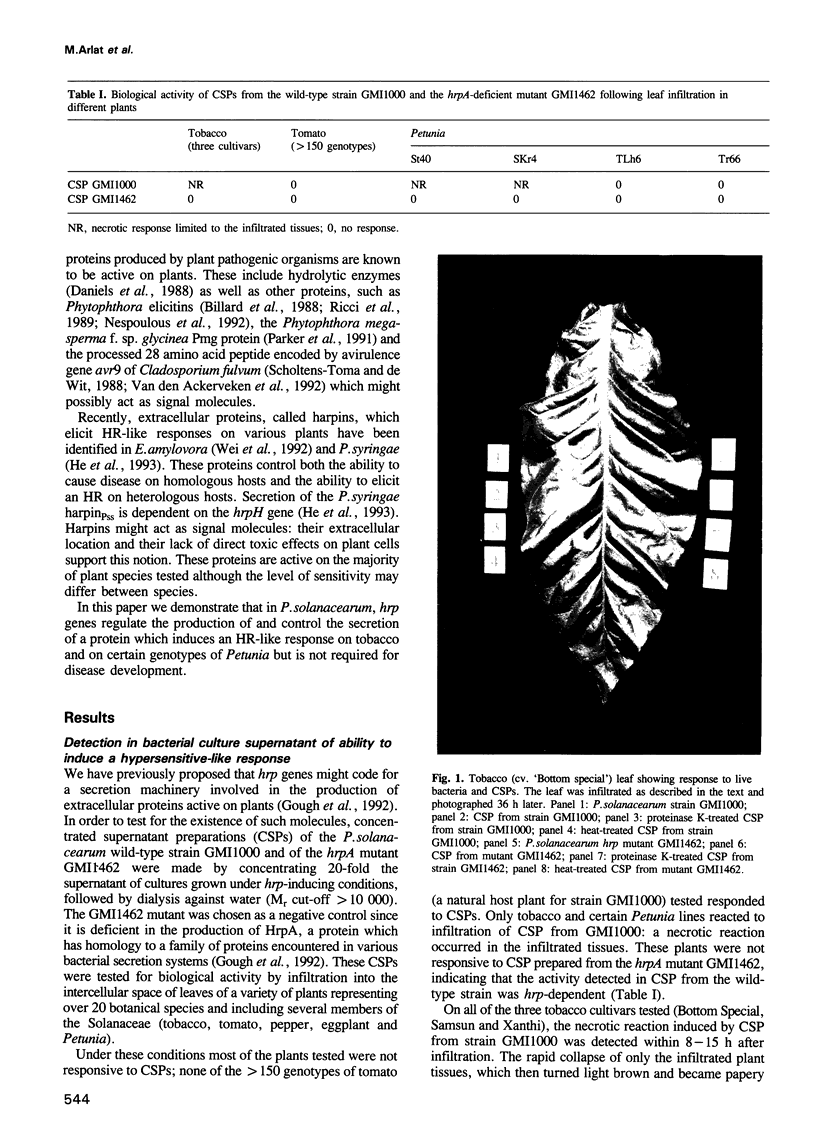
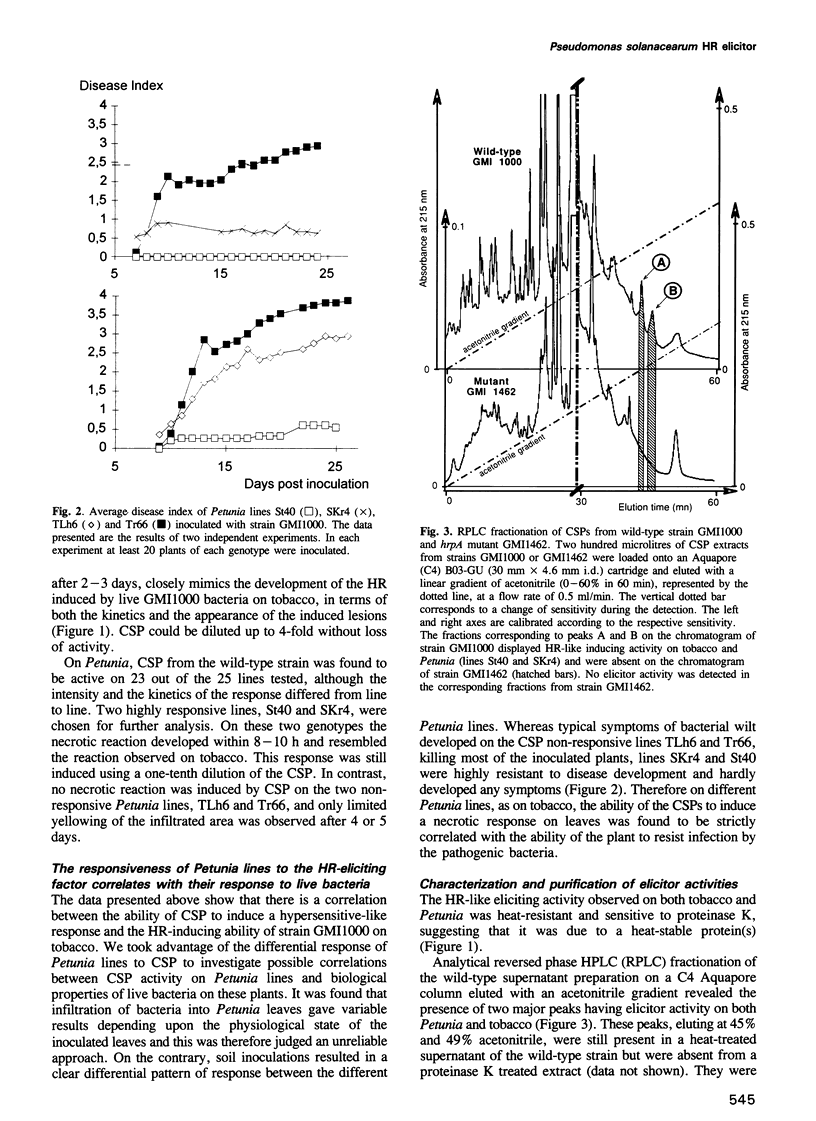
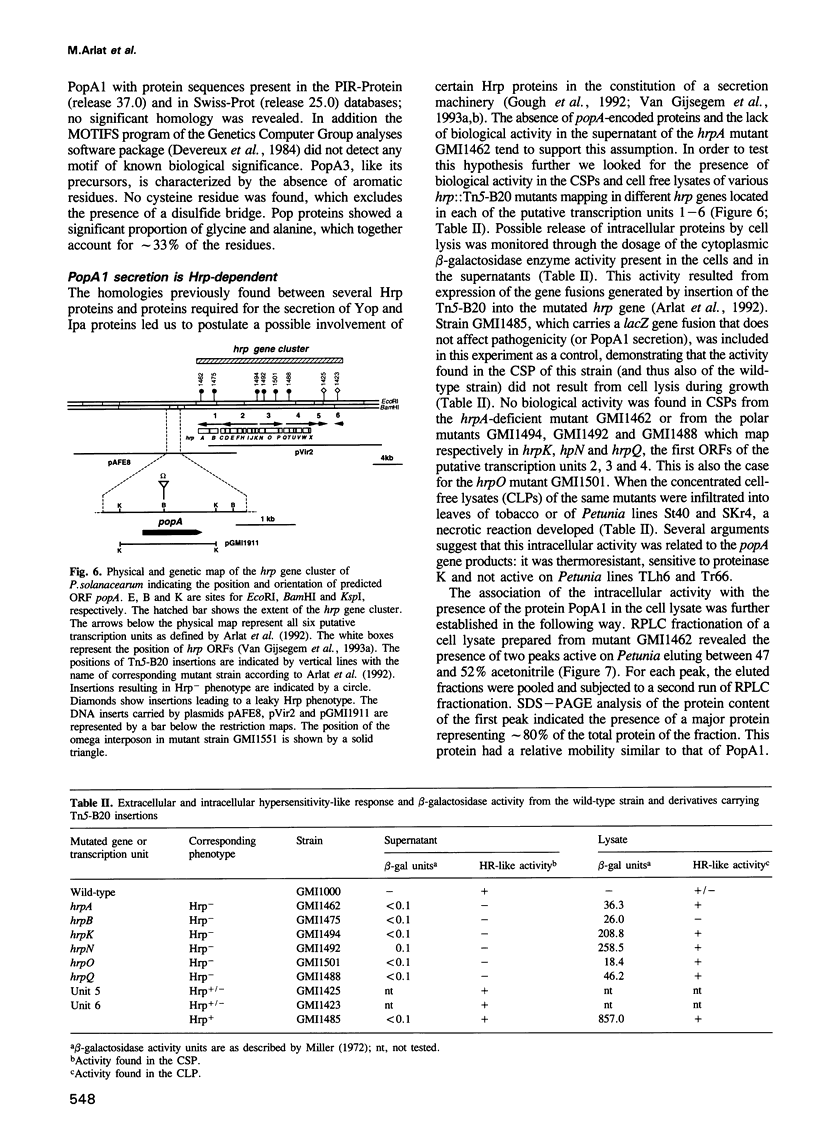
Abstract
This paper describes the identification of a new class of extracellular bacterial proteins, typified by PopA1 and its derivative PopA3, which act as specific hypersensitive response (HR) elicitors. These two heat-stable proteins, with HR-like elicitor activities on tobacco (non-host plant) but without activity on tomato (host plant), have been characterized from the supernatant of the plant pathogenic bacterium Pseudomonas solanacearum strain GMI1000. These two proteins induced the same pattern of response on Petunia, as a function of the genotypes tested. popA, the structural gene for PopA1, maps outside of the hrp gene cluster but belongs to the hrp regulon. The amino acid sequence of PopA1 does not show homology to any characterized proteins. Its secretion is dependent on hrp genes and is followed by stepwise removal of the 93 amino-terminal amino acids, producing the protein PopA3. Petunia lines responsive to PopA3 and its precursors were resistant to infection by strain GMI1000, whereas non-responsive lines were sensitive, suggesting that popA could be an avirulence gene. A popA mutant remained fully pathogenic on sensitive plants, indicating that this gene is not essential for pathogenicity. While lacking PopA1, this mutant, which remained avirulent on tobacco and on resistant Petunia lines, still produced additional extracellular necrogenic compounds. On the basis of both their structural features and the biological properties of the popA mutant, PopA1 and PopA3 clearly differ from hairpins characterized in other plant pathogenic bacteria.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allaoui A., Sansonetti P. J., Parsot C. MxiD, an outer membrane protein necessary for the secretion of the Shigella flexneri lpa invasins. Mol Microbiol. 1993 Jan;7(1):59–68. doi: 10.1111/j.1365-2958.1993.tb01097.x. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Arlat M., Gough C. L., Zischek C., Barberis P. A., Trigalet A., Boucher C. A. Transcriptional organization and expression of the large hrp gene cluster of Pseudomonas solanacearum. Mol Plant Microbe Interact. 1992 Mar-Apr;5(2):187–193. doi: 10.1094/mpmi-5-187. [DOI] [PubMed] [Google Scholar]
- Boivin C., Camut S., Malpica C. A., Truchet G., Rosenberg C. Rhizobium meliloti Genes Encoding Catabolism of Trigonelline Are Induced under Symbiotic Conditions. Plant Cell. 1990 Dec;2(12):1157–1170. doi: 10.1105/tpc.2.12.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher C. A., Van Gijsegem F., Barberis P. A., Arlat M., Zischek C. Pseudomonas solanacearum genes controlling both pathogenicity on tomato and hypersensitivity on tobacco are clustered. J Bacteriol. 1987 Dec;169(12):5626–5632. doi: 10.1128/jb.169.12.5626-5632.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney B. F., Denny T. P. A cloned avirulence gene from Pseudomonas solanacearum determines incompatibility on Nicotiana tabacum at the host species level. J Bacteriol. 1990 Sep;172(9):4836–4843. doi: 10.1128/jb.172.9.4836-4843.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis G. R., Biot T., Lambert de Rouvroit C., Michiels T., Mulder B., Sluiters C., Sory M. P., Van Bouchaute M., Vanooteghem J. C. The Yersinia yop regulon. Mol Microbiol. 1989 Oct;3(10):1455–1459. doi: 10.1111/j.1365-2958.1989.tb00129.x. [DOI] [PubMed] [Google Scholar]
- Curry K. A., Tomich C. S. Effect of ribosome binding site on gene expression in Escherichia coli. DNA. 1988 Apr;7(3):173–179. doi: 10.1089/dna.1988.7.173. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenselau S., Balbo I., Bonas U. Determinants of pathogenicity in Xanthomonas campestris pv. vesicatoria are related to proteins involved in secretion in bacterial pathogens of animals. Mol Plant Microbe Interact. 1992 Sep-Oct;5(5):390–396. doi: 10.1094/mpmi-5-390. [DOI] [PubMed] [Google Scholar]
- Forsberg A., Wolf-Watz H. The virulence protein Yop5 of Yersinia pseudotuberculosis is regulated at transcriptional level by plasmid-plB1-encoded trans-acting elements controlled by temperature and calcium. Mol Microbiol. 1988 Jan;2(1):121–133. [PubMed] [Google Scholar]
- Genin S., Gough C. L., Zischek C., Boucher C. A. Evidence that the hrpB gene encodes a positive regulator of pathogenicity genes from Pseudomonas solanacearum. Mol Microbiol. 1992 Oct;6(20):3065–3076. doi: 10.1111/j.1365-2958.1992.tb01764.x. [DOI] [PubMed] [Google Scholar]
- Gough C. L., Genin S., Lopes V., Boucher C. A. Homology between the HrpO protein of Pseudomonas solanacearum and bacterial proteins implicated in a signal peptide-independent secretion mechanism. Mol Gen Genet. 1993 Jun;239(3):378–392. doi: 10.1007/BF00276936. [DOI] [PubMed] [Google Scholar]
- Gough C. L., Genin S., Zischek C., Boucher C. A. hrp genes of Pseudomonas solanacearum are homologous to pathogenicity determinants of animal pathogenic bacteria and are conserved among plant pathogenic bacteria. Mol Plant Microbe Interact. 1992 Sep-Oct;5(5):384–389. doi: 10.1094/mpmi-5-384. [DOI] [PubMed] [Google Scholar]
- Hale T. L. Genetic basis of virulence in Shigella species. Microbiol Rev. 1991 Jun;55(2):206–224. doi: 10.1128/mr.55.2.206-224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S. Y., Huang H. C., Collmer A. Pseudomonas syringae pv. syringae harpinPss: a protein that is secreted via the Hrp pathway and elicits the hypersensitive response in plants. Cell. 1993 Jul 2;73(7):1255–1266. doi: 10.1016/0092-8674(93)90354-s. [DOI] [PubMed] [Google Scholar]
- High N., Mounier J., Prévost M. C., Sansonetti P. J. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J. 1992 May;11(5):1991–1999. doi: 10.1002/j.1460-2075.1992.tb05253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. C., He S. Y., Bauer D. W., Collmer A. The Pseudomonas syringae pv. syringae 61 hrpH product, an envelope protein required for elicitation of the hypersensitive response in plants. J Bacteriol. 1992 Nov;174(21):6878–6885. doi: 10.1128/jb.174.21.6878-6885.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. C., Xiao Y., Lin R. H., Lu Y., Hutcheson S. W., Collmer A. Characterization of the Pseudomonas syringae pv. syringae 61 hrpJ and hrpI genes: homology of HrpI to a superfamily of proteins associated with protein translocation. Mol Plant Microbe Interact. 1993 Jul-Aug;6(4):515–520. doi: 10.1094/mpmi-6-515. [DOI] [PubMed] [Google Scholar]
- Huang J. H., Schell M. A. DNA sequence analysis of pglA and mechanism of export of its polygalacturonase product from Pseudomonas solanacearum. J Bacteriol. 1990 Jul;172(7):3879–3887. doi: 10.1128/jb.172.7.3879-3887.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. Z., Schell M. A. Evidence that extracellular export of the endoglucanase encoded by egl of Pseudomonas solanacearum occurs by a two-step process involving a lipoprotein intermediate. J Biol Chem. 1990 Jul 15;265(20):11628–11632. [PubMed] [Google Scholar]
- Keen N. T. Gene-for-gene complementarity in plant-pathogen interactions. Annu Rev Genet. 1990;24:447–463. doi: 10.1146/annurev.ge.24.120190.002311. [DOI] [PubMed] [Google Scholar]
- Long S. R., Staskawicz B. J. Prokaryotic plant parasites. Cell. 1993 Jun 4;73(5):921–935. doi: 10.1016/0092-8674(93)90271-q. [DOI] [PubMed] [Google Scholar]
- Michiels T., Wattiau P., Brasseur R., Ruysschaert J. M., Cornelis G. Secretion of Yop proteins by Yersiniae. Infect Immun. 1990 Sep;58(9):2840–2849. doi: 10.1128/iai.58.9.2840-2849.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993 Mar;57(1):50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci P., Bonnet P., Huet J. C., Sallantin M., Beauvais-Cante F., Bruneteau M., Billard V., Michel G., Pernollet J. C. Structure and activity of proteins from pathogenic fungi Phytophthora eliciting necrosis and acquired resistance in tobacco. Eur J Biochem. 1989 Aug 15;183(3):555–563. doi: 10.1111/j.1432-1033.1989.tb21084.x. [DOI] [PubMed] [Google Scholar]
- Roberts D. P., Denny T. P., Schell M. A. Cloning of the egl gene of Pseudomonas solanacearum and analysis of its role in phytopathogenicity. J Bacteriol. 1988 Apr;170(4):1445–1451. doi: 10.1128/jb.170.4.1445-1451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmond G. P., Reeves P. J. Membrane traffic wardens and protein secretion in gram-negative bacteria. Trends Biochem Sci. 1993 Jan;18(1):7–12. doi: 10.1016/0968-0004(93)90080-7. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Measurements of the effects that coding for a protein has on a DNA sequence and their use for finding genes. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):551–567. doi: 10.1093/nar/12.1part2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gijsegem F., Genin S., Boucher C. Conservation of secretion pathways for pathogenicity determinants of plant and animal bacteria. Trends Microbiol. 1993 Aug;1(5):175–180. doi: 10.1016/0966-842x(93)90087-8. [DOI] [PubMed] [Google Scholar]
- Van den Ackerveken G. F., Van Kan J. A., De Wit P. J. Molecular analysis of the avirulence gene avr9 of the fungal tomato pathogen Cladosporium fulvum fully supports the gene-for-gene hypothesis. Plant J. 1992 May;2(3):359–366. doi: 10.1111/j.1365-313x.1992.00359.x. [DOI] [PubMed] [Google Scholar]
- Wandersman C. Secretion across the bacterial outer membrane. Trends Genet. 1992 Sep;8(9):317–322. doi: 10.1016/0168-9525(92)90264-5. [DOI] [PubMed] [Google Scholar]
- Wei Z. M., Laby R. J., Zumoff C. H., Bauer D. W., He S. Y., Collmer A., Beer S. V. Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science. 1992 Jul 3;257(5066):85–88. doi: 10.1126/science.1621099. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]