chestnut B, Landek MA. Molecular regulation of DNA damage-induced apoptosis in neurons of cerebral cortex. Cerebral Cortex. 2009;19:1273–1293. doi: 10.1093/cercor/bhn167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Gleichmann M, Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60:748–766. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: A dynamic duo in age-related neuronal plasticity and neurode-generative disorders. Trends in Neurosciences. 2004;27:589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Hahn PJ. Network perspectives on the mechanisms of deep brain stimulation. Neurobiology of Diseases. 2010;38:329–337. doi: 10.1016/j.nbd.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Lipton SA. Preventing Ca2+-mediated nitrosative stress in neurodegenerative diseases: Possible pharmacological strategies. Cell Calcium. 2010;47:190–197. doi: 10.1016/j.ceca.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Research. 1996;726:49–56. [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philosophical Transactions of the Royal Society of London. Series B, Biological sciences. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roze E, Saudou F, Caboche J. Pathophysiology of Huntington’s disease: From huntingtin functions to potential treatments. Current Opinion in Neurology. 2008;21:497–503. doi: 10.1097/WCO.0b013e328304b692. [DOI] [PubMed] [Google Scholar]
- Rybnikova E, Gluschenko T, Tulkova E, Churilova A, Jaroshevich O, Baranova K, et al. Preconditioning induces prolonged expression of transcription factors pCREB and NF-κB in the neocortex of rats before and following severe hypobaric hypoxia. Journal of Neurochemistry. 2008;106:1450–1458. doi: 10.1111/j.1471-4159.2008.05516.x. [DOI] [PubMed] [Google Scholar]
- Saha T, Rih JK, Roy R, Ballal R, Rosen EM. Transcriptional regulation of the base excision repair pathway by BRCA1. Journal of Biological Chemistry. 2010;285:19092–19105. doi: 10.1074/jbc.M110.104430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartori CR, Pelagio FC, Teixeira SA, Valentinuzzi VS, Nascimento AL, Rogerio F, et al. Effects of voluntary running on spatial memory and mature brain-derived neurotrophic factor expression in mice hippocampus after status epilepticus. Behavioural Brain Research. 2009;203:165–172. doi: 10.1016/j.bbr.2009.04.022. [DOI] [PubMed] [Google Scholar]
- Schabitz WR, Schwab S, Spranger M, Hacke W. Intraventricular brain-derived neurotrophic factor reduces infarct size after focal cerebral ischemia in rats. Journal of Cerebral Blood Flow and Metabolism. 1997;17:500–506. doi: 10.1097/00004647-199705000-00003. [DOI] [PubMed] [Google Scholar]
- Shen H, Tong L, Balazs R, Cotman CW. Physical activity elicits sustained activation of the cyclic AMP response element-binding protein and mitogen-activated protein kinase in the rat hippocampus. Neuroscience. 2001;107:219–229. doi: 10.1016/s0306-4522(01)00315-3. [DOI] [PubMed] [Google Scholar]
- Stetler RA, Gao Y, Zukin RS, Vosler PS, Zhang L, Zhang F, et al. Apurinic/apyrimidinic endonuclease APE1 is required for PACAP-induced neuroprotection against global cerebral ischemia. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3204–3209. doi: 10.1073/pnas.1000030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG, et al. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19:951–961. doi: 10.1002/hipo.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki Y, Sasaki T, Yagita Y, Okazaki S, Sugiyama Y, Oyama N, et al. Activation of NR2A receptors induces ischemic tolerance through CREB signaling. Journal of Cerebral Blood Flow and Metabolism. 2010;30:1441–1449. doi: 10.1038/jcbfm.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Xing Z, Vosler PS, Yin H, Li W, Zhang F, et al. Cellular NAD replenishment confers marked neuroprotection against ischemic cell death: Role of enhanced DNA repair. Stroke. 2008;39:2587–2595. doi: 10.1161/STROKEAHA.107.509158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman L, de Souza-Pinto NC, Mattson MP, Bohr VA. DNA base excision repair activities in mouse models of Alzheimer’s disease. Neurobiology of Aging. 2009;30:2080–2081. doi: 10.1016/j.neurobiolaging.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman L, Jo DG, Sorensen MM, de Souza-Pinto NC, Markesbery WR, Mattson MP, et al. Defective DNA base excision repair in brain from individuals with Alzheimer’s disease and amnestic mild cognitive impairment 5. Nucleic Acids Research. 2007;35:5545–5555. doi: 10.1093/nar/gkm605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DM, III, Bohr VA. The mechanics of base excision repair, and its relationship to aging and disease. DNA Repair (Amst) 2007;6:544–559. doi: 10.1016/j.dnarep.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Wilson DM, III, McNeill DR. Base excision repair and the central nervous system. Neuroscience. 2007;145:1187–1200. doi: 10.1016/j.neuroscience.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Woods JA, Young AJ, Gilmore IT, Morris A, Bilton RF. Measurement of menadione-mediated DNA damage in human lymphocytes using the comet assay. Free Radical Research. 1997;26:113–124. doi: 10.3109/10715769709097790. [DOI] [PubMed] [Google Scholar]
- Yang JL, Tadokoro T, Keijzers G, Mattson MP, Bohr VA. Neurons efficiently repair glutamate-induced oxidative DNA damage by a process involving CREB-mediated up-regulation of apurinic endonuclease 1. Journal of Biological Chemistry. 2010;285:28191–28199. doi: 10.1074/jbc.M109.082883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Weissman L, Bohr VA, Mattson MP. Mitochondrial DNA damage and repair in neurodegenerative disorders. DNA Repair (Amst) 2008;7:1110–1120. doi: 10.1016/j.dnarep.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Leister WH, Robinson RG, Barnett SF, feo-Jones D, Jones RE, et al. Discovery of 2,3,5-trisubstituted pyridine derivatives as potent Akt1 and Akt2 dual inhibitors. Bioorganic & Medicinal Chemistry Letters. 2005;15:905–909. doi: 10.1016/j.bmcl.2004.12.062. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nature Reviews Neurology. 2009;5:311–322. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]