Abstract
Brain tumors are a diverse group of neoplasms that often carry a poor prognosis for patients. Despite tremendous efforts to develop diagnostic tools and therapeutic avenues, the treatment of brain tumors remains a formidable challenge in the field of neuro-oncology. Physiological barriers including the blood-brain barrier result in insufficient accumulation of therapeutic agents at the site of a tumor, preventing adequate destruction of malignant cells. Furthermore, there is a need for improvements in brain tumor imaging to allow for better characterization and delineation of tumors, visualization of malignant tissue during surgery, and tracking of response to chemotherapy and radiotherapy. Multifunctional nanoparticles offer the potential to improve upon many of these issues and may lead to breakthroughs in brain tumor management. In this review, we discuss the diagnostic and therapeutic applications of nanoparticles for brain tumors with an emphasis on innovative approaches in tumor targeting, tumor imaging, and therapeutic agent delivery. Clinically feasible nanoparticle administration strategies for brain tumor patients are also examined. Furthermore, we address the barriers towards clinical implementation of multifunctional nanoparticles in the context of brain tumor management.
Keywords: brain tumor, nanotechnology, nanoparticles, theranostics, imaging, diagnosis, therapy, drug delivery
1. Introduction
With the rapid development of nanotechnology for biomedical applications, it is expected that newly developed particle systems can have a revolutionary impact on brain cancer diagnosis and therapy [1–10]. In general, nanotechnology involves the design, synthesis, and application of materials with at least one dimension in the size range of 1–100 nanometers [3]. Multifunctional nanoparticles containing optical, thermal, and magnetic properties are promising systems that offer new opportunities to overcome the limitations of current brain tumor management options in the clinic. In this review, we begin by introducing the prognostic and biologic features of brain tumors, followed by the major obstacles facing of brain tumor management. We then highlight recent advances and clinical applications of nanoparticles in brain therapeutics, focusing on (i) tumor imaging, (ii) therapy, and (iii) the combination of both imaging and therapeutic functions (i.e. theranostics). Furthermore, strategies for nanoparticle administration and regulation issues surrounding nanoparticle translation to clinic are discussed. Lastly, the barriers towards clinical implementation of these nanoparticles are discussed in order to bring better insight into strategies for developing the most feasible systems for treating brain tumor patients.
2. Brain tumors
Brain tumors, referring to a heterogeneous group of primary and metastatic neoplasms in the central nervous system, are life-threatening diseases characterized by the low survival rate [11]. The annual incidence of primary malignant brain tumors is approximately 24,000 cases [11, 12]. Malignant gliomas are primary tumors that are derived from glial origin and account for approximate 70% of new primary brain cancer diagnosis [12, 13]. Of these, glioblastoma multiforme (GBM), a grade IV astrocytoma according to the World Health Organization (WHO) classification, is the most common and aggressive form in nature [14]. Most patients with brain tumors eventually succumb to the disease despite the aggressive standard of care treatment approach. The median survival is only about three years for anaplastic astrocytomas and around 14.6 months for GBM patients [15, 16]. Brain metastases are another important class of tumors in the central nervous system originating mainly from systemic cancers in the lung, breast and skin [17]. Metastatic brain tumors occur at a high frequency with an estimated incidence of 100,000–170,000 cases in the USA annually [18].
Today, a multimodality treatment approach including surgical resection, radiotherapy, and chemotherapy is the current standard of care for malignant brain tumor patients [16]. It has been demonstrated that aggressive resection of a brain tumor and postoperative radiation lead to a significant survival advantage [16, 19]. Adjuvant chemotherapy can be administered at different time points as well [20, 21]. Cytotoxic and cytostatic agents are the two major categories of chemotherapy used to treat brain tumors. The mechanism of these agents involves direct tumor cell death, anti-angiogenesis, pro-differentiation, growth factor pathway disruption, and inhibition of tumor invasion. Temozolomide, an imidazotetrazine derivative, is the first line systemic chemotherapy agent used for patients with brain tumors [22–24]. Unconventional therapies including immunotherapy, gene therapy, and photodynamic therapy (PDT) are potential adjuvant treatments for brain tumors and are under clinical trials. These additive therapies have broadened the spectrum of therapeutic agents for brain tumors to antibodies, genetic material, and photosensitizers.
Furthermore, advancements in anatomical and functional imaging techniques for brain tumors play a critical role in management as it allows for early detection, diagnostic testing, surgical planning, and follow-up evaluation [25–30]. Imaging techniques including magnetic resonance imaging (MRI), computed tomography (CT), and positron-emission tomography (PET) are the most common modalities for brain tumor diagnosis, characterization and intraoperative imaging [31–34]. Other techniques such as fluorescence imaging have been developed for intraoperative fluorescence-guided tumor resection [35, 36]. These imaging modalities can help delineate the boundaries between neoplastic and normal tissue, helping doctors determine the most appropriate course of treatment.
3. Major obstacles in brain tumor treatment
Despite tremendous efforts to develop diagnostic tools and therapeutic avenues, the treatment of brain tumors remains a formidable challenge in the field of neuro-oncology. The major obstacles to the successful treatment of brain tumors include a) the structural complexity of the brain, b) the heterogeneous and invasive nature of many brain tumors, c) difficulty in identifying tumor margins and disseminated tumor burdens, d) insufficient accumulation of therapeutic agents at the site of a tumor, and e) acquired drug resistance to chemotherapy.
The brain, arguably the most complex system in the body, controls a multitude of functions including information processing, perception, motor control, arousal, homeostasis, motivation, as well as learning and memory. Due to the complexity of brain functions, the treatment of brain tumors requires both robust and highly selective elimination of all cancerous tissues including those that invade beyond the main tumor mass into the surrounding normal tissue. Highly skilled surgeons are presented with the difficult task of accurately identifying all the diseased tissue and resecting it from the brain while attempting to preserve surrounding normal, functional tissue. Even after extensive removal, brain tumors usually recur locally within centimeters of the resection margin [37].
Adjuvant treatments including chemotherapy for brain tumors only achieve modest clinical outcomes. The effectiveness of systemic delivery of therapeutic agents to brain tumors is hampered by several physiological barriers. Unlike other organs, the brain is protected by the blood-brain barrier (BBB) [38–40]. The BBB prevents the influx of harmful endogenous and exogenous molecules from the bloodstream but also becomes a major limiting factor for anti-brain tumor therapy. The BBB is composed of tight junctions between endothelial cells, pericytes, a basement membrane, as well as the feet of astrocytes [39]. Normal brain capillaries act as a continuous lipid layer and exhibit selective permeability based on molecular solubility and size. Deficiency of pinocytotic vesicles within the cerebral endothelial cells compromises cellular transcytosis and further contributes to the selectivity of the BBB [39]. Additionally, ATP-binding cassette transporters such as P-glycoprotein act as drug efflux transporters and their high expression limits substrate transportation across the BBB [41–44]. Only small lipophilic molecules, electro-neutral molecules, and nutrients under 400–600 Daltons in the blood can diffuse passively into the brain [38, 45–47].
The second barrier that blocks the passage of systemically administrated therapeutic agents is known as the blood-cerebrospinal fluid barrier (CSF) [1, 39]. It is formed by tightly bound choroid epithelial cells, which regulate molecule penetration within the interstitial fluid of the brain parenchyma. This barrier prevents most macromolecules from passing into the CSF through the bloodstream. In addition, the intact blood-CSF barrier is reinforced by active transport systems for weak organic acids, which are mainly located in the choroid plexus [48]. They are capable of actively removing therapeutic organic acids from the CSF and preventing their diffusion into the brain parenchyma [49, 50]. Examples include the organic anion transporter 3 (Oat 3), peptide transporter 2 (PEPT2), and P-glycoproteins (P-gp) [50, 51]. By working as outward efflux systems, these pumps are able to decrease the CSF concentration of several antibiotics (e.g. penicillins and cephalosporins), chemotherapeutic agents (e.g. methotrexate) [52], and HIV proteinase inhibitors (e.g. ritonavir and atazanavir) [53].
The blood-tumor barrier in the tumor forms a third barrier for transporting therapeutic agents [1, 38, 39]. Unlike normal brain capillaries, the tight junctions of endothelial cells in the tumor are significantly compromised. The high intratumoral interstitial pressure created by the leaky tumor vasculature limits drug penetration from the bloodstream into the tumor [54]. Moreover, different tumor microvessel populations and spatial variability in capillary functions present in the tumor area also lead to variability in penetration [38]. This can, for example, lead to heterogeneous distribution of drug molecules, which may significantly compromise therapeutic outcome.
4. Key features of nanoparticles for brain tumors
Here, we highlight the key features of nanoparticles including composition, unique physical properties, passive targeting abilities, as well as tunable surface functionality for active targeting. These features enable the detection of brain tumors in a sensitive and specific manner as well as the transportation of diagnostic or therapeutic agents across the BBB.
4.1 Versatile compositions and physical properties
Nanoparticles can be made from a variety of materials such as compositing polymers, lipids, proteins, metals, or semiconductors. A variety of nanoparticles with well-defined shapes such as solid spheres, rods, tubes, and other complex shapes can be designed and synthesized by top-down or bottom-up engineering techniques. Current nanoparticle platforms for brain tumors can be classified into three major categories including organic-based (e.g. liposomes, polymeric nanoparticles, micelles, dendrimers, and solid lipid nanoparticles), inorganic-based (e.g. iron oxide nanoparticles, gold nanoparticles, semiconductor nanocrystals, ceramic nanoparticles, and carbon nanotubes) and hybrid nanoparticles [55–68]. We list examples of currently available nanoparticle platforms for brain tumors in Table 1. Each nanoparticle system has the luxury of being individually tailored based on size, shape, and surface chemistry to meet the objectives of the proposed function (Table 1).
Table 1.
Examples of nanoparticle platforms for brain tumors
| Material | Particle Type | Structure Formed |
Size | Main component | Main Applications | Phase of development |
Ref. |
|---|---|---|---|---|---|---|---|
| Organic | Liposomes | Colloid vesicular strucutre |
10nm–1000 nm | Lipid | Drug carrier | Phase I, II Clinical Trials |
[63] |
| Micelles | Nanosphere; cylinder |
20nm–200 nm | Polymer | Drug carrier | Preclinical | [64] | |
| Polymeric nanoparticles |
Nanosphere; nanocapsules |
10nm–1000 nm | Polymer | Drug carrier | Preclinical | [59] | |
| Dendrimers | Branch | > 5 nm | poly(amidoamine) | Drug carrier | Preclinical | [62] | |
| Inorganic | Gold nanoparticles |
Nanosphere; nanorod; nanoshell |
1nm–100nm | Gold | Drug carrier; photothermal therapy; photoacoustic imaging |
Preclinical | [58,71] |
| Iron oxide nanoparticle |
Nanosphere | 10nm–50nm | Iron oxide | Drug carrier; magnetic hyperthermia; MRI |
Phase I, II Clinical Trials |
[57,60] | |
| Ferromagnetic discs |
Microdisk | lµm | Iron, nickel | Magnomechanical stimulation |
Preclinical | [61] | |
| Ceramic nanoparticle |
Nanosphere | 20nm–100nm | Silica | Drug carrier | Preclinical | [235] | |
| Quantum dots | Nanosphere; nanorod |
2–20nm | Cadmium selenide | Fluorescence imaging |
Preclinical | [66] | |
| Titanium dioxide nanocrystals |
Sphere | 5 nm | Titanium dioxide | Photodynamic therapy |
Preclinical | [67] | |
| Hybrid | More than one type of nanomaterials |
Barge or tanker |
Range depends on materials selected |
Core-metallic and polymeric; corona-single or multiple lipid layers |
Theranostics | Preclinical | [168] |
In general, nanoparticles have large surface to volume ratios that contribute to their high loading capacity. As drug delivery systems, nanoparticles have been shown to improve drug solubility, prolong blood circulation half-life, and control drug-release [3]. Either hydrophilic or hydrophobic therapeutic molecules can be incorporated into nanoparticles to improve the half-life of systemic circulation. Many nanoparticle delivery systems are designed to respond to various environmental stimuli such as pH and temperature, allowing for controlled therapeutic payload release [69, 70]. Furthermore, the payload can be extended to include imaging probes and contrast agents. For example, organic fluorescence probes can be incorporated into the nanoparticle structure for particle tracking. More importantly, nanoparticles are easily modified to meet the demands of the intended functionalities with the ability to combine multiple therapeutic agents and imaging probes onto a single platform.
Nanoparticles may also contain intrinsic optical, thermal, electrical, or magnetic properties can be utilized for imaging or therapeutic purposes. Colloidal gold nanoparticles have been of high interest because their low toxicity, dynamic surface chemistry, size, and shape allow for imaging properties to be attained [70–73]. Gold nanoparticles have high density and extinction coefficients, and therefore can be applied as the contrast agents for CT, dark field imaging and photoacoustic imaging. In addition, the nanorod or nanoshell shape of gold nanoparticles allow them to strongly absorb light in the near-infrared range due to surface plasmon resonance and to efficiently convert this energy into heat for photothermal therapy [74–76]. Magnetic nanoparticles such as iron oxide-based nanoparticles are another widely investigated inorganic-based nanoparticle system [10]. Magnetic nanoparticles can be used as contrast agents to produce hypointense regions on T2/T2*-weighted MR images. Magnetic nanomaterials can also either generate heat or mechanical force under an alternating magnetic field to destroy brain tumor cells [77, 78]. In comparison to traditional fluorophores, quantum dots made of semiconductor materials with quantum confinement have tunable narrow emission spectra and excellent photostability. They can be used as stable fluorescent probes for brain tumor diagnosis at the molecular level [79]. It has also been demonstrated that quantum dots can act as PDT therapeutic agents to induce cytotoxicity by creating free oxygen radicals under light. Additionally, titanium dioxide nanocrystals are a versatile photoreactive nanomaterial acting as the new generation of photosensitizer for PDT [67, 80].
More recently, “hybrid nanoparticles” have been developed to combine the capabilities of different nanomaterials into one platform [68]. Hybrid nanoparticles are synthesized from two or more types of nanomaterials and are generally formed with a metallic or polymeric core covered with a single or multiple lipid coronas to increase the biocompatibility of the system. As a multifunctional platform, they can serve in both diagnostic and therapeutic applications and will be discussed later in this review.
4.2 Passive targeting of brain tumors
The size of a nanoparticle is a fundamental characteristic that determines the passive targeting of and biodistribution within brain tumors. Nanoparticles within the size range of 10–100 nm take advantage of the hyper-vascularized, leaky, and compromised lymphatic drainage system in a brain tumor to passively target and access the intratumoral space while being denied access to healthy brain tissue [1, 6]. In other words, nanometer-sized particles accumulate selectively at the site of a brain tumor due to the enhanced permeability and retention (EPR) effect [81]. Such a phenomenon is not observed with small molecular weight compounds like chemotherapeutic agents that rely on free diffusion and therefore cannot discriminate between normal and diseased tissues [82]. The EPR effect in the brain tumor opens many opportunities for nanoparticles to function as diagnostic and therapeutic tools for brain cancer. However, this passive targeting mechanism has its inherent limitations. When given intravascularly, all nanoparticles are susceptible to opsonization and removal by cells of the reticuloendothelial system (RES) [83]. Only a small fraction of administered nanoparticles will therefore reach the tumor. The liver, spleen, lung, kidney, and bone marrow are the primary locations that nanoparticles are trapped [84, 85]. Therefore, to achieve an optimal EPR effect, nanoparticles should be less than 100 nm in diameter, and their surfaces should be biocompatible (i.e. hydrophilic and almost neutral in electric charge) to avoid removal by cells of the RES.
4.3 Tunable surface functionality for active targeting
Surface functionality, which regulates the interface of nanomaterials and biological systems, is an important factor determining the behavior and the biomedical application of nanomaterials both in vitro and in vivo [86]. By altering their physiochemical properties (i.e. surface charge, hydrophobicity) one can influence the half-life and localization of nanoparticles during the circulation. For example, the development of “stealth nanocarriers” that use hydrophilic polymers such as polyethylene glycol (PEG) for surface coating render nanoparticles with more resistance to protein adsorption and RES uptake [87]. PEGylation prolongs the circulation half-life of nanoparticles, subsequently increasing the chance of reaching distant brain tumor cells.
While the vasculature of a brain tumor may be disturbed to some extent, this disruption is not always consistent throughout a tumor and may not even be extensive in low-grade tumors. For example, high-grade brain tumors display much more vascular permeability than low-grade tumors, illustrating the variability of BBB integrity between different brain tumors [88, 89]. However, active targeting of a tumor site can be achieved even without a severely disrupted BBB. Receptors (e.g. transferrin and nicotinic acetylcholine receptor) and integrins (e.g. αVβ3 and Aminopeptidase N) are distributed on brain capillary endothelial cells or on proliferating endothelial cells within a tumor, sites that are in direct contact with circulating nanoparticles in the bloodstream [90–94].
Penetration into a tumor area can therefore be improved by simply targeting receptors that are normally expressed on brain capillary endothelial cells. A number of studies have demonstrated the ability to successfully enhance the delivery of therapeutic nanoparticles to a brain tumor by targeting transferrin receptors and nicotinic acetylcholine receptors expressed on endothelial cells. Transferrin has been incorporated onto the surface of micelles[95], solid lipid nanoparticles [96], superparamagnetic iron oxide (SPIO) nanoparticles [97], and dendrimers [62, 98] for targeting glioma, leading to enhancement in drug efficacy or tumor imaging. Nicotinic acetylcholine receptors have also been used as targets to enhance micelle penetration into the CNS to target glioblastoma [99–101]. Peptides targeting this receptor have been derived from snake neurotoxins including candoxin and Ophiophagus hannah toxin b [101, 102].
A number of biomolecules have targets that can also be found on proliferating endothelial cells within a tumor. For example, RGD peptide has been found to bind to αVβ3 integrin, which is expressed on the periphery of high-grade gliomas as well as on proliferating endothelial cells in the tumor vasculature [93]. This peptide has been incorporated into the design of a broad range of nanoparticles targeting brain tumors including micelles [103, 104], iron oxide nanoparticles [105, 106], gold nanoparticles [107], dendrimers [108], as well as other types of nanoparticles [109, 110]. Aminopeptidase N (CD 13) which is overexpressed on the tumor vasculature [94] has also been targeted with another tri-peptide (Asn-Gly-Arg (NGR) peptide) [111]. This NGR peptide has allowed for enhanced targeting of micelles [112, 113] and liposomes [114–116] to various brain tumors including glioma and neuroblastoma. Nucleolin is yet another specific marker for angiogenic endothelial cells within the tumor vasculature [117]. F3 peptide was found to bind to this receptor [117], and since this discovery, several nanoparticle systems targeting brain tumors have incorporated this targeting peptide into their design [118, 119].
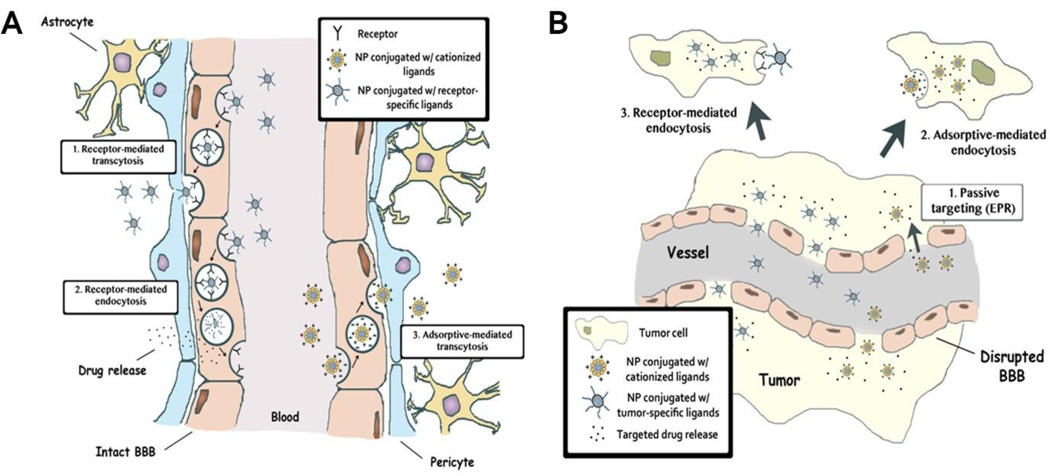
Once nanoparticles have bypassed the BBB, they must be able to target glioma cells in a specific manner. One of the methods to achieve this is to incorporate targeting moieties that bind to receptors that are overexpressed on these cells. Such receptors include αVβ3 integrin and CD 13, which have already been mentioned, as well as EGFR [120, 121], IL13Ra2 [122–124], and LRP1 [125–127]. Antibodies to EGFR or EGFRvIII have been incorporated into several nanoparticle systems targeted against glioma [128, 129]. Anti-IL13Rα2 antibodies [78] and IL13 peptide [130, 131] have also been conjugated to the surface of various nanoparticle systems, allowing for the targeting and destruction of glioma cells. LRP1 is a target of angiopep-2 and incorporation of this peptide into the structure of various nanoparticle systems has enhanced therapeutic delivery to brain tumor cells [132– 134]. As more glioma-specific receptors are identified, nanoparticles may be able to integrate multiple targeting moieties providing a dynamic platform for targeting heterogeneous brain tumors. Figure 1 summarizes the variety of mechanisms nanoparticles can use to target a brain tumor including the EPR effect, carrier-mediated transportation, receptor-mediated endocytosis, and adsorptive-mediated endocytosis.
Figure 1.
Transportation mechanisms of multifunctional nanoparticles into the brain tumor. (A) Transport of multifunctional nanoparticles across the BBB: 1) receptor-mediated transcytosis, 2) receptor-mediated endocytosis, 3) adsorptive-mediated transcytosis of nanoparticles with cationized ligands. (B) Mechanisms of transportation across the disrupted BBB and selective targeting of brain tumor cells: 1) passive targeting via the EPR effect, 2) adsorptive-mediated endocytosis or 3) receptor-mediated endocytosis. Both mechanisms offer a targeted delivery to brain cancer cells, sparing the normal tissue. NP: nanoparticle.
5. Diagnostic nanoparticles for brain tumors
Having a high-resolution image before surgery is especially important for GBM, which are characteristically invasive. Such invasiveness makes it quite difficult to accurately determine a clear tumor boundary by eye. Appropriate imaging of a tumor is critical for measuring the extent of tumor distribution preoperatively as well as for determining response to a treatment regimen postoperatively, both being necessary for the successful management of brain tumor patients. By far the most common method for imaging brain tumors is contrast-enhanced T1-weighted MRI [135]. However, this measurement technique can be greatly influenced by vascular leakage that may not be present throughout the entire tumor area [136]. Furthermore, an accurate view of postoperative response can be difficult to acquire, especially with phenomena such pseudoprogression after radiotherapy [137] and pseudoresponse when using anti-angiogenic therapies [138, 139]. Even though it is the contrast agent of choice, gadolinium is nephrotoxic, leading to nephrogenic systemic fibrosis in some patients [140]. Therefore, its use in patients with renal impairment is limited. Other MRI techniques to image a tumor include perfusion-weighted imaging, which can measure blood perfusion and permeability, and diffusion-weighted imaging, which can be helpful for grading gliomas [139, 141]. Other modalities such as PET imaging and magnetic resonance spectroscopy (MRS) can allow for the quantification of metabolic activity of tumor cells, potentially allowing for more sensitive measurements of response rates to therapy [139]. 11C-methionine is an example of a radiolabelled amino acid used in PET imaging that demonstrates enhanced uptake by tumor cells compared to normal brain tissue [139, 142]. It can be used to measure tumor progression as well as to discriminate between recurrence and radiation necrosis on a scan [139]. Nanoparticles have the potential to enhance the sensitivity and effectiveness of these various imaging. Here, we outline single and multimodal nanoparticles currently in development with an emphasis on MRI, photoacoustic, and intraoperative fluorescence imaging.
5.1 MRI contrast agents
One of the primary imaging modalities used in clinic to assess the extent of a brain tumor is MRI. MRI can provide high spatial resolution and is the usual standard for imaging brain tumors in a clinical setting. Gadolinium chelates such as Gd-DTPA are contrast agents typically used for MRI. Such molecules do not normally cross the BBB but can cause marked signal change in the brain tumor where the BBB is disrupted. They have a short half-life and require repeated injections with high dosages to achieve adequate visualization of a tumor [143, 144]. Accurate delineation of tumor boundaries and the quantification of tumor volume is limited due to the spatial variability of BBB disruption in a tumor area and technical difficulties caused by the false-positive contrast enhancement [145, 146].
Nanoparticle platforms are being developed as contrast agents to provide improvements over those currently used. Various nanoparticle constructs containing magnetic elements such as iron, gadolinium, and manganese are in development or have already made their way to a clinical setting for use as MRI contrast agents in the imaging of brain tumors [147]. These nanoparticles have been shown to increase signal enhancement for a long period of time and enhance visualization of the tumor border [148]. Iron oxide nanoparticles have been extensively studied as T2/T2* contrast agents for brain tumor imaging. Ferumoxytol, an ultra-small SPIO coated with polyglucose sorbitol carboxymethyl ether, has been used as the MRI contrast agent together with a standard gadolinium chelate for patients with recurrent high grade glioma receiving chemotherapy in phase I clinical trials (ClinicalTrials.gov identifier: NCT00769093). Quantitative imaging changes of brain tumor vascularity after anti-angiogenic therapy with bevacizumab versus steroid therapy with dexamethasone is being assessed by dual agent MRI study using gadolinium and ferumoxytol.
Many recent reports describe that targeting peptide modification of iron oxide nanoparticles can enhance uptake at a brain tumor site, resulting in better MRI contrast within a tumor [149, 150]. Sun et al. designed PEGylated SPIO nanoparticles possessing a surface-bound brain tumor targeting peptide chlorotoxin (CTX) that showed high selectively and binding affinity to membrane-bound matrix metalloproteinase-2 (MMP-2) in gliomas [149]. A significant negative contrast enhancement and higher T2 relaxivity was observed in cells when these targeted nanoparticles were used compared to cells that had been incubated with nanoparticles without surface-bound CTX. After retro-orbital injections into mice bearing subcutaneous 9L rat gliosarcoma tumors, the CTX bound nanoparticles served as a MRI contrast enhancement agent accumulating to a greater extent in the tumor when compared to non-CTX bound ones [149]. Although negative contrast agents such as iron oxide particles have been shown to enhance MR imaging of intracranial tumors, a major drawback is that they produce hypointense regions that can often be difficult to distinguish from resident brain iron signal [151]. T2 and T2* weighted imaging in general also provides images of lower resolution than most T1-weighted sequences [152, 153].
Positive contrast agents may therefore be of more benefit as they lead to bright signal enhancement on T1-weighted MRI [152–154]. Gadolinium nanoparticles possess positive contrast properties that enhance signal by accelerating longitudinal relaxation of water protons and promoting longer retention time at the tumor site. Faucher et al. designed ultra-small paramagnetic gadolinium oxide particles as a T1 contrast agent for brain tumors [153]. The longitudinal relaxivity of these nanoparticles was estimated to be 9.9 s−1 mM−1 compared to 4.1 s−1 mM−1 of the Gd-DTPA. These nanoparticles showed a high contrast enhancement in rat brain tumors, increasing over the span of 2 hours. Manganese oxide (MnO) nanoparticles are another group of T1-weighted contrast agents that have been developed for the imaging of brain tumors [151, 155, 156]. Just like gadolinium-based particles, these nanoparticles exert bright contrast on images and can selectively target the brain tumor. Na et al. designed MnO nanoparticles encapsulated in a PEG-phospholipid shell with conjugated Her-2/neu receptor antibody to target EGFRs that were expressed on certain types of breast cancer cells and brain tumors [155]. Using a mouse model of a breast cancer brain metastasis, the authors observed the selective enhancement of tumor cells using T1-weighted MRI. While the nonfunctionalized MnO particles also accumulated in the tumor due to disrupted tumor vasculature, only the functionalized nanoparticles accumulated at the site for an extended time (up to 24 hrs was reported) [155].
5.2 Optical imaging probes
A major prognostic factor of patients with malignant gliomas is the extent of removal of malignant tissue during surgery [157, 158]. Optical imaging can aid in the intraoperative resection of brain tumors [159], and nanoparticle-based imaging probes with absorption and fluorescence properties have been developed with such capabilities. Quantum dots with intrinsic fluorescent properties allow for cancer targeting and imaging [66]. Cai et al. developed RGD peptide-labeled quantum dots (QD705-RGD) with a maximum emission at 705 nm that could be used to image αVβ3 integrin positive tumor vasculature [66]. The greatest contrast was observed around 6 hour post-injection in a subcutaneous U87MG tumor, but no significant fluorescence signal was observed in non-modified QD705 injected mice, demonstrating the importance of the targeting RGD peptide [66]. Nie et al. described tumor-targeting deep-blue polyacrylamide nanoparticles with coomassie blue, PEG, and F3 peptide surface conjugation. These particles could target a tumor effectively and allow for visible color staining of neoplastic tissue. This simple approach could allow for color-guided tumor resection in real time without the need for extra equipment or special lighting conditions [160].
In addition to optical approaches reliant only on light, photoacoustic imaging in which the combination of light exposure and sound generation provides higher spatial resolution and deeper tissue penetration. This imaging modality is based on the ability of different types of probes to absorb light and generate transient acoustic signals [161–165]. Exogenous contrast agents such as gold nanoparticles with surface plasmon resonance in the near infrared (NIR) range can be used with this technique to enhance the visualization of different tissue types including brain tumors [65, 166–169]. Lu et al. designed a 40 nm hollow gold nanospheres for use with photoacoustic tomography (PAT) to successfully visualize the microvasculature of brain tumor margins [169]. In a follow-up study, Lu et al. used PAT to visualize the targeted accumulation of RGD peptide-modified gold nanoparticles in an intracranial U87 glioma tumor [167]. Quantitative studies demonstrated that the mean contrast-enhanced photoacoustic signal ratio of tumor-to-normal brain 24 hours after injection was about twice as high as that seen in pre-contrast images [167]. Gold nanoparticles with a high extinction coefficient can also serve as contrast agents for dark field light scattering imaging, which allow for ex vivo diagnosis [76]. Although nanoparticle platforms enable the use of light in the NIR range, the penetration of light through brain tissue is only in the millimeter range. Furthermore, non-invasive optical imaging of brain tumors is severely hampered due to the physical barrier created by the skull. Therefore, the application’s main utility is for the in vitro diagnosis and intraoperative localization of brain tumor tissue.
5.3 Nanoparticles for multimodality imaging
More and more studies are starting to design nanoparticles that incorporate the use of multiple imaging modalities [150, 168, 170–175]. Each imaging modality (e.g. MRI, PET, optical, and SPECT) has its own advantages and drawbacks. However, when used in combination, they may allow for earlier detection of cancer, better characterization of the molecular and metabolic features of a tumor, as well as better tracking of the effects of treatment. Thus, particles with multimodal imaging features may have a role in preoperative delineation of a tumor, intraoperative visualization of malignant tissue location, as well as follow-up during treatment with chemotherapy or radiotherapy.
Veiseh et al. developed a dual-imaging modality nanoparticle to overcome the BBB, allowing for MRI and near-infrared fluorescence (NIRF) imaging of brain tumors [170, 172]. In this study, an iron oxide nanoparticle coated with a PEG-grafted chitosan copolymer with the addition of CTX and fluorescent Cy5.5 was synthesized [170]. After intravenous injection of particles into a transgenic mouse model, ND2:SmoA1, which resembles human medulloblastoma, the authors observed the accumulation of nanoparticles in neoplastic tissue but did not observe any significant accumulation of the nanoprobes in healthy tissue. No contrast enhancement was observed with the nanoparticles that lacked the CTX targeting ligand. Signal intensity increased over the first 50 hrs after injection and then stayed stable for the next 70 hours [170]. Lee et al. designed an RGD-conjugated radiolabeled iron oxide nanoparticle for both MRI and PET imaging [171]. MRI signal intensity decreased significantly after tail vein injection of the nanoparticle conjugate compared to the non-targeted nanoparticle and to the nanoparticle co-administered with a αVβ3 integrin blocking agent. For PET imaging, these nanoparticles were labeled with 64Cu and provided greater signal-to-noise ratio. Again, the RGD conjugated nanoparticles displayed greater tumor uptake and better visualization on imaging [171].
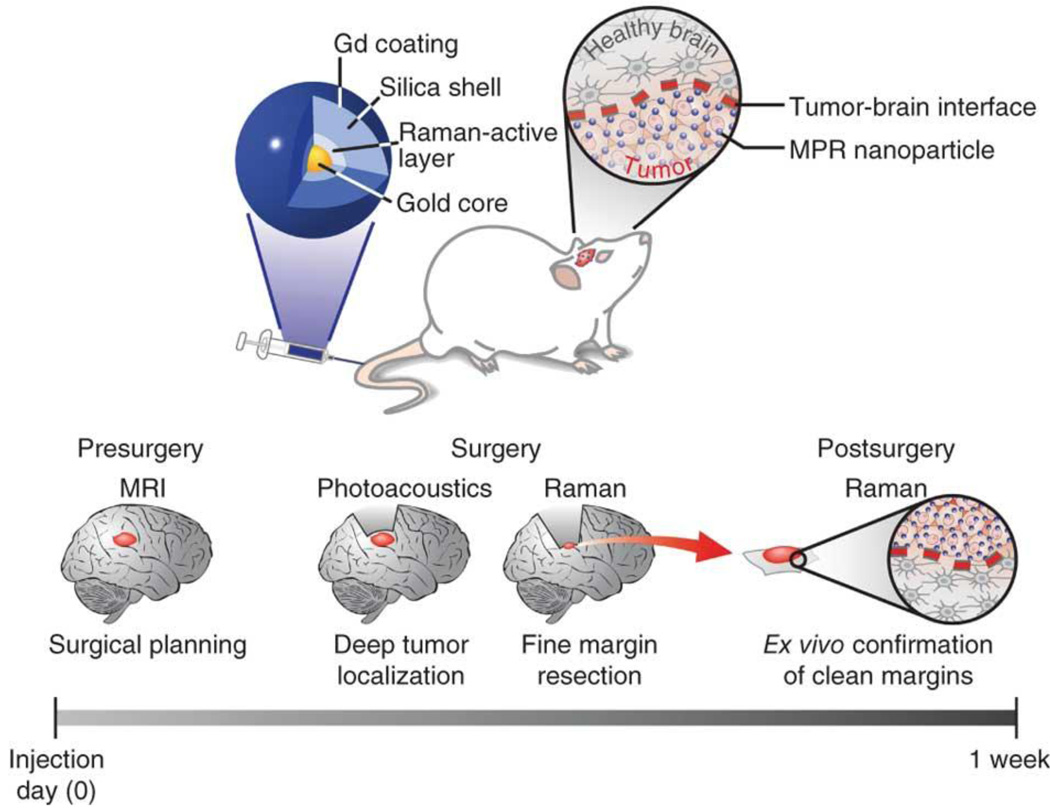
Nanoparticles with triple imaging modality capabilities have also been developed and may enhance the ability to visualize the extent of invasive tumors. Kircher et al. developed a gold nanoparticle-based platform with triple-modality MRI, photoacoutic, and Raman imaging capabilities (Figure 2) [168]. These particles were detected by all three modalities with at least picomolar sensitivity, and co-localization of tumor imaging was observed using all three modalities in an orthotopic U87MG mouse model. Furthermore, the authors demonstrated that tumor resection could be guided using the photoacoustic and Raman signal properties of these nanoparticles 24 hours after intravenous injection. Photoacoustic imaging was used to delineate malignant tissue in situ, and high-resolution intraoperative Raman images were taken during resection. Such a nanoparticle system could improve both preoperative and intraoperative resection of GBM tumors, which may lead to better patient outcomes. However, a current drawback for these particles is that the new instrumentation for endoscopic and intraoperative photoacoustic and Raman imaging that would be required for the approach is still under development [168]. Xie et al. demonstrated another triple-modality imaging using an iron oxide nanoparticle labeled with 64Cu-DOTA and Cy5.5 for MRI, PET and NIRF in a subcutaneous U87MG xenograft mouse model. These particles demonstrated extensive accumulation at the tumor site and led to enhanced visualization of the entire tumor using the combination of all three imaging modalities [176].
Figure 2.
Triple-modality MRI-Photoacoustic-Raman nanoparticles (MPRs). MPRs were injected intravenously into mice bearing an orthotopic brain tumor (top). The proposed clinical use is diagramed at the bottom of the illustration. Detectability of MPRs by MRI allowed for preoperative detection and surgical planning. Photoacoustic imaging, with its relatively high resolution and deep tissue penetration, was then able to guide bulk tumor resection intraoperatively. Raman imaging, with its high sensitivity and spatial resolution, can then be used to remove any residual microscopic tumor burden. The resected specimen can subsequently be examined using a Raman probe ex vivo to verify clear tumor margins. Reproduced with permission from Ref 168.
6. Therapeutic nanoparticles for brain tumors
Another vital role for nanoparticles is to serve as therapeutic agents, with the potential to overcome many of the hurdles conventional therapies face. Various nanoparticle systems have been explored as carriers to overcome the BBB for targeted treatment of brain tumors. Additionally, nanoparticles responding to external triggers such as an applied magnetic field and light offer new therapeutic avenues targeting malignant brain tumors. Here, we focus on novel therapeutic nanoparticles and highlight examples currently under clinical evaluation.
6.1 Nanoparticles as delivery systems
The advantages of nanoparticles as therapeutic carriers are: 1) improved therapeutic agent circulation, 2) targeted drug delivery, 3) controlled drug release, 4) high loading capacity, and 5) co-delivery of more than one therapeutic agent. Nanoparticle-based delivery systems for therapeutic agents such as chemotherapy drugs are expected to have a great clinical impact on brain tumor treatment [130, 177, 178]. Many hydrophobic agents that normally cannot cross the BBB can accumulate at a tumor site when incorporated into a nanocarrier [108, 179, 180]. As discussed previously, modification of the nanoparticle surface with targeting moieties that specifically bind to receptors overexpressed on the tumor vasculature and/or cancer cell membrane could provide an effective delivery system for brain tumor. In addition, controlled drug release approaches to the cancer cells could further enhance the specificity and efficacy of the therapeutic payload to the brain tumor.
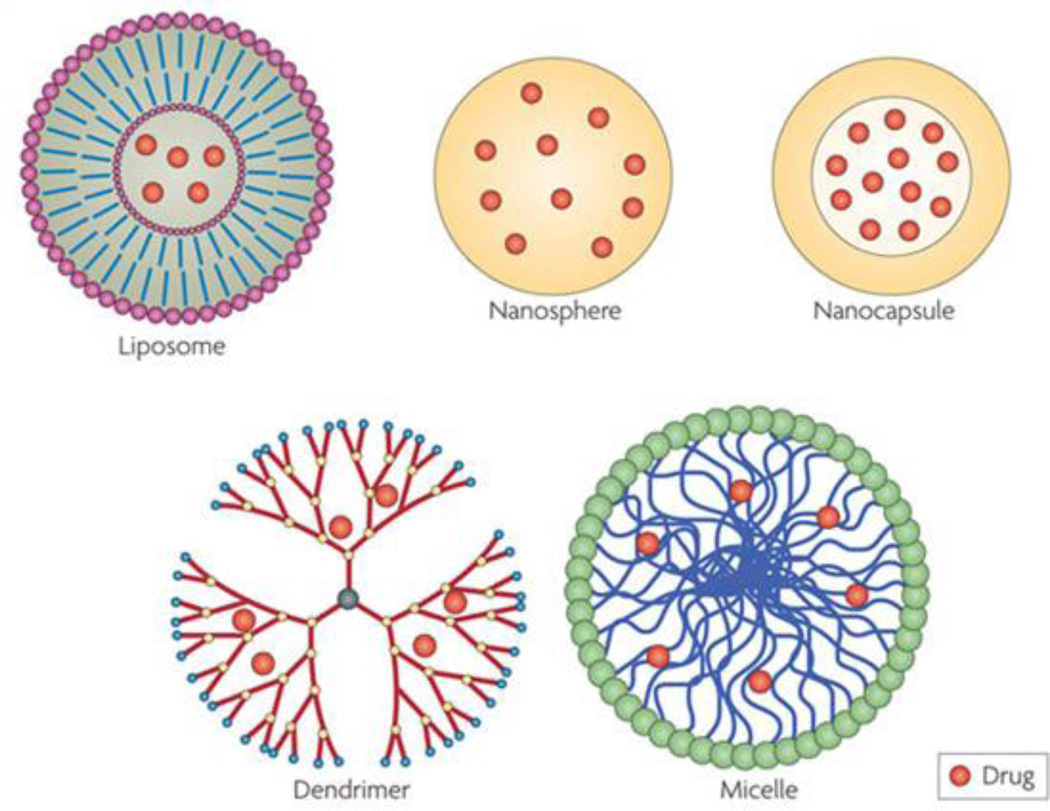
Liposomes, self-assembled spherical phospholipid bilayers, have received a lot of attention as the pharmaceutical carriers for brain tumor therapy (Figure 3) [181, 182]. Major advantages of liposomes include selective accumulate to brain tumors by passive and active targeting and improved pharmacokinectic effect [181, 183]. There are several liposome-based drug delivery systems under clinical trials. 2B3–101 is a PEGylated liposomal doxorubicin formulation conjugated with glutathione that allows for enhanced drug-delivery to the brain by specialized transporters on the BBB. A phase I/II clinical trial with 2B3–101 was started in 2011 in patients with glioma or brain metastases secondary to breast cancer (ClinicalTrials.gov identifier: NCT01386580). The purpose of the study was to determine the safety, tolerability and pharmacokinetics of 2B3–101 as a monotherapy and in combination with trastuzumab, a monoclonal antibody that interferes with the human epidermal growth factor receptor. A liposomal encapsulation of the camptothecin derivative and topoisomerase I inhibitor CPT-11 is another delivery system being examined in clinic trials (ClinicalTrials.gov Identifier: NCT00734682) [63, 177]. Currently, it is under investigation in a phase I trial to assess the safety, pharmacokinetics, and maximum tolerated dose in patients with recurrent high-grade gliomas who are wild type or heterozygous for the UGT1A1*28 gene. The structural stability, low drug-loading capacity, and scale-up methods are issues of liposomal systems that need to be further improved [184, 185].
Figure 3.
Schematic structure of different nanocarriers (liposomes, polymeric nanoparticles including nanospheres and nanocapsules, dendrimers, and micelles) for drug delivery to the brain tumor. Reproduced with permission from Ref 192.
Other organic-based nanoparticle including micelles, biodegradable polymeric nanoparticles, and dendrimers are also potential candidates for brain tumor treatment (Figure 3) [64, 99, 186]. Polymeric micelles are core-shell nanoshperes composed of self-assembling amphiphilic block copolymers [187]. Compared to liposomes, micelles with smaller sizes have high structure solubility to delivery hydrophobic drugs to malignant brain tumors. Guo et al. designed an aptamer-functionalized poly(ethylene glycol)-poly (D,L-lactic-co-glycolic acid) PEG-PLGA nanoparticle for anti-glioma delivery of paclitaxel (Ap-PTX-NP) [186]. AS1411, a DNA aptamer specific for nucleolin which was highly expressed in the plasma membrane of both cancer cells and endothelial cells in angiogenic blood vessels, was conjugated to the surface of PEG-PLGA nanoparticle with a final particle size of 156±54.8 nm in diameter. The aptamer-nucleolin interaction significantly enhanced the nanoparticle uptake into C6 glioma cells and resulted in enhanced cytotoxicity of the drug payload. Prolonged circulation and enhanced drug accumulation of Ap-PTX-NP were demonstrated in vivo in C6 glioma-bearing mice when compared with Taxol and the unmodified nanoparticledrug system.
Biodegradable polymeric nanoparticles with nanosphere and nanocapsule structures also show great promise for the delivery of BBB-impenetrable therapeutic agents [59, 188–191]. These systems are also useful in controlling drug payload release and protect drug from the surrounding environment [192]. Wang et al. evaluated the antitumor effect of 1% polysorbate-80 coated PBCA nanoparticles loaded with a nucleoside reductase inhibitor gemcitabine in vitro and in vivo in C6 glioma cells [188]. The nanoparticle formulation could inhibit the proliferation of glioma cells and extend the survival time in a rat brain tumor model. Ding et al. demonstrated another polymeric nanoplatform incorporating poly (β-L-malic acid) (PMLA) with an antisense oligonucleotide (AON) payload [59]. This system showed pH-regulated release of AON and significant inhibition of intracranial human glioma tumor growth by specifically blocking the synthesis of the tumor neovascular trimer protein laminin-411 [59].
Dendrimers are highly branched polymers with multivalent functional groups, allowing for the easy incorporation of therapeutic agents within their structure [193]. Several studies have demonstrated that dendrimers can overcome the BBB and increase drug accumulation at the site of a brain tumor [62, 194]. Controlled drug-release from dendrimers can be achieved using different types of stimulus-sensitive groups [62]. Li et al. developed a pH-sensitive dual-targeting dendrimer conjugated with transferrin and tamoxifen for treating brain gliomas [62]. The anticancer drug doxorubicin was covalently linked to the interior of the poly(amidoamine) (PAMAM) dendrimers via a pH-sensitive hydrazone bond. Only 6% drug release was observed at a pH of 7.4 while 32% release was observed at pH 4.5. Studies in an in vitro BBB model indicated that these dual-targeting dendrimers showed an enhanced transport efficiency when compared to the non-targeted and transferrin-targeted dendrimers [62].
Inorganic nanoparticles are physically and chemically more stable compared to the organic nanoparticles, allowing for long-term storage. The concept of using biocompatible inorganic nanocarriers such as iron oxide and gold nanoparticles to gain access to a tumor through the BBB is gaining the momentum for treating brain tumor. Most of these nanoparticles are protected by hydrophilic polymers, making them more water-soluble and biocompatible. Studies have shown that magnetic nanoparticles can accumulate within a tumor area after systemic administration with a locally applied magnetic field [57, 195, 196]. Chertok et al. showed that the iron oxide nanoparticle concentration in glioma tumors could be enhanced by 5-fold when an external magnetic field was applied [57]. Hua et al. demonstrated the effectiveness of a poly[aniline-co-N-(1-one-butyric acid) aniline] (SPAnH) coated iron oxide core based delivery system for the chemotherapy drug 1, 3-bis(2-chloroethyl)-1-nitrosourea (BCNU) in the treatment of glioma under external magnetic field [195]. In this system, the bound-BCNU on the nanoparticle was more thermally stable than free BCNU and could be concentrated in vitro and in vivo at targeted sites upon exposure to the external magnet [195]. Recently, gold nanoparticles with 5 nm core size have been explored as a drug delivery system to overcome the BBB for anti-glioma therapy [71]. Cheng et al. demonstrated that the hydrophobic PDT drug Pc 4 could be attached to the nanoparticle surface via a non-covalent attachment and a 10-fold improvement in the selectivity for a brain tumor was achieved in vivo through the use of epidermal growth factor peptide-modified gold nanoparticles. This selectivity was due to the EPR effect within the tumor and receptor-mediated endocytosis of the nanoparticle into the cancer cells [71].
6.2 Innovative therapeutic nanoparticles
6.2.1 Magnetic nanomaterials
Nanomaterials with magnetic properties that respond to magnetic fields in a non-invasive manner have been recently explored as therapeutic agents for brain tumors. To date, the most successful application of magnetic nanoparticles is for magnetic hyperthermia. A remarkable example is SPIO nanoparticles, which are in a phase II study for patients with recurrent GBM in Europe [197–199]. The principle of this approach is based on heat generated from the movement of magnetic nanoparticles under a high frequency alternating magnetic field. Due to the non-invasive nature of this therapy, inaccessible brain tumor tissues can be reached with magnetic nanoparticles. The therapeutic efficacy of magnetic nanoparticles is dependent on several factors including the targeting ability of the nanoparticles to tumor tissue, the magnetization and Curie temperature reached by the magnetic nanoparticle, as well as parameters of the magnetic field. Preclinical studies indicate that magnetic hyperthermia achieved by SPIO nanoparticles can effectively promote glioma cell death and increase survival [198]. The safety and efficacy of intratumoral hyperthermia using SPIOs coated with aminosilane under a 2.5–18 kA/m and 100 kHz alternating magnetic field in conjunction with radiotherapy has been investigated in patients with recurrent GBM [198–200]. Results of the study showed that the combination therapy is relatively safe and leads to longer overall survival following tumor recurrence compared to conventional therapies alone. However, an improvement of the nanoparticle composition and intratumoral distribution is required to achieve an optimal risk-benefit ratio in patients with glioma.
Recently, magnetic nanomaterials have been utilized under a low frequency magnetic field for the treatment of brain tumors [56, 61]. Kim et al. designed anti-IL13Rα2 biofunctionalized magnetic-vortex microdiscs for targeted brain cancer therapy [61]. Discs were composed of 20:80% iron-nickel permalloy coated by a 5 nm thick layer of gold and were 60 nm in thickness and 1 µm in diameter. Under an alternating magnetic field with low frequency, the discs oscillated, creating mechanical torque. The authors demonstrated that the system could selectively destroy N10 glioma cells via mechanical stimulus generated by the disc oscillations. The proposed therapeutic mechanism was that membrane integrity was compromised due to mechanical damage and programmed cell death was initiated due to changes in calcium equilibrium. It should be noted that the discs need to be further scaled down into the nanometer range and cell destruction should be tested in other brain cancer cell lines to better support applications for brain tumor treatment [61].
6.2.2 Photosensitive nanomaterials
Semiconductor nanomaterials such as titanium dioxide (TiO2) and quantum dots possess photosensitive properties and have recently been developed as therapeutic agents for light-mediated glioma treatment [67, 201]. One promising application of these photosensitive nanoparticles is to treat a brain tumor with PDT. Under light exposure, these particles absorb energy from light and transfer it to molecular oxygen, generating a variety of cytotoxic reactive oxygen species (ROS) to react with essential cellular components such as DNA, proteins, and lipids [67, 80]. Rozhkova et al. demonstrated that a 5 nm anti-IL13Rα2 functionalized 3,4-dihydroxyphenilacetic acid (DOPAC) modified TiO2 nanoparticle could specifically target brain cancer cells and initiate programmed cell death following visible light treatment [67]. Modification of TiO2 nanoparticles with electron donating enediol ligands enabled visible light harvesting and generated superoxides [67].
Another potential application of photosensitive nanoparticles is photothermal therapy. It has been demonstrated that gold nanoparticles including gold nanoshells and nanorods can convert absorbed NIR light to heat and induce cell death [58, 202]. Day et al. investigated a silica-gold nanoshell, with a 120 nm diameter silica core coated by a 10–20 nm thin layer of gold, as a photothermal cancer therapeutic agent for glioma therapy [58]. Under NIR laser light excitation, successful targeted ablation of glioma cells in vitro and in vivo was demonstrated in a subcutaneous implanted U373 glioma mouse model. However, photothermal therapy for noninvasive treatment of human brain tumors in an actual clinical setting could be difficult because light cannot penetrate the human skull. During surgery, the brain tumors become accessible and light can be introduced to the areas where the tumor cells have been resected. PDT using conventional photosensitizers has been tested on the clinical setting for brain tumors as an intraoperative adjuvant therapy (ClinicalTrials.gov Identifier: NCT01682746, NCT00118222, NCT01148966). Light can be placed at the surgical cavity through optical fibers to active the photosensitizers, which can guide the tumor resection as well as help kill any cells that had been left behind.
7. Theranostic nanoparticles for brain tumor treatment
The incorporation of multiple functions into a nanoparticle system would be highly beneficial for clinical translation. With the combination of imaging and carrying capabilities, nanoparticles could allow for the simultaneous delivery of therapeutic agents to the tumor area and real-time tracking of their biodistribution and fate in vivo [203, 204]. Innovative nanoparticles such as magnetic nanoparticles, gold nanoparticles, and quantum dots, which possess both imaging and therapy functions simultaneously, may serve as emerging theranostic nanoparticles [118, 149, 203, 205, 206]. Here, we focus on a few reports of nanoparticles that exemplify theranostic systems.
7.1 Combination of MRI and therapy
Liu et al. developed a theranostic system using iron oxide nanoparticles conjugated with the anticancer drug epirubicin for delivery and image tracking functions in a C6 tumor-bearing rat model [207]. In this study, the authors included focused ultrasound to disrupt the BBB at the tumor site to improve nanoparticle accumulation. Drug delivery to the brain tumor was further enhanced by magnetic targeting and monitored in real time. A 2.6 fold increase in relaxation rate with MRI was observed in the animals injected with the nanoparticles after focused ultrasound/magnetic targeting treatment compared to the nontreated groups [207].
Reddy et al. demonstrated an example of a theranostic system based on a polymeric nanoparticle formulation containing Photofrin® and iron oxide conjugated with F3 for MRI and PDT in a 9L glioma rat model [118]. Photofrin® was able to generate singlet oxygen (1O2) and induce the cytotoxicity under light exposure. The pharmacokinetics of the nanoparticles could be evaluated by MRI due to the magnetic properties of iron oxide. Compared to the untargeted nanoparticles, the F3 modified nanoparticles showed longer retention at the brain tumor area with a 2-fold increase of the contrast enhancement. The authors also demonstrated the targeted theranostic nanoparticles exhibited significant improvement of survival rates of mice bearing brain tumors after the PDT-mediated laser irradiation compared with the non-targeted theranostic nanoparticles or Photofrin® alone. Forty percent of animals in the targeted theranostic nanoparticle treated group were found to be tumor-free at the end of the study period [118].
In another study, Hadjipanayis et al. developed iron oxide nanoparticles with bound anti-EGFRvIII antibody for targeted glioma imaging and therapy [128]. Glioma cells demonstrated preferential uptake of these functionalized nanoparticles via receptor-mediated endocytosis, resulting in caspase-3 activation and strong T2-weighted contrast on MRI. In vivo studies revealed increased survival after intratumoral administration of these particles to animals implanted with EGFRvIII-expressing intracranial xenografts [147]. Additional reports have described the use of tumor-specific CTX-peptide conjugated iron oxide nanoparticles to track toxicity and inhibition of glioma cell invasion by MRI, further demonstrating theranostic applications of nanomaterials [172, 208].
7.2 Combination of optical imaging and therapy
Optical imaging has demonstrated ex vivo and intraoperative applications. Organic fluorescence imaging probes can label therapeutic nanoparticles and allow for in vitro and in vivo tracking of nanoparticle distribution. Recent studies have been focused on the potential theranostic application of optical nanoprobes such as quantum dots for brain tumors [205]. Jung et al. designed a multifunctional quantum dot-based siRNA delivery system for brain cancer cells [205]. siRNA was attached through two strategies: 1) enzymatic cleavable disulfide linkage for payload release and 2) a non-cleavable linkage for imaging and tracking. In vitro experiments indicated theranostic nanoparticles selectively inhibited the expression of EGFRvIII in U87 glioblastoma cells. The resulting nanoparticle uptake and localization in the cells were monitored by fluorescence imaging. The potential for targeted delivery was demonstrated at the cellular level by introducing active targeting ligands such as RGD peptide and HIV-derived Tat peptide onto these quantum dots [205]. As most of these combinations are still restricted to preclinical studies, further investigation is necessary in order to establish efficacy and a safety profile of the systems.
8. Administration strategies for nanoparticles
The diagnostic and therapeutic potential of nanoparticles could be improved for clinical translation if the administration method of these particles could 1) allow for specific distribution to and diffuse distribution within a tumor and 2) the neurotoxicity and systemic toxicity could be minimized. Here, we review relevant strategies for nanoparticle administration and discuss their pros and cons for treating brain cancer.
8.1 Systemic administration
Systemic administration of nanoparticles is a very convenient strategy for delivery, allowing for repetitive dosing. Despite the diversity of nanoparticle systems in development, most have the potential to target brain tumors through passive and active targeting mechanisms. As previously described, passive targeting occurs through the diffusion of nanosized particles through the disrupted BBB, a phenomenon known as the EPR [81]. Active targeting involves functionalizing nanoparticle surfaces with BBB and glioma-specific targeting moieties [1].
One obvious method for the delivery of nanoparticles is via intravenous (IV) injections. IV administration of nanoparticles has been used in countless reports including many of those mentioned previously. In the preclinical models, the nanoparticle-based therapeutics are often given to the animals in multiple doses with the injection frequency from every3 days to 2 weeks to control the tumor growth [179, 209]. The maximum tolerated dose for nanoparticle-based therapeutics is usually significantly greater than the free drug [210]. These therapeutics, with lower starting dose in phase I trials compared with the free drugs, are given to patients every three and four weeks (ClinicalTrials.gov Identifier: NCT01386580). Besides tuning of nanoparticle size and surface properties to influence intratumoral accumulation [211], external forces such as a magnetic field and focused ultrasound can also help capture systemically administered nanoparticles at the site of a tumor [207]. Low-frequency focused ultrasound provides local disruption of the BBB [207, 212, 213], and preclinical studies have shown that this technique can safely enhance the focal delivery of the therapeutic agents into brain tumors [212]. This ultrasound-mediated disruption of the BBB is transient and reversible without permanent neuronal injury or other undesired long-term effects [213]. Magnetic targeting, another noninvasive strategy to facilitate magnetic nanoparticle accumulation at a target site [214, 215], has been applied in clinical trials (ClinicalTrials.gov Identifier: NCT0005495, NCT00034333). Based on the FDA guidelines, exposure to magnetic field devices up to 8 Tesla for adults and 4 Tesla for children do not represent any safety concerns. Chertok et al. demonstrated that IV administered iron oxide particles to rodents with 9L-gliosarcoma could be monitored by MRI [57]. The authors demonstrated that magnetic targeting induced a 5-fold increase in the total exposure of glioma cells to the nanoparticles over non-targeted tumors and a 3.6 fold enhancement in target selectivity for accumulation in the tumor versus normal brain tissue [57]. Although some strategies have been developed for systemic administration to overcome the BBB, the overall percentage of systemically injected nanoparticles found in the brain is typically less than 1% [6, 190]. This non-specific accumulation of nanoparticles in normal tissues may cause severe adverse effects and increase mortality and morbidity in patients.
Intracarotid administration of nanoparticles appears to be a feasible means of ensuring that more nanoparticles accumulate within a brain tumor on the first pass. Han et al. examined the effect of implanted BCNU-loaded wafers and intracarotid perfusion of BCNU-loaded nanoparticles for glioma treatment in vivo [216]. BCNU-loaded nanoparticles were made from poly(D,L-lactic acid)(PLA) and coated with transferrin (Tf-PLA-BCNU). Rats with intracranial C6 glioma tumors had improved survival with treatment of Tf-PLA-BCNU delivered via intracarotid injections when compared with no treatment controls and even greater survival when intracarotid injections of these nanoparticles were done in conjunction with the implantation of BCNU-loaded wafers. Chertok et al. developed polyethyleneimine-modified magnetic nanoparticles and found that intratumoral levels of the nanoparticles after “active” magnetic capture were increased by 30-fold after intracarotid injections versus intravenous injections with the same magnetic capture technique [217].
The major downside of systemic delivery is the risk for accumulation of nanoparticles in non-target organs such as the liver, kidneys, spleen, and lungs. Nanoparticles such as iron oxide and gold nanoparticles are thought to be non-toxic to normal tissues, but the long-term consequences of nanoparticle deposition in the brain have not been fully addressed as of yet. A few studies have shown that nanoparticles can be cleared from the brain [218, 219]. Jain et al. studied the biodistribution and clearance of iron oxide nanoparticles in rats [218]. Changes in tissue iron levels including in the brain were analyzed over three weeks after IV injection. The iron concentration in the brain increased 24 hours after administration and then decreased after 3 days. Wang et al. studied the fates of gold nanorods in a rat model from 0.5 hours to 28 days after IV injection (0.6 µg g−1) [219]. Kinetic results demonstrated that gold nanorod accumulation in the brain declined from 0.08 ng g−1 to 0.02 ng g−1 within 7 days and remained at 0.02 ng g−1 after 28 days. However, the clearance mechanism of these nanoparticles in the brain remains unclear and will require further investigation.
8.2 Intracranial administration
Local administration of nanoparticles directly into a tumor site provides another option to overcome the BBB and circumvent non-specific systemic accumulation. Although this method of administration using degradable or non-degradable polymers has shown some ability to destroy tumor cells[220, 221], it is characterized by poor drug penetration and dosing limitations [222].
Convection enhanced delivery (CED), another method for intratumoral delivery of nanoparticles, appears to overcome these issues [222]. CED is a means of delivering therapeutic agents directly to the site of the tumor with the benefit of enhancing the distribution of molecules within tumor tissue. This method relies on pressure gradients driving bulk flow of nanoparticles and agents are delivered continuously via a catheter connected to a syringe pump which can be implanted during surgery [222]. In this case, higher drug concentrations and more widespread distribution can be achieved in a tumor compared to systemic administration with minimal systemic toxicity [222–224]. CED of therapeutic agents has even made its way into clinical trials, which can be applied for nanoparticle administration [63]. Noble et al. demonstrated a single CED infusion of 1.6 mg nanoliposomal CPT-11 significantly prolonged median survival over 100 days compared to 28.5 days of the free drug or 19.5 days of the control liposomes in an intracranial U87 xenograft model [63]. In addition, the prolonged exposure to nanoliposomal CPT-11 had no measurable central nervous system toxicity at any of the doses tested [63]. Dendrimers and iron oxide nanoparticles have also been delivered to brain tumors via CED [128, 225]. While local administration shows the effectiveness in treating brain tumors, the highly invasive nature of this method is still a major concern.
8.3 Cell-mediated delivery
Stem cells have been found to cross the BBB and have attracted significant attention because of their ability to target brain tumors [226–228]. Mesenchymal stem cells (MSCs) and neural stem cells (NSCs) exhibit tumor-tropic migration toward glioma cells in vitro and in vivo and may serve as a novel method for targeted delivery of nanoparticles to brain tumor cells [226, 229–234]. Versatile image enhancing nanoparticles and therapeutic nanoparticles can be loaded into these cellular carriers for better targeting effect.
Li et al. demonstrated that MSCs with bound silica nanorattle-doxorubicin nanoparticles could migrate towards U251 glioma cells both in vitro and in vivo. Increased apoptosis was observed after intratumoral injection of silica nanorattle-doxorubicin loaded MSCs when compared to injections of free doxorubicin [235]. Roger et al. demonstrated that marrow-isolated adult multilineage inducible cells loaded with lipid nanocapsules containing ferrociphenol could induce cytotoxicity in U87MG glioma cells [236]. This system was able to slow down tumor growth rate in vivo as well. Recently, Cheng et al. demonstrated the use of an FDA-approved NSC cell line to carry silica nanoparticle-doxorubicin conjugates (MSN-Dox) possessing pH-mediated drug release capabilities [237]. In vivo, MSN-Dox loaded-NSCs maintained their glioma-homing ability and could deliver doxorubicin conjugates to tumor cells. Both intratumoral and contralateral injections of the MSN-Dox loaded-NSCs were sufficient to achieve significant enhanced therapeutic efficacy compared to using MSN-Dox alone. Results thus far combining stem cells and therapeutic nanoparticles for the treatment of brain cancer are mainly based on local administration methods. Due to their ability to cross the BBB, systemic administration of these stem cell offers promise but warrants further investigation. It has been shown that murine NSCs injected into the systemic circulation via the tail vein of nude mice bearing CNS-1 glioblastoma reach the tumor burden within four days [226]. Furthermore, these administered NSCs did not appear in normal brain tissue. Although systemically delivered NSCs reach the brain tumor, the percentage of stem cells that localize in the brain tumor is currently undetermined [226]. It has been hypothesized that tropic cytokines released from the tumor site may bind to receptors on stem cells to trigger their migration. Of the various signaling proteins that have been suggested to regulate NSC migration, vascular endothelial growth factor that is highly expressed in gliomas is one of the leading candidates [238–240]. A more detailed understanding of the mechanism behind stem cell migration may provide us with the information necessary to increase their migratory efficiency and therefore increase the number of systemically delivered cells that reach a brain tumor.
Another prominent way in which cellular carriers and nanoparticles have been combined is for in vivo cellular tracking after administration. Magnetic iron oxide nanoparticles for example have been used to track the migration of stem cells towards tumors [233, 241, 242]. Wu et al. demonstrated the ability to track the course of migrating MSCs after intracranial injections. These cells were monitored over 14 days and distributed to the tumor border in a specific-manner [233]. Thus, such a system in combination with MR imaging may offer a more efficient way of delineating the boundaries of the tumor. Also, tracking the destination and distribution of stem cells will be important as the fate of these cells is of concern for clinical applications.
Although these cell carriers may have the ability to actively deliver the nanoparticles, the role of this innovative combination for brain tumors needs to be further investigated. Nanoparticle loading capacity, targeting efficiency, tumorgenecity originating from the stem cells, controlled nanoparticle release, and clearance are all concerns surrounding the use of this strategy.
9. Regulatory issues surrounding nanoparticle translation to clinic
Complex nanoparticle formulations have shown great promise in vitro and in animal models of glioma but the scale of production for these applications are relatively small compared to the size of the production necessary for a clinical trial. Manufacturing nanoparticles on a large scale for clinical trials poses additional challenges that include: 1) efficient and reliable production of the desired nanoparticles and 2) governmental regulations.
First, “scaling-up” of nanomedicines requires a detailed understanding of each component of the system in order to generate a manufacturing protocol that is reproducible and includes checkpoints for the analysis and characterization of products at different stages of the synthesis. Two preparation techniques are often considered: “top-down” and “bottom-up”. Top-down preparation utilizes larger pieces of material to generate the desired nanostructures while bottom-up preparation produces large complex structures through self-assembly of single-molecule components or polymerization of monomers [243]. Regardless of the preparation technique, it is important to select formulation processes (i.e. crosslinking, emulsification, sonication, etc.) and conditions (reaction time, temperature, pH, pressure, etc.) during the early stages of production that can be consistently reproduced on a larger scale and yield a consistent product that has the same structure and properties as the nanoparticles synthesized in the laboratory [244]. Furthermore, if it is required that the nanoparticles are sterile during administration, an appropriate sterilization technique must be selected because nanoparticles are susceptible to damage from gamma irradiation and autoclaving [245–247]. Moreover, in order to regulate the nanoparticles during synthesis, quick and reliable in-process analytic characterization is required to ensure that the manufacturing process does not alter the composition as well as compromise the quality and stability of the final product. Such techniques may include transmission electron microscopy, light scattering, analytic ultracentrifugation, electrophoresis, spectroscopy, and X-ray diffraction [244].
Second, because of the inherent complexity of nanoparticle systems, government regulation is often unclear and difficult to navigate. Today, there is no specific regulation for nanomedicines and therefore the FDA uses an adapted version of the current framework of regulatory standards. The FDA also recognizes that the unique properties of nanoparticles such as their size, robust physiochemical properties, and classification as a combination product make nanoparticles functionally different from their bulk counterparts. Due to this, the FDA agrees that specific regulatory guidelines for nanomedicines are warranted. To address this issue, the FDA established a Nanotechnology Task Force to create a detailed regulatory pathway for the production of safe, novel, and beneficial nanomedicines [248]. Since its conception in 2006, the FDA’s Nanotechnology Task Force has published several draft guidelines. Despite the progress, no official guidelines currently exist and there is a need for the FDA and other regulatory agencies to develop comprehensive guidelines in order to clarify and expedite the production of nanomedicines.
10. Future challenges and perspectives
The ultimate goal of multifunctional nanoparticles for brain tumors is to enhance patient survival and improve their quality of life. As discussed in this review, there are a number of nanomaterials with unique properties that can serve as imaging probes or therapeutic agents for brain tumors. Theranostic nanoparticles will open even more opportunities to create innovative nanomedicines for brain tumors. Despite the tremendous efforts thus far, only a few nanoparticle systems have been approved for clinic trials, illustrating that many obstacles still need to be overcome in order to translate such nanoparticles from the bench to the bedside.
One of major challenges is the development of an effective nanoparticle system that can overcome the BBB and allow for specific yet widespread targeting of brain cancer cells. However, the heterogeneity of malignant brain tumors and the physiological barriers surrounding the central nervous system make this an arduous task. Exclusively targeting overexpressed proteins and receptors on brain cancer cells remains challenging since normal cells may also express these cancer cell-associated targets to some degree. Nanoparticles incorporating multivalent targeting moieties hold the most promise for achieving optimal targeting after systemic administration. Alternatively, local delivery approaches such as CED can be applied for all nanoparticle systems to bypass the BBB and improve the distribution within a brain tumor. Moreover, recent advances in cellular based delivery offer a novel strategy for targeting brain tumors.
A second challenge associated with drug-carrying nanoparticles in brain tumor therapy is controlled drug release. Only after a nanoparticle-based delivery system reaches the tumor site should its therapeutic payload be released. Nanoparticles using non-covalent delivery strategies can preserve active drugs but often suffer from non-specific diffusion during transportation. Enzyme degradable or stimulus-sensitive chemical linkers should be considered to enhance the overall efficacy of the nanoparticle delivery system. Magnetic field, X-ray, light, and heat are promising external triggers which can control drug release in a spatial and temporal manner.
Safety is another concern nanoparticles face. While the unique chemical and physical properties of nanomaterials make them attractive for therapy and diagnosis, potential neurotoxicity and systemic toxicity are concerns related to implementing these particles in actual patients. Current clinical examples using nanoparticles for brain tumors are carried out on patients with recurrent high-grade glioma or patients with brain metastases, both diseases having very poor prognoses. To expand the application of nanoparticles to less aggressive forms of brain cancer is challenging. Although many nanoparticles show low acute systemic toxicity, the long-term side effects are not yet known. In addition, the clearance mechanism of nanoparticles after administration is not fully understood, which makes it unclear as to the long-term consequences of particle accumulation in various tissues. Toxicological profiles and clearance mechanisms in animal models will be needed in order to push the field forward. Other issues such as the long-term stability of nanoparticles, quality control in the synthesis process, and the ability to synthesize these materials on a large scale are practical problems hampering their clinical application. The future design of nanoparticles for brain tumors should take the above challenges into consideration.
Another important direction in the field of nanomedicine will be the development of particles with multiple imaging functionalities. Designing these nanoparticles will require focus on materials that show unique magnetic or optical properties. Integrating more than one imaging probe will further improve the sensitivity for detecting recurrence and tracking patient responses to treatment. Furthermore, they may also allow for new methods of imageguided surgery for brain tumor resection. The field of nanomedicine is also moving towards developing theranostic nanoparticles that combine therapeutic and diagnostic applications. Such systems will be extremely important since they may help in evaluating the tumor status directly in conjunction with therapy. Iron oxide nanoparticle and gold nanoparticles, possessing light- or magnetic field-mediated properties, are distinguished nanomaterials serving as intrinsic theranostic platforms.
Clinical implementation of nanoparticles for patients with brain tumors is still in its infancy. Developing successful systems requires knowledge in a multitude of areas including nanoparticle engineering, medicine, cancer biology, and pharmacokinetics. As our knowledge in these fields expands in the coming years, it will facilitate the creation of innovative and effective nanomaterials for curing patients with brain tumors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beduneau A, Saulnier P, Benoit JP. Active targeting of brain tumors using nanocarriers. Biomaterials. 2007;28:4947–4967. doi: 10.1016/j.biomaterials.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2002;54:631–651. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 3.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 4.Koo H, Huh MS, Sun IC, Yuk SH, Choi K, Kim K, Kwon IC. In vivo targeted delivery of nanoparticles for theranosis. Acc. Chem. Res. 2011;44:1018–1028. doi: 10.1021/ar2000138. [DOI] [PubMed] [Google Scholar]
- 5.Koo YE, Reddy GR, Bhojani M, Schneider R, Philbert MA, Rehemtulla A, Ross BD, Kopelman R. Brain cancer diagnosis and therapy with nanoplatforms. Adv. Drug Deliv. Rev. 2006;58:1556–1577. doi: 10.1016/j.addr.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Meyers JD, Doane T, Burda C, Basilion JP. Nanoparticles for imaging and treating brain cancer. Nanomedicine (Lond) 2013;8:123–143. doi: 10.2217/nnm.12.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohs AM, Provenzale JM. Applications of nanotechnology to imaging and therapy of brain tumors. Neuroimaging Clin. N. Am. 2010;20:283–292. doi: 10.1016/j.nic.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Orringer DA, Koo YE, Chen T, Kopelman R, Sagher O, Philbert MA. Small solutions for big problems: the application of nanoparticles to brain tumor diagnosis and therapy. Clin. Pharmacol. Ther. 2009;85:531–534. doi: 10.1038/clpt.2008.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang AZ. Nanoparticle drug delivery: focusing on the therapeutic cargo. Nanomedicine (Lond) 2012;7:1463–1465. doi: 10.2217/nnm.12.114. [DOI] [PubMed] [Google Scholar]
- 10.Xie J, Lee S, Chen X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010;62:1064–1079. doi: 10.1016/j.addr.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro. Oncol. 2012;14:v1–v49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wen PY, Kesari S. Malignant gliomas in adults. N. Engl. J. Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 13.DeAngelis LM. Brain tumors. N. Engl. J. Med. 2001;344:114–123. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 14.Scheithauer BW. Development of the WHO classification of tumors of the central nervous system: a historical perspective. Brain Pathol. 2009;19:551–564. doi: 10.1111/j.1750-3639.2008.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, Sabel M, Steinbach JP, Heese O, Reifenberger G, Weller M, Schackert G. Long-term survival with glioblastoma multiforme. Brain. 2007;130:2596–2606. doi: 10.1093/brain/awm204. [DOI] [PubMed] [Google Scholar]
- 16.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 17.Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK. The biology of brain metastases-translation to new therapies. Nat. Rev. Clin. Oncol. 2011;8:344–356. doi: 10.1038/nrclinonc.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J. Neurooncol. 2005;75:5–14. doi: 10.1007/s11060-004-8093-6. [DOI] [PubMed] [Google Scholar]
- 19.Chaichana KL, Zadnik P, Weingart JD, Olivi A, Gallia GL, Blakeley J, Lim M, Brem H, Quinones-Hinojosa A. Multiple resections for patients with glioblastoma: prolonging survival. J Neurosurg. 2013;118:812–820. doi: 10.3171/2012.9.JNS1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graham CA, Cloughesy TF. Brain tumor treatment: chemotherapy and other new developments. Semin. Oncol. Nurs. 2004;20:260–272. [PubMed] [Google Scholar]
- 21.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 22.Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, Fisher J. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin. Cancer Res. 2010;16:2443–2449. doi: 10.1158/1078-0432.CCR-09-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stupp R, Dietrich PY, Ostermann Kraljevic S, Pica A, Maillard I, Maeder P, Meuli R, Janzer R, Pizzolato G, Miralbell R, Porchet F, Regli L, de Tribolet N, Mirimanoff RO, Leyvraz S. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J. Clin. Oncol. 2002;20:1375–1382. doi: 10.1200/JCO.2002.20.5.1375. [DOI] [PubMed] [Google Scholar]
- 24.Yung WK. Temozolomide in malignant gliomas. Semin. Oncol. 2000;27:27–34. [PubMed] [Google Scholar]
- 25.Bahr W, Stoll P. Intraoperative histological evaluation of tumor resection borders without prolonging surgery. Int. J. Oral Maxillofac. Surg. 1992;21:90–91. doi: 10.1016/s0901-5027(05)80539-9. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman JM. New advances in brain tumor imaging. Curr. Opin. Oncol. 2001;13:148–153. doi: 10.1097/00001622-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Knauth M, Wirtz CR, Tronnier VM, Aras N, Kunze S, Sartor K. Intraoperative MR imaging increases the extent of tumor resection in patients with high-grade gliomas. AJNR Am. J. Neuroradiol. 1999;20:1642–1646. [PMC free article] [PubMed] [Google Scholar]
- 28.Poon WS, Schomacker KT, Deutsch TF, Martuza RL. Laser-induced fluorescence: experimental intraoperative delineation of tumor resection margins. J. Neurosurg. 1992;76:679–686. doi: 10.3171/jns.1992.76.4.0679. [DOI] [PubMed] [Google Scholar]
- 29.Ricci PE. Imaging of adult brain tumors. Neuroimaging Clin. N. Am. 1999;9:651–669. [PubMed] [Google Scholar]
- 30.Thurber GM, Figueiredo JL, Weissleder R. Detection limits of intraoperative near infrared imaging for tumor resection. J. Surg. Oncol. 2010;102:758–764. doi: 10.1002/jso.21735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hustinx R, Alavi A. SPECT and PET imaging of brain tumors. Neuroimaging. Clin. N. Am. 1999;9:751–766. [PubMed] [Google Scholar]
- 32.Makary M, Chiocca EA, Erminy N, Antor M, Bergese SD, Abdel-Rasoul M, Fernandez S, Dzwonczyk R. Clinical and economic outcomes of low-field intraoperative MRI-guided tumor resection neurosurgery. J. Magn. Reson. Imaging. 2011;34:1022–1030. doi: 10.1002/jmri.22739. [DOI] [PubMed] [Google Scholar]
- 33.Nabavi DG, Cenic A, Craen RA, Gelb AW, Bennett JD, Kozak R, Lee TY. CT assessment of cerebral perfusion: experimental validation and initial clinical experience. Radiology. 1999;213:141–149. doi: 10.1148/radiology.213.1.r99oc03141. [DOI] [PubMed] [Google Scholar]
- 34.Petrirena GJ, Goldman S, Delattre JY. Advances in PET imaging of brain tumors: a referring physician's perspective. Curr. Opin. Oncol. 2011;23:617–623. doi: 10.1097/CCO.0b013e32834aa752. [DOI] [PubMed] [Google Scholar]
- 35.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J. Natl. Cancer. Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tykocki T, Michalik R, Bonicki W, Nauman P. Fluorescence-guided resection of primary and recurrent malignant gliomas with 5-aminolevulinic acid. Preliminary results, Neurol. Neurochir. Pol. 2012;46:47–51. doi: 10.5114/ninp.2012.27212. [DOI] [PubMed] [Google Scholar]
- 37.Sneed PK, Gutin PH, Larson DA, Malec MK, Phillips TL, Prados MD, Scharfen CO, Weaver KA, Wara WM. Patterns of recurrence of glioblastoma multiforme after external irradiation followed by implant boost. Int. J. Radiat. Oncol. Biol. Phys. 1994;29:719–727. doi: 10.1016/0360-3016(94)90559-2. [DOI] [PubMed] [Google Scholar]
- 38.Groothuis DR. The blood-brain and blood-tumor barriers: A review of strategies for increasing drug delivery. Neuro-Oncology. 2000;2:45–59. doi: 10.1093/neuonc/2.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lesniak MS, Brem H. Targeted therapy for brain tumours. Nat. Rev. Drug Discov. 2004;3:499–508. doi: 10.1038/nrd1414. [DOI] [PubMed] [Google Scholar]
- 40.Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kakee A, Terasaki T, Sugiyama Y. Brain efflux index as a novel method of analyzing efflux transport at the blood-brain barrier. J. Pharmacol. Exp. Ther. 1996;277:1550–1559. [PubMed] [Google Scholar]
- 42.Demeule M, Regina A, Jodoin J, Laplante A, Dagenais C, Berthelet F, Moghrabi A, Beliveau R. Drug transport to the brain: key roles for the efflux pump P-glycoprotein in the blood-brain barrier. Vascul. Pharmacol. 2002;38:339–348. doi: 10.1016/s1537-1891(02)00201-x. [DOI] [PubMed] [Google Scholar]
- 43.Golden PL, Pollack GM. Blood-brain barrier efflux transport. J. Pharm. Sci. 2003;92:1739–1753. doi: 10.1002/jps.10424. [DOI] [PubMed] [Google Scholar]
- 44.Lee NY, Kang YS. The brain-to-blood efflux transport of taurine and changes in the blood-brain barrier transport system by tumor necrosis factor-alpha. Brain Res. 2004;1023:141–147. doi: 10.1016/j.brainres.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 45.Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J. Cell Biol. 1967;34:207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levin VA. Relationship of octanol/water partition coefficient and molecular weight to rat brain capillary permeability. J. Med. Chem. 1980;23:682–684. doi: 10.1021/jm00180a022. [DOI] [PubMed] [Google Scholar]
- 47.Pardridge WM. CNS drug design based on principles of blood-brain barrier transport. J. Neurochem. 1998;70:1781–1792. doi: 10.1046/j.1471-4159.1998.70051781.x. [DOI] [PubMed] [Google Scholar]
- 48.Spector R. Nature and consequences of mammalian brain and CSF efflux transporters: four decades of progress. J. Neurochem. 2010;112:13–23. doi: 10.1111/j.1471-4159.2009.06451.x. [DOI] [PubMed] [Google Scholar]
- 49.Nau R, Sorgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin. Microbiol. Rev. 2010;23:858–883. doi: 10.1128/CMR.00007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuroda M, Kusuhara H, Endou H, Sugiyama Y. Rapid elimination of cefaclor from the cerebrospinal fluid is mediated by a benzylpenicillin-sensitive mechanism distinct from organic anion transporter 3. J. Pharmacol. Exp. Ther. 2005;314:855–861. doi: 10.1124/jpet.105.085027. [DOI] [PubMed] [Google Scholar]
- 51.Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, Chang G. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shapiro WR, Young DF, Mehta BM. Methotrexate: distribution in cerebrospinal fluid after intravenous, ventricular and lumbar injections. N. Engl. J. Med. 1975;293:161–166. doi: 10.1056/NEJM197507242930402. [DOI] [PubMed] [Google Scholar]
- 53.Zastre JA, Chan GN, Ronaldson PT, Ramaswamy M, Couraud PO, Romero IA, Weksler B, Bendayan M, Bendayan R. Up-regulation of P-glycoprotein by HIV protease inhibitors in a human brain microvessel endothelial cell line. J. Neurosci. Res. 2009;87:1023–1036. doi: 10.1002/jnr.21898. [DOI] [PubMed] [Google Scholar]
- 54.Roose T, Netti PA, Munn LL, Boucher Y, Jain RK. Solid stress generated by spheroid growth estimated using a linear poroelasticity model. Microvasc. Res. 2003;66:204–212. doi: 10.1016/s0026-2862(03)00057-8. [DOI] [PubMed] [Google Scholar]
- 55.Kim BY, Rutka JT, Chan WC. Nanomedicine. N. Engl. J. Med. 2010;363:2434–2443. doi: 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- 56.Rozhkova EA. Nanoscale materials for tackling brain cancer: recent progress and outlook. Adv. Mater. 2011;23:H136–H150. doi: 10.1002/adma.201004714. [DOI] [PubMed] [Google Scholar]
- 57.Chertok B, Moffat BA, David AE, Yu FQ, Bergemann C, Ross BD, Yang VC. Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials. 2008;29:487–496. doi: 10.1016/j.biomaterials.2007.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Day ES, Thompson PA, Zhang L, Lewinski NA, Ahmed N, Drezek RA, Blaney SM, West JL. Nanoshell-mediated photothermal therapy improves survival in a murine glioma model. J. Neurooncol. 2011;104:55–63. doi: 10.1007/s11060-010-0470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ding H, Inoue S, Ljubimov AV, Patil R, Portilla-Arias J, Hu J, Konda B, Wawrowsky KA, Fujita M, Karabalin N, Sasaki T, Black KL, Holler E, Ljubimova JY. Inhibition of brain tumor growth by intravenous poly (beta-L-malic acid) nanobioconjugate with pH-dependent drug release [corrected] Proc. Natl. Acad. Sci. U S A. 2010;107:18143–18148. doi: 10.1073/pnas.1003919107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jordan A, Scholz R, Maier-Hauff K, van Landeghem FKH, Waldoefner N, Teichgraeber U, Pinkernelle J, Bruhn H, Neumann F, Thiesen B, von Deimling A, Felix R. The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. J. Neurooncol. 2006;78:7–14. doi: 10.1007/s11060-005-9059-z. [DOI] [PubMed] [Google Scholar]
- 61.Kim DH, Rozhkova EA, Ulasov IV, Bader SD, Rajh T, Lesniak MS, Novosad V. Biofunctionalized magnetic-vortex microdiscs for targeted cancer-cell destruction. Nat. Mater. 2010;9:165–171. doi: 10.1038/nmat2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Y, He H, Jia X, Lu WL, Lou J, Wei Y. A dual-targeting nanocarrier based on poly(amidoamine) dendrimers conjugated with transferrin and tamoxifen for treating brain gliomas. Biomaterials. 2012;33:3899–3908. doi: 10.1016/j.biomaterials.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 63.Noble CO, Krauze MT, Drummond DC, Yamashita Y, Saito R, Berger MS, Kirpotin DB, Bankiewicz KS, Park JW. Novel nanoliposomal CPT-11 infused by convection-enhanced delivery in intracranial tumors: pharmacology and efficacy. Cancer Res. 2006;66:2801–2806. doi: 10.1158/0008-5472.CAN-05-3535. [DOI] [PubMed] [Google Scholar]
- 64.Xin HL, Sha XY, Jiang XY, Zhang W, Chen LC, Fang XL. Anti-glioblastoma efficacy and safety of paclitaxel-loading Angiopep-conjugated dual targeting PEG-PCL nanoparticles. Biomaterials. 2012;33:8167–8176. doi: 10.1016/j.biomaterials.2012.07.046. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Q, Iwakuma N, Sharma P, Moudgil BM, Wu C, McNeill J, Jiang H, Grobmyer SR. Gold nanoparticles as a contrast agent for in vivo tumor imaging with photoacoustic tomography. Nanotechnology. 2009;20:395102. doi: 10.1088/0957-4484/20/39/395102. [DOI] [PubMed] [Google Scholar]
- 66.Cai W, Shin DW, Chen K, Gheysens O, Cao Q, Wang SX, Gambhir SS, Chen X. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett. 2006;6:669–676. doi: 10.1021/nl052405t. [DOI] [PubMed] [Google Scholar]
- 67.Rozhkova EA, Ulasov I, Lai B, Dimitrijevic NM, Lesniak MS, Rajh T. A high-performance nanobio photocatalyst for targeted brain cancer therapy. Nano Lett. 2009;9:3337–3342. doi: 10.1021/nl901610f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sailor MJ, Park JH. Hybrid nanoparticles for detection and treatment of cancer. Adv. Mater. 2012;24:3779–3802. doi: 10.1002/adma.201200653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sethuraman VA, Lee MC, Bae YH. A biodegradable pH-sensitive micelle system for targeting acidic solid tumors. Pharm. Res. 2008;25:657–666. doi: 10.1007/s11095-007-9480-4. [DOI] [PubMed] [Google Scholar]
- 70.Doane TL, Burda C. The unique role of nanoparticles in nanomedicine: imaging, drug delivery and therapy. Chem. Soc. Rev. 2012;41:2885–2911. doi: 10.1039/c2cs15260f. [DOI] [PubMed] [Google Scholar]
- 71.Cheng Y, Meyers JD, Agnes RS, Doane TL, Kenney ME, Broome AM, Burda C. J.P. Basilion, Addressing brain tumors with targeted gold nanoparticles: A new gold standard for hydrophobic drug delivery? Small. 2011;7:2301–2306. doi: 10.1002/smll.201100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giljohann DA, Seferos DS, Daniel WL, Massich MD, Patel PC, Mirkin CA. Gold nanoparticles for biology and medicine. Angew. Chem. Int. Ed. 2010;49:3280–3294. doi: 10.1002/anie.200904359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rana S, Bajaj A, Mout R, Rotello VM. Monolayer coated gold nanoparticles for delivery applications. Adv. Drug Deliv. Rev. 2012;64:200–216. doi: 10.1016/j.addr.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jain PK, Huang XH, El-Sayed IH, El-Sayed MA. Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 2008;41:1578–1586. doi: 10.1021/ar7002804. [DOI] [PubMed] [Google Scholar]
- 75.Bardhan R, Lal S, Joshi A, Halas NJ. Theranostic nanoshells: from probe design to imaging and treatment of cancer. Acc. Chem. Res. 2011;44:936–946. doi: 10.1021/ar200023x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Murphy CJ, Gole AM, Stone JW, Sisco PN, Alkilany AM, Goldsmith EC, Baxter SC. Gold nanoparticles in biology: beyond toxicity to cellular imaging. Acc. Chem. Res. 2008;41:1721–1730. doi: 10.1021/ar800035u. [DOI] [PubMed] [Google Scholar]
- 77.Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L, Muller RN. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008;108:2064–2110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 78.Kim DH, Rozhkova EA, Ulasov IV, Bader SD, Rajh T, Lesniak MS, Novosad V. Biofunctionalized magnetic-vortex microdiscs for targeted cancer-cell destruction. Nat. Mater. 2010;9:165–171. doi: 10.1038/nmat2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao X, Cui Y, Levenson RM, Chung LW, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 2004;22:969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 80.Dimitrijevic NM, Rozhkova E, Rajh T. Dynamics of localized charges in dopamine-modified TiO2 and their effect on the formation of reactive oxygen species. J. Am. Chem. Soc. 2009;131:2893–2899. doi: 10.1021/ja807654k. [DOI] [PubMed] [Google Scholar]
- 81.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Controlled Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 82.Bhojani MS, Van Dort M, Rehemtulla A, Ross BD. Targeted imaging and therapy of brain cancer using theranostic nanoparticles. Mol. Pharmaceut. 2010;7:1921–1929. doi: 10.1021/mp100298r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2007;2:469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 84.Khlebtsov N, Dykman L. Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem. Soc. Rev. 2011;40:1647–1671. doi: 10.1039/c0cs00018c. [DOI] [PubMed] [Google Scholar]
- 85.Yan HH, Wang L, Wang JY, Weng XF, Lei H, Wang XX, Jiang L, Zhu JH, Lu WY, Wei XB, Li C. Two-order targeted brain tumor imaging by using an optical/paramagnetic nanoprobe across the blood brain barrier. ACS Nano. 2012;6:410–420. doi: 10.1021/nn203749v. [DOI] [PubMed] [Google Scholar]
- 86.Kim ST, Saha K, Kim C, Rotello VM. The role of surface functionality in determining nanoparticle cytotoxicity. Acc. Chem. Res. 2013;46:681–691. doi: 10.1021/ar3000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gref R, Luck M, Quellec P, Marchand M, Dellacherie E, Harnisch S, Blunk T, Muller RH. 'Stealth' corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf. B Biointerfaces. 2000;18:301–313. doi: 10.1016/s0927-7765(99)00156-3. [DOI] [PubMed] [Google Scholar]
- 88.Provenzale JM, Wang GR, Brenner T, Petrella JR, Sorensen AG. Comparison of permeability in high-grade and low-grade brain tumors using dynamic susceptibility contrast MR imaging. AJR Am. J. Roentgenol. 2002;178:711–716. doi: 10.2214/ajr.178.3.1780711. [DOI] [PubMed] [Google Scholar]
- 89.Roberts HC, Roberts TPL, Brasch RC, Dillon WP. Quantitative measurement of microvascular permeability in human brain tumors achieved using dynamic contrast-enhanced MR imaging: Correlation with histologic grade. Am J. Neuroradiol. 2000;21:891–899. [PMC free article] [PubMed] [Google Scholar]
- 90.Kalaria RN, Homayoun P, Whitehouse PJ. Nicotinic cholinergic receptors associated with mammalian cerebral vessels. J. Auton. Nerv. Syst. 1994;49:S3–S7. doi: 10.1016/0165-1838(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 91.Macklin KD, Maus AD, Pereira EF, Albuquerque EX, Conti-Fine BM. Human vascular endothelial cells express functional nicotinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 1998;287:435–439. [PubMed] [Google Scholar]
- 92.Fishman JB, Rubin JB, Handrahan JV, Connor JR, Fine RE. Receptor-mediated transcytosis of transferrin across the blood-brain barrier. J. Neurosci. Res. 1987;18:299–304. doi: 10.1002/jnr.490180206. [DOI] [PubMed] [Google Scholar]
- 93.Bello L, Francolini M, Marthyn P, Zhang J, Carroll RS, Nikas DC, Strasser JF, Villani R, Cheresh DA, Black PM. Alpha(v)beta3 and alpha(v)beta5 integrin expression in glioma periphery. Neurosurgery. 2001;49:380–389. doi: 10.1097/00006123-200108000-00022. [DOI] [PubMed] [Google Scholar]
- 94.Pasqualini R, Koivunen E, Kain R, Lahdenranta J, Sakamoto M, Stryhn A, Ashmun RA, Shapiro LH, Arap W, Ruoslahti E. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 2000;60:722–727. [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang P, Hu L, Yin Q, Feng L, Li Y. Transferrin-modified c[RGDfK]-paclitaxel loaded hybrid micelle for sequential blood-brain barrier penetration and glioma targeting therapy. Mol. Pharm. 2012;9:1590–1598. doi: 10.1021/mp200600t. [DOI] [PubMed] [Google Scholar]
- 96.Jain A, Singhai P, Gurnany E, Updhayay S, Mody N. Transferrin-tailored solid lipid nanoparticles as vectors for site-specific delivery of temozolomide to brain. J. Nanopart. Res. 2013;15:1518. [Google Scholar]
- 97.Jiang W, Xie H, Ghoorah D, Shang Y, Shi H, Liu F, Yang X, Xu H. Conjugation of functionalized SPIONs with transferrin for targeting and imaging brain glial tumors in rat model. PLoS One. 2012;7:e37376. doi: 10.1371/journal.pone.0037376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.He H, Li Y, Jia XR, Du J, Ying X, Lu WL, Lou JN, Wei Y. PEGylated Poly(amidoamine) dendrimer-based dual-targeting carrier for treating brain tumors. Biomaterials. 2011;32:478–487. doi: 10.1016/j.biomaterials.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 99.Zhan C, Li B, Hu L, Wei X, Feng L, Fu W, Lu W. Micelle-based brain-targeted drug delivery enabled by a nicotine acetylcholine receptor ligand. Angew. Chem. Int. Ed. Engl. 2011;50:5482–5485. doi: 10.1002/anie.201100875. [DOI] [PubMed] [Google Scholar]
- 100.Zhan C, Wei X, Qian J, Feng L, Zhu J, Lu W. Co-delivery of TRAIL gene enhances the anti-glioblastoma effect of paclitaxel in vitro and in vivo. J. Controlled Release. 2012;160:630–636. doi: 10.1016/j.jconrel.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 101.Zhan C, Yan Z, Xie C, Lu W. Loop 2 of Ophiophagus hannah toxin b binds with neuronal nicotinic acetylcholine receptors and enhances intracranial drug delivery. Mol. Pharm. 2010;7:1940–1947. doi: 10.1021/mp100238j. [DOI] [PubMed] [Google Scholar]
- 102.Lou X, Liu Q, Tu X, Wang J, Teng M, Niu L, Schuller DJ, Huang Q, Hao Q. The atomic resolution crystal structure of atratoxin determined by single wavelength anomalous diffraction phasing. J. Biol. Chem. 2004;37:39094–39104. doi: 10.1074/jbc.M403863200. [DOI] [PubMed] [Google Scholar]
- 103.Zhan C, Gu B, Xie C, Li J, Liu Y, Lu W. Cyclic RGD conjugated poly(ethylene glycol)-co-poly(lactic acid) micelle enhances paclitaxel anti-glioblastoma effect. J. Controlled Release. 2010;143:136–142. doi: 10.1016/j.jconrel.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 104.Zhan C, Meng Q, Li Q, Feng L, Zhu J, Lu W. Cyclic RGD-polyethylene glycol-polyethylenimine for intracranial glioblastoma-targeted gene delivery. Chem. Asian J. 2012;7:91–96. doi: 10.1002/asia.201100570. [DOI] [PubMed] [Google Scholar]
- 105.Chen K, Xie J, Xu H, Behera D, Michalski MH, Biswal S, Wang A, Chen X. Triblock copolymer coated iron oxide nanoparticle conjugate for tumor integrin targeting. Biomaterials. 2009;30:6912–6919. doi: 10.1016/j.biomaterials.2009.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang F, Huang X, Zhu L, Guo N, Niu G, Swierczewska M, Lee S, Xu H, Wang AY, Mohamedali KA, Rosenblum MG, Lu G, Chen X. Noninvasive monitoring of orthotopic glioblastoma therapy response using RGD-conjugated iron oxide nanoparticles. Biomaterials. 2012;33:5414–5422. doi: 10.1016/j.biomaterials.2012.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Morales-Avila E, Ferro-Flores G, Ocampo-Garcia BE, De Leon-Rodriguez LM, Santos-Cuevas CL, Garcia-Becerra R, Medina LA, Gomez-Olivan L. Multimeric system of 99mTc-labeled gold nanoparticles conjugated to c[RGDfK(C)] for molecular imaging of tumor alpha(v)beta(3) expression. Bioconjug. Chem. 2011;22:913–922. doi: 10.1021/bc100551s. [DOI] [PubMed] [Google Scholar]
- 108.Zhang L, Zhu S, Qian L, Pei Y, Qiu Y, Jiang Y. RGD-modified PEG-PAMAM-DOX conjugates: in vitro and in vivo studies for glioma. Eur. J. Pharm. Biopharm. 2011;79:232–240. doi: 10.1016/j.ejpb.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 109.Jiang X, Sha X, Xin H, Xu X, Gu J, Xia W, Chen S, Xie Y, Chen L, Chen Y, Fang X. Integrin-facilitated transcytosis for enhanced penetration of advanced gliomas by poly(trimethylene carbonate)-based nanoparticles encapsulating paclitaxel. Biomaterials. 2013;34:2969–2979. doi: 10.1016/j.biomaterials.2012.12.049. [DOI] [PubMed] [Google Scholar]
- 110.Kopelman R, Lee Koo Y-E, Philbert M, Moffat BA, Ramachandra Reddy G, McConville P, Hall DE, Chenevert TL, Bhojani MS, Buck SM, Rehemtulla A, Ross BD. Multifunctional nanoparticle platforms for in vivo MRI enhancement and photodynamic therapy of a rat brain cancer. J. Magn. Magn. Mater. 2005;293:404–410. [Google Scholar]
- 111.Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 112.Zhao B-J, Ke X-Y, Huang Y, Chen X-M, Zhao X, Zhao B-X, Lu W-l, Lou J-N, Zhang X, Zhang Q. The antiangiogenic efficacy of NGR-modified PEG–DSPE micelles containing paclitaxel (NGR-M-PTX) for the treatment of glioma in rats. J. Drug Target. 2011;19:382–390. doi: 10.3109/1061186X.2010.504267. [DOI] [PubMed] [Google Scholar]
- 113.Liu C, Liu F, Feng L, Li M, Zhang J, Zhang N. The targeted co-delivery of DNA and doxorubicin to tumor cells via multifunctional PEI-PEG based nanoparticles. Biomaterials. 2013;34:2547–2564. doi: 10.1016/j.biomaterials.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 114.Chen Y, Wu JJ, Huang L. Nanoparticles targeted with NGR motif deliver c-myc siRNA and doxorubicin for anticancer therapy. Mol. Ther. 2010;18:828–834. doi: 10.1038/mt.2009.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pastorino F, Brignole C, Marimpietri D, Cilli M, Gambini C, Ribatti D, Longhi R, Allen TM, Corti A, Ponzoni M. Vascular damage and anti-angiogenic effects of tumor vessel-targeted liposomal chemotherapy. Cancer Res. 2003;63:7400–7409. [PubMed] [Google Scholar]
- 116.Pastorino F, Brignole C, Di Paolo D, Nico B, Pezzolo A, Marimpietri D, Pagnan G, Piccardi F, Cilli M, Longhi R, Ribatti D, Corti A, Allen TM, Ponzoni M. Targeting liposomal chemotherapy via both tumor cell-specific and tumor vasculature-specific ligands potentiates therapeutic efficacy. Cancer Res. 2006;66:10073–10082. doi: 10.1158/0008-5472.CAN-06-2117. [DOI] [PubMed] [Google Scholar]
- 117.Christian S, Pilch J, Akerman ME, Porkka K, Laakkonen P, Ruoslahti E. Nucleolin expressed at the cell surface is a marker of endothelial cells in angiogenic blood vessels. J. Cell Biol. 2003;163:871–878. doi: 10.1083/jcb.200304132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reddy GR, Bhojani MS, McConville P, Moody J, Moffat BA, Hall DE, Kim G, Koo YE, Woolliscroft MJ, Sugai JV, Johnson TD, Philbert MA, Kopelman R, Rehemtulla A, Ross BD. Vascular targeted nanoparticles for imaging and treatment of brain tumors. Clin. Cancer Res. 2006;12:6677–6686. doi: 10.1158/1078-0432.CCR-06-0946. [DOI] [PubMed] [Google Scholar]
- 119.Hu Q, Gu G, Liu Z, Jiang M, Kang T, Miao D, Tu Y, Pang Z, Song Q, Yao L, Xia H, Chen H, Jiang X, Gao X, Chen J. F3 peptide-functionalized PEG-PLA nanoparticles co-administrated with tLyp-1 peptide for anti-glioma drug delivery. Biomaterials. 2013;34:1135–1145. doi: 10.1016/j.biomaterials.2012.10.048. [DOI] [PubMed] [Google Scholar]
- 120.Fleming TP, Saxena A, Clark WC, Robertson JT, Oldfield EH, Aaronson SA, Ali IU. Amplification and/or overexpression of platelet-derived growth factor receptors and epidermal growth factor receptor in human glial tumors. Cancer Res. 1992;52:4550–4553. [PubMed] [Google Scholar]
- 121.Heimberger AB, Hlatky R, Suki D, Yang D, Weinberg J, Gilbert M, Sawaya R, Aldape K. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin. Cancer Res. 2005;11:1462–1466. doi: 10.1158/1078-0432.CCR-04-1737. [DOI] [PubMed] [Google Scholar]
- 122.Debinski W, Gibo DM, Hulet SW, Connor JR, Gillespie GY. Receptor for interleukin 13 is a marker and therapeutic target for human high-grade gliomas. Clin. Cancer Res. 1999;5:985–990. [PubMed] [Google Scholar]
- 123.Debinski W, Gibo DM, Slagle B, Powers SK, Gillespie GY. Receptor for interleukin 13 is abundantly and specifically over-expressed in patients with glioblastoma multiforme. Int. J. Oncol. 1999;15:481–486. doi: 10.3892/ijo.15.3.481. [DOI] [PubMed] [Google Scholar]
- 124.Joshi BH, Plautz GE, Puri RK. Interleukin-13 receptor alpha chain: a novel tumor-associated transmembrane protein in primary explants of human malignant gliomas. Cancer Res. 2000;60:1168–1172. [PubMed] [Google Scholar]
- 125.Bu G, Maksymovitch EA, Geuze H, Schwartz AL. Subcellular localization and endocytic function of low density lipoprotein receptor-related protein in human glioblastoma cells. J. Biol. Chem. 1994;269:29874–29882. [PubMed] [Google Scholar]
- 126.Herz J, Bock HH. Lipoprotein receptors in the nervous system. Annu. Rev. Biochem. 2002;71:405–434. doi: 10.1146/annurev.biochem.71.110601.135342. [DOI] [PubMed] [Google Scholar]
- 127.Yamamoto M, Ikeda K, Ohshima K, Tsugu H, Kimura H, Tomonaga M. Increased expression of low density lipoprotein receptor-related protein/alpha2-macroglobulin receptor in human malignant astrocytomas. Cancer Res. 1997;57:2799–2805. [PubMed] [Google Scholar]
- 128.Hadjipanayis CG, Machaidze R, Kaluzova M, Wang L, Schuette AJ, Chen H, Wu X, Mao H. EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res. 2010;70:6303–6312. doi: 10.1158/0008-5472.CAN-10-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kuo YC, Liang CT. Inhibition of human brain malignant glioblastoma cells using carmustine-loaded catanionic solid lipid nanoparticles with surface anti-epithelial growth factor receptor. Biomaterials. 2011;32:3340–3350. doi: 10.1016/j.biomaterials.2011.01.048. [DOI] [PubMed] [Google Scholar]
- 130.Madhankumar AB, Slagle-Webb B, Mintz A, Sheehan JM, Connor JR. Interleukin-13 receptor-targeted nanovesicles are a potential therapy for glioblastoma multiforme. Mol. Cancer Ther. 2006;5:3162–3169. doi: 10.1158/1535-7163.MCT-06-0480. [DOI] [PubMed] [Google Scholar]
- 131.Shultz MD, Duchamp JC, Wilson JD, Shu CY, Ge J, Zhang J, Gibson HW, Fillmore HL, Hirsch JI, Dorn HC, Fatouros PP. Encapsulation of a radiolabeled cluster inside a fullerene cage, (177)Lu(x)Lu((3-x))N@C(80): an interleukin-13-conjugated radiolabeled metallofullerene platform. J. Am. Chem. Soc. 2010;132:4980–4981. doi: 10.1021/ja9093617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Huang S, Li J, Han L, Liu S, Ma H, Huang R, Jiang C. Dual targeting effect of Angiopep-2-modified, DNA-loaded nanoparticles for glioma. Biomaterials. 2011;32:6832–6838. doi: 10.1016/j.biomaterials.2011.05.064. [DOI] [PubMed] [Google Scholar]
- 133.Ren J, Shen S, Wang D, Xi Z, Guo L, Pang Z, Qian Y, Sun X, Jiang X. The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials. 2012;33:3324–3333. doi: 10.1016/j.biomaterials.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 134.Xin H, Jiang X, Gu J, Sha X, Chen L, Law K, Chen Y, Wang X, Jiang Y, Fang X. Angiopep-conjugated poly(ethylene glycol)-co-poly(epsilon-caprolactone) nanoparticles as dual-targeting drug delivery system for brain glioma. Biomaterials. 2011;32:4293–4305. doi: 10.1016/j.biomaterials.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 135.Macdonald DR, Cascino TL, Schold SC, Cairncross JG. Response Criteria for Phase-Ii Studies of Supratentorial Malignant Glioma. J. Clin. Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 136.van den Bent MJ, Vogelbaum MA, Wen PY, Macdonald DR, Chang SM. End point assessment in gliomas: novel treatments limit usefulness of classical Macdonald's Criteria. J. Clin. Oncol. 2009;27:2905–2908. doi: 10.1200/JCO.2009.22.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9:453–461. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]
- 138.Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M, Ancukiewicz M, Mrugala MM, Plotkin S, Drappatz J, Louis DN, Ivy P, Scadden DT, Benner T, Loeffler JS, Wen PY, Jain RK. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dhermain FG, Hau P, Lanfermann H, Jacobs AH, van den Bent MJ. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. 2010;9:906–920. doi: 10.1016/S1474-4422(10)70181-2. [DOI] [PubMed] [Google Scholar]
- 140.Perazella MA. Gadolinium-contrast toxicity in patients with kidney disease: nephrotoxicity and nephrogenic systemic fibrosis. Curr Drug Saf. 2008;3:67–75. doi: 10.2174/157488608783333989. [DOI] [PubMed] [Google Scholar]
- 141.Arvinda HR, Kesavadas C, Sarma PS, Thomas B, Radhakrishnan VV, Gupta AK, Kapilamoorthy TR, Nair S. Glioma grading: sensitivity, specificity, positive and negative predictive values of diffusion and perfusion imaging. J. Neurooncol. 2009;94:87–96. doi: 10.1007/s11060-009-9807-6. [DOI] [PubMed] [Google Scholar]
- 142.Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem. Sci. 1999;24:68–72. doi: 10.1016/s0968-0004(98)01344-9. [DOI] [PubMed] [Google Scholar]
- 143.Ludemann L, Hamm B, Zimmer C. Pharmacokinetic analysis of glioma compartments with dynamic Gd-DTPA-enhanced magnetic resonance imaging. Magn. Reson. Imaging. 2000;18:1201–1214. doi: 10.1016/s0730-725x(00)00223-x. [DOI] [PubMed] [Google Scholar]
- 144.Knauth M, Wirtz CR, Aras N, Sartor K. Low-field interventional MRI in neurosurgery: finding the right dose of contrast medium. Neuroradiology. 2001;43:254–258. doi: 10.1007/pl00006047. [DOI] [PubMed] [Google Scholar]
- 145.Knauth M, Aras N, Wirtz CR, Dorfler A, Engelhorn T, Sartor K. Surgically induced intracranial contrast enhancement: potential source of diagnostic error in intraoperative MR imaging. AJNR Am. J. Neuroradiol. 1999;20:1547–1553. [PMC free article] [PubMed] [Google Scholar]
- 146.Reinges MH, Nguyen HH, Krings T, Hutter BO, Rohde V, Gilsbach JM. Course of brain shift during microsurgical resection of supratentorial cerebral lesions: limits of conventional neuronavigation. Acta. Neurochir. 2004;146:369–377. doi: 10.1007/s00701-003-0204-1. [DOI] [PubMed] [Google Scholar]
- 147.Neuwelt EA, Varallyay CG, Manninger S, Solymosi D, Haluska M, Hunt MA, Nesbit G, Stevens A, Jerosch-Herold M, Jacobs PM, Hoffman JM. The potential of ferumoxytol nanoparticle magnetic resonance imaging, perfusion, and angiography in central nervous system malignancy: a pilot study. Neurosurgery. 2007;60:601–611. doi: 10.1227/01.NEU.0000255350.71700.37. [DOI] [PubMed] [Google Scholar]
- 148.Varallyay P, Nesbit G, Muldoon LL, Nixon RR, Delashaw J, Cohen JI, Petrillo A, Rink D, Neuwelt EA. Comparison of two superparamagnetic viral-sized iron oxide particles ferumoxides and ferumoxtran-10 with a gadolinium chelate in imaging intracranial tumors. AJNR Am. J. Neuroradiol. 2002;23:510–519. [PMC free article] [PubMed] [Google Scholar]
- 149.Sun C, Fang C, Stephen Z, Veiseh O, Hansen S, Lee D, Ellenbogen RG, Olson J, Zhang M. Tumor-targeted drug delivery and MRI contrast enhancement by chlorotoxin-conjugated iron oxide nanoparticles. Nanomedicine (Lond) 2008;3:495–505. doi: 10.2217/17435889.3.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tomanek B, Iqbal U, Blasiak B, Abulrob A, Albaghdadi H, Matyas JR, Ponjevic D, Sutherland GR. Evaluation of brain tumor vessels specific contrast agents for glioblastoma imaging. Neuro. Oncol. 2012;14:53–63. doi: 10.1093/neuonc/nor183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kim T, Momin E, Choi J, Yuan K, Zaidi H, Kim J, Park M, Lee N, McMahon MT, Quinones-Hinojosa A, Bulte JWM, Hyeon T, Gilad AA. Mesoporous silica-coated hollow manganese oxide nanoparticles as positive T-1 contrast agents for labeling and MRI tracking of adipose-derived mesenchyrnal stem cells. J. Am. Chem. Soc. 2011;133:2955–2961. doi: 10.1021/ja1084095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Faucher L, Guay-Begin AA, Lagueux J, Cote MF, Petitclerc E, Fortin MA. Ultra-small gadolinium oxide nanoparticles to image brain cancer cells in vivo with MRI. Contrast Media Mol. Imaging. 2011;6:209–218. doi: 10.1002/cmmi.420. [DOI] [PubMed] [Google Scholar]
- 153.Faucher L, Tremblay M, Lagueux J, Gossuin Y, Fortin MA. Rapid synthesis of PEGylated ultrasmall gadolinium oxide nanoparticles for cell labeling and tracking with MRI. ACS Appl. Mater. Inter. 2012;4:4506–4515. doi: 10.1021/am3006466. [DOI] [PubMed] [Google Scholar]
- 154.Miladi I, Le Duc G, Kryza D, Berniard A, Mowat P, Roux S, Taleb J, Bonazza P, Perriat P, Lux F, Tillement O, Billotey C, Janier M. Biodistribution of ultra small gadolinium-based nanoparticles as theranostic agent: Application to brain tumors. J. Biomater. Appl. 2012 doi: 10.1177/0885328212454315. [DOI] [PubMed] [Google Scholar]
- 155.Na HB, Lee JH, An K, Park YI, Park M, Lee IS, Nam DH, Kim ST, Kim SH, Kim SW, Lim KH, Kim KS, Kim SO, Hyeon T. Development of a T1 contrast agent for magnetic resonance imaging using MnO nanoparticles. Angew. Chem. Int. Ed. 2007;46:5397–5401. doi: 10.1002/anie.200604775. [DOI] [PubMed] [Google Scholar]
- 156.Shin JM, Anisur RM, Ko MK, Im GH, Lee JH, Lee IS. Hollow manganese oxide nanoparticles as multifunctional agents for magnetic resonance imaging and drug delivery. Angew. Chem. Int. Ed. 2009;48:321–324. doi: 10.1002/anie.200802323. [DOI] [PubMed] [Google Scholar]
- 157.Bucci MK, Maity A, Janss AJ, Belasco JB, Fisher MJ, Tochner ZA, Rorke L, Sutton LN, Phillips PC, Shu HK. Near complete surgical resection predicts a favorable outcome in pediatric patients with nonbrainstem, malignant gliomas: results from a single center in the magnetic resonance imaging era. Cancer. 2004;101:817–824. doi: 10.1002/cncr.20422. [DOI] [PubMed] [Google Scholar]
- 158.Stupp R, Hegi ME, van den Bent MJ, Mason WP, Weller M, Mirimanoff RO, Cairncross JG. Changing paradigms--an update on the multidisciplinary management of malignant glioma. Oncologist. 2006;11:165–180. doi: 10.1634/theoncologist.11-2-165. [DOI] [PubMed] [Google Scholar]
- 159.Iqbal U, Albaghdadi H, Luo Y, Arbabi M, Desvaux C, Veres T, Stanimirovic D, Abulrob A. Molecular imaging of glioblastoma multiforme using anti-insulin-like growth factor-binding protein-7 single-domain antibodies. Br. J. Cancer. 2010;103:1606–1616. doi: 10.1038/sj.bjc.6605937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nie G, Hah HJ, Kim G, Lee YE, Qin M, Ratani TS, Fotiadis P, Miller A, Kochi A, Gao D, Chen T, Orringer DA, Sagher O, Philbert MA, Kopelman R. Hydrogel nanoparticles with covalently linked coomassie blue for brain tumor delineation visible to the surgeon. Small. 2012;8:884–891. doi: 10.1002/smll.201101607. [DOI] [PubMed] [Google Scholar]
- 161.Hoelen CG, de Mul FF, Pongers R, Dekker A. Three-dimensional photoacoustic imaging of blood vessels in tissue. Opt. Lett. 1998;23:648–650. doi: 10.1364/ol.23.000648. [DOI] [PubMed] [Google Scholar]
- 162.Kruger RA, Reinecke DR, Kruger GA. Thermoacoustic computed tomography-technical considerations. Medical Physics. 1999;26:1832–1837. doi: 10.1118/1.598688. [DOI] [PubMed] [Google Scholar]
- 163.Xu MH, Wang LHV. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006;77:041101. [Google Scholar]
- 164.Manohar S, Vaartjes SE, van Hespen JCG, Klaase JM, van den Engh FM, Steenbergen W, van Leeuwen TG. Initial results of in vivo non-invasive cancer imaging in the human breast using near-infrared photoacoustics. Opt. Express. 2007;15:12277–12285. doi: 10.1364/oe.15.012277. [DOI] [PubMed] [Google Scholar]
- 165.Yang X, Stein EW, Ashkenazi S, Wang LV. Nanoparticles for photoacoustic imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009;1:360–368. doi: 10.1002/wnan.42. [DOI] [PubMed] [Google Scholar]
- 166.Schultz DA. Plasmon resonant particles for biological detection. Curr. Opin. Biotechnol. 2003;14:13–22. doi: 10.1016/s0958-1669(02)00015-0. [DOI] [PubMed] [Google Scholar]
- 167.Lu W, Melancon MP, Xiong C, Huang Q, Elliott A, Song S, Zhang R, Flores LG, 2nd, Gelovani JG, Wang LV, Ku G, Stafford RJ, Li C. Effects of photoacoustic imaging and photothermal ablation therapy mediated by targeted hollow gold nanospheres in an orthotopic mouse xenograft model of glioma. Cancer Res. 2011;71:6116–6121. doi: 10.1158/0008-5472.CAN-10-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kircher MF, de la Zerda A, Jokerst JV, Zavaleta CL, Kempen PJ, Mittra E, Pitter K, Huang RM, Campos C, Habte F, Sinclair R, Brennan CW, Mellinghoff IK, Holland EC, Gambhir SS. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat. Med. 2012;18:829–834. doi: 10.1038/nm.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lu W, Xiong C, Zhang G, Huang Q, Zhang R, Zhang JZ, Li C. Targeted photothermal ablation of murine melanomas with melanocyte-stimulating hormone analog-conjugated hollow gold nanospheres. Clin. Cancer Res. 2009;15:876–886. doi: 10.1158/1078-0432.CCR-08-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Veiseh O, Sun C, Fang C, Bhattarai N, Gunn J, Kievit F, Du K, Pullar B, Lee D, Ellenbogen RG, Olson J, Zhang M. Specific targeting of brain tumors with an optical/magnetic resonance imaging nanoprobe across the blood-brain barrier. Cancer Res. 2009;69:6200–6207. doi: 10.1158/0008-5472.CAN-09-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lee HY, Li Z, Chen K, Hsu AR, Xu CJ, Xie J, Sun SH, Chen XY. PET/MRI dual-modality tumor imaging using arginine-glycine-aspartic (RGD) - Conjugated radiolabeled iron oxide nanoparticles. J. Nucl. Med. 2008;49:1371–1379. doi: 10.2967/jnumed.108.051243. [DOI] [PubMed] [Google Scholar]
- 172.Veiseh O, Sun C, Gunn J, Kohler N, Gabikian P, Lee D, Bhattarai N, Ellenbogen R, Sze R, Hallahan A, Olson J, Zhang M. Optical and MRI multifunctional nanoprobe for targeting gliomas. Nano Lett. 2005;5:1003–1008. doi: 10.1021/nl0502569. [DOI] [PubMed] [Google Scholar]
- 173.Lee JH, Jun YW, Yeon SI, Shin JS, Cheon J. Dual-mode nanoparticle probes for high-performance magnetic resonance and fluorescence imaging of neuroblastoma. Angew. Chem. Int. Ed. 2006;45:8160–8162. doi: 10.1002/anie.200603052. [DOI] [PubMed] [Google Scholar]
- 174.Chen K, Li ZB, Wang H, Cai W, Chen X. Dual-modality optical and positron emission tomography imaging of vascular endothelial growth factor receptor on tumor vasculature using quantum dots. Eur. J. Nucl. Med. Mol. Imaging. 2008;35:2235–2244. doi: 10.1007/s00259-008-0860-8. [DOI] [PubMed] [Google Scholar]
- 175.Cai W, Chen K, Li ZB, Gambhir SS, Chen X. Dual-function probe for PET and near-infrared fluorescence imaging of tumor vasculature. J. Nucl. Med. 2007;48:1862–1870. doi: 10.2967/jnumed.107.043216. [DOI] [PubMed] [Google Scholar]
- 176.Xie J, Chen K, Huang J, Lee S, Wang J, Gao J, Li X, Chen X. PET/NIRF/MRI triple functional iron oxide nanoparticles. Biomaterials. 2010;31:3016–3022. doi: 10.1016/j.biomaterials.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Krauze MT, Noble CO, Kawaguchi T, Drummond D, Kirpotin DB, Yamashita Y, Kullberg E, Forsayeth J, Park JW, Bankiewicz KS. Convection-enhanced delivery of nanoliposomal CPT-11 (irinotecan) and PEGylated liposomal doxorubicin (Doxil) in rodent intracranial brain tumor xenografts. Neuro. Oncol. 2007;9:393–403. doi: 10.1215/15228517-2007-019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Krol S, Macrez R, Docagne F, Defer G, Laurent S, Rahman M, Hajipour MJ, Kehoe PG, Mahmoudi M. Therapeutic benefits from nanoparticles: the potential significance of nanoscience in diseases with compromise to the blood brain barrier. Chem. Rev. 2013;113:1877–1903. doi: 10.1021/cr200472g. [DOI] [PubMed] [Google Scholar]
- 179.Cui Y, Xu Q, Chow PK, Wang D, Wang CH. Transferrin-conjugated magnetic silica PLGA nanoparticles loaded with doxorubicin and paclitaxel for brain glioma treatment. Biomaterials. 2013;34:8511–8520. doi: 10.1016/j.biomaterials.2013.07.075. [DOI] [PubMed] [Google Scholar]
- 180.Yoo HS, Lee EA, Park TG. Doxorubicin-conjugated biodegradable polymeric micelles having acid-cleavable linkages. J. Controlled Release. 2002;82:17–27. doi: 10.1016/s0168-3659(02)00088-3. [DOI] [PubMed] [Google Scholar]
- 181.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 182.Torchilin VP. Multifunctional nanocarriers. Adv. Drug Deliv. Rev. 2012;64:302–315. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 183.Alexis F, Pridgen EM, Langer R, Farokhzad OC. Nanoparticle technologies for cancer therapy. Handb. Exp. Pharmacol. 2010:55–86. doi: 10.1007/978-3-642-00477-3_2. [DOI] [PubMed] [Google Scholar]
- 184.Wagner A, Vorauer-Uhl K. Liposome technology for industrial purposes. J. Drug Deliv. 2011;2011:591325. doi: 10.1155/2011/591325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yang H. Nanoparticle-mediated brain-specific drug delivery, imaging, and diagnosis. Pharm. Res. 2010;27:1759–1771. doi: 10.1007/s11095-010-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Guo J, Gao X, Su L, Xia H, Gu G, Pang Z, Jiang X, Yao L, Chen J, Chen H. Aptamer-functionalized PEG-PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials. 2011;32:8010–8020. doi: 10.1016/j.biomaterials.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 187.Gong J, Chen MW, Zheng Y, Wang SP, Wang YT. Polymeric micelles drug delivery system in oncology. J. Controlled Release. 2012;159:312–323. doi: 10.1016/j.jconrel.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 188.Wang CX, Huang LS, Hou LB, Jiang L, Yan ZT, Wang YL, Chen ZL. Antitumor effects of polysorbate-80 coated gemcitabine polybutylcyanoacrylate nanoparticles in vitro and its pharmacodynamics in vivo on C6 glioma cells of a brain tumor model. Brain Res. 2009;1261:91–99. doi: 10.1016/j.brainres.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 189.Ling Y, Wei K, Zou F, Zhong SZ. Temozolomide loaded PLGA-based superparamagnetic nanoparticles for magnetic resonance imaging and treatment of malignant glioma. Intl. J. Pharm. 2012;430:266–275. doi: 10.1016/j.ijpharm.2012.03.047. [DOI] [PubMed] [Google Scholar]
- 190.Costantino L, Boraschi D. Is there a clinical future for polymeric nanoparticles as brain-targeting drug delivery agents? Drug Discov. Today. 2012;17:367–378. doi: 10.1016/j.drudis.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 191.Lu W, Sun Q, Wan J, She ZJ, Jiang XG. Cationic albumin-conjugated pegylated nanoparticles allow gene delivery into brain tumors via intravenous administration. Cancer Res. 2006;66:11878–11887. doi: 10.1158/0008-5472.CAN-06-2354. [DOI] [PubMed] [Google Scholar]
- 192.Orive G, Anitua E, Pedraz JL, Emerich DF. Biomaterials for promoting brain protection, repair and regeneration. Nat. Rev. Neurosci. 2009;10:682–692. doi: 10.1038/nrn2685. [DOI] [PubMed] [Google Scholar]
- 193.Mignani S, El Kazzouli S, Bousmina M, Majoral JP. Expand classical drug administration ways by emerging routes using dendrimer drug delivery systems: A concise overview. Adv. Drug Deliv. Rev. 2013 doi: 10.1016/j.addr.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 194.Dhanikula RS, Argaw A, Bouchard JF, Hildgen P. Methotrexate loaded polyether-copolyester dendrimers for the treatment of gliomas: Enhanced efficacy and intratumoral transport capability. Mol. Pharm. 2008;5:105–116. doi: 10.1021/mp700086j. [DOI] [PubMed] [Google Scholar]
- 195.Hua MY, Liu HL, Yang HW, Chen PY, Tsai RY, Huang CY, Tseng IC, Lyu LA, Ma CC, Tang HJ, Yen TC, Wei KC. The effectiveness of a magnetic nanoparticle-based delivery system for BCNU in the treatment of gliomas. Biomaterials. 2011;32:516–527. doi: 10.1016/j.biomaterials.2010.09.065. [DOI] [PubMed] [Google Scholar]
- 196.Chertok B, David AE, Yang VC. Brain tumor targeting of magnetic nanoparticles for potential drug delivery: effect of administration route and magnetic field topography. J. Controlled Release. 2011;155:393–399. doi: 10.1016/j.jconrel.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wankhede M, Bouras A, Kaluzova M, Hadjipanayis CG. Magnetic nanoparticles: an emerging technology for malignant brain tumor imaging and therapy. Expert Rev. Clin. Pharmacol. 2012;5:173–186. doi: 10.1586/ecp.12.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Silva AC, Oliveira TR, Mamani JB, Malheiros SM, Malavolta L, Pavon LF, Sibov TT, Amaro E, Jr., Tannus A, Vidoto EL, Martins MJ, Santos RS, Gamarra LF. Application of hyperthermia induced by superparamagnetic iron oxide nanoparticles in glioma treatment. Int. J. Nanomed. 2011;6:591–603. doi: 10.2147/IJN.S14737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, Orawa H, Budach V, Jordan A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neurooncol. 2011;103:317–324. doi: 10.1007/s11060-010-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.van Landeghem FKH, Maier-Hauff K, Jordan A, Hoffmann KT, Gneveckow U, Scholz R, Thiesen B, Bruck W, von Deimling A. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials. 2009;30:52–57. doi: 10.1016/j.biomaterials.2008.09.044. [DOI] [PubMed] [Google Scholar]
- 201.Samia AC, Chen X, Burda C. Semiconductor quantum dots for photodynamic therapy. J. Am. Chem. Soc. 2003;125:15736–15737. doi: 10.1021/ja0386905. [DOI] [PubMed] [Google Scholar]
- 202.Bernardi RJ, Lowery AR, Thompson PA, Blaney SM, West JL. Immunonanoshells for targeted photothermal ablation in medulloblastoma and glioma: an in vitro evaluation using human cell lines. J. Neurooncol. 2008;86:165–172. doi: 10.1007/s11060-007-9467-3. [DOI] [PubMed] [Google Scholar]
- 203.McCarthy JR, Weissleder R. Multifunctional magnetic nanoparticles for targeted imaging and therapy. Adv. Drug Deliv. Rev. 2008;60:1241–1251. doi: 10.1016/j.addr.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kim J, Lee JE, Lee SH, Yu JH, Lee JH, Park TG, Hyeon T. Designed fabrication of a multifunctional polymer nanomedical platform for simultaneous cancer-targeted imaging and magnetically guided drug delivery. Adv. Mater. 2008;20:478–483. [Google Scholar]
- 205.Jung JJ, Solanki A, Memoli KA, Kamei K, Kim H, Drahl MA, Williams LJ, Tseng HR, Lee K. Selective inhibition of human brain tumor cells through multifunctional quantum-dot-based siRNA delivery. Angew. Chem. Intl. Ed. 2010;49:103–107. doi: 10.1002/anie.200905126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Foy SP, Manthe RL, Foy ST, Dimitrijevic S, Krishnamurthy N, Labhasetwar V. Optical imaging and magnetic field targeting of magnetic nanoparticles in tumors. ACS Nano. 2010;4:5217–5224. doi: 10.1021/nn101427t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Liu HL, Hua MY, Yang HW, Huang CY, Chu PC, Wu JS, Tseng IC, Wang JJ, Yen TC, Chen PY, Wei KC. Magnetic resonance monitoring of focused ultrasound/magnetic nanoparticle targeting delivery of therapeutic agents to the brain. Proc. Natl. Acad. Sci. U S A. 2010;107:15205–15210. doi: 10.1073/pnas.1003388107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Veiseh O, Gunn JW, Kievit FM, Sun C, Fang C, Lee JS, Zhang M. Inhibition of tumor-cell invasion with chlorotoxin-bound superparamagnetic nanoparticles. Small. 2009;5:256–264. doi: 10.1002/smll.200800646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Siegal T, Horowitz A, Gabizon A. Doxorubicin encapsulated in sterically stabilized liposomes for the treatment of a brain-tumor model - biodistribution and therapeutic efficacy. J. Neurosurg. 1995;83:1029–1037. doi: 10.3171/jns.1995.83.6.1029. [DOI] [PubMed] [Google Scholar]
- 210.Caron WP, Clewell H, Dedrick R, Ramanathan RK, Davis WL, Yu N, Tonda M, Schellens JH, Beijnen JH, Zamboni WC. Allometric scaling of pegylated liposomal anticancer drugs. J. Pharmacokinet. Pharmacodyn. 2011;38:653–669. doi: 10.1007/s10928-011-9213-5. [DOI] [PubMed] [Google Scholar]
- 211.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 212.Etame AB, Diaz RJ, Smith CA, Mainprize TG, Hynynen K, Rutka JT. Focused ultrasound disruption of the blood-brain barrier: a new frontier for therapeutic delivery in molecular neurooncology. Neurosurg. Focus. 2012;32:E3. doi: 10.3171/2011.10.FOCUS11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hynynen K, McDannold N, Vykhodtseva N, Raymond S, Weissleder R, Jolesz FA, Sheikov N. Focal disruption of the blood-brain barrier due to 260-kHz ultrasound bursts: a method for molecular imaging and targeted drug delivery. J. Neurosurg. 2006;105:445–454. doi: 10.3171/jns.2006.105.3.445. [DOI] [PubMed] [Google Scholar]
- 214.Shapiro B, Dormer K, Rutel IB. A two-magnet system to push therapeutic nanoparticles. AIP Conf. Proc. 2010;1311:77–88. doi: 10.1063/1.3530064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kong SD, Lee J, Ramachandran S, Eliceiri BP, Shubayev VI, Lal R, Jin S. Magnetic targeting of nanoparticles across the intact blood-brain barrier. J. Controlled Release. 2012;164:49–57. doi: 10.1016/j.jconrel.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Han L, Ren Y, Long LX, Zhong Y, Shen CH, Pu PY, Yuan XB, Kang CS. Inhibition of C6 glioma in vivo by combination chemotherapy of implantation of polymer wafer and intracarotid perfusion of transferrin-decorated nanoparticles. Onco. Rep. 2012;27:121–128. doi: 10.3892/or.2011.1459. [DOI] [PubMed] [Google Scholar]
- 217.Chertok B, David AE, Yang VC. Polyethyleneimine-modified iron oxide nanoparticles for brain tumor drug delivery using magnetic targeting and intra-carotid administration. Biomaterials. 2010;31:6317–6324. doi: 10.1016/j.biomaterials.2010.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Jain TK, Reddy MK, Morales MA, Leslie-Pelecky DL, Labhasetwar V. Biodistribution clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol. Pharm. 2008;5:316–327. doi: 10.1021/mp7001285. [DOI] [PubMed] [Google Scholar]
- 219.Wang LM, Li YF, Zhou LJ, Liu Y, Meng L, Zhang K, Wu XC, Zhang LL, Li B, Chen CY. Characterization of gold nanorods in vivo by integrated analytical techniques: their uptake, retention, and chemical forms. Anal. Bioanal. Chem. 2010;396:1105–1114. doi: 10.1007/s00216-009-3302-y. [DOI] [PubMed] [Google Scholar]
- 220.Guerin C, Olivi A, Weingart JD, Lawson HC, Brem H. Recent advances in brain tumor therapy: local intracerebral drug delivery by polymers. Invest. New Drugs. 2004;22:27–37. doi: 10.1023/b:drug.0000006172.65135.3e. [DOI] [PubMed] [Google Scholar]
- 221.Raza SM, Pradilla G, Legnani FG, Thai QA, Olivi A, Weingart JD, Brem H. Local delivery of antineoplastic agents by controlled-release polymers for the treatment of malignant brain tumours. Expert Opin. Biol. Ther. 2005;5:477–494. doi: 10.1517/14712598.5.4.477. [DOI] [PubMed] [Google Scholar]
- 222.Allard E, Passirani C, Benoit JP. Convection-enhanced delivery of nanocarriers for the treatment of brain tumors. Biomaterials. 2009;30:2302–2318. doi: 10.1016/j.biomaterials.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 223.Groothuis DR, Ward S, Itskovich AC, Dobrescu C, Allen CV, Dills C, Levy RM. Comparison of 14C-sucrose delivery to the brain by intravenous, intraventricular, and convection-enhanced intracerebral infusion. J. Neurosurg. 1999;90:321–331. doi: 10.3171/jns.1999.90.2.0321. [DOI] [PubMed] [Google Scholar]
- 224.Vavra M, Ali MJ, Kang EW, Navalitloha Y, Ebert A, Allen CV, Groothuis DR. Comparative pharmacokinetics of 14C-sucrose in RG-2 rat gliomas after intravenous and convection-enhanced delivery. Neuro. Oncol. 2004;6:104–112. doi: 10.1215/S1152851703000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wu G, Barth RF, Yang WL, Kawabata S, Zhang LW, Green-Church K. Targeted delivery of methotrexate to epidermal growth factor receptor-positive brain tumors by means of cetuximab (IMC-C225) dendrimer bioconjugates. Mol. Cancer Ther. 2006;5:52–59. doi: 10.1158/1535-7163.MCT-05-0325. [DOI] [PubMed] [Google Scholar]
- 226.Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM, Breakefield XO, Snyder EY. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc. Natl. Acad. Sci. U S A. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Madsen SJ, Baek SK, Makkouk AR, Krasieva T, Hirschberg H. Macrophages as cell-based delivery systems for nanoshells in photothermal therapy. Ann. Biomed. Eng. 2012;40:507–515. doi: 10.1007/s10439-011-0415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hickey WF. Leukocyte traffic in the central nervous system: the participants and their roles. Semin. Immun. 1999;11:125–137. doi: 10.1006/smim.1999.0168. [DOI] [PubMed] [Google Scholar]
- 229.Benedetti S, Pirola B, Pollo B, Magrassi L, Bruzzone MG, Rigamonti D, Galli R, Selleri S, Di Meco F, De Fraja C, Vescovi A, Cattaneo E, Finocchiaro G. Gene therapy of experimental brain tumors using neural progenitor cells. Nat. Med. 2000;6:447–450. doi: 10.1038/74710. [DOI] [PubMed] [Google Scholar]
- 230.Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J, Andreeff M, Lang FF. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 231.Nakamura K, Ito Y, Kawano Y, Kurozumi K, Kobune M, Tsuda H, Bizen A, Honmou O, Niitsu Y, Hamada H. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004;11:1155–1164. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- 232.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 233.Wu X, Hu J, Zhou LF, Mao Y, Yang B, Gao L, Xie R, Xu F, Zhang D, Liu J, Zhu JH. In vivo tracking of superparamagnetic iron oxide nanoparticle-labeled mesenchymal stem cell tropism to malignant gliomas using magnetic resonance imaging. J. Neurosurg. 2008;108:320–329. doi: 10.3171/JNS/2008/108/2/0320. [DOI] [PubMed] [Google Scholar]
- 234.Auffinger B, Morshed R, Tobias A, Cheng Y, Ahmed AU, Lesniak MS. Drug-loaded nanoparticle systems and adult stem cells: a potential marriage for the treatment of malignant glioma? Oncotarget. 2013;4:378–396. doi: 10.18632/oncotarget.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Li LL, Guan YQ, Liu HY, Hao NJ, Liu TL, Meng XW, Fu CH, Li YZ, Qu QL, Zhang YG, Ji SY, Chen L, Chen D, Tang FQ. Silica nanorattle-doxorubicin-anchored mesenchymal stem cells for tumor-tropic therapy. ACS Nano. 2011;5:7462–7470. doi: 10.1021/nn202399w. [DOI] [PubMed] [Google Scholar]
- 236.Roger M, Clavreul A, Huynh NT, Passirani C, Schiller P, Vessieres A, Montero-Menei C, Menei P. Ferrociphenol lipid nanocapsule delivery by mesenchymal stromal cells in brain tumor therapy. Intl. J. Pharm. 2012;423:63–68. doi: 10.1016/j.ijpharm.2011.04.058. [DOI] [PubMed] [Google Scholar]
- 237.Cheng Y, Morshed R, Cheng SH, Tobias A, Auffinger B, Wainwright DA, Zhang L, Yunis C, Han Y, Chen CT, Lo LW, Aboody KS, Ahmed AU, Lesniak MS. Nanoparticle-programmed self-destructive neural stem cells for glioblastoma targeting and therapy. Small. 2013 doi: 10.1002/smll.201301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ahmed AU, Thaci B, Alexiades NG, Han Y, Qian S, Liu F, Balyasnikova IV, Ulasov IY, Aboody KS, Lesniak MS. Neural stem cell-based cell carriers enhance therapeutic efficacy of an oncolytic adenovirus in an orthotopic mouse model of human glioblastoma. Mol. Ther. 2011;19:1714–1726. doi: 10.1038/mt.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Heidenreich R, Machein M, Nicolaus A, Hilbig A, Wild C, Clauss M, Plate KH, Breier G. Inhibition of solid tumor growth by gene transfer of VEGF receptor-1 mutants. Intl. J. Cancer. 2004;111:348–357. doi: 10.1002/ijc.20260. [DOI] [PubMed] [Google Scholar]
- 240.Schmidt NO, Przylecki W, Yang W, Ziu M, Teng Y, Kim SU, Black PM, Aboody KS, Carroll RS. Brain tumor tropism of transplanted human neural stem cells is induced by vascular endothelial growth factor. Neoplasia. 2005;7:623–629. doi: 10.1593/neo.04781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Berman SMC, Walczak P, Bulte JWM. Tracking stem cells using magnetic nanoparticles, Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011;3:343–355. doi: 10.1002/wnan.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Liu G, Wang Z, Lu J, Xia C, Gao F, Gong Q, Song B, Zhao X, Shuai X, Chen X, Ai H, Gu Z. Low molecular weight alkyl-polycation wrapped magnetite nanoparticle clusters as MRI probes for stem cell labeling and in vivo imaging. Biomaterials. 2011;32:528–537. doi: 10.1016/j.biomaterials.2010.08.099. [DOI] [PubMed] [Google Scholar]
- 243.Feng SS, Mu L, Win KY, Huang G. Nanoparticles of biodegradable polymers for clinical administration of paclitaxel. Curr. Med. Chem. 2004;11:413–424. doi: 10.2174/0929867043455909. [DOI] [PubMed] [Google Scholar]
- 244.Desai N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012;14:282–295. doi: 10.1208/s12248-012-9339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kanjickal D, Lopina S, Evancho-Chapman MM, Schmidt S, Donovan D. Effects of sterilization on poly(ethylene glycol) hydrogels. J. Biomed. Mater. Res. A. 2008;87:608–617. doi: 10.1002/jbm.a.31811. [DOI] [PubMed] [Google Scholar]
- 246.Bozdag S, Dillen K, Vandervoort J, Ludwig A. The effect of freeze-drying with different cryoprotectants and gamma-irradiation sterilization on the characteristics of ciprofloxacin HCl-loaded poly(D,L-lactide-glycolide) nanoparticles. J. Pharm. Pharmacol. 2005;57:699–707. doi: 10.1211/0022357056145. [DOI] [PubMed] [Google Scholar]
- 247.Kempner ES. Effects of high-energy electrons and gamma rays directly on protein molecules. J. Pharm. Sci. 2001;90:1637–1646. doi: 10.1002/jps.1114. [DOI] [PubMed] [Google Scholar]
- 248.Ventola CL. The nanomedicine revolution: part 3: regulatory and safety challenges. P T. 2012;37:631–639. [PMC free article] [PubMed] [Google Scholar]