Abstract
Objective
Decision support systems linked to administrative databases provide a unique opportunity to monitor adherence to guidelines and target disease management strategies towards patients not receiving guideline-based therapy. The objective of this study was to evaluate the discrepancy between actual asthma treatments prescribed by primary care physicians compared to those recommended by evidence-based guidelines using a decision support tool linked to a provincial health administrative database.
Design
The drug and medical services information of individuals with asthma was identified from the provincial health database and was pushed through an asthma decision support system (ADSS). Recommendations aimed at optimising asthma treatment were generated on two index dates, 15 September 2007 (index date 1) and 15 March 2008 (index date 2).
Setting
Primary care settings in a large Canadian metropolitan area.
Participants
Individuals with asthma and provincial health insurance primary and secondary outcome measures: well controlled asthma.
Results
16 803 eligible individuals were identified on index date 1, and 18 103 on index date 2. The distribution of recommendation categories was similar on both index dates. 94% were classified as well controlled and 7% as not well controlled. Among well-controlled individuals, the largest proportion was in the maintain treatment category (63.8%), followed by the maintain/decrease treatment category (28.2%) and the decrease treatment category (2.7%). Almost all individuals who were not well controlled had the recommendation to increase treatment (88%) with a small proportion in the refer category (1%).
Conclusions
The ADSS was able to identify subgroups of patients from an administrative database that could benefit from a medication review and possible change. Decision support systems linked to an administrative database can be used to identify individuals with uncontrolled asthma or prescriptions that deviate from recommended treatment. When connected to the point of care, this can provide an opportunity for physicians to intervene early.
Keywords: Epidemiology
Strengths and limitations of this study.
The availability of a provincial administrative database and decision support system allowed us to assess guideline adherence, and to identify subgroups of individuals at risk of poor outcomes.
The administrative database only includes individuals who are provincially insured, and therefore discrepancies could not be examined for individuals with private insurance.
The proportion of individuals with poor asthma control may have been underestimated as control status was evaluated over a 3-month period.
Introduction
Asthma poses a significant burden on healthcare resources and costs,1 and results in reduced individual functioning and quality of life.2 3 Over the past 10 years, there have been tremendous improvements in the scientific understanding of asthma and its treatment, and these findings have been made available to clinicians through the development of clinical practice guidelines. Despite achieving such sentinel milestones in asthma care, over 50%4 5 of individuals remain poorly controlled in the USA and Canada, with similar estimates worldwide.6 This has translated into direct and indirect costs of 654 million and 7.2 billion dollars (equivalent to US$ in 2008) in Canada and the USA, respectively.7
Healthcare organisations worldwide have been charged with improving asthma outcomes over the next 2–3 years, with the aim of reducing hospitalisations and deaths related to asthma.8 Several barriers for optimal management result in poor outcomes for asthma,9 including clinician-related (non-adherence to guidelines), patient-related (non-adherence to treatment) and treatment-related barriers (cost, complexity of treatment). In moving towards improving clinical outcomes, potentially modifiable barriers must be identified and targeted through appropriate interventions. A mechanism is needed to identify problematic asthma management so that gaps in care and barriers can be further evaluated and managed.
One potentially modifiable barrier is the gap between optimal versus actual asthma management as reflected by the lack of adoption of guidelines by clinicians or non-adherence of patients to recommended care.10 11 Much of the costs of asthma care are related to poor disease control due to the underuse of effective prophylactic therapies, and inadequate monitoring of disease control.7 At a population level, there are few mechanisms available for tracking disease-management indicators for asthma to evaluate the current application of guidelines. Several studies have evaluated divergence from asthma guidelines,12 13 but have not been able to accurately estimate non-adherence to guidelines among a representative sample of individuals. Evaluations of adherence have mostly relied on chart reviews and clinician or patient reports which are difficult to complete for a large number of patients across several healthcare settings.14–16
Decision support systems are designed to facilitate uptake of evidence-based guidelines with the expectation that adherence to such guidelines will improve health outcomes.17 Typically, decision support systems are used at the point of care. Such systems, however, may also have an alternate benefit of allowing population monitoring of adherence to disease management guidelines when the decision support algorithms are linked to administrative databases. By pushing through administrative health data including diagnoses, healthcare utilisation and medication information, algorithms can be used to generate recommendations for optimising treatment. In turn, patterns of underoptimisation of treatment can be identified to monitor adherence to guidelines and target specific physician and patient subgroups with disease management interventions.
The implementation of an asthma decision support system (ADSS) linked to provincial health insurance information represents a novel approach and facilitates the evaluation of the gap between recommended and actual treatment. We have developed a new methodology for assessing the quality of asthma management and asthma control in the population. Using evidence-based decision-support systems developed to guide physicians using computerised physician order entry and electronic medical record systems, we developed a programme for sequentially entering, assessing and extracting individual and summarised population-level quality monitoring and control status indicators. Using population-level administrative data for over 16 000 asthma patients, we then used this programme to evaluate asthma status and quality of adherence to national guidelines in a Quebec population on two randomly selected days in fall 2007 and spring 2008. This information is needed for asthma management, and can be used for identifying opportunities to target interventions and improve asthma outcomes.
In this study, we examined the discrepancy between actual asthma treatments as recorded in the provincial administrative database compared with those recommended by evidence-based guidelines as defined in the ADSS on two index dates.
Methods
Study population
The drug and medical services information of patients cared for by primary care physicians (PCPs) participating in the Medical Office of the 21st Century (MOXXI) study18 in a large metropolitan area was used to evaluate adherence to asthma treatment guidelines. PCPs were identified by professional association master lists and contacted by letter and telephone to determine their interest in participating in the MOXXI project. Patients of these physicians were identified from the Quebec provincial health database (RAMQ) medical service claims, physician and beneficiary files. PCPs who accepted provided consent for the research team to receive patient anonymised administrative data.
All patients with an International Classification of Diseases (ICD)-9 code for asthma, with no prior diagnosis for chronic obstructive pulmonary disease (COPD), and who were ≥5 years old were identified from RAMQ based on algorithms validated in prior research.19 For the purposes of this study, only patients with drug coverage by RAMQ for 75% of the year were included to ensure that all drugs dispensed were captured.
The provincial drug and administrative database (RAMQ)
The RAMQ beneficiary demographic database provided data on individual age, gender and mortality, and census data provided information on income and education.20 Information on each drug dispensed was obtained from the prescription claims database and included the drug name, quantity, date and duration for each prescription. The medical services claims database provided information on the beneficiary, date, type, provider and location of service delivery (eg, inpatient, emergency, clinic) for all medical services remunerated on a fee-for-service basis.
Study procedure: evaluating the gap between actual and recommended asthma treatment using the ADSS
The ADSS is integrated into the MOXXI electronic prescribing drug management application with patient information retrieved by real-time integration with the beneficiary, prescription and medical services claims files of the RAMQ. Using information from the prescription drug management platform, the ADSS uses the profile of existing drugs and health problems to customise recommended changes in asthma drug therapy. For this study, recommendations aimed at optimising asthma treatment were generated on two index dates, 15 September 2007 (index date 1) and 15 March 2008 (index date 2), representing peak times for asthma symptoms.
In the ADSS, asthma control is determined based on the overuse of short-acting β agonists (SABA) and visits to the emergency department (ED) for a respiratory problem over a 3-month period before the index date. Based on a previously validated algorithm, a patient is considered to be not well controlled if the sum of the quantity of all SABA medications dispensed to the patient within the past 3 months exceeds 250 dosesi 21 and/or they visited an ED for a respiratory-related problem in the past 3 months. Only asthma drugs that were (1) prescribed and dispensed within 1 year of the index date and (2) active (ie, based on prescription algorithms, it is likely that the person has a supply of the medication) or expired within 30 days prior to the index date were considered when generating the recommendations.
Patient-specific recommendations related to drug therapy are translated into preformatted prescriptions in the drug management platform. The ADSS is structured to support the Canadian Consensus guidelines for Asthma Management.22 Recommendations are categorised based on control status. For individuals in control, recommendations generated are one of three categories: maintain treatment, decrease treatment or maintain or decrease treatment. Recommendations also include options for action plan prescriptions for patients who are in control. For individuals who were not well controlled, recommendations are either to increase treatment or to refer to a specialist. Within each recommendation category, physicians are presented with specific recommendations for medications and doses to achieve the desired level of drug treatment.
Data analysis
Results were calculated for each index date. Descriptive statistics were used to characterise the study population and to evaluate differences between individuals with and without RAMQ coverage for prescription drugs. For individuals with RAMQ coverage, the proportion of individuals under each recommendation category was evaluated among individuals classified as ‘well controlled’ and ‘not well controlled’, and descriptive statistics were used to compare the characteristics of patients across categories. Multivariable logistic regression was used to estimate the probability of being classified in control or not well controlled as a function of sociodemographic characteristics and healthcare utilisation.
Results
Study population and insured compared to non-insured
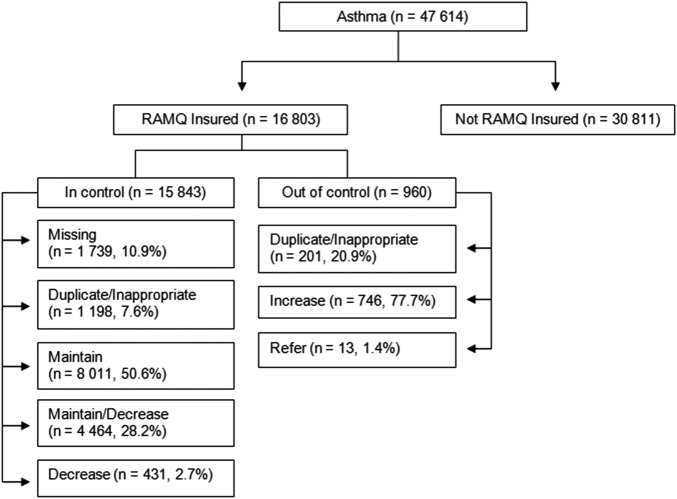
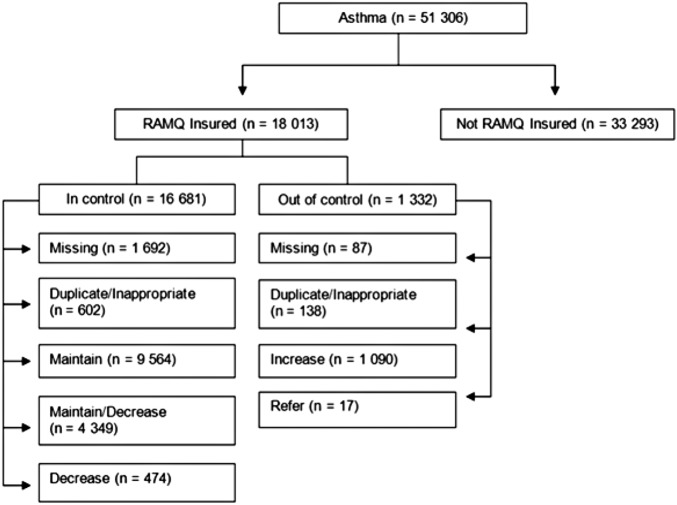
A total of 47 614 individuals with an asthma diagnosis were identified on index date 1, after removing individuals with a prior diagnosis of COPD (6018) and those ≤ 5years old (figure 1). Thirty-five per cent of individuals were RAMQ insured for prescription drugs at least 75% of the year prior to the index date, for both dates. On index date 2, 51 306 individuals with an asthma diagnosis were identified (figure 2). Approximately the same proportion of individuals was classified as well controlled on index date 1 (93%) and index date 2 (94%). As the distribution of individual characteristics, control status and recommendation categories was similar on both index dates, we only report the results from index date 2 from this point on (table 1).
Figure 1.
The number of individuals in each RAMQ insurance, control status, and recommendation category for index date 1 (15 September 2007).
Figure 2.
The number of individuals in each RAMQ insurance, control status, and recommendation category for index date 2(15 March 2008).
Table 1.
Characteristics of study participants with and without provincial health coverage (RAMQ) on index day 2*
| RAMQ coverage | No RAMQ coverage | |
|---|---|---|
| n=18 013 | n=33 293 | |
| Age mean (SD) | 38.3 (21.8) | 30.81 (17.5) |
| Age n (%) | ||
| ≤17 | 3963 (22.0) | 10 273 (30.9) |
| 18–39 | 5129 (28.6) | 9926 (29.8) |
| 40–59 | 5254 (29.2) | 11 277 (33.9) |
| ≥60 | 3637 (20.2) | 1817 (5.5) |
| Sex n (% female) | 11 035 (61.3) | 18 665 (56.1) |
| Income n (%) * | ||
| Low SES | 3490 (19.4) | 2665 (8.0) |
| Middle SES | 13 148 (73.0) | 25 947 (78.0) |
| High SES | 1230 (6.8) | 4298 (13.0) |
| Healthcare utilisation over 1 year prior to 15 March 2008 | ||
| Medical physician visits† n (%) | ||
| 0 | 1736 (9.6) | 3855 (11.6) |
| 1 | 1998 (11.1) | 4453 (13.4) |
| 2 | 1895 (10.5) | 4154 (12.5) |
| 3 or more | 12 384 (68.8) | 20 831 (62.6) |
| Emergency department visits n (%) | ||
| 0 | 10 435 (57.9) | 22 738 (68.0) |
| 1 | 3139 (17.4) | 5445 (16.4) |
| 2 | 1698 (9.4) | 2416 (7.3) |
| 3 or more | 2741 (15.2) | 2694 (8.1) |
| Emergency department visits for asthma n (%) | 1313 (7.3) | 1644 (4.9) |
| Hospitalisation (days) | ||
| 0 | 14 890 (82.7) | 29 445 (88.4) |
| 1 | 1340 (7.4) | 2072 (6.2) |
| 2 | 445 (2.5) | 658 (2.0) |
| 3 or more | 1338 (7.4) | 1118 (3.4) |
| Comorbidity n (%) | ||
| Depression | 1400 (7.77) | 1724 (5.2) |
| Anxiety | 1913 (10.62) | 2361 (7.1) |
*Around 1% of missing values for each category; all differences between RAMQ and non-RAMQ insured are significant, p<0.01.
†Ambulatory and specialty care.
SES, socioeconomic status.
Individuals who were RAMQ insured were on average older (mean=38±22) as compared to non-RAMQ insured individuals (mean=31±18) and had a greater percentage of individuals ≥ 60 years old; also, a larger proportion was female (61% vs 56%) and in the lower socioeconomic status (SES) category (21% vs 6%). A greater proportion of RAMQ insured patients had three or more ED (16% vs 9%) and hospital visits (8% vs 3%) 1 year prior to the index date, and a diagnostic code for anxiety (11 compared to 7%) or depression (8 compared to 5%).
Control status and recommendation categories
Among the 18 013 individuals who were RAMQ insured for prescription drugs, 93% were classified as well controlled and 7% as not well controlled over 3 months prior to the index date (figure 1).
Sixty-three per cent of individuals who were not well controlled were in the ≥40 age group and 26% in the low SES category compared to 49% and 19%, respectively, in the well-controlled group. These individuals also had a higher Charlson Comorbidity Index of 2.11 as compared to 1.6 among those well controlled. A larger proportion of individuals among those who were not well controlled had a diagnostic code for depression, anxiety, mental illness and a cardiac-related condition. Among those who were not well controlled, 69% (n=667) had at least one ED visit, and 74% a medical visit associated with a respiratory problem (in the past year). In comparison, 13% (n=2039) of those well controlled had at least one ED visit, and 52% a medical visit related to a respiratory problem in the past year.
Fifty-three per cent of patients in the not well controlled group had an active prescription for an inhaled corticosteroid (ICS), 20% a combination therapy, and 14% as compared to 36%, 10% and 6% in the well controlled group. Sixty-three per cent and 42% of not well and well controlled, respectively, had an active prescription for a fast-acting β agonist. At index date 1, all individuals who were not well controlled had asthma drugs as compared to 9.2% of those well controlled who had no asthma drugs dispensed.
Table 2 presents the incremental regression coefficients for the demographic, healthcare utilisation and comorbidity variables hypothesised to be associated with control status. Healthcare utilisation, including ≥3 days of hospitalisation (OR=4.58) and ≥3 visits to the ED (for reasons other than a respiratory problem; OR=2.32), was found to be most strongly associated with control status. Being male (OR=0.85), from a low SES (OR=1.9) and in the 40–59 age group increased the odds of having asthma that was not well controlled.
Table 2.
Multivariable logistic regression models for identifying individuals controlled and not well controlled
| Variable| | OR (95% CI) control status |
|---|---|
| Age mean (SD) | |
| ≤17 | Reference |
| 18–39 | 0.56 (0.44 to 0.72) |
| 40–59 | 2.19 (1.73 to 2.77) |
| ≥ 60 | 1.19 (1 to 1.42) |
| Sex n (% female) | |
| Male | Reference |
| Female | 85 (0.74 to 0.98) |
| Income n (%)* | |
| High SES | Reference |
| Middle SES | 1.44 (1.04 to 1.98) |
| Low SES | 1.90 (1.35 to 2.68) |
| Healthcare utilisation over 1 year prior to 15 March 2008 | |
| Medical physician *visits n (%) | |
| 0 | Reference |
| 1 | 0.73 (0.47 to 1.2) |
| 2 | 0.82 (0.53 to 1.28) |
| ≥3 | 1.62 (1.162.27) |
| Emergency department visits (other than resp) n (%) | |
| 0 | Reference |
| 1 | 1.38 (1.14 to 1.66) |
| 2 | 1.46 (1.16 to 1.84) |
| ≥3 | 2.32 (1.94 to 2.8) |
| Hospitalisation (days) | |
| 0 | Reference |
| 1 | 2.24 (1.55 to 3.27) |
| 2 | 2.88 (1.79 to 4.6) |
| 3 or more | 4.58 (3.36 to 6.22) |
| Comorbidity n (%) | |
| Charlson comorbidity index | 1.04 (1.01 to 1.08) |
| Anxiety | |
| No | Reference |
| Yes | 1.26 (1.05 to 1.52 ) |
*General practitioner and specialist.
SES, socioeconomic status.
Recommendation category by control group
The distribution of individuals across recommendation categories is presented in table 3.
Table 3.
Comparison of characteristics of individuals in each recommendation category (based on primary recommendation)
| In control N=14 989 |
Not well controlled N=1245 |
||||||
|---|---|---|---|---|---|---|---|
| Maintain n=9564 | Maintain/decrease n=4349 | Decrease n=474 | Duplicate/inappropriate n=602 | Increase n=1090 | Refer n=17 | Duplicate/inappropriate n=138 | |
| Age mean (SD) | 41.8 (19.2) | 38.2 (22.7) | 44.8 (21.6) | 45.9 (20.3) | 40.4 (21.5) | 57.1 (9.3) | 46.6 (16.0) |
| Age n (%) | |||||||
| ≤17 | 919 (9.6) | 1115 (25.6) | 74 (15.6) | 68 (11.3) | 189 (17.3) | 0 | 6 (4.4) |
| 18–39 | 3561 (37.2) | 996 (22.9) | 86 (18.1) | 123 (20.4) | 310 (28.4) | 0 | 33 (23.9) |
| 40–59 | 2987 (31.2) | 1269 (29.2) | 195 (41.1) | 260 (43.2) | 372 (34.1) | 10 (58.8) | 79 (57.2) |
| ≥60 | 2097 (21.9) | 969 (22.3) | 119 (25.1) | 151 (25.1) | 219 (20.1) | 7 (41.2) | 20 (14.5) |
| Sex n (% female) | 6073 (63.5) | 2659 (61.1) | 303 (63.9) | 381 (63.3) | 709 (65.0) | 12 (70.6) | 101 (73.2) |
| Income n (%) * | |||||||
| Low SES | 1684 (17.6) | 923 (21.2) | 117 (24.7) | 156 (25.9) | 237 (21.7) | 4 (23.5) | 43 (31.2) |
| Middle SES | 7028 (73.5) | 3161 (72.7) | 330 (69.6) | 420 (69.8) | 802 (73.6) | 13 (76.5) | 90 (65.2) |
| High SES | 763 (8.0) | 228 (5.2) | 25 (5.3) | 22 (3.6) | 47 (4.3) | 0 | 5 (3.6) |
| Medical visits mean (SD) past year | |||||||
| All | 8.78 (13.1) | 9.68 (13.8) | 12.62 (13.3) | 12.87 (13.4) | 16.52 (22.2) | 29.29 (21.3) | 24.99 (26.1) |
| Ambulatory | 7.72 (9.6) | 8.31 (9.2) | 10.89 (9.5) | 11.13 (9.5) | 13.53 (15.0) | 19.94 (10.0) | 20.01 (18.1) |
| Hospitalised | 1.07 (6.8) | 1.37 (7.7) | 1.73 (7.4) | 1.73 (7.6) | 2.99 (11.6) | 9.35 (16.4) | 4.98 (13.3) |
| Medical visits n (%) past year | |||||||
| Physician | |||||||
| 0 | 1036 (10.8) | 265 (6.1) | 14 (3.0) | 22 (3.6) | 62 (5.7) | 0 | 7 (5.1) |
| 1 | 1048 (10.96) | 451 (10.4) | 31 (6.5) | 40 (6.6) | 76 (7.0) | 0 | 5 (3.6) |
| 2 | 1000 (10.5) | 486 (11.2) | 41 (8.6) | 26 (4.3) | 81 (7.4) | 0 | 2 (1.4) |
| 3 or more | 6480 (67.8) | 3147 (72.4) | 388 (81.9) | 514 (85.4) | 871 (79.9) | 17 (100) | 124 (89.9) |
| ER visits | |||||||
| 0 | 5995 (62.7) | 2501 (57.5) | 240 (50.6) | 289 (48.0) | 200 (18.4) | 1 (5.9) | 25 (18.1) |
| 1 | 1565 (16.4) | 790 (18.2) | 89 (18.8) | 118 (19.6) | 221 (20.3) | 3 (17.6) | 21 (15.2) |
| 2 | 846 (8.8) | 414 (9.5) | 59 (12.4) | 63 (10.5) | 172 (15.8) | 1 (5.9) | 9 (6.5) |
| 3 or more | 1158 (12.1) | 644 (14.8) | 86 (18.1) | 132 (21.9) | 497 (45.6) | 12 (70.6) | 83 (60.2) |
| ED visits for respiratory problems | |||||||
| 0 | 8781 (91.8) | 3792 (87.2) | 394 (83.1) | 491 (81.6) | 294 (27.0) | 4 (23.5) | 38 (27.5) |
| 1 | 593 (6.2) | 402 (9.2) | 52 (11.0) | 64 (10.6) | 450 (41.3) | 4 (23.5) | 27 (19.6) |
| 2 | 142 (1.5) | 105 (2.4) | 15 (3.2) | 25 (4.2) | 188 (17.2) | 3 (17.65) | 22 (15.9) |
| 3 or more | 48 (0.5) | 50 (1.2) | 13 (2.7) | 22 (3.7) | 158 (14.5) | 6 (35.3) | 51 (37.0) |
| ED visits NOT for respiratory problems | |||||||
| 0 | 6268 (65.5) | 2712 (62.4) | 265 (55.9) | 326 (54.2) | 456 (41.8) | 4 (23.5) | 45 (32.6) |
| 1 | 1535 (16.1) | 742 (17.1) | 94 (19.8) | 118 (19.6) | 205 (18.8) | 3 (17.6) | 29 (21.0) |
| 2 | 746 (7.8) | 370 (8.5) | 49 (10.3) | 58 (9.6) | 117 (10.7) | 3 (17.6) | 14 (10.1) |
| 3 or more | 1015 (10.6) | 525 (12.1) | 66 (13.9) | 100 (16.6) | 312 (28.6) | 7 (41.2) | 50 (36.2) |
| Hospitalisation (days) | |||||||
| 0 | 8046 (84.1) | 3581 (82.3) | 356 (75.1) | 449 (74.6) | 774 (71.0) | 5 (29.4) | 78 (56.5) |
| 1 | 697 (7.3) | 318 (7.3) | 39 (8.2) | 62 (10.3) | 100 (9.2) | 3 (17.6) | 17 (12.3) |
| 2 | 215 (2.2) | 107 (2.5) | 20 (4.2) | 23 (3.8) | 44 (4.0) | 1 (5.9) | 3 (2.2) |
| 3 or more | 606 (6.3) | 343 (7.9) | 59 (12.4) | 68 (11.3) | 172 (15.8) | 8 (47.1) | 40 (29.0) |
| Hospitalisation for respiratory problems (days) | |||||||
| 0 | 9370 (98.0) | 4210 (96.8) | 447 (94.3) | 563 (93.5) | 990 (90.8) | 14 (82.4) | 109 (79.0) |
| 1 | 100 (1.0) | 60 (1.4) | 7 (1.5) | 20 (3.3) | 33 (3.0) | 0 | 7 (5.1) |
| 2 | 32 (0.3) | 32 (0.74) | 4 (0.8) | 5 (0.8) | 14 (1.3) | 0 | 3 (2.2) |
| 3 or more | 62 (0.6) | 47 (1.1) | 16 (3.4) | 14 (2.3) | 53 (4.9) | 3 (17.6) | 19 (13.8) |
| Asthma medications mean (SD) range past year | |||||||
| FABA | 0.61 (1.7) | 2.93 (3.8) | 4.32 (5.2) | 4.95 (5.1) | 2.50 (4.4) | 5.00 (5.2) | 6.82 (6.8) |
| ICS | 0.2 (0.7) | 2.3 (2.9) | 1.4 (2.6) | 3.6 (3.8) | 1.4 (2.4) | 0.9 (1.7) | 3.5 (3.9) |
| Leukotrienes | 0.1 (1.4) | 0.4 (3.0) | 6.7 (10.0) | 1.5 (4.8) | 0.8 (4.4) | 3.3 (5.1) | 3.9 (11.5) |
| Combination therapy | 0.0 (0.4) | 1.2 (2.9) | 5.1 (4.9) | 2.18 (3.9) | 1.0 (2.7) | 7.7 (4.5) | 3.0 (4.3) |
| Other | 0.2 (1.8) | 0.8 (3.4) | 2.9 (6.8) | 2.36 (3.9) | 1.8 (17.0) | 2.1 (2.5) | 4.45 (6.6) |
| Control status n (%) | |||||||
| Overuse FABA | 0 | 0 | 0 | 0 | 1 (0.1) | 0 | 0 |
| ER visits for Asthma | 0 | 0 | 0 | 0 | 1076 (98.7) | 17 (100) | 135 (97.8) |
| ER or FABA | 0 | 0 | 0 | 0 | 1076 (98.7) | 17 (100) | 135 (97.8) |
| Comorbidity Index | 1.6 (1.5) | 1.6 (1.5) | 1.8 (1.6) | 1.9 (1.9) | 1.8 (2.0) | 2.2 (1.4) | 2.6 (2.5) |
Less than 1% of missing values for each category.
ED, emergency department; FABA, fast-acting β agonist; SES, socioeconomic status.
For 8% (1198/15843) of those in control, and 21% (201/960) of those who were not well controlled, a recommendation could not be determined by the ADSS either because the patient (1) had dispensed prescriptions for an inappropriate combination of medications that the ADSS could not reconcile to provide an appropriate recommendation (eg, a long-acting β-adrenoceptor agonist with two prescriptions for combination therapy) or (2) dispensed two medications that resulted in a duplication of therapy. For those who were not well controlled, those in the duplicate/inappropriate category had a larger proportion in the lower SES, a higher comorbidity index and more frequent ambulatory and hospital visits.
Among well-controlled individuals, the largest proportion was in the maintain treatment category (63.8%), followed by the maintain/decrease treatment category (28.2%) and the decrease treatment category (2.7%). Almost all the individuals who were not well controlled had the recommendation to increase treatment (88%) with a small proportion in the refer category (1%). Reasons for the low referral to specialty care needs to be closely examined, and may be related to the uncertainty of PCPs of when to refer patients, and/or patients may not go to see the specialists once referred.23 Regardless of the recommendation category, the largest proportion of individuals was in the 40–59 age group, except for the maintain treatment category that had a larger proportion of individuals in the 18–39 age group. The middle SES was the largest for all recommendation groups and the proportion of females was the same across all categories. Individuals in the refer category were on average older than those in the other categories, but comparable on many of the other characteristics.
Discussion
The purpose of this study was to evaluate the discrepancy between current asthma management and recommended guidelines using the provincial administrative databases and an ADSS. The present study represents an example of how decision support systems can be used to monitor guideline adherence, and to identify individuals at risk of poor outcomes to provide targeted interventions. To our knowledge, this is the first time that a decision support system has been used to evaluate disease management at a population level.
As expected, individuals who were provincially insured were on average older, from a lower SES and a higher proportion used healthcare services. A larger proportion compared to those non-provincially insured also had a diagnosis code for anxiety and depression.
The algorithms used to identify individuals with asthma and evaluate control status were validated in previous work.24 25 The majority of well-controlled patients were on an appropriate quantity of asthma treatment. We found, however, that ∼31% of those well controlled could benefit from a medication review and potentially lower doses of asthma medications.
The majority of individuals who were not well controlled had the recommendation to increase treatment, and for these individuals there was an opportunity to change therapy according to the existing guidelines.26 The SMART inhaler helps address needs for increase in therapy, as it allows patients to use their as-needed medication because of declining asthma control—as is very often the case—evolving exacerbations will possibly be treated at an early stage and a further worsening of asthma may possibly be prevented. The SMART inhaler is not a recommended yet part of Canadian guidelines; however, with emerging evidence of its benefits for marinating control compared to other alternatives,27 28 it will be included in the next version of guidelines and become more commonly prescribed for Canadian patients. Individuals who were not well controlled were in the 40–59 age range and had a more complex health profile with greater comorbidity, including a higher proportion with a diagnosis of anxiety or depression as compared to those who were well controlled. The logistic regression analysis in our study also supported these conclusions. These individuals represent a more vulnerable subgroup of the asthma population and place a greater burden on the healthcare system, given the higher proportion that had an ED visit or hospitalisation. As such, they require a closer monitoring and review of medication to reach doses sufficient to maintain asthma control, or to review reasons for failed treatment.
In this study, we were not able to generate a recommendation for a larger proportion of individuals who were not well controlled compared to those who were well controlled either because they were dispensed prescriptions for an inappropriate combination of medications that the ADSS could not reconcile to provide an appropriate recommendation, or they were dispensed two medications that resulted in a duplication of therapy. These cases in themselves represent a segment of the asthma population that requires a closer review of their prescribed medication.
The generation of asthma recommendations at a population level using an administrative database allows individuals not receiving treatment based on guidelines to be identified. We found that many individuals with non-controlled asthma visit a physician three or more times per year, and potentially represent missed opportunities to optimise treatment. Possible reasons for our findings may include the lack of knowledge of PCPs of guidelines in general, especially for more complicated cases. It may also be, however, that patients are not going to see the same physician, or are switching physicians to ensure access to SABAs. In such situations, physicians may be reluctant to conduct a complete medication review if they do not perceive themselves as the primary provider for the patient.
Other physician concerns may be the reluctance to prescribe ICS and/or concern regarding polypharmacy with multiple inhalers.29 This is where the role of pharmacists is important as they can see individuals’ entire medication dispensing history and have been shown to be effective in managing asthma patients, in particular if supported by an ADSS.30
Previous studies have also found that physicians do not adopt guidelines in their practice because of the perceived appropriateness of the guidelines.13 31 Surveys have shown that they believe that guidelines do not take into account the heterogeneity of asthma and do not account for individual patient variations in response to treatment,32 as well as other factors that impact on response to asthma therapy such as age and comorbidities.
Further, patient non-adherence to prescribed therapy and not having prescribed medications filled may also explain the findings from our study. Patient beliefs about the negative impact and benefits of their medications,33 their confidence in managing their asthma and not seeking care early enough to prevent exacerbations have all been identified as contributors to poor outcomes for asthma.34
Mechanisms to identify patients who need a closer follow-up and evaluation have been identified as an important need for primary healthcare.3 34 35 Future initiatives can include linking administrative databases to decision support systems that can help identify individuals who need closer monitoring and follow-up and allow for targeted services such as visit reminders sent to patients or to their care provider. The ongoing implementation of electronic health records and patient health portals will facilitate this approach. Information can be fed back to physicians and pharmacists to improve patient management, and initiate care early on, before individuals experience deteriorations in health.
Conclusions
This study demonstrated how a decision support system linked to an administrative database could be used to identify individuals in the population who require a review of asthma treatment. Such an approach can help identify individuals with uncontrolled asthma or prescriptions that deviate from recommended treatment to intervene early. This study provides a model for monitoring adherence to guidelines for other chronic conditions such as hypertension and diabetes.
Limitations
Our approach for identifying individuals with asthma and assessing asthma status may have underestimated the percentage out of control in our study. We examined asthma control on two index dates, and went back 3 months prior to the index date to assess control status. A more sensitive algorithm that treats control as a time varying covariate would most likely provide a more accurate evaluation of control status.
Also, our estimation of the percentage of well-controlled individuals may be an overestimate compared to previous studies because of our method of defining asthma control. A previous study conducted in the UK,36 and another using a US administrative database37 assumed that two puffs of an SABA per day, the equivalent of 180 puffs over 3 months, would be the threshold for asthma control. With this measure of asthma control, the authors reported that 72% of patients were well controlled in the UK study and 56% in the US study. This estimate is substantially below the measure of 250 puffs we used in this study, and most likely explains why we found a larger proportion of individuals who were well controlled.
Also, because we used administrative data and not clinical information from an electronic medical record to generate recommendations, we were not able to use asthma severity and relapse as part of the asthma control algorithm. Two previous studies used composite measures of asthma control including (1) no recorded hospital attendance for asthma (including admission or ED visit, out of hours, or outpatient department attendance); (2) no prescription for oral corticosteroid and (3) no consultation, hospital admission or ED attendance for lower respiratory tract infection requiring antibiotics.36 37 These two studies found 72%,36 and 56%37 of individuals were well-controlled, which is lower than the proportion we found in our study (94%).
In addition, at the time that the ADSS was being developed, the SMART treatments that allow for the same inhaler to be used as a preventative and rescue inhaler were not commonly used or part of the guidelines. Therefore, they were not programmed as part of the ADSS and not included in the recommendations.
Further, the ADSS does not distinguish between a SABA nebuliser and an MDI. Finally, the use of decision support during clinical encounters allows not only for a patient-reported assessment of symptoms at the time when recommendations are generated, but also for a more accurate assessment of asthma control. We were also limited to generating recommendations for those who were provincially insured, which represents a more vulnerable segment of the population.
Supplementary Material
Contributors: SA, RT and NW were involved in conception and design, analysis and interpretation of data, drafting of the article and revising it critically for important intellectual content and final approval of the version to be published.
Funding: Health Canada and the Canadian Institute of Health Research (grant number MCT67814) supported the development of the asthma decision support system.
Competing interests: SA is supported by a Fonds de la Recherche en Santé du Quebec (FRSQ) research career award..
Ethics approval: McGill University institutional review board (IRB) approval was obtained for this study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Additional data regarding the study sample characteristics and guidelines generated from the decision support system can be provided on request from the corresponding author.
In total, 250 doses is based on the most commonly prescribed SABA salbutamol 100 μg, two inhalations at a time, or the equivalent for other fast-acting bronchodilators in the past 3 months.
References
- 1.Shenolikar R, Song X, Anderson JA, et al. Costs of asthma among US working adults. Am J Manag Care 2011;17:409–16 [PubMed] [Google Scholar]
- 2.Schatz M, Zeiger RS, Yang SJ, et al. The relationship of asthma impairment determined by psychometric tools to future asthma exacerbations. Chest 2012;141:66–72 [DOI] [PubMed] [Google Scholar]
- 3.Urrutia I, Aguirre U, Pascual S, et al. Impact of anxiety and depression on disease control and quality of life in asthma patients. J Asthma 2012;49:201–8 [DOI] [PubMed] [Google Scholar]
- 4.Colice GL, Ostrom NK, Geller DE, et al. The CHOICE survey: high rates of persistent and uncontrolled asthma in the United States. Ann Allergy Asthma Immunol 2012;108:157–62 [DOI] [PubMed] [Google Scholar]
- 5.Glaxo Wellcome Asthma in Canada A Landmark Survey, 2000:1–31 [Google Scholar]
- 6.Bousquet J, Mantzouranis E, Cruz AA, et al. Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization consultation on severe asthma. J Allergy Clin Immunol 2010;126:926–38 [DOI] [PubMed] [Google Scholar]
- 7.Krahn MD, Berka C, Langlois P, et al. Direct and indirect costs of asthma in Canada, 1990 3465. CMAJ 1996;154:821–31 [PMC free article] [PubMed] [Google Scholar]
- 8.National Center for Health Statistics. Healthy people. 2010. Final Review. Hyattsville, MD, 2012. [Google Scholar]
- 9.Maciejewski ML, Chen SY, Au DH. Adult asthma disease management: an analysis of studies, approaches, outcomes, and methods. Respir Care 2009;54:878–86 [DOI] [PubMed] [Google Scholar]
- 10.Legorreta AP, Christian-Herman J, O'Connor RD, et al. Compliance with National Asthma Management Guidelines and Specialty Care: a health mantenance organization experience. Arch Intern Med 1998;158:457–64 [DOI] [PubMed] [Google Scholar]
- 11.Adams RJ, Fuhlbrigge A, Guilbert T, et al. Inadequate use of asthma medication in the United States: results of the asthma in America national population survey. J Allergy Clin Immunol 2002;110:58–64 [DOI] [PubMed] [Google Scholar]
- 12.Flor-Escriche X, Rodriguez-Mas M, Espiau M, et al. Compliance with guidelines in the treatment of asthma exacerbations in primary care. Ther Adv Respir Dis 2011;5:369–75 [DOI] [PubMed] [Google Scholar]
- 13.Rank MA, Liesinger JT, Ziegenfuss JY, et al. The impact of asthma medication guidelines on asthma controller use and on asthma exacerbation rates comparing 1997–1998 and 2004–2005. Ann Allergy Asthma Immunol 2012;108:9–13 [DOI] [PubMed] [Google Scholar]
- 14.Cazzola M, Segreti A, Bettoncelli G, et al. Change in asthma and COPD prescribing by Italian general practitioners between 2006 and 2008. Prim Care Respir J 2011;20:291–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnlind MH, Wettermark B, Nokela M, et al. Regional variation and adherence to guidelines for drug treatment of asthma. Eur J Clin Pharmacol 2010;66:187–98 [DOI] [PubMed] [Google Scholar]
- 16.Lougheed MD, Olajos-Clow J, Szpiro K, et al. Multicentre evaluation of an emergency department asthma care pathway for adults. CJEM 2009;11:215–29 [DOI] [PubMed] [Google Scholar]
- 17.Roshanov PS, Misra S, Gerstein HC, et al. Computerized clinical decision support systems for chronic disease management: a decision-maker-researcher partnership systematic review. Implement Sci 2011;6:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamblyn R, Huang A, Perreault R, et al. The medical office of the twenty-first century (MOXXI): evaluation of the effectiveness of computerised decision support in reducing inappropriate prescribing in primary care. CMAJ 2003;169:549–56 [PMC free article] [PubMed] [Google Scholar]
- 19.Kawasumi Y, Abrahamowicz M, Ernst P, et al. Development and validation of a predictive algorithm to identify adult asthmatics from medical services and pharmacy claims databases. Health Serv Res 201;46:939–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamblyn RM, Laprise R, Hanley JA, et al. Adverse events associated with prescription drug cost-sharing among poor and elderly persons. JAMA 2001;285:421–9 [DOI] [PubMed] [Google Scholar]
- 21.Schatz M, Zeiger RS, Vollmer WM, et al. Validation of a beta-agonist long-term asthma control scale derived from computerized pharmacy data. J Allergy Clin Immunol 2006;117:995–1000 [DOI] [PubMed] [Google Scholar]
- 22.Lemiere C, Bai T, Balter M, et al. Adult Asthma Consensus Guidelines update 2003. Can Respir J 2004;11(Suppl A):9A–18A [DOI] [PubMed] [Google Scholar]
- 23.Wu AW, Young Y, Skinner EA, et al. Quality of Care and Outcomes of Adults With Asthma Treated by Specialists and Generalists in Managed Care. Arch Intern Med 2001;161:2554–60. [DOI] [PubMed] [Google Scholar]
- 24.Spitzer WO, Suissa S, Ernst P, et al. The use of beta-agonists and the risk of death and near death from asthma. N Engl J Med 1992;326:501–6 [DOI] [PubMed] [Google Scholar]
- 25.Wilchesky M, Tamblyn RM, Huang A. Validation of diagnostic codes in medical services claims data. Can J Clin Pharmacol 2001;8:39. [DOI] [PubMed] [Google Scholar]
- 26.Becker A, Lemiere C, Berube D, et al. Summary of recommendations from the Canadian Asthma Consensus guidelines, 2003. CMAJ 2005;173(Suppl 6):S3–11 [PMC free article] [PubMed] [Google Scholar]
- 27.Boursquet J, Boulet LP, Peters MJ, et al. Budesonide/formoterol for maintenance and relief in uncontrolled asthma vs. high-dose salmeterol/fluticasone. Respir Med 2007;101:2437–46 [DOI] [PubMed] [Google Scholar]
- 28.Kuna P, Peters M, Manjra A, et al. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. Int J Clin Pract 2007;61:725–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Navaratnam P, Jayawant SS, Pedersen CA, et al. Asthma pharmacotherapy prescribing in the ambulatory population of the United States: evidence of nonadherence to national guidelines and implications for elderly people. J Am Geriatr Soc 2008;56:1312–17 [DOI] [PubMed] [Google Scholar]
- 30.Armour C, Bosnic-Anticevich S, Brillant M, et al. Pharmacy Asthma Care Program (PACP) improves outcomes for patients in the community. Thorax 2007;62:496–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bracha Y, Brottman G, Carlson A. Physicians, guidelines, and cognitive tasks. Eval Health Prof 2011;34:309–35 [DOI] [PubMed] [Google Scholar]
- 32.Yeh KW, Chen SH, Chiang LC, et al. Survey of asthma care in Taiwan: a comparison of asthma specialists and general practitioners. Ann Allergy Asthma Immunol 2006;96:593–9 [DOI] [PubMed] [Google Scholar]
- 33.Brunton SA, Graham LM, Stoloff SW. Primary care management of patients with asthma. J Fam Pract 2011;60(Suppl 5):S1–8 [PubMed] [Google Scholar]
- 34.Razykov I, Newton EG, Lober J, et al. Daily SMS reminders for asthma treatment adherence: a comment on Strandbygaard et al . Respir Med 2010;104:1234–5; author reply1236 [DOI] [PubMed] [Google Scholar]
- 35.Martens JD, van der Weijden T, Winkens RA, et al. Feasibility and acceptability of a computerised system with automated reminders for prescribing behaviour in primary care. Int J Med Inform 2008;77:199–207 [DOI] [PubMed] [Google Scholar]
- 36.Price D, Martin RJ, Barnes N, et al. Prescribing practices and asthma control with hydrofluroroalkane-beclomethasone and fluticasone: a real-world observational study. J allergy Clin Immunol 2010;126:511–18 [DOI] [PubMed] [Google Scholar]
- 37.Colice G, Martin RJ, Israel E, et al. Asthma outcomes and costs of therapy with extrafine beclomethasone and fluticason. J Allergy Clin Immunol 2013;132:45–54 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.