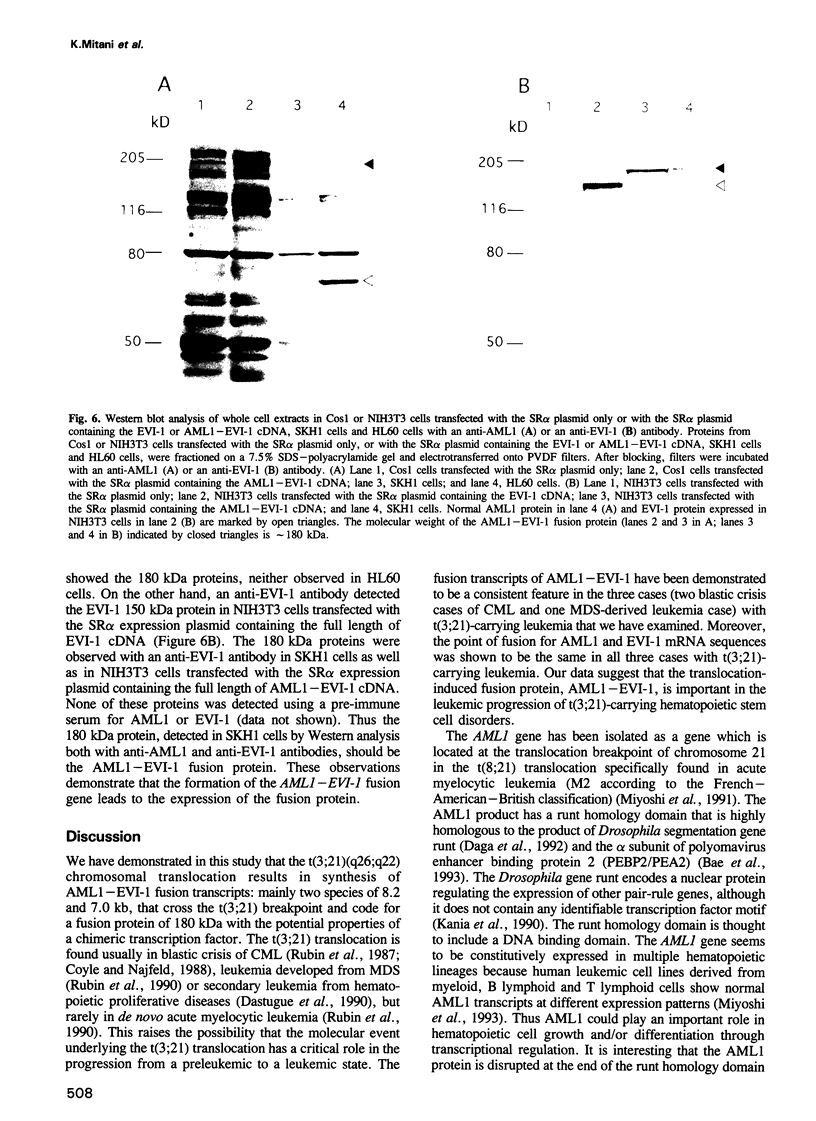
Abstract
The t(3;21)(q26;q22) translocation, which is one of the consistent chromosomal abnormalities found in blastic crisis of chronic myelocytic leukemia (CML), is thought to play an important role in the leukemic progression of CML to an acute blastic crisis phase. The AML1 gene, which is located at the translocation breakpoint of the t(8;21)(q22;q22) translocation found in acute myelocytic leukemia, was also rearranged by the t(3;21)(q26;q22) translocation. Screening of a cDNA library of the t(3;21)-carrying leukemic cell line cells (SKH1) resulted in the isolation of two potentially complete AML1-EVI-1 chimeric cDNAs of 6 kb. Two species of AML1-EVI-1 fusion transcripts of 8.2 and 7.0 kb were detected in SKH1 cells. These cells expressed the 180 kDa AML1-EVI-1 fusion protein containing an N-terminal half of AML1 including a runt homology domain which is fused to the entire zinc finger EVI-1 protein. The AML1-EVI-1 fusion transcript was consistent in all three cases of the t(3;21)-carrying leukemia examined by RNA-based PCR. These findings strongly suggest that the t(3;21) translocation results in the formation of a new class of chimeric transcription factor which could contribute to the leukemic progression of CML through interference with cell growth and differentiation.
Full text
PDF


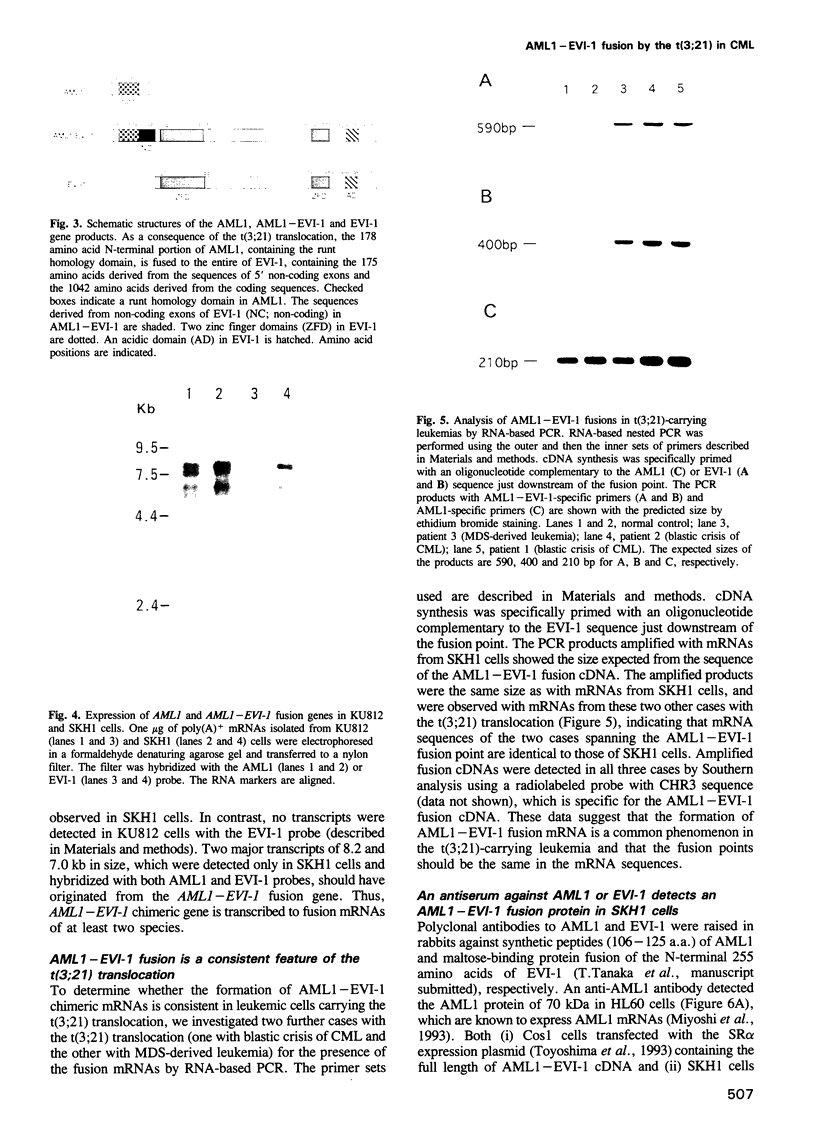


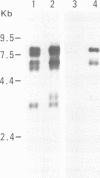
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahuja H., Bar-Eli M., Arlin Z., Advani S., Allen S. L., Goldman J., Snyder D., Foti A., Cline M. The spectrum of molecular alterations in the evolution of chronic myelocytic leukemia. J Clin Invest. 1991 Jun;87(6):2042–2047. doi: 10.1172/JCI115234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alimena G., De Cuia M. R., Diverio D., Gastaldi R., Nanni M. The karyotype of blastic crisis. Cancer Genet Cytogenet. 1987 May;26(1):39–50. doi: 10.1016/0165-4608(87)90131-2. [DOI] [PubMed] [Google Scholar]
- Bae S. C., Yamaguchi-Iwai Y., Ogawa E., Maruyama M., Inuzuka M., Kagoshima H., Shigesada K., Satake M., Ito Y. Isolation of PEBP2 alpha B cDNA representing the mouse homolog of human acute myeloid leukemia gene, AML1. Oncogene. 1993 Mar;8(3):809–814. [PubMed] [Google Scholar]
- Bartholomew C., Ihle J. N. Retroviral insertions 90 kilobases proximal to the Evi-1 myeloid transforming gene activate transcription from the normal promoter. Mol Cell Biol. 1991 Apr;11(4):1820–1828. doi: 10.1128/mcb.11.4.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew C., Morishita K., Askew D., Buchberg A., Jenkins N. A., Copeland N. G., Ihle J. N. Retroviral insertions in the CB-1/Fim-3 common site of integration activate expression of the Evi-1 gene. Oncogene. 1989 May;4(5):529–534. [PubMed] [Google Scholar]
- Chen Z., Morgan R., Baer M. R., Ligorsky R., Sandberg A. A. Translocation (3;21) characterizes crises in myeloid stem cell disorders. Cancer Genet Cytogenet. 1991 Dec;57(2):153–159. doi: 10.1016/0165-4608(91)90146-l. [DOI] [PubMed] [Google Scholar]
- Cleary M. L. Oncogenic conversion of transcription factors by chromosomal translocations. Cell. 1991 Aug 23;66(4):619–622. doi: 10.1016/0092-8674(91)90105-8. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Coyle T., Najfeld V. Translocation (3;21) in Philadelphia chromosome-positive chronic myelogenous leukemia prior to the onset of blast crisis. Am J Hematol. 1988 Jan;27(1):56–59. doi: 10.1002/ajh.2830270113. [DOI] [PubMed] [Google Scholar]
- Daga A., Tighe J. E., Calabi F. Leukaemia/Drosophila homology. Nature. 1992 Apr 9;356(6369):484–484. doi: 10.1038/356484b0. [DOI] [PubMed] [Google Scholar]
- Daley G. Q., Van Etten R. A., Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990 Feb 16;247(4944):824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- Dastugue N., Pris J., Colombies P. Translocation t(3;21)(q26;q22) in acute myeloblastic leukemia secondary to polycythemia vera. Cancer Genet Cytogenet. 1990 Feb;44(2):275–276. doi: 10.1016/0165-4608(90)90057-h. [DOI] [PubMed] [Google Scholar]
- Feinstein E., Cimino G., Gale R. P., Canaani E. Initiation and progression of chronic myelogenous leukemia. Leukemia. 1992;6 (Suppl 1):37–43. [PubMed] [Google Scholar]
- Fialkow P. J., Jacobson R. J., Papayannopoulou T. Chronic myelocytic leukemia: clonal origin in a stem cell common to the granulocyte, erythrocyte, platelet and monocyte/macrophage. Am J Med. 1977 Jul;63(1):125–130. doi: 10.1016/0002-9343(77)90124-3. [DOI] [PubMed] [Google Scholar]
- Foti A., Ahuja H. G., Allen S. L., Koduru P., Schuster M. W., Schulman P., Bar-Eli M., Cline M. J. Correlation between molecular and clinical events in the evolution of chronic myelocytic leukemia to blast crisis. Blood. 1991 Jun 1;77(11):2441–2444. [PubMed] [Google Scholar]
- Heisterkamp N., Stephenson J. R., Groffen J., Hansen P. F., de Klein A., Bartram C. R., Grosveld G. Localization of the c-ab1 oncogene adjacent to a translocation break point in chronic myelocytic leukaemia. Nature. 1983 Nov 17;306(5940):239–242. doi: 10.1038/306239a0. [DOI] [PubMed] [Google Scholar]
- Kakizuka A., Miller W. H., Jr, Umesono K., Warrell R. P., Jr, Frankel S. R., Murty V. V., Dmitrovsky E., Evans R. M. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RAR alpha with a novel putative transcription factor, PML. Cell. 1991 Aug 23;66(4):663–674. doi: 10.1016/0092-8674(91)90112-c. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Look A. T., Baltimore D. The human t(1;19) translocation in pre-B ALL produces multiple nuclear E2A-Pbx1 fusion proteins with differing transforming potentials. Genes Dev. 1991 Mar;5(3):358–368. doi: 10.1101/gad.5.3.358. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Murre C., Sun X. H., Baltimore D. A new homeobox gene contributes the DNA binding domain of the t(1;19) translocation protein in pre-B ALL. Cell. 1990 Feb 23;60(4):547–555. doi: 10.1016/0092-8674(90)90658-2. [DOI] [PubMed] [Google Scholar]
- Kania M. A., Bonner A. S., Duffy J. B., Gergen J. P. The Drosophila segmentation gene runt encodes a novel nuclear regulatory protein that is also expressed in the developing nervous system. Genes Dev. 1990 Oct;4(10):1701–1713. doi: 10.1101/gad.4.10.1701. [DOI] [PubMed] [Google Scholar]
- Kishi K. A new leukemia cell line with Philadelphia chromosome characterized as basophil precursors. Leuk Res. 1985;9(3):381–390. doi: 10.1016/0145-2126(85)90060-8. [DOI] [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Witte O. N. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984 Jul;37(3):1035–1042. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Miyoshi H., Kozu T., Shimizu K., Enomoto K., Maseki N., Kaneko Y., Kamada N., Ohki M. The t(8;21) translocation in acute myeloid leukemia results in production of an AML1-MTG8 fusion transcript. EMBO J. 1993 Jul;12(7):2715–2721. doi: 10.1002/j.1460-2075.1993.tb05933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H., Shimizu K., Kozu T., Maseki N., Kaneko Y., Ohki M. t(8;21) breakpoints on chromosome 21 in acute myeloid leukemia are clustered within a limited region of a single gene, AML1. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10431–10434. doi: 10.1073/pnas.88.23.10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinaga N., Yonehara S., Tomita Y., Kuwata T. Insensitivity to interferon of two subclones of human endometrial carcinoma cell line, HEC-1. Int J Cancer. 1983 Jan 15;31(1):21–28. doi: 10.1002/ijc.2910310105. [DOI] [PubMed] [Google Scholar]
- Morishita K., Parganas E., Douglass E. C., Ihle J. N. Unique expression of the human Evi-1 gene in an endometrial carcinoma cell line: sequence of cDNAs and structure of alternatively spliced transcripts. Oncogene. 1990 Jul;5(7):963–971. [PubMed] [Google Scholar]
- Morishita K., Parganas E., Matsugi T., Ihle J. N. Expression of the Evi-1 zinc finger gene in 32Dc13 myeloid cells blocks granulocytic differentiation in response to granulocyte colony-stimulating factor. Mol Cell Biol. 1992 Jan;12(1):183–189. doi: 10.1128/mcb.12.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita K., Parganas E., Parham D. M., Matsugi T., Ihle J. N. The Evi-1 zinc finger myeloid transforming gene is normally expressed in the kidney and in developing oocytes. Oncogene. 1990 Sep;5(9):1419–1423. [PubMed] [Google Scholar]
- Morishita K., Parganas E., William C. L., Whittaker M. H., Drabkin H., Oval J., Taetle R., Valentine M. B., Ihle J. N. Activation of EVI1 gene expression in human acute myelogenous leukemias by translocations spanning 300-400 kilobases on chromosome band 3q26. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3937–3941. doi: 10.1073/pnas.89.9.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita K., Parker D. S., Mucenski M. L., Jenkins N. A., Copeland N. G., Ihle J. N. Retroviral activation of a novel gene encoding a zinc finger protein in IL-3-dependent myeloid leukemia cell lines. Cell. 1988 Sep 9;54(6):831–840. doi: 10.1016/s0092-8674(88)91175-0. [DOI] [PubMed] [Google Scholar]
- Nichols J., Nimer S. D. Transcription factors, translocations, and leukemia. Blood. 1992 Dec 15;80(12):2953–2963. [PubMed] [Google Scholar]
- Nourse J., Mellentin J. D., Galili N., Wilkinson J., Stanbridge E., Smith S. D., Cleary M. L. Chromosomal translocation t(1;19) results in synthesis of a homeobox fusion mRNA that codes for a potential chimeric transcription factor. Cell. 1990 Feb 23;60(4):535–545. doi: 10.1016/0092-8674(90)90657-z. [DOI] [PubMed] [Google Scholar]
- Nucifora G., Birn D. J., Erickson P., Gao J., LeBeau M. M., Drabkin H. A., Rowley J. D. Detection of DNA rearrangements in the AML1 and ETO loci and of an AML1/ETO fusion mRNA in patients with t(8;21) acute myeloid leukemia. Blood. 1993 Feb 15;81(4):883–888. [PubMed] [Google Scholar]
- Perkins A. S., Fishel R., Jenkins N. A., Copeland N. G. Evi-1, a murine zinc finger proto-oncogene, encodes a sequence-specific DNA-binding protein. Mol Cell Biol. 1991 May;11(5):2665–2674. doi: 10.1128/mcb.11.5.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitts T. H. Translocations, master genes, and differences between the origins of acute and chronic leukemias. Cell. 1991 Nov 15;67(4):641–644. doi: 10.1016/0092-8674(91)90057-6. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973 Jun 1;243(5405):290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- Rubin C. M., Larson R. A., Anastasi J., Winter J. N., Thangavelu M., Vardiman J. W., Rowley J. D., Le Beau M. M. t(3;21)(q26;q22): a recurring chromosomal abnormality in therapy-related myelodysplastic syndrome and acute myeloid leukemia. Blood. 1990 Dec 15;76(12):2594–2598. [PubMed] [Google Scholar]
- Rubin C. M., Larson R. A., Bitter M. A., Carrino J. J., Le Beau M. M., Diaz M. O., Rowley J. D. Association of a chromosomal 3;21 translocation with the blast phase of chronic myelogenous leukemia. Blood. 1987 Nov;70(5):1338–1342. [PubMed] [Google Scholar]
- Schneider N. R., Bowman W. P., Frenkel E. P. Translocation (3;21)(q26;q22) in secondary leukemia. Report of two cases and literature review. Ann Genet. 1991;34(3-4):256–263. [PubMed] [Google Scholar]
- Solomon E., Borrow J., Goddard A. D. Chromosome aberrations and cancer. Science. 1991 Nov 22;254(5035):1153–1160. doi: 10.1126/science.1957167. [DOI] [PubMed] [Google Scholar]
- Toyoshima H., Kozutsumi H., Maru Y., Hagiwara K., Furuya A., Mioh H., Hanai N., Takaku F., Yazaki Y., Hirai H. Differently spliced cDNAs of human leukocyte tyrosine kinase receptor tyrosine kinase predict receptor proteins with and without a tyrosine kinase domain and a soluble receptor protein. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5404–5408. doi: 10.1073/pnas.90.12.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Thé H., Lavau C., Marchio A., Chomienne C., Degos L., Dejean A. The PML-RAR alpha fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell. 1991 Aug 23;66(4):675–684. doi: 10.1016/0092-8674(91)90113-d. [DOI] [PubMed] [Google Scholar]
- von Lindern M., Fornerod M., van Baal S., Jaegle M., de Wit T., Buijs A., Grosveld G. The translocation (6;9), associated with a specific subtype of acute myeloid leukemia, results in the fusion of two genes, dek and can, and the expression of a chimeric, leukemia-specific dek-can mRNA. Mol Cell Biol. 1992 Apr;12(4):1687–1697. doi: 10.1128/mcb.12.4.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]