Abstract
Decreased voluntary wheel running has recently been proposed as a preclinical pain measure for inflammatory pain, but whether this reflects pain evoked by use of the affected limbs is unknown. To assess the role of inflammation site as a determinant of this measure, complete Freund’s adjuvant (CFA), formalin, or equivolume vehicle was subcutaneously injected into the plantar surface of the hindpaws (bilateral) or L1 dorsum dermatome (leaving paws unaffected) of male Sprague Dawley rats. CFA-induced hindpaw mechanical allodynia (P<0.001) did not correlate with reduced voluntary wheel running. Intraplantar formalin did not attenuate voluntary running, despite eliciting robust licking/writhing/flinching behavior and hindpaw mechanical allodynia (P<0.001). Subcutaneous L1 dorsum dermatome formalin, but not CFA, induced licking/writhing/flinching behavior (P<0.001), but neither induced hindpaw mechanical allodynia or attenuated voluntary running. That voluntary running is decreased by hindpaw, but not L1 dorsum CFA, implies that the behavior is a measure of CFA-induced pain evoked by use of the affected limbs, rather than supraspinal pain processing that is independent of inflammation site. Furthermore, the results suggest that interpretation of voluntary wheel running data cannot simply be explained by correlation with mechanical allodynia.
Perspective
Whether decreased voluntary running is dependent on inflammation site is unknown. We show that intraplantar, but not L1 dorsum, CFA suppressed voluntary running and formalin induced licking/writhing/flinching behavior but no effect on voluntary running. These data suggest that suppressed voluntary running by CFA likely reflects pain evoked by use of the affected limbs.
Keywords: Peripheral inflammation, allodynia, pain assessment, spontaneous pain, reflex
Introduction
Over the past decade, the preclinical study of pain has come under considerable scrutiny due to limited success in translating basic science into clinical therapies. One particular issue raised is the narrow range of outcome measures applied in preclinical pain models 24, 25, 27, 36. Whereas clinical studies examine spontaneous pain (although the pathophysiological accuracy of this terminology has recently been challenged 3), paroxysmal and evoked pain, preclinical studies have almost exclusively depended on reflex measures 27. The sole reliance on reflex measures has been criticized, as these are unlikely to capture the full spectrum of systemic adaptations that underlie chronic pain 25, 36. As a result, alternative measures have been developed that may reflect supraspinal pain processing, such as conditioned place aversion 15 and the mouse grimace scale 17. Recently, decreased running activity has been observed in inflammatory, acetic acid and osteoarthritis pain models, partially correlating with other indicators of inflammation 7, 21, 30. However, these studies have not identified whether attenuated voluntary running activity is reflective of pain is evoked in the affected paw due to the motion and weight bearing activity of running, or of supraspinal pain processing that is independent of inflammation site.
To assess the role of inflammation site as a determinant of decreased voluntary running, we compared voluntary running wheel activity between rats that had received subcutaneous injections of formalin or complete Freund’s adjuvant (CFA) into either the hindpaws (bilateral) or L1 dorsum dermatome. CFA or formalin injections into the dorsal lumbar surface of the back within the L5 dermatome are sufficient to induce hindpaw sensitivity 14. Hence, injections into the L1 dermatome, would likely induce nociceptive hypersensitivity at the injection site, but would leave the plantar surface of the hind and fore paws unaffected since the spinal projections of the L1 dermatome afferents and the forepaw and hindpaw afferents do not overlap (unlike the L5 dermatome). Thus, nociceptive activation of L1 dermatome afferents is less likely to produce paw sensitization. CFA was examined here since it has been the subject of previous investigation of voluntary wheel running in mice 7. Formalin was employed as a comparator, since the underlying mechanisms of formalin-induced allodynia substantially differ from those of CFA 10, 19. These differences, such as paw edema 19, may influence running ability.
Materials and methods
Subjects
Pathogen-free male Sprague–Dawley rats (300–325 g, Harlan Laboratories, Madison, WI) were single housed with temperature (23 ± 0.3 °C) and light (12:12 light:dark cycle; lights on at 07:00) controls. All groups were n = 6. Rats had ad libitum access to water and standard chow and were acclimated to the colony for 1 week before experimentation. Von Frey testing and licking/writhing/flinching behavior assessment occurred during the first 3 hours of lights on, while voluntary wheel running was performed during the first hour of lights off. No animals were excluded in this study for any reason. The Institutional Animal Care and Use Committee of the University of Colorado at Boulder approved all procedures.
Formalin or complete Freund’s adjuvant injections
Subcutaneous injections of dilute formalin or CFA are commonly used methods to induce persistent or chronic inflammatory nociceptive stimulation in animal studies of pain 29, 34. To determine whether hindpaw hypersensitivity was required to decrease voluntary running activity, injections of CFA containing heat killed Mycobacterium tuberculosis (50% in 0.9%, w/v pyrogen-free saline [1:1 paraffin oil and mannide monooleate:saline emulsion]; Sigma, St. Louis, MO), formalin (4% in 0.9%, w/v pyrogen-free saline; Sigma, St. Louis, MO), or equivolume vehicle were made bilaterally into the hindpaws (100 μL for CFA per injection; 50 μL for formalin per injection), with the needle directed between the toes and the tip placed subcutaneously (s.c.) into the plantar surface, or subcutaneously on the dorsal lumbar surface of the back (200 μL for CFA; 100 μL for formalin) at the region identified by Takahashi et al. 32, 33 to be within the L1 dermatome. The rats were lightly held in toweling and rapidly injected. Doses and volumes of CFA and formalin represent those commonly reported in pain studies 4, 14, 20. All injections were performed on Day 0 between 09:00 h–10:00 h. Except for all Baseline measurements, voluntary wheel running assessment began the night of injections (Night 1), von Frey assessments the following day (Day 1), and licking/writhing/flinching behaviors were recorded for the first hour immediately after injections.
Voluntary wheel running
To ensure acquisition of running behavior, all rats were allowed voluntary, unrestricted access to in-cage running wheels for 3 days. From nights 4–7 during acquisition, running was restricted to the first hour of the dark cycle by unlocking the wheel at 19:00 h, and relocking at 20:00 h, in which stable running during the 3 nights prior to injection was observed. Voluntary wheel running was recorded for the first hour of the dark cycle prior to (0) and up to 7 nights after injection. Wheel revolutions were recorded digitally using Vital View software (Mini Mitter, Bend, OR) and distance was calculated by multiplying number of revolutions by wheel circumference (1.081 m). Running time was calculated by summing the number of minutes in which wheel revolutions were > 0.
Von Frey test for mechanical allodynia
Testing was conducted blind with respect to group assignment. Rats received at least three 60 min habituations to the test environment prior to behavioral testing. The von Frey test 6 was performed at the distal region of the heel in the hindpaws, within the region of sciatic innervation as previously described in detail 5, 23. Importantly this test site was posterior to the formalin/CFA injections site, avoiding possible confounds of tissue damage and hypoalgesia observed previously 10. Assessments were made prior to (baseline) and on days 1, 2, 3, 4 and 7 post injection. A logarithmic series of 10 calibrated Semmes-Weinstein monofilaments (von Frey hairs; Stoelting, Wood Dale, IL) were applied randomly to the left vs. right hindpaws to define the threshold stimulus intensity required to elicit a paw withdrawal response. Log stiffness of the hairs ranged from manufacturer designated 3.61 (0.407 g) to 5.18 (15.136 g) filaments. The behavioral responses were used to calculate absolute threshold (the 50 % probability of response) by fitting a Gaussian integral psychometric function using a maximum-likelihood fitting method 12, 35, as described previously 22, 23. This fitting method allows parametric analyses that otherwise would not be statistically appropriate 22, 23. All assessments took place between 09:00 h and 11:00 h.
Licking/writhing/flinching behaviors
Following injection, rats were observed for pain-evoked behavior. A time-sampling procedure assessed the rats’ behavior every 30 s using a weighted scoring system 1, 34 modified for behaviors relevant to the site of injection 14, with 0 = no nociceptive behaviors displayed, 1 = flinching, writhing or lying with an arched or curved back, or 2 = attempting to lick or scratch the injection site. Animals were observed for 5-min periods and the pain scores averaged across 10 observations. Aside from those assessments made during the first hour immediately after injections, all subsequent assessments took place between 09:00 h and 10:00 h. Voluntary wheel running was not assessed during licking/writhing/flinching behaviors to avoid the confound of response competition, or rats running on the accessible wheel in an attempt to escape the injection (unpublished observations by collaborators B. Greenwood, M Fleshner).
Statistics
A power analysis was performed using G*Power 8 with power (1 − β) set at 0.80 and α= 0.05, which indicated that n = 5/group was sufficient to reach statistical significance (P < 0.05). Data from the von Frey test were analyzed as the interpolated 50% thresholds (absolute threshold). For voluntary running and mechanical allodynia, baseline values were compared between groups using a one-way ANOVA. Differences between treatment groups were determined using repeated measures 2-way ANOVA, with group, distance and time as main effects, followed by Sidak’s post hoc test where appropriate. Licking/writhing/flinching behavior was analyzed using non-parametric t-tests with a Holm-Sidak correction for multiple comparisons. A Pearson correlation was performed between voluntary wheel running and von Frey thresholds. Total running time differences between treatment groups were analyzed by paired Students t-test. Statistical comparisons are indicated on the figures for clarity and ± standard error of the mean. Statistical significance was set at P < 0.05.
Results
Bilateral hindpaw intraplantar CFA, but not formalin, attenuates voluntary running
Voluntary wheel running
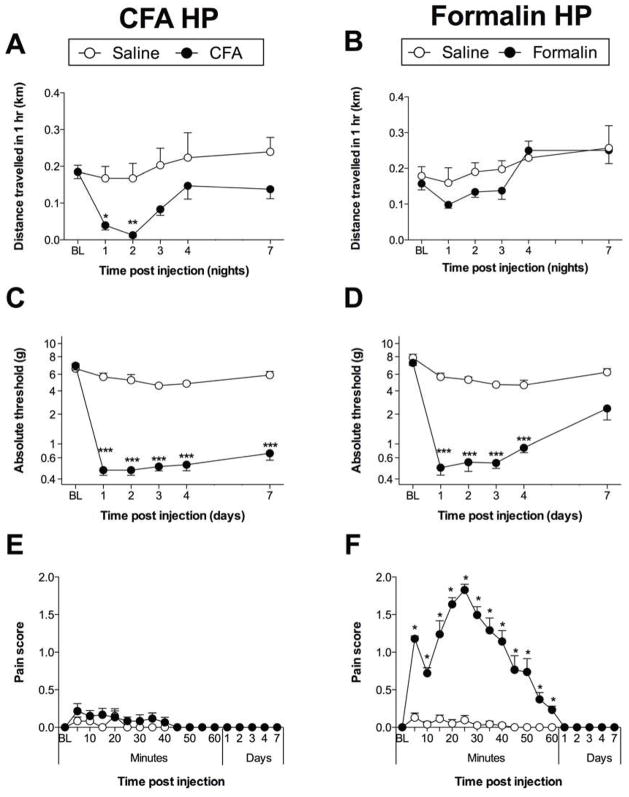
Voluntary wheel running was recorded for the first hour of the dark cycle for rats that received bilateral intraplantar injections of CFA or formalin and respective vehicle controls. No differences in voluntary wheel running were observed between groups prior to injection. CFA attenuated voluntary wheel running (time × treatment: F4,40 = 0.7022; P = 0.60; time: F4,40 = 6.775; P < 0.001; treatment: F1,10 = 7.878; P < 0.05; Figure 1A). However, these data provide the first evidence that formalin was not sufficient to suppress voluntary running (time × treatment: F4,40 = 1.120; P = 0.36; time: F4,40 = 9.139; P < 0.001; treatment: F1,10 = 0.9272; P = 0.36; Figure 1B). CFA significantly attenuated voluntary running during nights 1 (P < 0.05) and 2 (P < 0.01) post-injection, compared to vehicle control, while formalin did not.
Figure 1.
Bilateral intraplantar (A) CFA attenuated voluntary wheel running during nights 1 (P < 0.05) and 2 (P < 0.01) post-injection. (B) formalin did not attenuate wheel running. Bilateral intraplantar (C) CFA or (D) formalin induced hindpaw mechanical allodynia (P < 0.001). Licking/writhing/flinching behaviors (E) were not induced by bilateral intraplantar CFA, but (F) were induced by bilateral intraplantar formalin (P < 0.05). n = 6/group. *P < 0.05; **P < 0.01; ***P < 0.001.
Low threshold mechanical allodynia
Similar to that described previously 7, the duration of CFA-induced mechanical allodynia exceeded that of attenuated voluntary running. In contrast to the transient suppression and absence of suppression of voluntary running by intraplantar CFA and formalin, respectively, both intraplantar CFA (time × treatment: F4,40 = 1.027; P = 0.41; time: F4,40 = 2.269; P = 0.07; treatment: F1,10 = 658.5; P < 0.001; Figure 1C) and formalin (time × treatment: F4,40 = 5.925; P < 0.001; time: F4,40 = 10.00; P < 0.001; treatment: F1,10 = 339.2; P < 0.001; Figure 1D) caused a reduction in von Frey threshold across days when bilaterally injected into the hindpaws. CFA-induced mechanical allodynia was still present at day 7 post-injection compared with saline control (P < 0.001), while formalin-induced mechanical allodynia was no longer statistically significant. No correlation was found between voluntary wheel running and paw withdrawal reflex (r = 0.61; P = 0.2).
Licking/writhing/flinching behavior
Intraplantar CFA did not produce licking/writhing/flinching behaviors (Figure 1E), in contrast to intraplantar formalin (P < 0.05 for 5 mins to 60 mins; Figure 1F).
L1 dorsum dermatome CFA or formalin does not attenuate voluntary running
All prior studies have examined voluntary wheel running when inflammation occurred in the hindlimbs 7, 30, which are directly impacted by the activity of running. Hence, this experiment aimed to determine whether the CFA-induced decrease in voluntary running is indicative of inflammatory pain specific to the anatomical region directly impacted during wheel running, versus supraspinal pain processing that is independent of inflammation site; that is, having characteristics of evoked pain. Formalin was also tested at this site to compare with bilateral intradermal formalin.
Voluntary wheel running
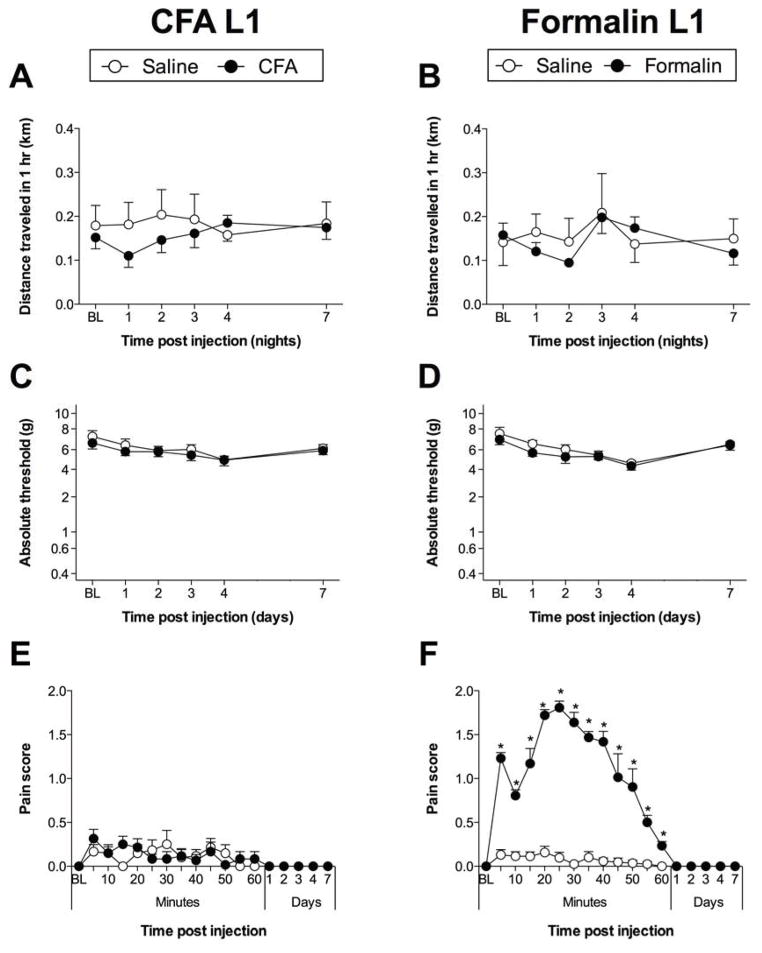
In contrast to results from bilateral intraplantar CFA, subcutaneous injection of CFA into the L1 dorsal dermatome did not attenuate voluntary running (time × treatment: F4,40 = 1.716; P = 0.17; time: F4,40 = 0.8265; P = 0.52; treatment: F1,10 = 0.3636; P = 0.56; Figure 2A). Subcutaneous injection of formalin into the L1 dorsal dermatome did not attenuate voluntary running (time × treatment: F4,40 = 1.312; P = 0.28; time: F4,40 = 4.590; P < 0.01; treatment: F1,10 = 0.1262; P = 0.73; Figure 2B).
Figure 2.
S.c. injection of (A) CFA, or (B) formalin into the L1 dorsum dermatome was not associated with a significant decrease in voluntary running wheel activity. Hindpaw withdrawal thresholds were unaffected by s.c. injection of either (C) CFA or (D) formalin into the L1 dorsum dermatome. Licking/writhing/flinching behaviors (E) were not induced by s.c. CFA injections into L1 dorsum dermatome, but (F) were present following s.c. formalin into the L1 dorsum dermatome (P < 0.001). n = 6/group. ***P < 0.001.
Low threshold mechanical allodynia
No hindpaw mechanical allodynia was induced by either CFA (time × treatment: F4,40 = 0.8915; P = 0.89; time: F4,40 = 2.981; P < 0.05; treatment: F1,10 = 0.4420; P = 0.52; Figure 2C) or formalin (time × treatment: F4,40 = 0.7061; P = 0.60; time: F4,40 = 9.537; P < 0.001; treatment: F1,10 = 0.8496; P = 0.38; Figure 2D) into the L1 dorsal dermatome.
Licking/writhing/flinching behavior
CFA did not induce licking/writhing/flinching behaviors (Figure 2E), while formalin injection induced classic biphasic, transient licking/writhing/flinching behavior (P < 0.05 for 5 mins to 60 mins; Figure 2F), indicating that formalin injection at this alternate site was sufficient to cause pain.
Attenuated voluntary running is associated with decreased total running time
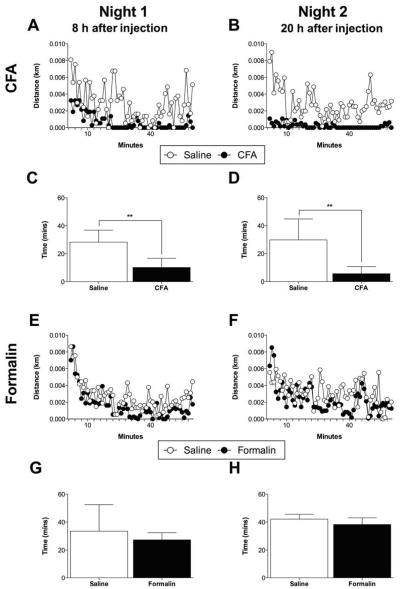
In order to examine the pattern of voluntary running during the 1-hour test session on nights 1 and 2, mean distance travelled was presented for CFA and formalin (Figure 3A, B, E, F). Those rats injected with CFA into the hindpaw ran only moderately during the first 20 minutes of Night 1, and then very little during the remaining 40 minutes during Night 1 and for all of Night 2 (Figure 3A, B). By contrast, those rats injected with formalin into the hindpaw, or CFA or formalin into the L1 dorsal dermatome exhibited continuous bouts of running during the 1-hour test session on both nights (Figure 3C, D, E, F). Total running time was also calculated and was decreased during Nights 1 and 2 (P < 0.01; Figure 3C, D), while formalin did not (Figure 3G, H). These data demonstrate that suppressed voluntary wheel running due to intraplantar CFA is due to a significant sedentary period of time, rather than a slow, but continuous rate of running.
Figure 3.
Mean distance travelled over the first hour of the dark cycle is presented for bilateral intraplantar (A, B) CFA and (E, F) formalin during Nights 1 and 2. CFA reduced total running time during (C) Night 1 (P < 0.01) and (D) Night 2 (P < 0.01). (G, H) Formalin administration failed to suppress total running time. n = 6/group. **P < 0.01.
Discussion
In this study, we demonstrate that voluntary running is decreased when inflammation is induced by CFA in the hindpaws, but not by CFA injection into the L1 dorsum dermatome. Hence these data demonstrate for the first time that inflammatory pain induced by CFA in the L1 dorsum dermatome is not sufficient to reduce voluntary wheel running; rather, CFA-induced inflammation must involve the hindpaws, suggestive that reduction of wheel running reflects evoked pain from the act of running. Our data with rats extend the one prior observation in mice that voluntary wheel running is attenuated by bilateral intraplantar CFA 7. As expected, intraplantar or L1 dorsum dermatome CFA did not produce licking/writhing/flinching behaviors 14. For the first time, the impact of a standard bilateral intraplantar formalin dose on voluntary running was also tested and shown to be insufficient to significantly attenuate this behavior, despite induction of robust licking/writhing/flinching behavior and hindpaw mechanical allodynia. Despite a similar induction of hindpaw mechanical allodynia by CFA and formalin, these substances induce very different voluntary wheel running profiles. This failure is striking in that it clearly demonstrates that voluntary wheel running cannot be predicted by hindpaw mechanical allodynia or licking/writhing/flinching behavior. Finally, we analyzed the timecourse of distance travelled during the 1-hour test session on nights 1 and 2, in which total running time was significantly reduced by CFA.
With the predominant use of reflex-evoked outcome measures being raised as a limitation of preclinical pain research 24, 25, 27, 36, voluntary running was introduced on the assumption that it would incorporate supraspinal processing beyond reflex responses. The present study extends the interpretation of this behavior to suggest that suppressed voluntary wheel running is a measure of pain evoked by use of the affected limbs. Results from other studies also support this conclusion: voluntary running is only attenuated when hindpaw inflammation is bilateral 7, 30. The requirement for bilateral induction of pain may limit translation of this outcome measure to the predominantly unilateral nerve injury models, which is supported by data from our laboratory (unpublished) demonstrating that voluntary running does not correlate with the magnitude of mechanical allodynia induced by unilateral graded chronic constriction injury 11.
While intraplantar formalin and CFA induced comparable hindpaw mechanical allodynia during days 1–4 post-injection, reliable suppression of voluntary running was only induced by CFA. It is possible that this disparity is reflective of differing mechanisms, as formalin is associated with peripheral nerve damage and microglial activation in the spinal lumbar dorsal horn, while CFA is not 10, 19. Another, though not mutually exclusive, explanation is that voluntary wheel running is a sensitive measure of paw inflammation, such as edema. Formalin at an even higher percentage and volume (5%, 100 μL) has been shown to induce significantly less paw edema and redness than the same CFA dose employed here 19. Therefore, changes in weight-bearing, which closely correlated with intraplantar CFA-attenuated wheel running in a previous study 7, should be compared with formalin-impaired voluntary wheel running in the future. It has been previously reported that after intraplantar formalin (5%, 50 μL), rats fail to respond to baseline von Frey thresholds when testing is performed on dorsum surface of the paw (possibly due to a lack of deep tissue allodynia), which was posited to be linked to tissue destruction 10. However, such hypoalgesia was not observed in another study where vigorous paw withdrawals due to von Frey stimulation at the 5% formalin plantar injection site 31. As hypoalgesia is not present at the injection site 31, tissue damage is not likely to explain differences in running wheel activity between CFA and formalin, since this behavior involves the entire plantar surface of the foot, including the sensitized mechanical allodynia test site. While the mechanisms underlying the disparate effects of CFA and formalin on voluntary running require further characterization, it is clear is that two pain-evoking stimuli (CFA, formalin) fail to create concordant effects on voluntary wheel-running when administered to the hindpaws. Hence, the interpretation of suppressed (or, correlatively, enhanced) voluntary wheel running behavior by a manipulation under study is far less clear than a simple correlation with other pain behaviors. However, the behavior is likely linked to pain, rather than inflammation per se, as a non-antiinflammatory analgesic (morphine) was previously found to restore CFA-attenuated voluntary running 7.
Another discrepancy was observed between CFA-induced hindpaw mechanical allodynia and decreased voluntary wheel running, in which the former persisted longer than the latter. While this lack of correlation has been observed previously 7, the reasons behind the differential behavioral endpoint outcomes cannot be fully explained, given an incomplete understanding of the neurobehavioral processes responsible for voluntary running 26. However, it could be speculated that the rewarding nature of wheel running and/or the inherent tendency of rats to be physically active overrides residual mechanical allodynia due to intraplantar CFA or formalin 26.
Central sensitization has been implicated in phase II of formalin-induced licking/writhing/flinching behaviors 37. In addition to prolonged hindpaw allodynia, spinal microglia p38 MAPK, an enzyme linked to microglial activation and central sensitization, is enduringly activated by intraplantar formalin, and pharmacological blockade of p38 abolished phase II licking/writhing/flinching behaviors 9, 10, 13, 18, 19. Therefore, the failure of robust licking/writhing/flinching behaviors to predict voluntary wheel running activity is striking, as it suggests that wheel running may not be a sensitive measure of central sensitization. That voluntary wheel running assessment occurred approximately 8 hours after the resolution of licking/writhing/flinching behaviors, due to the potential confound of response competition, does not limit these conclusions, since central sensitization persists beyond initial licking/flinching behaviors 9, 10, 18, 19, 37.
Our voluntary running wheel protocol departs from the protocols of prior studies at several key points. In contrast to Cobos et al. 7, rats were housed in the cage containing the running wheel for the duration of the study to avoid stress 2. Voluntary running was also recorded at night to accommodate nocturnality. Exercise has been shown to attenuate pain behavior in rodents 16, 28, which may confound subsequent measurements. Hence, we employed a shorter acquisition phase than that previously used 30.
We conclude that changes in voluntary wheel running does not correlate with hindpaw mechanical allodynia and is not predictive of prior licking/writhing/flinching behavior. We further conclude that select inflammatory challenges, such as CFA, must involve the hindpaw in order for an impact on voluntary wheel running to be observed. Yet, inflammation of the hindpaw is not sufficient to impact voluntary wheel running given that no reliable reduction in wheel running was observed in response to formalin. Why these striking disparities exist requires further study, but what is clear is that interpretation of voluntary wheel running data cannot simply be explained by a correlation with mechanical allodynia.
Footnotes
Disclosures: This work was supported by NIH Grants DA024044, DE107782 and DA023132. PMG is an NHMRC CJ Martin Fellow [ID:1054091]. The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abbott FV, Ocvirk R, Najafee R, Franklin KB. Improving the efficiency of the formalin test. Pain. 1999;83:561–569. doi: 10.1016/S0304-3959(99)00168-2. [DOI] [PubMed] [Google Scholar]
- 2.Balcombe JP, Barnard ND, Sandusky C. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci. 2004;43:42–51. [PubMed] [Google Scholar]
- 3.Bennett GJ. What is spontaneous pain and who has it? J Pain. 2012;13:921–929. doi: 10.1016/j.jpain.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Capone F, Aloisi AM. Refinement of pain evaluation techniques. The formalin test. Ann Ist Super Sanita. 2004;40:223–229. [PubMed] [Google Scholar]
- 5.Chacur M, Milligan ED, Gazda LS, Armstrong C, Wang H, Tracey KJ, Maier SF, Watkins LR. A new model of sciatic inflammatory neuritis (SIN): induction of unilateral and bilateral mechanical allodynia following acute unilateral peri-sciatic immune activation in rats. Pain. 2001;94:231–244. doi: 10.1016/S0304-3959(01)00354-2. [DOI] [PubMed] [Google Scholar]
- 6.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 7.Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ. Inflammation-induced decrease in voluntary wheel running in mice: a nonreflexive test for evaluating inflammatory pain and analgesia. Pain. 2012;153:876–884. doi: 10.1016/j.pain.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior research methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 9.Fu KY, Light AR, Maixner W. Relationship between nociceptor activity, peripheral edema, spinal microglial activation and long-term hyperalgesia induced by formalin. Neuroscience. 2000;101:1127–1135. doi: 10.1016/s0306-4522(00)00376-6. [DOI] [PubMed] [Google Scholar]
- 10.Fu KY, Light AR, Maixner W. Long-lasting inflammation and long-term hyperalgesia after subcutaneous formalin injection into the rat hindpaw. J Pain. 2001;2:2–11. doi: 10.1054/jpai.2001.9804. [DOI] [PubMed] [Google Scholar]
- 11.Grace PM, Hutchinson MR, Manavis J, Somogyi AA, Rolan PE. A novel animal model of graded neuropathic pain: utility to investigate mechanisms of population heterogeneity. J Neurosci Methods. 2010;193:47–53. doi: 10.1016/j.jneumeth.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 12.Harvey LO., Jr Efficient estimation of sensory thresholds. Behav Res Methods Instrum Comput. 1986;18:623–632. [Google Scholar]
- 13.Ji RR, Gereau RWt, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston IN, Maier SF, Rudy JW, Watkins LR. Post-conditioning experience with acute or chronic inflammatory pain reduces contextual fear conditioning in the rat. Behav Brain Res. 2012;226:361–368. doi: 10.1016/j.bbr.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12:1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuphal KE, Fibuch EE, Taylor BK. Extended swimming exercise reduces inflammatory and peripheral neuropathic pain in rodents. J Pain. 2007;8:989–997. doi: 10.1016/j.jpain.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AM, Ferrari MD, Craig KD, Mogil JS. Coding of facial expressions of pain in the laboratory mouse. Nature methods. 2010;7:447–449. doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- 18.Li K, Lin T, Cao Y, Light AR, Fu KY. Peripheral formalin injury induces 2 stages of microglial activation in the spinal cord. J Pain. 2010;11:1056–1065. doi: 10.1016/j.jpain.2010.01.268. [DOI] [PubMed] [Google Scholar]
- 19.Lin T, Li K, Zhang FY, Zhang ZK, Light AR, Fu KY. Dissociation of spinal microglia morphological activation and peripheral inflammation in inflammatory pain models. J Neuroimmunol. 2007;192:40–48. doi: 10.1016/j.jneuroim.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loram LC, Grace PM, Strand KA, Taylor FR, Ellis A, Berkelhammer D, Bowlin M, Skarda B, Maier SF, Watkins LR. Prior exposure to repeated morphine potentiates mechanical allodynia induced by peripheral inflammation and neuropathy. Brain Behav Immun. 2012;26:1256–1264. doi: 10.1016/j.bbi.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller LL, Picker MJ, Umberger MD, Schmidt KT, Dykstra LA. Effects of alterations in cannabinoid signaling, alone and in combination with morphine, on pain-elicited and pain-suppressed behavior in mice. The Journal of pharmacology and experimental therapeutics. 2012;342:177–187. doi: 10.1124/jpet.112.191478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milligan ED, Mehmert KK, Hinde JL, Harvey LO, Martin D, Tracey KJ, Maier SF, Watkins LR. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res. 2000;861:105–116. doi: 10.1016/s0006-8993(00)02050-3. [DOI] [PubMed] [Google Scholar]
- 23.Milligan ED, O’Connor KA, Nguyen KT, Armstrong CB, Twining C, Gaykema RP, Holguin A, Martin D, Maier SF, Watkins LR. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J Neurosci. 2001;21:2808–2819. doi: 10.1523/JNEUROSCI.21-08-02808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- 25.Mogil JS, Crager SE. What should we be measuring in behavioral studies of chronic pain in animals? Pain. 2004;112:12–15. doi: 10.1016/j.pain.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 26.Novak CM, Burghardt PR, Levine JA. The use of a running wheel to measure activity in rodents: relationship to energy balance, general activity, and reward. Neuroscience and biobehavioral reviews. 2012;36:1001–1014. doi: 10.1016/j.neubiorev.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rice AS, Cimino-Brown D, Eisenach JC, Kontinen VK, Lacroix-Fralish ML, Machin I, Mogil JS, Stohr T. Animal models and the prediction of efficacy in clinical trials of analgesic drugs: a critical appraisal and call for uniform reporting standards. Pain. 2008;139:243–247. doi: 10.1016/j.pain.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 28.Stagg NJ, Mata HP, Ibrahim MM, Henriksen EJ, Porreca F, Vanderah TW, Philip Malan T., Jr Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: role of endogenous opioids. Anesthesiology. 2011;114:940–948. doi: 10.1097/ALN.0b013e318210f880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stein C, Millan MJ, Herz A. Unilateral inflammation of the hindpaw in rats as a model of prolonged noxious stimulation: alterations in behavior and nociceptive thresholds. Pharmacol Biochem Behav. 1988;31:445–451. doi: 10.1016/0091-3057(88)90372-3. [DOI] [PubMed] [Google Scholar]
- 30.Stevenson GW, Mercer H, Cormier J, Dunbar C, Benoit L, Adams C, Jezierski J, Luginbuhl A, Bilsky EJ. Monosodium iodoacetate-induced osteoarthritis produces pain-depressed wheel running in rats: implications for preclinical behavioral assessment of chronic pain. Pharmacol Biochem Behav. 2011;98:35–42. doi: 10.1016/j.pbb.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sweitzer SM, Colburn RW, Rutkowski M, DeLeo JA. Acute peripheral inflammation induces moderate glial activation and spinal IL-1beta expression that correlates with pain behavior in the rat. Brain Res. 1999;829:209–221. doi: 10.1016/s0006-8993(99)01326-8. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi Y, Chiba T, Kurokawa M, Aoki Y. Dermatomes and the central organization of dermatomes and body surface regions in the spinal cord dorsal horn in rats. J Comp Neurol. 2003;462:29–41. doi: 10.1002/cne.10669. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi Y, Chiba T, Kurokawa M, Aoki Y, Takahashi K, Yamagata M. Stereoscopic structure of sensory nerve fibers in the lumbar spine and related tissues. Spine (Phila Pa 1976) 2003;28:871–880. doi: 10.1097/01.BRS.0000058717.43888.B9. [DOI] [PubMed] [Google Scholar]
- 34.Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- 35.Treutwein B, Strasburger H. Fitting the psychometric function. Percept Psychophys. 1999;61:87–106. doi: 10.3758/bf03211951. [DOI] [PubMed] [Google Scholar]
- 36.Vierck CJ, Hansson PT, Yezierski RP. Clinical and pre-clinical pain assessment: are we measuring the same thing? Pain. 2008;135:7–10. doi: 10.1016/j.pain.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Yaksh TL, Ozaki G, McCumber D, Rathbun M, Svensson C, Malkmus S, Yaksh MC. An automated flinch detecting system for use in the formalin nociceptive bioassay. Journal of applied physiology. 2001;90:2386–2402. doi: 10.1152/jappl.2001.90.6.2386. [DOI] [PubMed] [Google Scholar]