Abstract
Introduction
During the past decade, the volume of percutaneous coronary intervention (PCI) in China has risen by more than 20-fold. Yet little is known about patterns of care and outcomes across hospitals, regions and time during this period of rising cardiovascular disease and dynamic change in the Chinese healthcare system.
Methods and analysis
Using the China PEACE (Patient-centered Evaluative Assessment of Cardiac Events) research network, the Retrospective Study of Coronary Catheterisation and Percutaneous Coronary Intervention (China PEACE-Retrospective CathPCI Study) will examine a nationally representative sample of 11 900 patients who underwent coronary catheterisation or PCI at 55 Chinese hospitals during 2001, 2006 and 2011. We selected patients and study sites using a two-stage cluster sampling design with simple random sampling stratified within economical-geographical strata. A central coordinating centre will monitor data quality at the stages of case ascertainment, medical record abstraction and data management. We will examine patient characteristics, diagnostic testing patterns, procedural treatments and in-hospital outcomes, including death, complications of treatment and costs of hospitalisation. We will additionally characterise variation in treatments and outcomes by patient characteristics, hospital, region and study year.
Ethics and dissemination
The China PEACE collaboration is designed to translate research into improved care for patients. The study protocol was approved by the central ethics committee at the China National Center for Cardiovascular Diseases (NCCD) and collaborating hospitals. Findings will be shared with participating hospitals, policymakers and the academic community to promote quality monitoring, quality improvement and the efficient allocation and use of coronary catheterisation and PCI in China.
Registration details
Keywords: Catheterization, Angiography, Angioplasty, China
Strengths and limitations of this study.
Hospitalisations were sampled from a nationally representative hospital network for the years 2001, 2006 and 2011 to examine the influence of major changes in the Chinese healthcare system and the creation of percutaneous coronary intervention (PCI) guidelines by Chinese medical societies.
To elevate the quality of abstracted data, the study uses data collection techniques regularly employed by international clinical trials such as integrated central and on-site monitoring as well as source document checking.
Findings will be shared with hospitals and policymakers to improve healthcare quality.
Patient outcomes are limited to in-hospital outcomes, and data collection is limited to information available in medical records.
Introduction
China, a country with a rapidly rising prevalence of cardiovascular disease,1–3 is concomitantly expanding access to advanced cardiovascular procedures. During the past decade, the volume of percutaneous coronary intervention (PCI) has increased more than 20-fold.4 5 This expansion has occurred in the context of rising rates of health insurance coverage and healthcare costs.6 7 The optimal deployment of advanced care strategies in settings of constrained resources is a common challenge faced by many low-income and middle-income countries.8
Although the overall volume of coronary catheterisation and PCI procedures performed in China is known,9–12 there are gaps in knowledge about the details of their use and associated outcomes. Nationally representative data are lacking, and studies have generally included only a small number of hospitals in circumscribed geographical areas or single time periods.5 13–15 Longitudinal data on coronary catheterisation and PCI have largely been derived from a single centre.16 We therefore know relatively little about patient selection, use of specific procedural technologies and adjunctive therapies, and patient outcomes, including their variation over time and by site of care. We know even less about the influence of major Chinese health reforms and evolving standards for accreditation of physicians and hospitals that perform coronary catheterisation and PCI.17
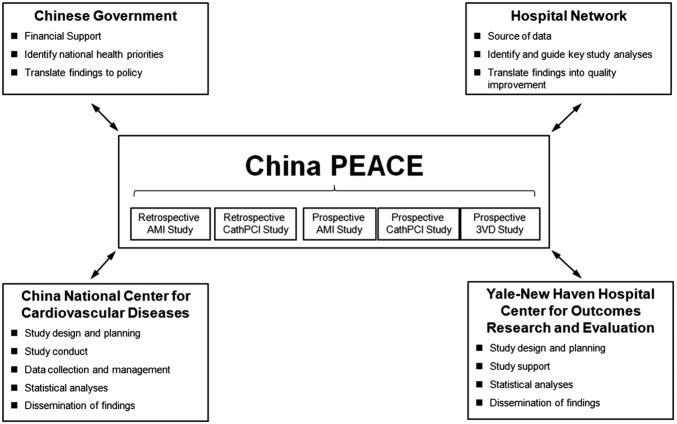
To address these gaps in knowledge and as a prelude to national quality improvement efforts, we have leveraged the China PEACE (Patient-centered Evaluative Assessment of Cardiac Events) research network to perform the China PEACE Retrospective Study of Coronary Catheterisation and Percutaneous Coronary Intervention (China PEACE-Retrospective CathPCI Study). China PEACE is a collaborative effort among the China National Center for Cardiovascular Diseases (NCCD); the Yale-New Haven Hospital Center for Outcomes Research and Evaluation; the Chinese government; and a national network of Chinese hospitals (figure 1). The goal of the network is to generate new knowledge relevant to practice and policy and to translate this knowledge into action to improve care and outcomes for patients with cardiovascular disease.
Figure 1.
The China PEACE initiative. Key partners include the Chinese government, collaborating hospitals, the China National Center for Cardiovascular Diseases and the Yale-New Haven Hospital Center for Outcomes Research and Evaluation. The China PEACE-Retrospective CathPCI Study is one of five initial studies from the China PEACE initiative. The topic areas for these five projects concern acute myocardial infarction, coronary catheterisation/percutaneous coronary intervention and multivessel coronary artery disease. Future studies will focus on cerebrovascular disease and other cardiovascular conditions. 3VD, triple-vessel coronary artery disease; AMI, acute myocardial infarction; PCI, percutaneous coronary intervention.
The China PEACE-Retrospective CathPCI Study will examine a nationally representative sample of almost 12 000 patients who underwent coronary catheterisation or PCI from 55 Chinese hospitals during 2001, 2006 and 2011. This approach will permit the study of care patterns and outcomes across hospitals, regions and time during a dynamic period of healthcare reform. The study is largely descriptive, and rather than test a specific hypothesis, seeks to provide a foundation for future quality improvement and research. Specific aims of the China PEACE-Retrospective CathPCI Study are: (1) to describe the characteristics of patients undergoing coronary catheterisation or PCI in China including their demographic, socioeconomic and clinical attributes; (2) to characterise patterns of treatment including the use of procedural technologies and adjunctive therapies; (3) to describe in-hospital outcomes such as mortality, treatment complications, length of stay and hospital charges; (4) to characterise differences in treatment and outcomes by patient characteristics, hospital, region and year of study and (5) to examine adherence to quality measures for PCI. Research findings will be shared with study hospitals and the Chinese government to improve the selection of patients for these procedures and their associated outcomes. In this paper, we describe study methodology, abstracted data elements, planned statistical analyses and initial enrolment of sites.
Methods and analysis
Design overview
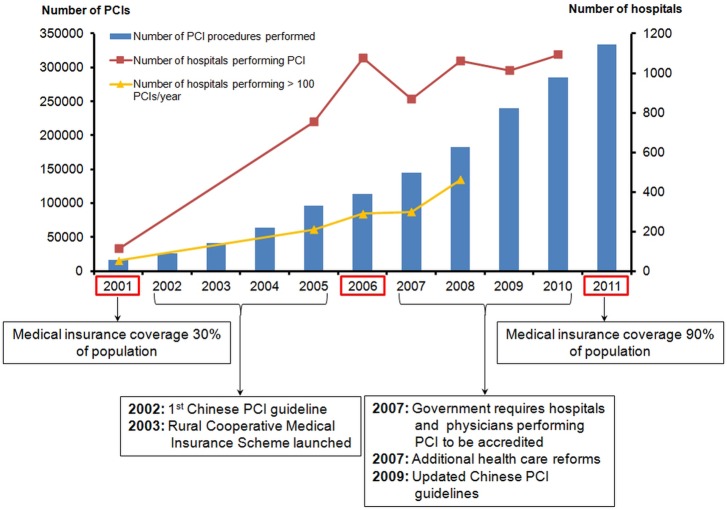
We defined our study cohort based only on in-hospital coronary catheterisations, as these procedures are not commonly performed on an outpatient basis in China. We included patients undergoing coronary catheterisation for any indication. We sampled eligible hospitalisations for 2001, 2006 and 2011 from a network of nationally representative hospitals. We chose these three time periods to reflect the influence of major changes in the Chinese healthcare system and the creation of PCI guidelines by Chinese medical societies (figure 2).
Figure 2.
Chinese trends in PCI volume from 2001 to 2011. Figure demonstrates the increase in PCI volume by year and the increase in the number of hospitals performing >100 PCIs per year. Notable events pertinent to the expansion in PCI access with time are highlighted below the graph. In 2003, the Rural Cooperative Medical Care System expanded health insurance to low-income rural residents. The healthcare reform of 2007 substantially expanded health spending for the rural and urban population. We sampled eligible hospitalisations for 2001, 2006 and 2011. PCI, percutaneous coronary intervention.
To study 10-year trends in patient characteristics, treatment patterns and outcomes nationally and within regions of different socioeconomic development, we drew a representative, stratified sample of patient discharges for each year. We intentionally drew a larger sample for 2011 to obtain more precise estimates of the current performance and variation in treatment patterns and outcomes among hospitals.
The central ethics committee at the China NCCD approved the China PEACE-Retrospective CathPCI Study. All collaborating hospitals accepted the central ethics approval except for five hospitals, which obtained local approval by internal ethics committees. The study is registered at http://www.clinicaltrials.gov (NCT01624896).
The Chinese government, which provided financial support for the study, had no role in its design or conduct; in the collection, management, analysis and interpretation of the data; or in the preparation or approval of the manuscript.
Sampling design
We chose hospitals to reflect the diverse sites of care that perform coronary catheterisation and PCI. Candidate hospitals were limited to urban areas, as catheterisation capability is restricted almost exclusively to these regions. We identified urban areas using official administrative divisions, in which a region is considered urban if it is part of a downtown or suburban area within a direct-controlled municipality (Beijing, Tianjin, Shanghai and Chongqing) or 1 of 283 prefectural-level cities. In total, Mainland China is composed of 287 urban regions. As financial and medical resources are not identical throughout Mainland China, we separately identified hospitals in each of its three official economical-geographical regions, that is, Eastern, Central and Western. As Central and Western urban regions have similar per capita income and health services capacity, we combined Central and Western regions into one stratum.18 We then identified hospitals separately for two study strata: Eastern-urban and Central/Western-urban.
We identified cases for study inclusion using a stratified two-stage cluster sampling design (figure 3). In the first stage, we identified hospitals using a simple random sampling procedure in each stratum. The sampling framework consisted of the highest-level hospitals in each of the predefined urban regions with the potential capability of performing PCI (833 hospitals in 287 urban regions). Hospital level is officially defined by the Chinese government based on clinical resource capacity, for example, secondary hospitals have at least 100 inpatient beds and the capacity to provide acute medical care and preventive care services to populations of at least 100 000, while tertiary hospitals are large referral centres in provincial capitals and major cities.15 19 We excluded military hospitals, prison hospitals, specialised hospitals without a cardiovascular disease division, and traditional Chinese medicine hospitals. We selected representative hospitals from 2011 to reflect current practices and traced this same hospital cohort backwards to 2006 and 2001 to describe temporal trends. As hospital numbers have grown by approximately 18% over the past decade,20 21 the cohort should be most representative of national treatment patterns and outcomes in 2011.
Figure 3.
The China PEACE-Retrospective CathPCI Study flow chart and associated quality assurance strategies. The flow chart should be read from top to bottom. CRF, case report form; Q & A, questions and answers.
In the second stage, we drew cases based on the local hospital database for patients who underwent coronary catheterisation or PCI at each sampled hospital using systematic random sampling procedures. For each stratum, we determined the sample size required to achieve a 1.5% precision for describing the percentage of patients experiencing in-hospital complications, which we estimated at 5%.9 22–25 To achieve a precision of 1.5% with an α of 0.05 in each stratum, assuming an intraclass correlation of 0.02 and design effect of 2.2, we would need to sample 1750 records among hospitals with an average cluster size of 60. This cluster size appeared reasonable based on national administrative data26 and our previous survey of treatment for acute coronary syndromes at more than 1000 hospitals in 2010, which demonstrated that the median annual PCI volume was approximately 300 cases. Assuming a participation rate of 85% among selected hospitals, we approached 35 hospitals for participation in each stratum, for a total of 70 hospitals. We doubled cluster sizes for 2011 to improve precision in the description of hospital-level treatment patterns and outcomes. Consequently, the total expected sample volume with the above assumptions was approximately 3500 cases in 2001, 3500 cases in 2006 and 7000 cases in 2011. A more detailed description of the sampling strategy is provided in the supplemental material within the online web appendix.
Data collection
We trained staff at participating hospitals to identify all hospitalisations with coronary catheterisation or PCI procedures from their respective local hospital databases for 2001, 2006 and 2011. After we sampled cases at each hospital, we assigned each case a unique study ID. We then required local investigators to obtain the original record and transmit a scanned copy to the coordinating centre. To facilitate this process, the coordinating centre provided each study site with a high-speed scanner. To verify compliance with the case-finding strategy, research staff from the coordinating centre visited 60% of the sites to repeat the case-finding process, confirm that the list of hospitalisations undergoing coronary catheterisation or PCI was complete, and assist in acquiring the sampled cases (figure 3). We chose to visit study sites that were expected to contribute a large volume of cases and have potential difficulties in complying with the case-finding strategy. These sites provided 73% of sampled cases.
Following receipt of the scanned record, research staff at the China NCCD ensured the completeness and quality with which each medical record was copied. We required incomplete or poorly scanned records to be rescanned and retransmitted (figure 3). We instructed study sites to include all parts of the medical record, including the face sheet, admission note, daily progress notes, procedure notes, medication administration record, diagnostic procedure reports, laboratory test results, physician orders, nursing notes and discharge summary.
The China PEACE-Retrospective CathPCI Study adhered to rigorous standards for abstraction. Before initiating the chart review, each abstractor received 2 weeks of training that included an introduction to the study, coronary heart disease and its subtypes, components of the inpatient medical record including specialised sections such as catheterisation reports, and the China PEACE-Retrospective CathPCI Study data dictionary. We provided all material, including the data dictionary, in Chinese. After training, we certified individuals who were able to abstract five sample medical records with greater than 98% accuracy. Inexperienced abstractors began with exclusively typewritten rather than handwritten medical records. In addition, we randomly audited approximately 5% of the abstracted records. If the records were not abstracted with 98% accuracy, all medical records in the audited batch were considered unqualified and were re-reviewed by a different abstractor. Abstractors were required to maintain this benchmark of 98% accuracy to retain certification. We used abstractors with formal medical training to identify data elements requiring more advanced medical knowledge, such as the development of postprocedural complications including bleeding or cardiac tamponade. A physician was always present in the room with abstractors or was available online to answer questions as they arose. We assigned medical records belonging to the same hospital and year to a broad group of reviewers to avoid potential residual disparities in quality among abstractors.
Data management
We systematically perform ongoing data cleaning. Data managers regularly query data for invalid and illogical values as well as for duplicate record entries. They identify potential invalid values by searching for outliers in continuous data distributions. Records with identical study identification numbers, hospital identification numbers, medical record identification numbers and dates of discharge trigger a search for duplicate records. Once a data query is made, concerns are resolved after tracing and reviewing the relevant records.
All data have been treated as protected health information and are thus securely stored in an encrypted and password protected database at the main coordinating centre.
Data elements
We examined the English language and Chinese literature for relevant studies to create a list of candidate variables. We supplemented these elements with variables used in the CathPCI Registry of the American College of Cardiology National Cardiovascular Data Registry (NCDR). CathPCI is an outcome-based registry and quality improvement programme that focuses on patients who undergo coronary catheterisation and PCI in the USA. Incorporating variables from CathPCI will permit cross-country comparisons in resource utilisation and adherence to quality metrics. We also incorporated elements from the case report form used in the China PEACE Retrospective Study of Acute Myocardial Infarction27 (clinicaltrials.gov identifier NCT01624883) to permit comparison of hospitalisations for acute myocardial infarction between these two China PEACE studies. Major categories of data elements are described in table 1.
Table 1.
China PEACE-Retrospective CathPCI Study data elements
| Category | Example elements |
|---|---|
| Patient demographics | Age, sex, ethnicity, postal code, occupation and insurance status |
| Medical history | Diabetes, hypertension, hyperlipidaemia, vascular disease and prior revascularisation |
| Initial cardiac status | Heart rate, blood pressure, Killip class, heart failure and cardiac arrest |
| Lab values | Troponin, CK, CK-MB, BNP, sodium, BUN, creatinine, WBC count and haemoglobin |
| Diagnostic procedures | Coronary catheterisation, echocardiogram, CT angiogram, stress testing and chest radiograph |
| Medications including dose | Antiplatelet therapy, anticoagulant therapy, beta blocker, ACE inhibitor/ARB, statin, and traditional Chinese medicines |
| Auxiliary imaging | Intravascular ultrasound and fractionated-flow reserve |
| Coronary flow dynamics | Preprocedural TIMI flow and postprocedural TIMI flow |
| Revascularisation | Fibrinolysis, PCI (access, coronary anatomy, bypass graft anatomy, stent number, stent type, stent length, contrast dose and closure device) and CABG surgery |
| Mechanical support | Intra-aortic balloon pump, left ventricular assist device and ECMO |
| Outcomes including in-hospital complications | Death, myocardial infarction, heart failure, shock, arrhythmia, stroke, bleeding, transfusion, infection, coronary perforation and coronary dissection |
ARB, angiotensin receptor blocker; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CABG, coronary artery bypass graft; CK, creatine kinase; ECMO, extracorporeal membrane oxygenation; PCI, percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction; WBC, white blood cell.
In table 2, we show data relevant to performance measures during the first 24 h of hospitalisation and at hospital discharge for patients with acute myocardial infarction and those undergoing PCI for stable coronary artery disease.
Table 2.
China PEACE-Retrospective CathPCI Study performance measures
| First 24 h | Discharge |
|---|---|
| Aspirin | Aspirin* |
| Time to primary PCI | Thienopyridine* |
| Time to fibrinolysis | β blocker |
| ACE inhibitor or ARB for LV systolic dysfunction | |
| Statin* | |
| Smoking cessation counselling | |
| Cardiac rehabilitation referral |
All performance measures apply to patients with acute myocardial infarction. Performance measures with asterisks also apply to patients undergoing PCI for stable coronary artery disease.
ARB, angiotensin receptor blocker; LV, left ventricle; PCI, percutaneous coronary intervention.
Where possible, we collected data that would allow us to construct the core quality measures for acute myocardial infarction used and reported by the Centers for Medicare & Medicaid Services in the USA as well as the quality measures for PCI from the NCDR.28 Two physician investigators at each participating hospital also completed a survey, modelled on the annual survey of hospitals performed by the American Hospital Association, of its major structural and organisational characteristics during the study period.29 Key variables assessed include bed size, teaching status and capability of performing coronary artery bypass graft surgery.
Statistical analyses
We will report summary statistics for patient characteristics, use of diagnostic tests, treatments received and in-hospital outcomes, including complications of care and hospitalisation costs across study sites. Weighting will reflect the reciprocal of sampling probability. For each aim, we will use standard parametric and non-parametric techniques for observational data, including t tests, χ2 tests, Wilcoxon rank sum tests and generalised linear models. Because patient characteristics, treatments and outcomes may be correlated within hospitals, analyses will account for the effect of clustering. To examine and adjust for differences between comparison groups, we will use linear, logistic, Cox proportional hazard and Poisson models with a generalised estimating equation approach and hierarchical models, where appropriate. We will develop models to stratify patients according to their risk of adverse outcomes. We will assess the relationship of candidate variables to in-hospital outcomes using appropriate statistical techniques for the dependent variable. We will further refine the list of candidate variables based on their clinical relevance.
Progress to date
As of December 2012, 55 hospitals agreed to participate in the study. Fifteen hospitals did not participate because they did not provide inpatient services for coronary heart disease (5 hospitals), were incapable of performing coronary catheterisation and PCI throughout the study period (8 hospitals), or refused to participate (2 hospitals). Of the 55 participating hospitals, 29 were located in the Eastern economical-geographical stratum and 26 were located in the Central/Western economical-geographical stratum. The distribution of sites by province and region is shown in figure 4.
Figure 4.
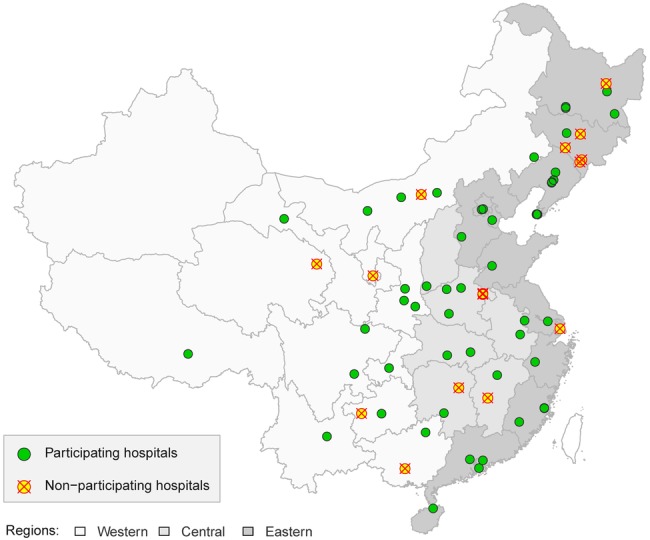
Geographical distribution of participating hospitals in the China PEACE-Retrospective CathPCI Study. Of the 70 sampled hospitals, 15 were unable or unwilling to participate and 55 provided cases for the study.
In parallel with national trends, the number of hospitals providing cases increased with each subsequent year of study. In 2001, 26 of the 55 participating hospitals were capable of performing PCI, of which 24 submitted medical records. Of the two hospitals that did not submit records, one did not keep records in 2001 and the other had its records destroyed by a fire. Both of these institutions most likely had a very limited capacity for PCI in 2001, as evidenced by their performance of only 16 and 28 respective cases of coronary catheterisation or PCI in 2006. A total of 44 hospitals performed PCI in 2006, all of which submitted records to the study. Fifty-four hospitals performed catheterisation and PCI in 2011. As with 2006, all hospitals that performed PCI in 2011 submitted records.
We collected all medical records for abstraction by December 2012. The examination of census databases from participating hospitals yielded 58 008 hospitalisations for coronary catheterisation and PCI (3270 in 2001, 12 875 in 2006 and 41 863 in 2011). Of these 58 008 hospitalisations, we sampled 12 477 (22%; 1444 (44%) in 2001, 3046 (24%) in 2006 and 7987 (19%) in 2011). Of these 12 477 sampled hospitalisations, we acquired medical records for 11 900 (95%); 577 (5%) medical records could not be found due to poor archiving. We began data abstraction of these medical records in March 2013 and expect abstraction to be completed in January 2014.
Discussion
Through the use of a national research network, the China PEACE-Retrospective CathPCI Study is developing a repository of data that describes the current and former use of coronary catheterisation and PCI in China. This project will provide answers to questions about contemporary practice patterns including variation among institutions and temporal trends in procedure use and adjunctive therapy. To elevate the quality of abstracted data, the study uses data collection techniques regularly employed by international clinical trials such as integrated central and on-site monitoring as well as source document checking. The China PEACE-Retrospective CathPCI Study also elicits the active participation of hospitals across China to ensure that study results disseminate broadly for the purpose of quality improvement. Findings will be shared with government to help research findings inform policy. The study is designed to guide the efficient allocation and high-quality use of advanced cardiovascular interventions in the context of a rapidly growing cardiovascular disease burden and dynamically changing healthcare system.
The China PEACE-Retrospective CathPCI Study falls under the larger China PEACE effort, which aims to create a national research network dedicated to improving cardiovascular outcomes. China PEACE is built on a platform of collaboration and coordination between clinicians, researchers and policymakers. With this framework in mind, study sites do not merely transmit information to the coordinating centre, but are full partners and consumers of the knowledge generated. In this way, China PEACE has similarities to the NCDR CathPCI Registry of the American College of Cardiology, which provides participating hospitals with regular performance reports, including analysis of process measures and in-hospital outcomes.
The Chinese government, a partner in China PEACE, will use study results to develop policies intended to strengthen the clinical performance of the hospitals. The goal is not simply to identify poor performing institutions, but to use a shared learning approach to elevate care delivery at all participating hospitals and produce knowledge that is broadly applicable across sites of care, including those external to the China PEACE network. In the future, we hope to directly involve patients and caregivers in designing study questions and disseminating findings.
We anticipate that the results of the China PEACE-Retrospective CathPCI study will illuminate the effects of recent healthcare reforms in China on the use of coronary catheterisation and PCI. Although China has adopted policies to enhance quality of care and dissemination of novel technologies over the past decades,17 they have been applied differentially across the country.26 After the first PCI was performed in China in 1985,5 there followed a period of unregulated dissemination.30 31 In 2000, most patients paid for PCI themselves, and there was widespread variation in access to the technology.6 7 Access was subsequently improved in 2003 with the implementation of the Rural Cooperative Medical Care Scheme that expanded insurance access to the low-income rural population. This government initiative was followed by additional healthcare reforms in the latter half of the decade that further expanded insurance support for rural and urban residents.6 7 In this context, the Ministry of Health established processes for accreditation of interventional centres in 2007 to promote minimal quality standards for PCI.17 Through our nationally representative and multiyear sample of catheterisation and PCI, we will have the capacity to evaluate the temporal influence of these policies on practice patterns and their variability by hospital and region. We may identify signals of potentially inefficient allocation of resources such as the use of drug-eluting stents in situations where bare-metal stents are similarly efficacious. Results may prove useful to future policymaking and guideline development.
We also expect that the knowledge generated from the China PEACE-Retrospective CathPCI Study will be useful internationally. Many low-income and middle-income countries are undergoing a similar epidemiological transition and experiencing comparable challenges associated with a growing number of persons with chronic cardiovascular disease and rapidly rising healthcare costs in the setting of limited healthcare resources.32 The experience with China PEACE may help guide other countries in their desire to learn about trajectories and variation in the use of novel health technologies. Developed nations are also facing common challenges around healthcare reform and the optimal utilisation of advanced interventions.33 The development of novel approaches to care in China may have relevance for more wealthy countries that are grappling with escalating costs and trying to determine whether reduction in resource expenditures can be achieved without compromising patient outcomes.
The China PEACE-Retrospective CathPCI Study is distinguished from many retrospective analyses by its use of data quality control strategies that are common in the performance of multicentre clinical trials. The study has devoted significant attention to data quality at multiple stages, including case ascertainment, data abstraction and data management. For example, research staff visited study sites to identify all hospitalisations involving coronary catheterisation or PCI from local hospital databases and physically found all medical records for sampled cases whenever possible. In addition, all abstractors underwent standardised training at the coordinating centre and continued to maintain high standards for accuracy to remain certified. Medical records reviewed by abstractors who fail to meet continuing recertification requirements are always reabstracted. These and other quality control and assurance strategies are the result of the NCCD's previous experience in conducting cardiovascular clinical trials in China.
This study has some limitations, including those inherent to its retrospective design. Findings depend on the accuracy and completeness of the medical records, and the abstraction process. However, these limitations would apply to all retrospective studies. Moreover, the identification of poor documentation of key variables is crucial to future quality improvement measurements. Our study is not designed to track outcomes following hospitalisation or the experience of patients. These variables, however, will be collected in the recently launched Prospective Study of PCI from the China PEACE platform. Finally, in our sampled hospital cohort, catheterisation and PCI volume were smaller than expected for the year 2001, in particular, as the number of hospitals capable of performing PCI and the PCI volume per centre were relatively low. However, the sample remains nationally representative in the last year and can still provide acceptable precision in describing 10-year trends (supplemental material within the online web appendix).
The China PEACE-Retrospective CathPCI Study, one of the first studies to be launched from the China PEACE platform, seeks to assess the characteristics, treatments and outcomes of patients who receive diagnostic catheterisation and PCI in a large, nationally representative sample of hospitals in China. It provides a unique opportunity to compare variation in care and to assess trajectories in practice in the context of an era of rapid adoption of new cardiovascular technologies and constrained healthcare resources. Findings are intended to guide the more efficient use of coronary catheterisation and PCI in a manner that improves health outcomes across diverse geographical settings and sites of care within China.
Dissemination
The Chinese government, which provided financial support for the study, had no role in its design or conduct; in the collection, management, analysis and interpretation of the data; or in the preparation or approval of this manuscript.
Study findings will be shared freely with participating hospitals and the Chinese government. To improve healthcare quality, data will be presented in the format of regular performance reports that include analysis of process measures and in-hospital outcomes. Findings will also be disseminated via peer-reviewed publications. Requests for collaboration will be welcomed.
Supplementary Material
Acknowledgments
The authors appreciate the multiple contributions made by study teams at the China Oxford Centre for International Health Research and the Yale-New Haven Hospital Center for Outcomes Research and Evaluation in the realms of study design and operations. They are grateful for the support provided by the Chinese government.
Footnotes
Contributors: JL and KD made substantial contributions to study conception and design and to the drafting and critical revision of the manuscript for important intellectual content. XL, ZL and S-LTN made substantial contributions to the study conception and design and critical revision of the manuscript for important intellectual content. HMK and LJ made substantial contributions to study conception and design, drafting and critical revision of the manuscript for important intellectual content, and provided administrative, technical and material support, including study supervision.
Funding: This project was partly supported by the Research Special Fund for Public Welfare Industry of Health (201202025) from National Health and Family Planning Commission of China, and the International Science & Technology Cooperation Program (2010DFB33140) from the Ministry of Science and Technology of China. At the time this study was initiated, Dr. Dharmarajan was supported by grant HL007854 from the National Heart, Lung, and Blood Institute; he was also supported as a Centers of Excellence Scholar in Geriatric Medicine at Yale by the John A. Hartford Foundation and the American Federation for Aging Research. HMK is supported by grant U01 HL105270-03 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute.
Competing interests: HMK reports being the recipient of research grants from Medtronic and from Johnson & Johnson, through Yale University, to develop methods of clinical trial data sharing, and the chair of a cardiac scientific advisory board for UnitedHealth.
Ethics approval: The central ethics committee at the China National Center for Cardiovascular Diseases (NCCD) approved the China PEACE-Retrospective-CathPCI Study. All collaborating hospitals accepted the central ethics approval except for five hospitals, which obtained the local approval from internal ethics committees. The study is registered at http://www.clinicaltrials.gov (NCT01624896).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.He J, Gu D, Wu X, et al. Major causes of death among men and women in China. N Engl J Med 2005;353:1124–34 [DOI] [PubMed] [Google Scholar]
- 2.Moran A, Gu D, Zhao D, et al. Future cardiovascular disease in China: Markov model and risk factor scenario projections from the coronary heart disease policy model-China. Circ Cardiovasc Qual Outcomes 2010;3:243–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang G, Kong L, Zhao W, et al. Emergence of chronic non-communicable diseases in China. Lancet 2008;372:1697–705 [DOI] [PubMed] [Google Scholar]
- 4.Gao R. The development of percutaneous cardiovascular intervention in China. http://www.cmt.com.cn/detail/39179.html (accessed 17 Sep 2013).
- 5.Gao R. Current status of percutaneous coronary intervention in China. Heart 2010;96:415–18 [DOI] [PubMed] [Google Scholar]
- 6.Cao Q, Shi L, Wang H, et al. Report from China: health insurance in China—evolution, current status, and challenges. Int J Health Serv 2012;42:177–95 [DOI] [PubMed] [Google Scholar]
- 7.Hu S, Tang S, Liu Y, et al. Reform of how health care is paid for in China: challenges and opportunities. Lancet 2008;372:1846–53 [DOI] [PubMed] [Google Scholar]
- 8.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Section of Interventional Cardiology, Chinese Society of Cardiology A data analysis of the third national coronary intervention registry. Chin J Cardiol 2002;30:719–23 [Google Scholar]
- 10.Lu SZ, Song XT, Chen YD, et al. Findings from registry of percutaneous coronary intervention in inland of China. Zhonghua Xin Xue Guan Bing Za Zhi 2009;37:26–9 [PubMed] [Google Scholar]
- 11.Lu SZ, Song XT, Chen YD, et al. Beyond the numerals: primary reports from Registry of PCI In China (ROPIC). Zhonghua Xin Xue Guan Bing Za Zhi 2006;34:966–70 [PubMed] [Google Scholar]
- 12.Yuan F, Song XT, Lu SZ. Percutaneous coronary intervention in mainland China in 2008: register results. Zhonghua Xin Xue Guan Bing Za Zhi 2010;38:629–32 [PubMed] [Google Scholar]
- 13.Song XT, Du MY, Yuan F, et al. Cost-utility analysis of percutaneous coronary intervention in 13 cities of China. Zhonghua Xin Xue Guan Bing Za Zhi 2010;38:484–7 [PubMed] [Google Scholar]
- 14.Yu LT, Zhu J, Mister R, et al. The Chinese registry on reperfusion strategies and outcomes in ST-elevation myocardial infarction. Zhonghua Xin Xue Guan Bing Za Zhi 2006;34:593–7 [PubMed] [Google Scholar]
- 15.Gao R, Patel A, Gao W, et al. Prospective observational study of acute coronary syndromes in China: practice patterns and outcomes. Heart 2008;94:554–60 [DOI] [PubMed] [Google Scholar]
- 16.Liu SW, Xu B, Chen J, et al. Trends in in-hospital outcome after percutaneous coronary intervention in the drug-eluting stents era. Clin Cardiol 2010;33:516–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ministry of Health of the People's Republic of China Regulation about cardiovascular interventional techniques 2007. http://www.moh.gov.cn/mohbgt/pw10712/200804/18725.shtml (accessed 17 Sep 2013).
- 18.National Bureau of Statistics of China 2010 China statistical yearbook. http://www.stats.gov.cn/tjsj/ndsj/2010/indexeh.htm (accessed 17 Sep 2013).
- 19.Ministry of Health of the People's Republic of China The performance evaluation standards for general hospitals (revised version). http://www.moh.gov.cn/cmsresources/mohbgt/cmsrsdocument/doc6535.pdf (accessed 17 Sep 2013).
- 20.Ministry of Health of the People's Republic of China China public health statistical yearbook 2003. Beijing: Peking Union Medical College Publishing House, 2003 [Google Scholar]
- 21.Ministry of Health of the People's Republic of China China public health statistical yearbook 2012. Beijing: Peking Union Medical College Publishing House, 2012 [Google Scholar]
- 22.Venkitachalam L, Kip KE, Selzer F, et al. Twenty-year evolution of percutaneous coronary intervention and its impact on clinical outcomes: a report from the National Heart, Lung, and Blood Institute–sponsored, multicenter 1985–1986 PTCA and 1997–2006 dynamic registries. Circ Cardiovasc Interv 2009;2:6–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Li CX, Wang HC, et al. Efficacy and safety of Firebird sirolimus-eluting stent in treatment of complex coronary lesions in Chinese patients: one-year clinical and eight-month angiographic outcomes from the FIREMAN registry. Chin Med J 2011;124:817–24 [PubMed] [Google Scholar]
- 24.Koh A, Khin L, Choi L, et al. Percutaneous coronary intervention in Asians- are there differences in clinical outcome? BMC Cardiovasc Disord 2011;11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chinese PLA Cardiology Society Analysis of the third (from 2003 to 2004) PLA coronary intervention therapy registry. Med J Chin People's Liberation Army 2006;31:60–4 [Google Scholar]
- 26.Huo Y. Current status and development of percutaneous coronary intervention in China. J Zhejiang Univ Sci B 2010;11:631–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dharmarajan K, Li J, Li X, et al. The China Patient-Centered Evaluative Assessment of Cardiac Events (China PEACE) retrospective study of acute myocardial infarction: study design. Circ Cardiovasc Qual Outcomes 2013;6:732–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Cardiovascular Data Registry NCDR quality metrics and measures. https://www.ncdr.com/WebNCDR/home/metrics-and-measures (accessed 17 Sep 2013).
- 29.American Hospital Association AHA annual survey database fiscal year 2011. http://www.ahadataviewer.com/book-cd-products/aha-survey/ (accessed 17 Sep 2013).
- 30.Chinese Society of Cardiology, Chinese Society of Cardiology Editorial Board 2002 Chinese guideline for percutaneous coronary intervention. Zhonghua Xin Xue Guan Bing Za Zhi 2002;30:707–18 [Google Scholar]
- 31.Chinese Society of Cardiology, Chinese Society of Cardiology Editorial Board 2009 Chinese guideline for percutaneous coronary intervention. Zhonghua Xin Xue Guan Bing Za Zhi 2009;37:4–2519671346 [Google Scholar]
- 32.Nugent R. Chronic diseases in developing countries: health and economic burdens. Ann N Y Acad Sci 2008;1136:70–9 [DOI] [PubMed] [Google Scholar]
- 33.Blumenthal D, Dixon J. Health-care reforms in the USA and England: areas for useful learning. Lancet 2012;380:1352–7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.