Abstract
Objective
To compare placental lesions for stillbirth cases and live birth controls in a population-based study.
Methods
Pathological examinations were performed on placentas from singleton pregnancies using a standard protocol. Data were analyzed overall and within gestational age groups at delivery.
Results
Placentas from 518 stillbirths and 1,200 live births were studied. Single umbilical artery was present in 7.7% of stillbirths and 1.7% of live births, velamentous cord insertion was present in 5% of stillbirths and 1.1% of live births, diffuse terminal villous immaturity was present in 10.3% of stillbirths and 2.3% of live births, inflammation (eg, acute chorioamnionitis of placental membranes) was present in 30.4% of stillbirths and 12% of live births, vascular degenerative changes in chorionic plate was present in 55.7% of stillbirths and 0.5% of live births, retroplacental hematoma was present in 23.8% of stillbirths and 4.2% of live births, intraparenchymal thrombi was present in 19.7% of stillbirths and 13.3% of live births, parenchymal infarction was present in 10.9% of stillbirths and 4.4% of live births, fibrin deposition was present in 9.2% of stillbirths and 1.5% of live births, fetal vascular thrombi was present in 23% of stillbirths and 7% of live births, avascular villi was present in 7.6% of stillbirths and 2.0% of live births, and hydrops was present in 6.4% of stillbirths and 1.0% of live births. Among stillbirths, inflammation and retroplacental hematoma were more common in placentas from early deliveries, while thrombotic lesions were more common in later gestation. Inflammatory lesions were especially common in early live births.
Conclusion
Placental lesions were highly associated with stillbirth compared to live births. All lesions associated with stillbirth were found in live births but often with variations by gestational age at delivery. Knowledge of lesion prevalence within gestational age groups in both stillbirths and live birth controls contributes to an understanding of the association between placental abnormality and stillbirth.
Précis
Placental lesions are highly associated with stillbirth compared to live births but all lesions associated with stillbirths are found in live births; prevalence varies by gestational age at delivery.
Introduction
Stillbirth, defined as fetal death at 20 weeks of gestation or later, occurs in about 1 in 160 births in the United States, a rate higher than in many other developed countries (1-3). Examination of the complex pathogenic interplay between the mother, fetus and placenta that leads to stillbirth has generally focused on maternal and fetal disorders (4-7). Examination of placental pathology to elucidate its contribution to stillbirth has been limited by insufficient sample size, lack of appropriate controls, ill-defined nomenclature, and non-standardized placental examination protocols (8-15). Thus, we sought to compare macroscopic and microscopic abnormalities of the umbilical cord, membranes, and placenta in singleton stillbirths and live births, both overall and within groups defined by gestational age at delivery, in a large, multisite, population-based case-control study with a standardized placental examination protocol.
Materials and Methods
A population-based case-control study of stillbirth was conducted by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Stillbirth Collaborative Research Network (SCRN). Participants were enrolled at delivery between March 2006 and September 2008. Eligible women were residents of five catchment areas that were defined by state and county boundaries and included the state of Rhode Island and portions of Massachusetts, Georgia, Texas, and Utah. The study was conducted at 59 hospitals recruited to ensure access to at least 90% of the stillbirths and live births among residents of each catchment area. These hospitals delivered a combined total of more than 80,000 infants per year to catchment area residents.
Details of methods and study design (16) and sample size considerations (17) have previously been published. We attempted to enroll all women with stillbirth (cases) and a representative sample of women with live birth (controls) residing in the five SCRN catchment areas during the enrollment period. Women delivering live births prior to 32 weeks of gestation were oversampled to ensure adequate numbers for stratified analyses (16): all live births delivered at 20 to 23 weeks were selected for potential enrollment, and live births between 24 to 31 weeks were selected at random using selection probabilities that were pre-specified by week of gestation to provide numbers similar to those for stillbirths at each gestational week. African American women delivering live births at or beyond 32 weeks of gestation were also oversampled. The study was approved by the Institutional Review Boards of each clinical site and the Data Coordinating and Analysis Center, and all mothers gave written informed consent.
Stillbirths were defined as infants born at or after 20 weeks of gestation with Apgar scores of 0 at 1 and 5 minutes and no signs of life by direct observation. However, fetal deaths at 18 or 19 weeks without good dating were also included in the study so as to include all potential cases at or after 20 weeks of gestation (16). Gestational age was determined by the best clinical estimate using multiple sources including history of assisted reproductive technology with documentation of the day of embryo transfer (if available), first day of the last menstrual period, and results of obstetric ultrasonography (18). Deliveries resulting from the termination of a live fetus were excluded.
For both stillbirths and live births, the SCRN case-control protocol included a maternal interview, medical record abstraction, placental pathology examination, and maternal and fetal biospecimen collection, and for stillbirths a postmortem examination (16). These methods were described in earlier publications (16, 19-20). During several workshops, network pathologists developed and adopted common placental examination protocols, observational criteria, and data collection forms focusing on those characteristics thought most likely to be associated with fetal death (19-20). The placental examination protocol (19) included initial digital imaging under specified lighting, macroscopic examination, collection of frozen and ambient temperature samples of the cord, membranes and the placental disc, and microscopic examination of sections collected according to a specific sampling protocol. The examiners were not blinded to stillbirth/live birth status since they also were performing clinical postmortem examinations (autopsies) and placental evaluations along with the research investigation.
A minimum of five full thickness placental tissue samples were obtained, one at the umbilical cord insertion and four others determined by random numbers that specified the axes and spacing of sampling (19). We employed this strategy to avoid systematic differences in sampling locations among pathologists and to ensure dispersion of the tissue sampling sites between the umbilical cord insertion and the periphery of the placenta. Distal and proximal umbilical cord sections and a membrane roll also were collected.
Placental disorders were characterized into three broad categories based on mechanism: developmental, inflammatory, and circulatory. Standard definitions (21) were used for macroscopic and microscopic findings. The following terms were used when appropriate: focal, present in one area on one single slide; multifocal/patchy, present in more than one area, in multiple slides, or both; and diffuse, when the distribution of lesions involved the full thickness of the placental disc and involved all sections to a similar degree. For inflammatory lesions, we defined the maternal compartment as consisting of the free chorioamnion, decidua and chorionic plate of the placental disc which includes the chorion and amnion layers. The fetal compartment included umbilical cord and vessels as well as fetal vessels in the chorionic plate.
The analyses were weighted for oversampling and other aspects of the study design as well as for differential consent using SUDAAN software, Version 11.0.0 (22). Construction of the weights for the overall study has been previously described (16). For analysis of placental examination findings, an additional multiplicative weighting component was created to account for differential losses of placental specimens for study protocol examination. Analysis was restricted to placentas from singleton gestations. In addition, placentas from mummified stillbirths, and fragmented placentas were excluded from analysis. Weighted distributions of characteristics were compared for those enrolled (weighted for the study design and differential consent) and those analyzed (weighted for study design, differential consent, and differential losses to placental examination), using a modified independent-sample test comparing enrollees who were included compared with not included in the analysis. To examine variation in results by gestational age at delivery, we analyzed data within gestational age groups: <240/7, 24-316/7, 32-366/7, and ≥370/7 weeks. For these analyses, weights were rescaled within group to reflect effective sample sizes for within-group comparisons.
Odds ratios (OR) and 95% confidence intervals (CI) were calculated from univariate logistic regression models. If there were no live births (or no stillbirths) with a given placental characteristic, we reported an upper bound for the p-value by identifying one observation having the smallest weight in the reference category and recoding the characteristic as present. All tests were performed at a nominal significance level of α=0.05, without correction for multiple comparisons. All single degree of freedom tests were 2-sided.
Results
Figure 1 describes study enrollment. Seventy percent of eligible stillbirth pregnancies and 63% of eligible live birth pregnancies were enrolled. Of the 620 singleton stillbirth pregnancies and 1,871 singleton live birth pregnancies enrolled, 613 stillbirth mothers and 1,747 live birth mothers consented to placental examination. Ninety-five stillbirth placentas and 547 live birth placentas were excluded from analysis because of inadequate examination (typically due to placentas having been inadvertently discarded), fragmentation, or mummification, leaving 518 stillbirth placentas and 1,200 live birth placentas analyzed for this report.
Figure 1. Study enrollment and inclusion in placenta case-control analyses.
This analysis compares placental examination results from singleton stillbirth and live birth pregnancies. A pregnancy was categorized as a stillbirth pregnancy if there were any stillbirths delivered and as a live birth pregnancy if all live births were delivered. A fetal death was defined by Apgar scores of 0 at 1 and 5 minutes and no signs of life by direct observation. Fetal deaths were classified as stillbirths if the best clinical estimate of gestational age at death was 20 or more weeks. Fetal deaths at 18 and 19 weeks without good dating were also included as stillbirths.
*Review of only slides or a report from a non-SCRN pathologist, or the placenta having been discarded in labor and delivery before it could be collected by the study staff.
†Mummified stillborn babies are those with Grade IV-V maceration among fragmented babies and Grade V maceration among intact babies.
‡Fragmented placenta only, n=66; mummified stillborn only, n=6; both, n=2.
Table 1 compares weighted distributions of demographic characteristics between those singleton births enrolled and those included in the analysis, for stillbirths and for live births. For both stillbirths and live births, women who enrolled and women whose placentas were analyzed were similar with regard to maternal age at delivery, race or ethnicity, maternal education, marital status, and insurance/method of payment, whereas there were small but statistically significant differences between those enrolled and those analyzed in gestational age at delivery. Differences between included stillbirths and live births were similar to those reported previously for the main study (17). The most dramatic difference between the included stillbirths and included live births was for the weighted distributions of gestational age at delivery, which are consistent with well known (e.g., from vital statistics) population differences. Results for univariate associations with stillbirth for demographic and other baseline factors (not shown) were similar to those reported for the main study (17).
Table 1. Weighted Characteristics of Enrolled Singleton Pregnancies and Those Included in the Placental Case-Control Analysis.
| Characteristic - weighted n (%)* | Singleton Stillbirths | Singleton Live Births | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Enrolled | Included | P† | Enrolled | Included | P† | |
|
|
|
|||||
| Unweighted sample size | n=620 | n=518 | n=1871 | n=1200 | ||
| Weighted sample size | nw=622 | nw=518 | nw=1412 | nw=966 | ||
|
| ||||||
| Maternal age at delivery, years | ||||||
| <20 | 84 (13.5) | 74 (14.3) | 0.617 | 148 (10.5) | 100 (10.3) | 0.653 |
| 20-34 | 433 (69.7) | 358 (69.2) | 1070 (75.8) | 737 (76.3) | ||
| 35-39 | 76 (12.3) | 62 (11.9) | 163 (11.5) | 106 (11.0) | ||
| 40+ | 28 ( 4.6) | 24 ( 4.6) | 30 ( 2.1) | 23 ( 2.4) | ||
| Maternal race/ethnicity | ||||||
| Non-Hispanic white | 209 (33.6) | 177 (34.2) | 0.260 | 641 (45.4) | 435 (45.0) | 0.702 |
| Non-Hispanic black | 146 (23.6) | 115 (22.2) | 168 (11.9) | 112 (11.6) | ||
| Hispanic | 227 (36.5) | 195 (37.8) | 498 (35.3) | 351 (36.4) | ||
| Other | 39 ( 6.3) | 30 ( 5.8) | 104 ( 7.4) | 68 ( 7.0) | ||
| Maternal education, grade | ||||||
| 0-11 (none/primary/some secondary) | 141 (24.6) | 116 (24.1) | 0.679 | 248 (18.6) | 167 (18.3) | 0.441 |
| 12 (completed secondary) | 171 (29.9) | 141 (29.5) | 349 (26.1) | 249 (27.2) | ||
| 13+ (college) | 261 (45.6) | 223 (46.4) | 740 (55.3) | 500 (54.6) | ||
| Marital status/cohabitating | ||||||
| Not married or cohabitating | 146 (25.3) | 124 (25.7) | 0.899 | 206 (15.4) | 143 (15.6) | 0.340 |
| Cohabitating | 150 (26.0) | 124 (25.7) | 325 (24.2) | 211 (23.0) | ||
| Married | 280 (48.7) | 234 (48.7) | 811 (60.4) | 565 (61.5) | ||
| Insurance/method of payment | ||||||
| No insurance | 38 ( 6.2) | 33 ( 6.4) | 0.8 | 51 ( 3.6) | 42 ( 4.4) | 0.143 |
| Any public/private assistance | 332 (53.7) | 274 (53.2) | 692 (49.0) | 475 (49.2) | ||
| VA/commercial health insurance/HMO | 248 (40.2) | 208 (40.4) | 668 (47.3) | 449 (46.5) | ||
| Gestational age at delivery‡ | ||||||
| 18-19 | 17 ( 2.7) | 11 ( 2.1) | 0.04 | 0 ( 0.0) | 0 ( 0.0) | 0.028 |
| 20-23 | 210 (33.8) | 164 (31.7) | 5 ( 0.3) | 3 ( 0.3) | ||
| 24-27 | 92 (14.8) | 76 (14.7) | 9 ( 0.6) | 5 ( 0.5) | ||
| 28-31 | 76 (12.3) | 66 (12.7) | 13 ( 0.9) | 7 ( 0.7) | ||
| 32-36 | 120 (19.3) | 105 (20.2) | 116 ( 8.2) | 74 ( 7.7) | ||
| 37+ | 106 (17.1) | 96 (18.6) | 1270 (89.9) | 878 (90.9) | ||
VA, Veterans Administration HMO, health maintenance organization.
Weighted n's, percentages and p-values are shown for all enrolled singletons (analysis weights) and for those included in the analysis (adequate placental exam, placenta not fragmented, and stillbirth not mummified) (placenta weights). The analysis weights take into account the study design and differential consent based on characteristics recorded on all eligible pregnancies that were screened for the study. The placenta weights include an additional multiplicative component which takes into account differential availability of an adequate placental exam based on characteristics that were recorded on enrolled pregnancies. Overall unweighted and weighted sample sizes are also provided. The overall weighted sample sizes and weighted n's for individual categories are not integers, but are shown rounded to the nearest integer. Overall sample sizes vary slightly by characteristic included in the table.
Modified independent-sample test comparing those included compared with those not included (see methods).
For gestational age groups among live births, the corresponding unweighted n's are: n=74 at 20-23 weeks, n=41 at 24-27 weeks, n=39 at 28-31 weeks, n=79 at 32-36 weeks, and n=967 at 37+ weeks.
Table 2 shows the prevalence of selected placental findings by type of lesion, comparing stillbirths to live births. Since 90% of singleton live births occurred at term, the results of these overall comparisons would be similar to comparisons of all stillbirths with term live births. The most common placental finding in stillbirths was acute chorioamnionitis of the free membranes (30.4%), also found in 12.0% of live births (OR 3.20, 95% CI 2.39-4.28). Similarly, acute chorioamnionitis of the chorionic plate was more common in stillbirth placentas at 23.2% versus 11.9% for live births (OR 2.24, 95% CI 1.65-3.04). Other findings commonly found in stillbirths were retroplacental hematoma, 23.8% versus 4.2% (OR 7.08, 95% CI 4.83-10.38), fetal vascular thrombi in the chorionic plate, 23.0% versus 7.0% (OR 3.99, 95% CI 2.84-5.61), and intraparenchymal thrombi, 19.7% versus 13.3% (OR 1.60, 95% CI 1.20-2.13). The other placental findings in stillbirths were found less frequently, but many were found significantly more often in cases than controls.
Table 2. Selected Placental Findings for Singleton Pregnancies, Stillbirth Compared With Live Birth.
| CHARACTERISTICS | Stillbirth nw=518 % | Live Birth nw=966 % | Odds Ratio (95% CI) | P |
|---|---|---|---|---|
|
| ||||
| DEVELOPMENTAL DISORDERS | ||||
|
| ||||
| Umbilical Cord | ||||
|
| ||||
| Single umbilical artery | 7.7 | 1.7 | 4.80 (2.67, 8.62) | <.001 |
|
| ||||
| Velamentous insertion* | 5.0 | 1.1 | 4.50 (2.18, 9.27) | <.001 |
|
| ||||
| Furcate insertion* | 1.8 | 3.6 | 0.50 (0.23, 1.11) | 0.089 |
|
| ||||
| Placental Membranes | ||||
|
| ||||
| Circummarginate insertion† | 12.2 | 10.6 | 1.16 (0.83, 1.63) | 0.375 |
|
| ||||
| Circumvallate insertion† | 2.4 | 1.4 | 1.77 (0.81, 3.85) | 0.152 |
|
| ||||
| Fetal Villous Capillaries | ||||
|
| ||||
| Terminal villous immaturity (diffuse) | 10.3 | 2.3 | 4.95 (2.95, 8.29) | <.001 |
|
| ||||
| Terminal villous hypoplasia (diffuse) | 3.3 | 1.8 | 1.83 (0.87, 3.86) | 0.111 |
|
| ||||
| INFLAMMATORY DISORDERS | ||||
|
| ||||
| Maternal Inflammatory Response | ||||
|
| ||||
| Acute chorioamnionitis – placental membranes | 30.4 | 12.0 | 3.20 (2.39, 4.28) | <.001 |
|
| ||||
| Acute chorioamnionitis – chorionic plate | 23.2 | 11.9 | 2.24 (1.65, 3.04) | <.001 |
|
| ||||
| Fetal Inflammatory Response | ||||
|
| ||||
| Acute funisitis | 9.5 | 3.3 | 3.09 (1.94, 4.93) | <.001 |
|
| ||||
| Acute umbilical cord arteritis (one or more arteries) | 3.3 | 1.9 | 1.81 (0.90, 3.63) | 0.096 |
|
| ||||
| Acute umbilical cord phlebitis | 4.9 | 3.1 | 1.61 (0.92, 2.83) | 0.093 |
|
| ||||
| Chorionic plate acute vasculitis | 7.9 | 5.2 | 1.56 (1.00, 2.44) | 0.05 |
|
| ||||
| Chorionic plate vascular degenerative changes | 5.7 | 0.5 | 13.06 (4.68, 36.42) | <.001 |
|
| ||||
| Villitis | ||||
|
| ||||
| Acute diffuse villitis | 0.7 | 0.1 | 5.55 (0.68, 45.17) | 0.109 |
|
| ||||
| Chronic diffuse villitis | 1.6 | 0.5 | 3.24 (1.06, 9.87) | 0.04 |
|
| ||||
| CIRCULATORY DISORDERS | ||||
|
| ||||
| Maternal Circulatory Disorders | ||||
|
| ||||
| Retroplacental hematoma | 23.8 | 4.2 | 7.08 (4.83, 10.38) | <.001 |
|
| ||||
| Parenchymal infarction‡ | ||||
| Focal | 13.9 | 11.3 | 1.44 (1.04, 2.00) | <.001 |
| Multifocal | 10.9 | 4.4 | 2.89 (1.92, 4.35) | |
| Diffuse | 2.8 | 0.1 | 42.76 (5.57, 327.97) | |
|
| ||||
| Intraparenchymal thrombus | 19.7 | 13.3 | 1.60 (1.20, 2.13) | 0.001 |
|
| ||||
| Perivillous/intervillous fibrin/fibrinoid deposition (diffuse) | 9.2 | 1.5 | 6.69 (3.65, 12.27) | <.001 |
|
| ||||
| Fetal Circulatory Disorders | ||||
|
| ||||
| Fetal vascular thrombi in the chorionic plate | 23.0 | 7.0 | 3.99 (2.84, 5.61) | <.001 |
|
| ||||
| Avascular villi§ | ||||
| Focal | 7.6 | 4.9 | 1.78 (1.16, 2.73) | <.001 |
| Multifocal | 7.6 | 2.0 | 4.28 (2.53, 7.21) | |
| Diffuse | 4.0 | 0.0 | not defined* | |
|
| ||||
| Edema (Placental Hydrops) | 6.4 | 1.0 | 6.56 (3.35, 12.85) | <.001 |
nw = weighted sample size; CI, confidence interval.
Includes umbilical cords with both velamentous and furcate insertion (stillbirths, 0.4%; live births, 0.1%).
Includes membranes with both circummarginate and circumvallate insertion (stillbirths, 0.6%; live births, 0.5%).
Reference group is no parenchymal infarction.
Reference group is no avascular villi.
In Table 3 we show the prevalence of the placental findings and odds ratios for deliveries within gestational age groups. If there were no live births (or no stillbirths) with the placental finding, an upper bound for the p-value is shown in place of the odds ratio. Results that are bolded are statistically significant.
Table 3. Selected Placental Findings for Singleton Pregnancies, Stillbirth Compared With Live Birth within Groups Defined by Gestational Age at Delivery (Completed Weeks).
| CHARACTERISTICS | Delivery <24 Weeks | Delivery 24-31 Weeks | Delivery 32-36 Weeks | Delivery ≥37 Weeks | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| SB nw=175 % | LB nw=74 % | OddsRatio (95% CI) | SB nw=142 % | LB nw=47 % | Odds Ratio (95% CI) | SB nw=105 % | LB nw=74 % | Odds Ratio (95% CI) | SB nw=96 % | LB nw=878 % | Odds Ratio (95% CI) | |
|
| ||||||||||||
| DEVELOPMENTAL DISORDERS | ||||||||||||
|
| ||||||||||||
| Umbilical Cord | ||||||||||||
|
| ||||||||||||
| Single umbilical artery | 7.5 | 4.2 | 1.87 (0.47, 7.50) | 8.9 | 0.0 | p<0.001* | 9.2 | 1.8 | 5.69 (1.17, 27.57) | 4.9 | 1.7 | 2.93 (1.03, 8.37) |
|
| ||||||||||||
| Velamentous insertion† | 7.0 | 0.0 | p<0.019* | 2.5 | 6.0 | 0.40 (0.09, 1.82) | 7.6 | 1.9 | 4.32 (0.51, 36.31) | 2.1 | 1.0 | 2.06 (0.44, 9.68) |
|
| ||||||||||||
| Furcate insertion† | 1.2 | 4.1 | 0.29 (0.04, 1.92) | 0.8 | 0.0 | p<0.446* | 3.5 | 3.7 | 0.95 (0.18, 5.10) | 2.6 | 3.6 | 0.72 (0.17, 3.11) |
|
| ||||||||||||
| Placental Membranes | ||||||||||||
|
| ||||||||||||
| Circummarginate‡ | 9.6 | 13.9 | 0.65 (0.26, 1.62) | 14.7 | 6.0 | 2.70 (0.97, 7.53) | 13.2 | 10.1 | 1.35 (0.52, 3.51) | 12.0 | 10.7 | 1.13 (0.60, 2.13) |
|
| ||||||||||||
| Circumvallate‡ | 2.7 | 6.2 | 0.42 (0.10, 1.67) | 3.6 | 3.7 | 0.96 (0.17, 5.43) | 1.2 | 1.6 | 0.73 (0.04, 11.98) | 1.6 | 1.3 | 1.24 (0.27, 5.70) |
|
| ||||||||||||
| Fetal Villous Capillaries | ||||||||||||
|
| ||||||||||||
| Terminal villous immaturity (diffuse) | 11.9 | 7.9 | 1.57 (0.59, 4.13) | 9.0 | 2.7 | 3.61 (0.97, 13.44) | 7.3 | 3.2 | 2.41 (0.59, 9.93) | 12.3 | 2.2 | 6.35 (2.90, 13.94) |
|
| ||||||||||||
| Terminal villous hypoplasia (diffuse) | 1.4 | 0.0 | p<0.467* | 5.8 | 11.5 | 0.48 (0.14, 1.66) | 5.1 | 2.9 | 1.82 (0.28, 11.58) | 1.0 | 1.6 | 0.60 (0.08, 4.68) |
|
| ||||||||||||
| INFLAMMATORY DISORDERS | ||||||||||||
|
| ||||||||||||
| Maternal Inflammatory Response | ||||||||||||
|
| ||||||||||||
| Acute chorioamnionitis – placental membranes | 43.0 | 79.1 | 0.20 (0.10, 0.38) | 22.1 | 32.3 | 0.59 (0.32, 1.12) | 21.6 | 14.6 | 1.61 (0.54, 4.79) | 29.2 | 11.3 | 3.24 (1.97, 5.32) |
|
| ||||||||||||
| Acute chorioamnionitis – chorionic plate | 35.7 | 82.3 | 0.12 (0.06, 0.24) | 12.5 | 23.2 | 0.47 (0.23, 0.98) | 14.7 | 18.0 | 0.79 (0.29, 2.14) | 25.7 | 11.0 | 2.79 (1.68, 4.64) |
|
| ||||||||||||
| Fetal Inflammatory Response | ||||||||||||
|
| ||||||||||||
| Acute funisitis | 22.2 | 42.9 | 0.38 (0.20, 0.72) | 3.9 | 19.1 | 0.17 (0.06, 0.46) | 0.7 | 1.8 | 0.39 (0.03, 4.38) | 4.1 | 3.1 | 1.34 (0.45, 4.02) |
|
| ||||||||||||
| Acute umbilical cord arteritis (one or more arteries) | 9.0 | 30.3 | 0.23 (0.10, 0.52) | 0.0 | 12.4 | p<0.001* | 0.9 | 1.5 | 0.61 (0.05, 7.11) | 0.8 | 1.7 | 0.47 (0.06, 3.65) |
|
| ||||||||||||
| Acute umbilical cord phlebitis | 10.2 | 25.7 | 0.33 (0.15, 0.73) | 1.4 | 11.2 | 0.11 (0.02, 0.55) | 0.9 | 3.5 | 0.26 (0.03, 2.40) | 5.0 | 2.9 | 1.75 (0.64, 4.79) |
|
| ||||||||||||
| Chorionic plate acute vasculitis | 16.7 | 61.4 | 0.13 (0.07, 0.24) | 2.8 | 14.9 | 0.17 (0.05, 0.54) | 1.8 | 4.0 | 0.43 (0.07, 2.84) | 5.7 | 5.0 | 1.15 (0.47, 2.82) |
|
| ||||||||||||
| Chorionic plate vascular degenerative changes | 5.6 | 9.1 | 0.59 (0.17, 2.12) | 6.9 | 0.0 | p<0.002* | 5.6 | 0.0 | p<0.027* | 4.2 | 0.5 | 9.16 (2.19, 38.39) |
|
| ||||||||||||
| Villitis | ||||||||||||
|
| ||||||||||||
| Acute diffuse villitis | 0.5 | 0.0 | p<0.908* | 1.8 | 0.7 | 2.86 (0.29, 28.41) | 0.0 | 0.0 | not defined* | 0.0 | 0.1 | p<0.231* |
|
| ||||||||||||
| Chronic diffuse villitis | 0.6 | 0.0 | p<0.997* | 1.8 | 6.3 | 0.27 (0.06, 1.32) | 2.1 | 0.0 | p<0.257* | 2.4 | 0.5 | 5.41 (0.97, 30.26) |
|
| ||||||||||||
| CIRCULATORY DISORDERS | ||||||||||||
|
| ||||||||||||
| Maternal Circulatory Disorders | ||||||||||||
|
| ||||||||||||
| Retroplacental hematoma | 36.2 | 36.6 | 0.98 (0.53, 1.83) | 23.2 | 9.2 | 2.99 (1.01, 8.88) | 20.2 | 3.9 | 6.28 (1.76, 22.37) | 6.1 | 4.1 | 1.53 (0.62, 3.81) |
|
| ||||||||||||
| Parenchymal Infarction§ | ||||||||||||
| Focal | 9.4 | 7.1 | 1.44 (0.48, 4.32) | 12.6 | 8.8 | 1.59 (0.50, 5.05) | 19.9 | 6.9 | 3.99 (1.36, 11.66) | 17.5 | 11.7 | 1.68 (0.94, 2.99) |
| Multifocal | 6.6 | 1.7 | 4.13 (0.52, 32.67) | 16.2 | 22.4 | 0.80 (0.35, 1.82) | 14.8 | 5.1 | 4.02 (1.21, 13.38) | 7.3 | 4.1 | 1.96 (0.87, 4.44) |
| Diffuse | 0.0 | 0.0 | not defined* | 9.0 | 0.0 | p<0.0001* | 1.8 | 0.0 | p<0.216* | 0.0 | 0.1 | p<0.112* |
|
| ||||||||||||
| Intraparenchymal thrombus | 15.1 | 12.0 | 1.30 (0.55, 3.12) | 18.0 | 16.1 | 1.14 (0.51, 2.58) | 22.7 | 10.9 | 2.40 (0.97, 5.97) | 27.3 | 13.5 | 2.41 (1.45, 3.98) |
|
| ||||||||||||
| Perivillous/intervillous fibrin/fibrinoid deposition (diffuse) | 8.2 | 12.0 | 0.66 (0.25, 1.70) | 12.5 | 4.8 | 2.85 (0.80, 10.16) | 11.5 | 2.6 | 4.94 (1.03, 23.70) | 3.8 | 1.3 | 2.92 (0.78, 10.90) |
|
| ||||||||||||
| Fetal Circulatory Disorders | ||||||||||||
|
| ||||||||||||
| Fetal vascular thrombi in the chorionic plate | 15.6 | 18.0 | 0.84 (0.35, 2.00) | 22.2 | 10.9 | 2.32 (0.87, 6.21) | 25.9 | 12.3 | 2.48 (1.05, 5.86) | 34.6 | 6.4 | 7.69 (4.56, 12.96) |
|
| ||||||||||||
| Avascular villi | ||||||||||||
| Focal | 5.5 | 8.8 | 0.66 (0.19, 2.26) | 5.3 | 0.0 | p<0.003* | 8.5 | 4.4 | 2.06 (0.51, 8.37) | 13.5 | 5.0 | 3.08 (1.64, 5.78) |
| Multifocal | 7.0 | 6.2 | 1.20 (0.37, 3.84) | 12.7 | 3.7 | 4.34 (0.91, 20.70) | 4.1 | 5.0 | 0.87 (0.22, 3.50) | 4.8 | 1.7 | 3.13 (1.02, 9.57) |
| Diffuse | 6.2 | 0.0 | p<0.022* | 5.5 | 0.0 | p<0.003* | 1.8 | 0.0 | p<0.307* | 0.0 | 0.0 | not defined* |
|
| ||||||||||||
| Edema (Placental Hydrops) | 10.9 | 12.7 | 0.84 (0.36, 1.98) | 5.0 | 4.3 | 1.18 (0.34, 4.06) | 0.9 | 2.4 | 0.35 (0.04, 3.45) | 6.4 | 0.8 | 8.12 (2.71, 24.35) |
Data are boldface where p<.05.
SB, stillbirth; LB, live birth; nw, weighted sample size; CI = confidence interval
Comparisons for which a placental characteristic is absent for one or both groups (SB and LB). A p-value provided in this circumstance is an upper bound as described in the text.
Includes umbilical cords with both velamentous and furcate insertion (<24 weeks, SB, 0.0%; LB, 0.0%; 24-31 weeks, SB, 0.0%, LB 0.0%; 32-36 weeks, SB 0.9%, LB 0.0%; ≥37 weeks, SB, 1.1%, LB 0.2%).
Includes membranes with both circummarginate and circumvallate insertion (<24 weeks, SB, 0.0%; LB, 2.2%; 24-31 weeks, SB, 1.9%, LB 1.3%; 32-36 weeks, SB 0.0%, LB 1.6%; ≥37 weeks, SB, 0.7%, LB 0.4%).
Reference group is no parenchymal infarction.
Reference group is no avascular villi.
As noted in Table 2, single umbilical artery was more prevalent among stillbirths (7.7% versus 1.7%). When examined by gestational age in Table 3, the differences were significant for deliveries at 24 weeks of gestation and later. Overall, velamentous cord insertion was found nearly five times as frequently (5.0% versus 1.1% [OR 4.50, 95% CI 2.18-9.27]) among all stillbirths, but a gestation-specific statistically significant difference was observed only among deliveries before 24 weeks (7.0% versus 0%). The frequency of furcate cord insertion did not differ between cases and controls. Circumvallate membrane insertion with or without circummargination occurred with comparable frequency overall and within gestational age groups. Terminal villous immaturity was positively associated with stillbirth both overall [OR 4.95, 95% CI (2.95 - 8.29)] and for deliveries at ≥37 weeks [OR 6.35, 95% CI (2.90 -13.94)].
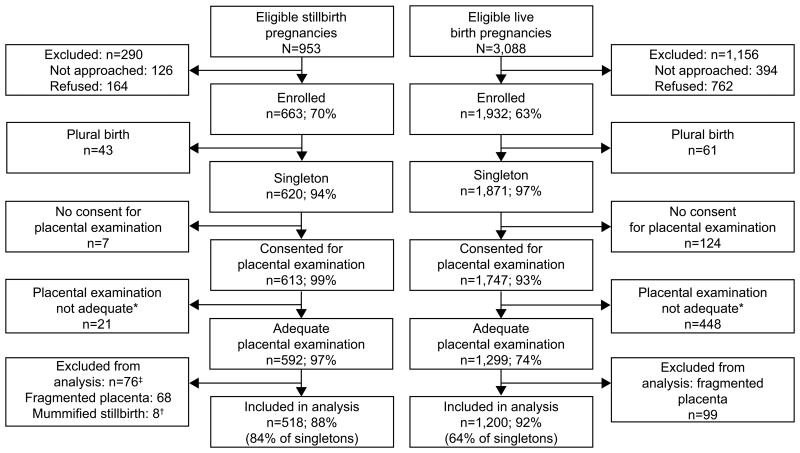
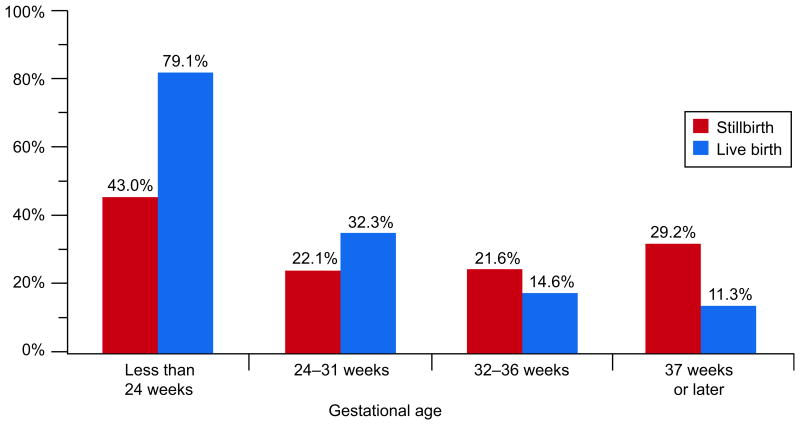
Chorioamnionitis was associated with stillbirth when all stillbirths were compared with live births. However, the relationship varied by gestational age (Table 3). The prevalence of acute chorioamnionitis was high in both stillbirths (43.0%) and live births (79.1%) delivered before 24 weeks, but was greater in live births (OR 0.20, 95% CI 0.10-0.38). In births occurring at term, acute chorioamnionitis was more common in stillbirths than in live births, 29.2% versus 11.3% (OR 3.24, 95% CI 1.97-5.32). Figure 2A illustrates these differences, and demonstrates the declining prevalence of chorioamnionitis in live births and a U-shaped pattern in stillbirths. Figure 2B shows similar patterns for acute chorioamnionitis in the chorionic plate. For this lesion, a significantly greater prevalence among live births also occurred at 24-31 weeks.
Figure 2A.
Acute chorioamnionitis of the placental membranes by gestational age at delivery.
Figure 2B.
Acute chorioamnionitis of the chorionic plate by gestational age at delivery.
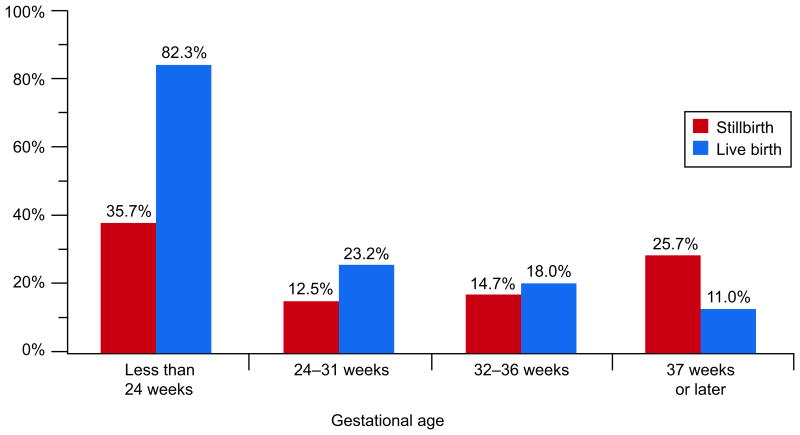
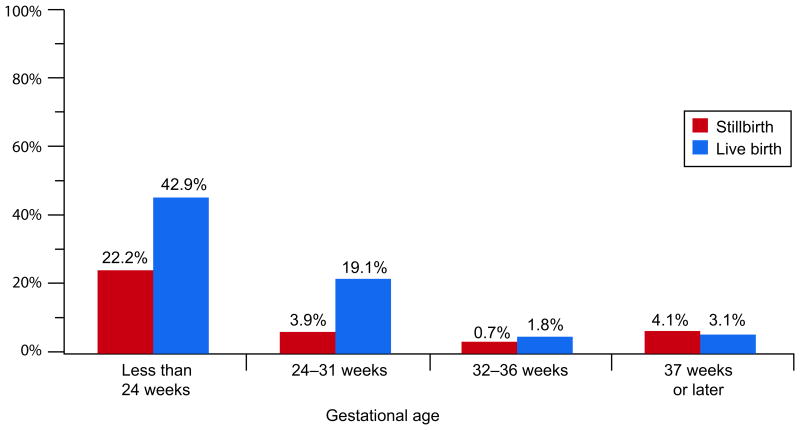
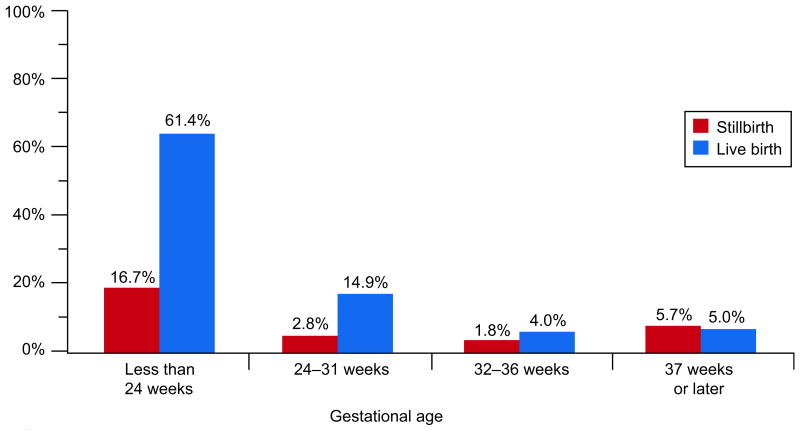
The gestational age-related patterns for acute funisitis, acute umbilical cord arteritis, acute umbilical cord phlebitis, and acute vasculitis of the chorionic plate were similar to each other. These lesions were common (42.9%, 30.3%, 25.7%, and 61.4%, respectively) in live births delivered before 24 weeks, and also at 24-31 weeks (ranging from 11.2% to 19.1%). In stillbirths delivered before 24 weeks, these lesions were also common (22.2%, 9.0%, 10.2%, and 16.7%, respectively) but had much lower prevalences thereafter. The prevalences were significantly higher in live births than in stillbirths at both <24 and 24-31 weeks. Figures 3A and 3B illustrate these relationships for acute funisitis and acute vasculitis of the chorionic plate. Chorionic plate vascular degenerative changes were more common in stillbirths than live births in every gestational age group at 24 weeks or later.
Figure 3A.
Acute funisitis by gestational age at delivery.
Figure 3B.
Acute vasculitis of the chorionic plate by gestational age at delivery.
Acute and chronic diffuse inflammation of the chorionic villi were rare in stillbirths and live births. None of the differences were significant overall or within gestational age groups.
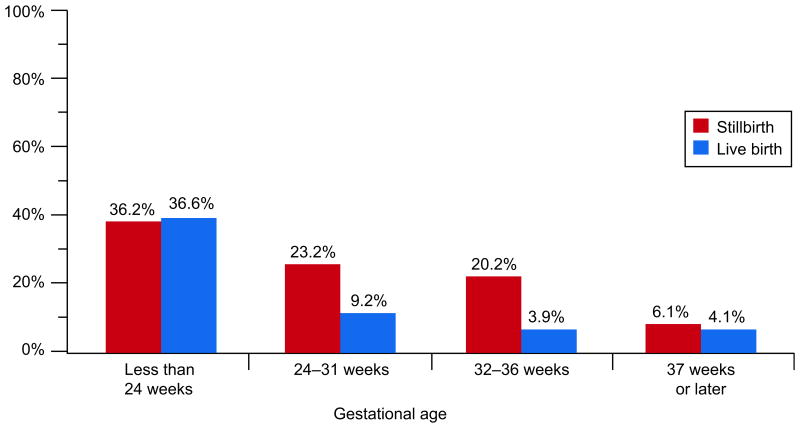
The prevalence of retroplacental hematoma among stillbirths was significantly greater than in controls, 23.8% v 4.2% (OR 7.08, 95% CI 4.83-10.38). However, the association differed by gestational age. Retroplacental hematoma was found in about 36% of both cases and controls delivered before 24 weeks. In later gestational age groups, the prevalence of this finding was higher among stillbirths than controls (Figure 4). Each of the other placental findings related to maternal circulatory disorders (parenchymal infarction, intraparenchymal thrombus, and diffuse perivillous and intravillous fibrin deposition with or without a fibrinoid component) was significantly more common among stillbirths compared with live births. These findings tended to be more prevalent in the stillbirths compared to controls for deliveries at 32 weeks or later.
Figure 4.
Retroplacental hematoma by gestational age at delivery.
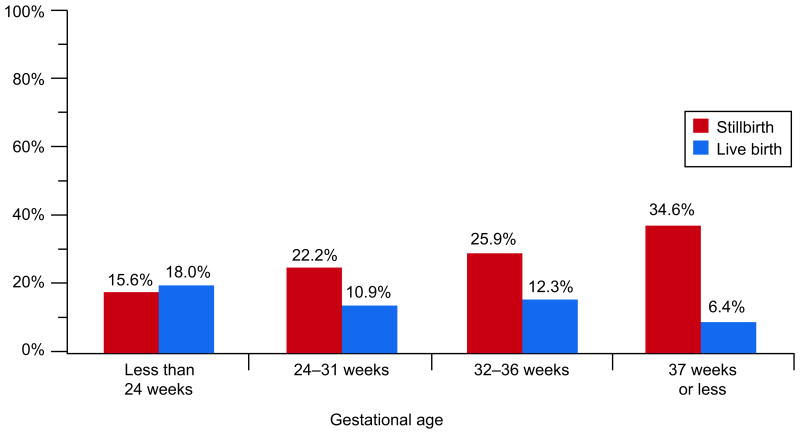
Each of the placental findings related to fetal circulatory disorders (thrombosis, avascular villi, hydrops) was more common in stillbirths than controls. For example, thrombosis in the fetal blood vessels in the chorionic plate was more prevalent among stillbirths than among live born controls, 23.0% versus 7.0% (OR 3.99, 95% CI 2.84-5.61). These differences were more pronounced at later gestational ages where many of the relationships were significant. Figure 5 depicts the increasing prevalence of fetal vascular thrombi in the chorionic plate of stillbirths as gestational age at delivery increased, compared to the decreasing prevalence in live births.
Figure 5.
Fetal vascular thrombi of the chorionic plate by gestational age at delivery.
Across all gestational ages, the prevalence of placental edema was significantly greater among stillbirths compared to controls, 6.4% versus 1.0% (OR 6.56, 95% CI 3.35-12.85), but reached gestation-specific statistical significance only among pregnancies ending at ≥37 weeks, 6.4% versus 0.8% (OR 8.12, 95% CI 2.71-24.35).
Discussion
The most common placental findings in stillbirths were inflammatory and thrombotic lesions and retroplacental hematoma. Notably, the prevalence of any specific placental finding in stillbirths rarely was higher than 30%, reflecting the heterogeneity of placental conditions associated with stillbirth.
Acute chorioamnionitis and other inflammatory lesions were common in both stillbirths and live births placentas, especially before 24 weeks. We believe that placental inflammation, commonly caused by infection (23) is important in the causal pathway for both stillbirth and preterm birth (24-25) and likely precipitates labor leading to preterm delivery (26,27). While there are a number of mechanisms by which placental inflammation might result in fetal death, at the earliest gestations the mechanism most likely involves inability of some fetuses to tolerate infection-precipitated labor (28). At viable gestations, cesarean delivery performed for fetal distress, would result in a live birth. Regardless, because placental inflammation is associated with preterm labor, it would be incorrect to interpret the differences in the placental findings with odds ratios below 1 as being protective against stillbirth.
Retroplacental hematoma was another finding commonly noted in both stillbirths and live births. At <24 weeks for both stillbirths and live births, the prevalence was about 36%; thus retroplacental hematoma likely contributed to both stillbirths and preterm delivery. Perhaps, as with chorioamnionitis, the retroplacental hematoma initiated preterm labor that only some fetuses could tolerate.
In contrast, several findings had greater prevalences among stillbirths delivered at 24 weeks or more. The prevalence of parenchymal infarction in stillbirths was greater than 35% at 24-31 and 32-36 weeks. The prevalences of intraparenchymal and fetal vascular thrombi in the chorionic plate were both about 15% for stillbirths delivered < 24 weeks, compared with prevalences of 27% and 35% at ≥37 weeks.
A number of other less prevalent findings were also important in differentiating stillbirths and live births, and their relationships also varied by gestational age at delivery. These included single umbilical artery, diffuse terminal villous immaturity, avascular villi and placental edema. Since lesions such as single umbilical artery may be determined by ultrasound examination prior to delivery, their discovery during pregnancy, accompanied by increased fetal surveillance, may be useful in preventing stillbirth (29).
Results comparing stillbirths and live births require careful interpretation. Because most live births occur at term, the overall odds ratios essentially compare all stillbirths, most of which are preterm, with term live births. However, examination within gestational age subgroups is required for a fuller understanding, although with certain limitations. For analyses within groups by gestational age at delivery, we acknowledged from the outset that preterm live births are by definition abnormal in some fashion and may not be representative of all fetuses alive at that gestational age.
The data in this paper will allow clinicians to interpret placental findings of stillbirths and provide meaningful explanations to stillbirth parents. First, there were no findings exclusively found in stillbirths. This strongly suggests that there often is a degree of uncertainty about cause and effect. By referring to lesion prevalence, the clinician can comment about the frequency of the finding. Using the odds ratios, and understanding the caveats related to preterm stillbirths discussed above, the clinician can explain the strength of the association between the placental lesion and stillbirth. I Therefore, the data presented in this paper should be highly useful to clinicians and parents.
The placental findings associated with stillbirth have been evaluated in a number of studies (8-15). In most of these, there was no control group so the authors could not determine the true association of placental pathology to stillbirth. Other studies lack standardized placental examination protocols, fail to consider the gestational age of the stillbirth, and use of convenience samples that may not reflect the general population of stillbirths and live births. Our study attempted to address these weaknesses
The major strengths of our study were the inclusion of live birth controls and use of a standardized placental pathology protocol to allow comparison of lesions between stillbirths and live births. Strengths also include the population-based design and large sample size. Limitations include the number of live births with placentas not available for study. Also, pathologists were not blinded to stillbirth/live birth status because of the need to perform both clinical and research placental examinations.
In summary, common findings in both early stillbirths and live births were inflammation and retroplacental hematoma. In contrast, parenchymal infarction and thrombosis were more common in stillbirths delivered later in pregnancy. Many other histologic findings were more common in stillbirth placentas than placentas from live births. However, knowledge of lesion prevalence within gestational age groups in both stillbirths and live birth controls, tempered with an appreciation of the limitations in the preterm comparisons, contributes to an understanding of the association between placental abnormality and stillbirth.
Acknowledgments
The authors thank the following members of the National Institute of Child Health and Human Development Scientific Advisory and Safety Monitoring Board for their review of the study protocol, materials, and progress: Reverend Phillip Cato, Ph.D.; James W. Collins Jr., M.D., M.P.H.; Terry Dwyer, M.D., M.P.H.; William P. Fifer, Ph.D.; John Ilekis, Ph.D.; Marc Incerpi, M.D.; George Macones, M.D., M.S.C.E.; Richard M. Pauli, M.D., Ph.D.; Raymond W. Redline, M.D.; Elizabeth Thom, Ph.D. (chair), as well as all of the other physicians, study coordinators, research nurses, and patients who participated in the Stillbirth Collaborative Research Network.
Supported by grants (HD45925, HD45944, HD45952, HD45953, HD45954, and HD45925) from the NICHD.
Footnotes
The views expressed in this article are those of the authors and do not necessarily reflect the views of the National Institutes of Health or the Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD).
For a list of members in the Eunice Kennedy Shriver National Institute of Child Health and Human Development Stillbirth Collaborative Research Network, see the Appendix online at http://links.lww.com/xxx.
References
- 1.Graafmans WC, Richardus JH, Macfarlane A, et al. EuroNatal Working Group. Comparability of published perinatal mortality rates in Western Europe: the quantitative impact of differences in gestational age and birthweight criteria. BJOG. 2001;108(12):1237–1245. doi: 10.1111/j.1471-0528.2001.00291.x. [DOI] [PubMed] [Google Scholar]
- 2.MacDorman MF, Kirmeyer S. Fetal and perinatal mortality, United States, 2005. Natl Vital Stat Rep. 2009;57(8):1–19. [PubMed] [Google Scholar]
- 3.Cousens S, Blencowe H, Stanton C, et al. National, regional, and worldwide estimates of stillbirth rates in 2009 with trends since 1995: a systematic analysis. Lancet. 2011;377(9774):1319–1330. doi: 10.1016/S0140-6736(10)62310-0. [DOI] [PubMed] [Google Scholar]
- 4.Saller DN, Jr, Lesser KB, Harrel U, Rogers BB, Oyer CE. The clinical utility of the perinatal autopsy. JAMA. 1995 Feb 22;273(8):663–5. doi: 10.1001/jama.273.8.663. [DOI] [PubMed] [Google Scholar]
- 5.Faye-Petersen OM, Guinn DA, Wenstrom KD. Value of perinatal autopsy. Obstet Gynecol. 1999;94(6):915–920. doi: 10.1016/s0029-7844(99)00468-8. [DOI] [PubMed] [Google Scholar]
- 6.Magee J. Investigation of stillbirth. Pediatr Develop Pathol. 2001;4(1):1–22. doi: 10.1007/s100240010121. [DOI] [PubMed] [Google Scholar]
- 7.ACOG Committee. Evaluation of Stillbirths and Neonatal Deaths. 2007 Oct;110(4):963–966. doi: 10.1097/01.AOG.0000263934.51252.e0. [DOI] [PubMed] [Google Scholar]
- 8.Larsen LG, Graem N. Morphological findings and value of placental examination at fetal and perinatal autopsy. APMIS. 1999 Mar;107(3):337–45. doi: 10.1111/j.1699-0463.1999.tb01562.x. [DOI] [PubMed] [Google Scholar]
- 9.Ogunyemi D, Jackson U, Buyske S, Risk a. Clinical and pathologic correlates of stillbirths in a single institution. Acta Obstet Gynecol Scand. 1998 Aug;77(7):722–728. [PubMed] [Google Scholar]
- 10.Burke CJ, Tannenberg AET. Intrapartum stillbirths in hospital unrelated to uteroplacental vascular insufficiency. Pediatric and developmental pathology. 2007;10(1):35–40. doi: 10.2350/06-02-0042.1. [DOI] [PubMed] [Google Scholar]
- 11.Erwich JJ, Korteweg FJ, Gordijn SJ, Timmer a, Holm JP, Ravisé JM. A placental cause of intra-uterine fetal death depends on the perinatal mortality classification system used. Placenta. 2008 Jan;29(1):71–80. doi: 10.1016/j.placenta.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Amir H, Weintraub A, Aricha-Tamir B, Apel-Sarid L, Holcberg G, Sheiner E. A piece in the puzzle of intrauterine fetal death: pathological findings in placentas from term and preterm intrauterine fetal death pregnancies. J Matern Fetal Neonatal Med. 2009;22(9):759–764. doi: 10.3109/14767050902929396. [DOI] [PubMed] [Google Scholar]
- 13.Flenady V, Pinar H, Frøen JF, Torabi R, Saastad E, Guyon G, et al. An evaluation of classification systems for stillbirth. BMC Pregnancy and Childbirth. 2009;9:1–34. doi: 10.1186/1471-2393-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heazell AEP, Martindale EA. Can post-mortem examination of the placenta help determine the cause of stillbirth? J Obstet Gynaecol. 2009;29(3):225–8. doi: 10.1080/01443610802716042. [DOI] [PubMed] [Google Scholar]
- 15.Helgadóttir LB, Turowski G, Skjeldestad FE, Jacobsen AF, Sandset PM, Roald B, Jacobsen EM. Classification of stillbirths and risk factors by cause of death--a case-control study. Obstet Gynecol Scand. 2013;92:325–333. doi: 10.1111/aogs.12044. [DOI] [PubMed] [Google Scholar]
- 16.Parker CB, Hogue CJ, Koch MA, Willinger M, Reddy UM, Thorsten VR, Dudley DJ, Silver RM, Coustan D, Saade GR, Conway D, Varner MW, Stoll B, Pinar H, Bukowski R, Carpenter M, Goldenberg R Stillbirth Collaborative Research Network. Stillbirth Collaborative Research Network: design, methods and recruitment experience. Paediatr Perinatol Epidemiol. 2011 Sep;25(5):425–35. doi: 10.1111/j.1365-3016.2011.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stillbirth Collaborative Research Network Writing Group. Bukowski R, Carpenter M, Conway D, Coustan D, Dudley DJ, Goldenberg RL, Rowland Hogue CJ, Koch MA, Parker CB, Pinar H, Reddy UM, Saade GR, Silver RM, Stoll BJ, Varner MW, Willinger M. Association between stillbirth and risk factors known at pregnancy confirmation. JAMA. 2011;306(22):2469–2479. doi: 10.1001/jama.2011.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carey JC, Klebanoff MA, Hauth JC, et al. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. N Engl J Med. 2000;342:534–540. doi: 10.1056/NEJM200002243420802. [DOI] [PubMed] [Google Scholar]
- 19.Pinar H, Koch M, Hawkins H, Heim-Hall J, Shehata B, Thorsten VR, Carpenter M, Lowichik A, Reddy UM. The Stillbirth Collaborative Research Network (SCRN) Placental and Umbilical Cord Examination Protocol. Am J Perinatol. 2011;28(10):781–92. doi: 10.1055/s-0031-1281509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinar H, Koch MA, Hawkins H, Heim-Hall J, Abramowsky CR, Thorsten VR, Carpenter MW, Zhou HH, Reddy UM. The Stillbirth Collaborative Research Network Postmortem Examination Protocol. Am J Perinatol. 2012;29(3):187–202. doi: 10.1055/s-0031-1284228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baergen R. Manual of Benirschke and Kaufmann's Pathology of the human placenta. New York: Springer; 2005. [Google Scholar]
- 22.Research Triangle Institute. SUDAAN Language Manual. 1 and 2. Research Triangle Park, NC: Research Triangle Institute; 2012. Release 11. [Google Scholar]
- 23.Goldenberg RL, Thompson C. The infectious origins of stillbirth. Am J Obstet Gynecol. 2003;189(3):861–873. doi: 10.1067/s0002-9378(03)00470-8. [DOI] [PubMed] [Google Scholar]
- 24.Goldenberg RL, Culhane JF, Johnson DC. Clinics in Perinatology. 3. Vol. 32. Elsevier; 2005. Sep, Maternal infection and adverse fetal and neonatal outcomes; pp. 523–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldenberg RL, McClure EM, Saleem S, Reddy UM. Infection-related stillbirths. Lancet. 2010;375(9724):1482–90. doi: 10.1016/S0140-6736(09)61712-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romero R. Preterm labor, intrauterine infection, and the fetal inflammatory response syndrome. NeoReviews. 2002;3(5):e73–e85. [Google Scholar]
- 27.Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Seminars in Reprod Med. 2007;25(1):21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldenberg RL, McClure EM, Saleem S, Reddy U. Reducing infection – related stillbirth: Program and research implications. Lancet. 2010;375:1482–90. doi: 10.1016/S0140-6736(09)61712-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy-Kaulbeck L, Dodds L, Joseph KS. Single umbilical artery risk factors and pregnancy outcomes. Obstet Gynecol. 2010;116:863–50. doi: 10.1097/AOG.0b013e3181f0bc08. [DOI] [PubMed] [Google Scholar]
- 30.Varli IH, Kublickas M, Papadogiannakis N, Petersson K. Chorioamnionitis without foetal inflammatory response is associated with stillbirth in early preterm pregnancies. J Matern Fetal Neonatal Med. 2013;26(10):953–959. doi: 10.3109/14767058.2013.766706. [DOI] [PubMed] [Google Scholar]
- 31.Varli IH, Petersson K, Kublickas M, Papadogiannakis N. Both acute and chronic placental inflammation are overrepresented in term stillbirths: a case-control study. Infect Dis Obstet Gynecol. 2012;2012:293867. doi: 10.1155/2012/293867. Epub 2012 Aug 26. [DOI] [PMC free article] [PubMed] [Google Scholar]