Abstract
This cross-sectional field study documented the effect of long-term t'ai chi, meditation, or aerobic exercise training versus a sedentary lifestyle on executive function. It was predicted that long-term training in t'ai chi and meditation plus exercise would produce greater benefits to executive function than aerobic exercise. T'ai chi and meditation plus exercise include mental and physical training. Fifty-four volunteers were tested: t'ai chi (n=10); meditation+exercise (n=16); aerobic exercisers (n=16); and sedentary controls (n=12). A one-factor (group), one-covariate (age) multivariate analysis of covariance was performed. Significant main effects of group and age were found (group, 67.9%, p<0.001; age, 76.3%, p=0.001). T'ai chi and meditation practitioners but not aerobic exercisers outperformed sedentary controls on percent switch costs (p=0.001 and p=0.006, respectively), suggesting that there may be differential effects of training type on executive function.
Introduction
As human life expectancy has lengthened in the developed world, successful aging has become a public health concern. Quality of life, cognitive capacity, and physiologic status are all affected by aging.1 Aging effects include declines in key physiologic systems (cardiovascular, neuromotor, and cognitive), and complicating effects of damage to those systems. Maximal oxygen uptake (VO2 Max), a well-established proxy for cardiovascular health, declines at a rate of 1% per year after the age of 20.2 Reaction time (RT) on neuropsychologic tests of cognitive capacity has been shown to decline by a factor of 1.5 during the 25th–65th years of life.3 Furthermore, evidence suggests that the seventh decade is a critical point when significantly greater decrements begin to occur.4,5 After the age of 65, walking and other locomotor skills necessary for independent, daily living require increasing cognitive resources to perform.6 Can these declines be mitigated?
This cross-sectional field study observed the aerobic and executive function capacity of self-selected long-term exercisers in three groups (t'ai chi, meditation plus exercise, and aerobic exercise) versus sedentary controls living in Eugene, Oregon. Investigation of training regimens that may offset the effects of aging on physiologic function has been under way for decades. Of particular interest are regimens that may extend mid-life (30–65 years) cognitive capacity into the seventh and eighth decades of life. Aerobic exercise and meditation are two available health regimens shown to positively affect executive function in young and older adults.7–13
Executive function processes involved in tasks of daily living include the ability to respond appropriately to novel situations, make choices, set goals, coordinate and modify task sequences, inhibit inappropriate responses, and switch between tasks or instructions appropriately.4,14 Key executive components underlying these operations are (1) inhibition, (2) updating, (3) shifting between instruction sets,15 and working memory.14 Salthouse et al.4 found that degradation of the inhibition and updating components of executive function may mediate age-related cognitive decline (i.e., memory, percept formation, association processes).
A small number of studies have divided exercise types into those requiring aerobic exertion alone and aerobic exertion plus consistent mental exertion. Exercise requiring consistent mental exertion (e.g., soccer, dance) was associated with better performance on tests of executive attention16,17 Exercise requiring consistent mental exertion generally also requires cortical coordination of complex movement sequences.18
Aspects of executive function that are upregulated by moderate exercise include speed of information processing as indexed by visual and auditory electroencephalographic (EEG) P300 event-related potential (ERP) latency and reaction-time decreases, and correlated accuracy increases in oddball tasks; and (2) enhancement of executive attention processes, indexed by RT and accuracy on Erikson flanker tasks in older adults.7 Additionally, research shows that consistent aerobic exercise regimens increase the thickness of frontal, parietal, and temporal cortices in humans.19 A recent meta-analysis showed that intense aerobic exercise as quantified by standard cardiovascular measures is not required to accrue cognitive benefits from exercise. Light-to-moderate exercise and dose frequency are key factors.20
Meditation has also been shown to enhance executive function. Meditation is defined as concentrated mental focus on a sound, image, set of syllables (chant or mantra), or activity (walking, sitting). All conflicting stimuli are pushed out of awareness.21 Thus, meditation requires mental effort to inhibit distracting thoughts or sensations. Chan and Woollacott10 found that long-term practitioners of meditation showed less interference on the Stroop task than nonpracticing controls. Importantly, many meditation practitioners also engage in yoga and other moderate exercise activities. Indeed, one of the most utilized and studied meditation practices, mindfulness-based stress reduction, explicitly incorporates Hatha yoga into its training regimen. Hatha yoga is a form of moderate exercise requiring complex coordination of body segments.22 Not surprisingly, during subject recruitment for this study, no sedentary meditators were found. All meditation practitioners reported habitual participation in moderate exercise of some kind. Thus, a contributing factor in meditation's benefit to executive function might be cardiovascular and metabolic modifications resulting from a lifestyle typically including moderate aerobic exertion.
Another health training regimen that shows promise for benefiting executive function is t'ai chi. T'ai chi is a form of moderate exercise23 that has been shown to be superior to aerobic walking for cardiovascular function.24 T'ai chi is also a form of moving meditation25 requiring memorization of complex movement sequences,26 and thus motor learning. Motor learning requires executive function.27 T'ai chi also requires constant application of attention for optimal performance.26 Because t'ai chi requires moderate exercise, motor learning, consistent application of attention, and complex coordination of movement, it is possible that frequent practice would yield similar or greater benefits to executive function compared to aerobic exercise alone. Indeed, a recent uncontrolled study showed that 10 weeks of t'ai chi training was associated with better task-switch performance in community-dwelling elders.28
This cross-sectional field study of long-term t'ai chi, meditation, and aerobic exercise practitioners versus sedentary controls utilized a complex visuospatial task switch (VSTS) test to assess executive function. This test required three main components of executive function: (1) inhibition, (2) updating, and (3) shifting between instruction sets. Subjects were also tested with the Rockport 1-mile walk, a well-validated assessment of estimated VO2 Max, to document cardiovascular status.29 It was expected that the training groups would outperform sedentary controls on estimated VO2 Max. As a result, it was also expected they would outperform sedentary controls on executive function measures.7,30,31 It was predicted that long-term t'ai chi and meditation practice would be associated with greater benefits to executive function than aerobic exercise alone, because both combine mental and physical exertion. All three training groups were predicted to significantly outperform sedentary controls on executive function. Further, it was hypothesized that age would have less effect on this study's key measures in health regimen practitioners compared to sedentary controls.
Materials and Methods
Participants
Participants were recruited from Eugene, Oregon. Inclusion criteria were (1) no self-reported neurological or physical disorders, (2) living independently, and (3) aged 20–75. The large age range was chosen so the effects of aging could be documented over the adult lifespan on the key measures. It was expected that group membership would be associated with larger effects than age.3,5,8,32–34 Sedentary participants were required to have (1) a generally inactive lifestyle for 5 or more years, and (2) no prior experience with meditation or t'ai chi. Training group practitioners were required to (1) have practiced at least 5 years or more, three times per week, 30 minutes per session. All participants had self-selected into their preferred level and type of exercise activity. Fifty-nine participants enrolled in the study. Because acute exercise has been shown to improve cognitive performance,17,35 cognitive testing was done before exercise testing. Two participants unable to use a computer effectively were excluded, since the executive attention test was administered via computer. Two subjects did not complete the testing. One subject who presented with bipolar disorder was excluded. Fifty-four subjects were thus included in this analysis (female=27). Group composition was (1) 10 t'ai chi (female=3), (2) 16 meditation plus exercise (female=6), (3) 16 aerobic fitness (female=8), and (4) 12 sedentary (female=10) participants. Body–mass index (BMI), which has been associated with cognitive capacity,36,37 was calculated for each participant. BMI is mass in kilograms/height in meters squared (Table 1).2Participant recruitment and experimental protocol were approved by the University of Oregon Institutional Review Board. Participants gave informed consent and were compensated for participation.
Table 1.
Participant Physiologic Scores
| Age | VO2 Max | BMI | ||||||
|---|---|---|---|---|---|---|---|---|
| Group | n | Females | M | SD | M | SD | M | SD |
| T'ai chi | 10 | 3 | 55.4 | 12.99 | 34.14 | 6.34 | 29.3 | 3.77 |
| Meditation | 16 | 6 | 48.63 | 15.00 | 41.83 | 9.04 | 23.3 | 3.53 |
| Aerobics | 16 | 8 | 44.09 | 16.2 | 45.66 | 9.67 | 23.78 | 2.62 |
| Sedentary | 12 | 2 | 46.92 | 12.81 | 28.68 | 5.76 | 27.93 | 6.37 |
Global ranges: age, 22–75; BMI, 18.50–37.90; estimated VO2 Max, 17.23–60.00.
VO2 Max, estimated maximal oxygen uptake; BMI, body–mass index; SD, standard deviation; M, mean.
Testing
Aerobic capacity: Rockport 1-mile walk29
The Rockport 1-mile walk was utilized to obtain estimated VO2 Max, controlling for age, weight, and gender (http://www.exrx.net/Calculators/Rockport.html).29
Executive attention
Executive attention test structure has been shown to affect RT. To maximize test difficulty, a complex visuospatial task switch test (Mayr Laboratory, University of Oregon, 2009) was utilized. This test required working memory, inhibition, shifting, and updating,15 was noncued, and utilized alternating runs of two rules.38 Stimulus appearance was randomized. More complex tests produce longer RTs.32 Noncued paradigms have been shown to be more difficult than cued paradigms.39 Short response-stimulus intervals have been shown to produce greater switch costs than longer response to stimulus intervals.40 The Mayr VSTS utilized a short response to stimulus interval (10 msec). Measures were switch RT and switch capacity (percent local switch costs). Percent local switch costs are calculated by subtracting no-switch from switch RT, then dividing by no-switch RT to control for the speed accuracy trade-off. Local switch costs index the capacity to switch tasks quickly and accurately.39,41,42 Lower switch costs index greater switch capacity.17
Paradigm
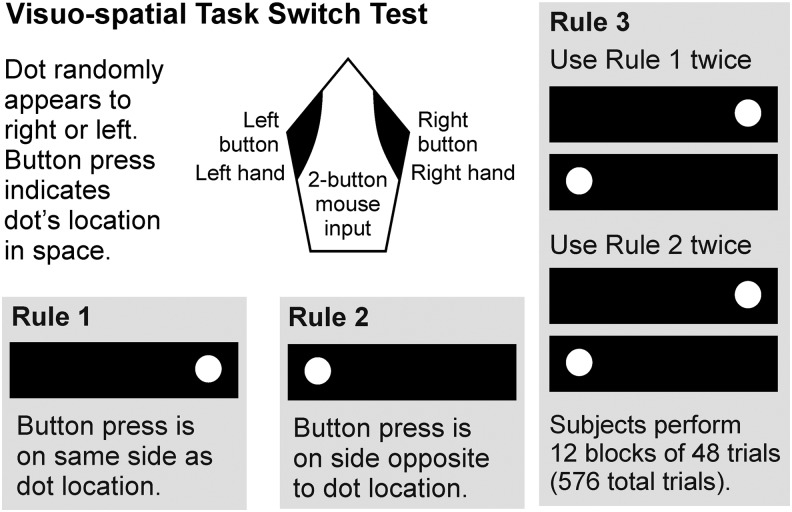
Participants were trained to indicate the location of a randomly appearing dot within a fixation rectangle using two different response rules (Fig. 1). For Rule 1, the button-press response was compatible with the dot's location in space, but for Rule 2, was incompatible. For Switch trials (Rule 3), participants switched between Rule 1 and 2 on every other trial.
FIG. 1.
Visuospatial task switch paradigm (Mayr Laboratory, University of Oregon).
Stimuli were displayed on a computer monitor located ∼24 inches in front of the participant. Participants were trained to respond as quickly and accurately as possible. For Rule 3, participants were provided with visual feedback regarding errors. They corrected their error and continued the trial block. Participants practiced until they achieved 85% accuracy. Rules 1 and 2 consisted of 48 trials in two blocks. Rule 3 (the actual task switch) consisted of 12 blocks of 48 trials/block.
Multivariate cross-sectional design
In multivariate designs, multiple dependent variables are measured on subjects who are assigned membership in carefully defined groups.43 The study was a one-factor (group), one-covariate (age) multivariate analysis of covariance (MANCOVA) with groups: (1) t'ai chi, (2) meditation+exercise, (3) aerobic exercise, and (4) sedentary controls who had never engaged in the training modalities. Age and group were expected to have significant effect sizes on VO2 Max, switch reaction time, and percent local switch costs. Each training group reported statistically similar lifetime hours of moderate aerobic exertion. This allowed the investigators to equate physical exercise effects. Covarying age allowed assessing its effect on the dependent measures. The difference between the training groups was type of attentional focus and complexity of motor output required.16,17
Data analysis
A multivariate analysis of covariance (MANCOVA) with one factor (group) and one covariate (age), and Levene's test for homogeneity of variance were performed. Alpha was 0.05 for the MANCOVA. A bivariate correlation was run on all variables. To control for α slippage for multiple analyses, a Bonferroni correction was applied and α was set at 0.0125. Dependent measures were (1) estimated VO2 Max, (2) switch reaction time, and (3) percent local switch costs. Because whole system functionality was evaluated, error trials were included in reaction time means. To document error effects on RT, mean RTs of error trials (milliseconds), accurate trials (milliseconds), and post-error trials (milliseconds) were compared in a one-way analysis of variance (ANOVA) with trial type as factor and trial time in milliseconds as the dependent measure. To isolate predictors of switch costs, a regression with group, age, BMI, VO2 Max, error trials, accurate trials, percent accuracy, and post-error trials as predictors of percent local switch costs was run. It was predicted that all training groups would outperform sedentary controls. All analyses were run with PSAW Statistics 19 (IBM, Chicago, IL).
Results
Overall results
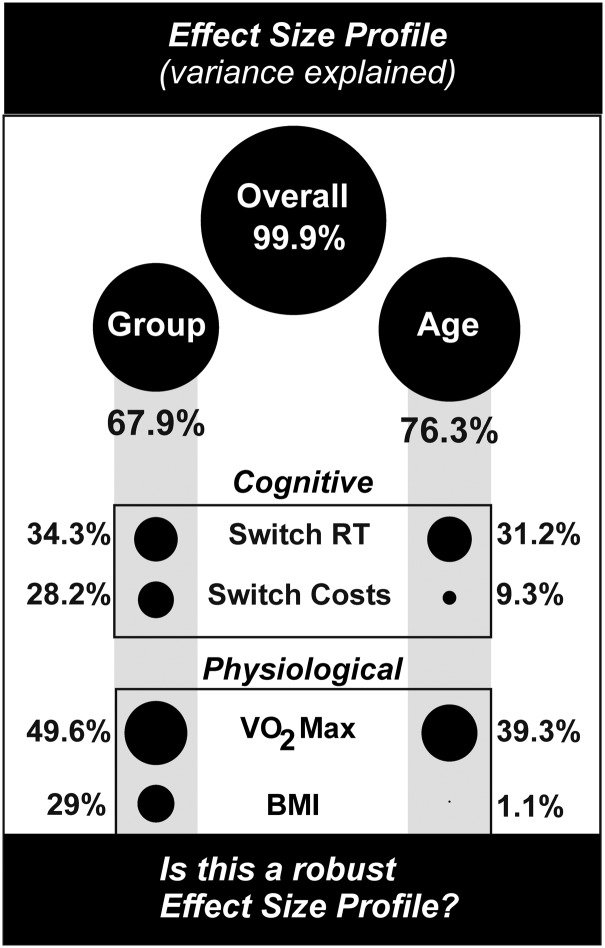
As expected, training group and age showed significant effect sizes on key outcome measures. The main MANCOVA omnibus was significant (Wilk's lambda (Λ) (F(24, 26)=1417.561, p<0.001.) Our overall partial η2 was .999, indicating that 99.9% of the variance in outcome measures were explained. Since key variables related to executive function—aerobic capacity and age—were included, this is not surprising. Group membership explained ∼68% of variance (Wilk's lambda (Λ) (F(72, 78.562)=2.321, p<0.001, partial η2=.679), indicating the presence of possible training effects. Though groups did not differ on age (p=0.295), age explained 76% of total variance (Wilk's lambda (Λ) F(24, 26)=3.488, p=0.001, partial η2=.763), suggesting that normal aging was a key factor affecting the outcome. Clearly these two have overlapping variance, as would be expected, since the cell populations that produce the output are affected by both age and training effects.7,30,33 Means, standard deviations, and variance explained for key factors and variables are presented in Figure 2. Age and group membership had similar effect sizes on switch RT (group, 34.3%; age, 31.2%) and VO2 Max (group, 49.6%; age, 39.3%), but not on percent switch costs (group, 28%; age, 9%) (Fig. 2, Table 2, and Table 3). Error trials (β=.012, t(44)=−.520, p>0.05) did not significantly predict percent local switch costs and were included in the analysis. Mean accuracy was not significantly different between the groups (p=0.425). A one-way ANOVA with BMI as the dependent variable and group as the factor showed significant differences between groups ((F(3, 50)=6.569, p=0.001.
FIG. 2.
Effect-size profiles. Effect of group and age on key system variables. RT, reaction time; VO2 Max, maximal oxygen uptake; BMI, body–mass index.
Table 2.
Participant Cognitive Scores
| SwRT | SwCosts | ||||
|---|---|---|---|---|---|
| Group | n | M | SD | M | SD |
| T'ai chi | 10 | 453.94 | 110.84 | 14.13 | 11.97 |
| Meditation | 16 | 477.41 | 188.88 | 401.23 | 122.83 |
| Aerobics | 16 | 489.63 | 96.89 | 400.84 | 53.90 |
| Sedentary | 12 | 654.3 | 154.56 | 654.3 | 154.56 |
Global ranges: switch reaction time, 301.88–1104.1; % local switch costs,−0.19 to 57.87.
SwRT, switch reaction time (msec); SwCosts, % local switch costs.
Table 3.
Effect Size by Group, Age, and Subject Measures
| Physiologic measures | Cognitive measures | |||
|---|---|---|---|---|
| IV, CoV | Est. VO2 Max | BMI | SwRT | SwCosts |
| IV-Group | 49.60** | 29.00* | 34.30** | 28.20* |
| CoV-Age | 39.30* | 1.10 | 31.20** | 9.30* |
p<0.05. **p<0.001.
Effect size=partial η2.
IV, quasi-independent variable; CoV, covariate; Est. VO2, estimated VO2 Max (mL O2/kg/min); BMI, body–mass index (kg/m2); SwRT, switch reaction trial time (RT) (msec); SwCosts, % local switch costs (switch RT – no-switch RT/no-switch RT).
Post-hoc results
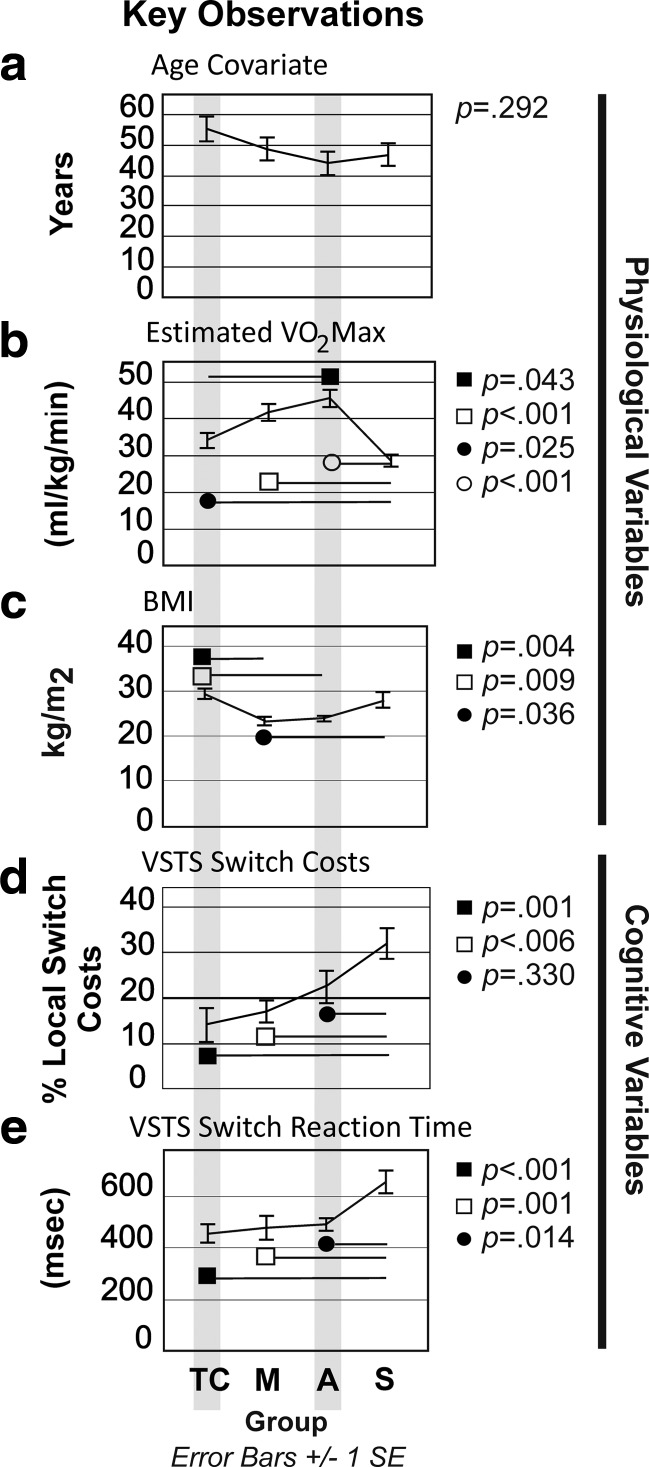
Our groups did not differ on age (Fig. 3a). T'ai chi (p=0.025), meditation (p<0.001), and aerobic fitness (p<0.001) practitioners outperformed sedentary controls on estimated VO2 Max. Aerobic exercisers outperformed t'ai chi practitioners (p=0.043) on VO2 Max (Fig. 3a). This may be due to the wide range of exertion required by different t'ai chi styles (i.e., Chen versus Yang style, long- versus short-form).23,44 VO2 Max was negatively correlated with age (r=−.539, p<0.001). BMI differed between groups (p=0.002). T'ai chi and sedentary controls showed significantly higher BMI than meditators (p=0.004 and p=0.036, respectively). T'ai chi BMI was significantly higher than aerobic exercisers (p=0.009). Aerobic exercisers and sedentary controls did not differ on BMI.
FIG. 3.
Key observations by group. (a) Age covariate. (b) Estimated maximal oxygen uptake (VO2 Max). (c) Body–mass index (BMI). (d) Visuospatial task switch (VSTS) costs. (e) VSTS reaction time. Error bars are±1 standard error (SE). TC, t'ai chi; M, meditation; A, aerobic; S, sedentary.
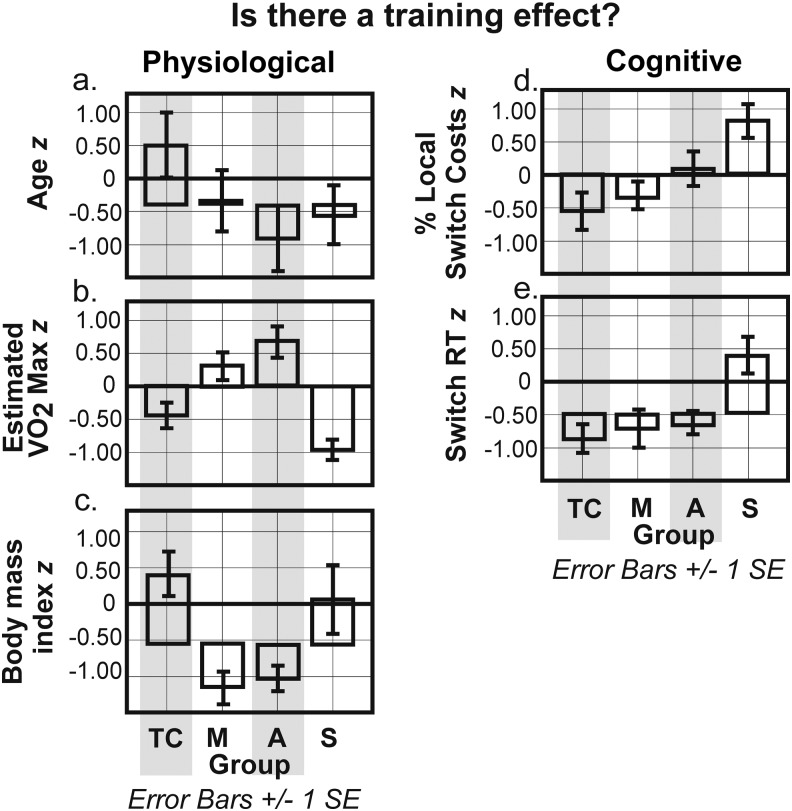
T'ai chi and meditation groups outperformed sedentary controls on percent switch costs: t'ai chi: p=0.001; meditators: p=0.006). Aerobic exercisers and sedentary controls did not differ (Fig. 3c). Training groups outperformed sedentary controls on switch RT: t'ai chi (p<0.001), meditation (p=0.001), aerobic exercisers (p=0.014) (Fig. 3d). There were no differences between training groups on switch RT. Group was significantly and positively correlated with both switch RT (p=0.003, r=.400) and percent switch costs (p<0.001; r=.468) (Table 4). Thus, aerobic exercisers and sedentary controls demonstrated longer RTs and higher switch costs than t'ai chi and meditation practitioners. Figure 4 shows z-score comparisons by key variable.
Table 4.
Correlations Between Key Measures
| Grp | Age | VO2 | BMI | SwRT | SwCosts |
|---|---|---|---|---|---|
| Grp | −.206 | −.142 | −.037 | .400* | .468* |
| Age | −.539** | .003 | .433* | .181 | |
| VO2 | −.386* | −.508** | −.289 | ||
| BMI | .144 | −.010 | |||
| SwRT | .660** | ||||
| SwCosts |
*p<0.0125, **p<0.001.
Grp, group (t'ai chi=1, meditation=2, aerobic=3, sedentary=4); Age (years); estimated VO2 Max, estimated maximal oxygen uptake (mL O2/kg/min); BMI, body–mass index (kg/m2); SwRT, switch reaction trial time (msec); SwCosts, % local switch costs (switch RT–no-switch RT/no-switch RT).
FIG. 4.
Possible training effects on physiologic and cognitive capacity by key variables. Error bars are±1 standard error (SE). VO2 Max, estimated maximal oxygen uptake; RT, reaction time; T, t'ai chi; M, meditation; A, aerobic; S, sedentary.
Discussion
This cross-sectional field study of the effects of long-term t'ai chi, meditation+exercise, or aerobic exercise training versus a sedentary lifestyle utilized a VSTS test5 to assess complex executive function. It was hypothesized that self-reported long-term practice of these modalities would be inversely associated with local switch costs: an index of executive function. First it was asked if the groups differed on aerobic capacity. If they did, those with higher VO2 Max could be expected to outperform individuals with lower VO2 Max on executive and cardiovascular indices.
It was then asked if groups differed on key executive function measures: switch RT and switch capacity (percent local switch costs). It was predicted that (1) training groups would outperform sedentary controls on estimated VO2 Max, and (2) long-term t'ai chi and meditation plus exercise training would produce the greatest benefits to the executive function measures. These would be followed by aerobic exercisers, then sedentary controls. Furthermore, we hypothesized that age effects would be offset by training effects.
As expected, each of our training groups outperformed sedentary controls on estimated VO2 Max. RT and switch cost benefits conferred by long-term moderate exercise were associated with the training groups, but not with sedentary controls. Even though groups were equated on age, and age significantly affected all outcome measures, post-hoc results showed that these effects fell most detrimentally on the sedentary controls: slower RT, higher switch costs, and lower VO2 Max. Furthermore, age should have affected executive function equally in each group,7 yet the training groups outperformed sedentary controls when controlling for age. This suggests that the executive function differences between groups may be associated with training effects. In line with previous studies on switch RT,7 all the training groups outperformed sedentary controls. However, on the more stringent switch costs measure, only t'ai chi and meditation groups significantly outperformed sedentary controls.
As noted, even though groups did not differ significantly on age (p=0.295), age was associated with a large effect size in outcome measures. Age effects generally went in the direction expected: longer RTs and lower cardiovascular capacity (estimated VO2 Max). However, an inspection of the distribution of age scores by group showed t'ai chi and meditation groups were older than the aerobic fitness or sedentary groups, so larger age effects associated with these two groups' scores should be seen. However, on average, younger aerobic fitness practitioners demonstrated higher switch costs than older t'ai chi and meditation practitioners. Finally, group was positively correlated with switch costs (p<.001, r=.468). T'ai chi was dummy coded 1, meditation 2, aerobic fitness 3, and sedentary controls, 4. These results show that higher group number is correlated with higher switch costs. This convergent evidence suggests that moderate aerobic exercise requiring consistent mental exertion is associated with greater benefits to attentional tasks requiring coordination of inhibition, updating, shifting, and working memory than aerobic exercise alone.
However, limitations in the data set could contribute to this outcome. The sample size of this study is small. Therefore, a lack of statistical power could contribute to the modest differences between the aerobic and sedentary groups on switch RT. There was no specific screening for mild cognitive disorders, which may have impacted the neuropsychologic outcomes. Self-selection into these groups related to socio-economic status, and alleles of moderators of executive function such as catechol-O-methyltransferase (COMT) and brain-derived neurotrophic factor (BDNF),30,31,45 could be driving the results. Finally, though data were collected on which type of exercise or t'ai chi each person practiced, the samples collected of each were not large enough to generalize these outcomes to the larger population of each of these types. Certainly different intensities of practice across participants could affect the outcome measures. Indeed, the literature suggests that VO2 Max is differentially affected by exercise intensity (i.e., low-to-high-exertion requirements).46 A longitudinal study comparing training effects on VO2 Max and executive attention correlated with BDNF and COMT alleles could begin to address some of these limitations.
In spite of these limitations, this evidence suggests that practice of t'ai chi and meditation+exercise may be associated with equal or better performance on a stringent measure of executive function than practice of aerobic fitness alone. This is good news for clinicians and individuals alike. Health care professionals need different types of evidence-based health practices to offer clients. If clients are able to select from regimens based on personal inclinations and preferences, it is possible that participation in such regimens would become habitual, ensuring cognitive and physical benefits, lower health care costs, and greater quality of life into later years.
Acknowledgments
This work was supported by a Francisco J. Varela Research Award, Mind & Life Institute, 2007, and an NIH T-32 Systems Training Grant Appointment, Institute of Neuroscience, University of Oregon, 2008. Andrew Lovering, PhD contributed valuable advice on estimated VO2 Max.
Disclosure Statement
No competing financial interests exist.
References
- 1.Murdaugh C. Health-related quality of life as an outcome in organizational research. Med Care 1997;35:N541–N548 [DOI] [PubMed] [Google Scholar]
- 2.Powers SK, Howley ET. Exercise Physiology: Theory and Application to Fitness and Performance, 5th edition. Boston: McGraw Hill, 2004 [Google Scholar]
- 3.Verhaeghen P, Cerella J. Aging, executive control, and attention: A review of meta-analyses. Neurosci Biobehav Rev 2002;26:849–857 [DOI] [PubMed] [Google Scholar]
- 4.Salthouse TA, Atkinson TM, Berish DE. Executive functioning as a potential mediator of age-related cognitive decline in normal adults. J Exp Psychol Gen 2003;132:566–594 [DOI] [PubMed] [Google Scholar]
- 5.Mayr U. Age differences in the selection of mental sets: The role of inhibition, stimulus ambiguity, and response-set overlap. Psychol Aging 2001;16:96–109 [DOI] [PubMed] [Google Scholar]
- 6.Shumway-Cook A, Woollacott M. Motor Control: Translating Research into Clinical Practice. Philadelphia: Lippincott, Williams & Wilkins, 2011 [Google Scholar]
- 7.Ratey JJ, Loehr JE. The positive impact of activity on cognition during adulthood: A review of underlying mechanisms, evidence and recommendations. Rev Neurosci 2011;2212:171–195 [DOI] [PubMed] [Google Scholar]
- 8.Etnier JL, Chang YK. The effect of physical activity on executive function: A brief commentary on definitions, measurement issues, and the current state of the literature. J Sport Exerc Psychol 2009;31:469–483 [DOI] [PubMed] [Google Scholar]
- 9.Tang SW, Chu E, Hui T, et al. Influence of exercise on serum brain-derived neurotrophic factor concentrations in healthy human subjects. Neurosci Lett 2008;431:62–65 [DOI] [PubMed] [Google Scholar]
- 10.Chan D, Woollacott MH. Effects of level of meditation experience on attentional focus: Is the efficiency of executive or orientation networks improved? J Altern Complement Med 2007;36:651–657 [DOI] [PubMed] [Google Scholar]
- 11.Brefczynski-Lewis JA, Lutz A, Schaefer HS, et al. Neural correlates of attentional expertise in long-term meditation practitioners. Proc Natl Acad Sci 2007;104:11483–11488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slagter HA, Lutz A, Greischar LL, et al. Mental training affects distribution of limited brain resources. PLoS Biol 2007;5:1228–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hölzel BK, Carmody J, Vangel M, et al. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res 2011;191:36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert SJ, Burgess PW. Executive function. Curr Biol 2008;18:R110–R114 [DOI] [PubMed] [Google Scholar]
- 15.Miyake A, Friedman NP, Emerson MJ, et al. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn Psychol 2000;41:49–100 [DOI] [PubMed] [Google Scholar]
- 16.Voss MW, Kramer AF, Basak C, et al. Are expert athletes ‘expert’ in the cognitive laboratory? A meta-analytic review of cognition and sport exercise. Appl Cogn Psychol 2009;24:812–826 [Google Scholar]
- 17.Pesce C, Audiffren M. Does acute exercise switch off switch costs? A study with younger and older athletes. J Sport Exerc Physiol 2011;33:609–626 [DOI] [PubMed] [Google Scholar]
- 18.Blasing B, Puttke M, Schack T. The Neurocognition of Dance: Mind, Movement and Motor Skills. New York: Psychology Press, 2010 [Google Scholar]
- 19.Kramer AF, Colcombe SJ, McAuley E, et al. Fitness, aging and neurocognitive function. Neurobiol Aging 2005;26S:S124–S127 [DOI] [PubMed] [Google Scholar]
- 20.Etnier JL, Nowell PM, Landers DM, Sibley BA. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res Rev 2006;52:119–130 [DOI] [PubMed] [Google Scholar]
- 21.Lutz A, Dunne JD, Davidson RJ. Meditation and the neuroscience of consciousness: An introduction. In: Zelazo PD, Moscovitch M, Thompson E, eds. The Cambridge Handbook of Consciousness. Cambridge, UK: Cambridge University Press, 2007 [Google Scholar]
- 22.Smith BW, Shelley BM, Dalen J, et al. A pilot study comparing the effects of mindfulness-based and cognitive-behavioral stress reduction. J Altern Complement Med 2008;14:251–258 [DOI] [PubMed] [Google Scholar]
- 23.Li JX, Hong Y. Chan KM . Tai chi: Physiological characteristics and beneficial effects on health. Br J Sports Med 2001;35:148–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Audette JF, Jin YS, Newcomer R, et al. Tai chi versus brisk walking in elderly women. Age Aging 2006;35:388–393 [DOI] [PubMed] [Google Scholar]
- 25.Luskin F. Transformative practices for integrating mind-body-spirit. J Altern Complement Med 2004;10(Suppl 1):S15–S23 [DOI] [PubMed] [Google Scholar]
- 26.Gatts S. A Tai Chi Chuan training model to improve balance control in older adults. Curr Aging Sci 2008;1:68–70 [DOI] [PubMed] [Google Scholar]
- 27.Halsband U, Lange RK. Motor learning in man: A review of functional and clinical studies. J Physiol Paris 2006;99:414–424 [DOI] [PubMed] [Google Scholar]
- 28.Matthews MM, Williams HG. Can Tai chi enhance cognitive vitality? A preliminary study of cognitive executive control in older adults after a Tai chi intervention. J S C Med Assoc 2008;104:255–257 [PubMed] [Google Scholar]
- 29.Kline GM, Porcari JP, Hintermeister R, et al. Estimation of VO2 Max from a one mile track walk, gender, age, and body weight. Med Sci Sports Exerc 1987;19:253–259 [PubMed] [Google Scholar]
- 30.Vaynman S, Gomez-Pinilla F. Revenge of the “sit”: How lifestyle impacts neuronal and cognitive health through molecular systems that interface energy metabolism with neuronal plasticity. J Neurosci Res 2006;84:699–716 [DOI] [PubMed] [Google Scholar]
- 31.Dishman RK, Berthoud HR, Booth FW, et al. The neurobiology of exercise. Obesity 2006;14:345–356 [DOI] [PubMed] [Google Scholar]
- 32.Bryan J, Luszcz MA. Measurement of executive function: Considerations for detecting adult age differences. J Clin Exp Neuropsychol 2000;22:40–55 [DOI] [PubMed] [Google Scholar]
- 33.Hillman HH, Weiss EP, Hagberg JM, Hatfiled BD. The relationship of age and cardiovascular fitness to cognitive and motor processes. Psychophysiology 2002;39:303–312 [DOI] [PubMed] [Google Scholar]
- 34.Colcombe SJ, Kramer AF, Erickson KI, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci 2004;101:3316–3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davranche K, Audiffren M. Facilitating effects of exercise on information processing. J Sports Sci 2004;22:419–428 [DOI] [PubMed] [Google Scholar]
- 36.Gunstad J, Lhotsky A, Wendell CR, et al. Longitudinal examination of obesity and cognitive function: Results from the Baltimore longitudinal study of aging. Neuroepidemiology 2010;34:222–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabia S, Nabi H, Kivimaki M, et al. Health behaviors from early to late midlife as predictors of cognitive function: The Whitehall II study. Am J Epidemiol 2009;170:428–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Altmann EM, Gray WD. An integrated model of cognitive control in task switching. Psychol Rev 2008;115:602–639 [DOI] [PubMed] [Google Scholar]
- 39.Monsell S, Sumner P, Waters H. Task-set reconfiguration with predictable and unpredictable task switches. Mem Cognit 2003;31:327–342 [DOI] [PubMed] [Google Scholar]
- 40.Karayanidis F, Coltheart M, Michie PT, Murphy K. Electrophysiological correlates of anticipatory and poststimulus components of task-switching. Psychophysiology 2003;40:329–348 [DOI] [PubMed] [Google Scholar]
- 41.Wylie G, Allport A. Task switching and the measurement of “switch costs.” Psychol Res 2000;63:212–233 [DOI] [PubMed] [Google Scholar]
- 42.Milan EG, Gonzalez A, Sanabria D, et al. The nature of residual cost in regular switch response factors. Acta Psychol (Amst) 2006;122:45–57 [DOI] [PubMed] [Google Scholar]
- 43.Stevens JP. Applied Multivariate Statistics for the Social Sciences, 4th edition. Mahwah, NJ: Lawrence Erlbaum Associates, 2002 [Google Scholar]
- 44.Wolf SL, Coogler C, Tingsen X. Exploring the basis for Tai Chi Chuan as a therapeutic exercise approach. Arch Phys Med Rehabil 1997;78:886–892 [DOI] [PubMed] [Google Scholar]
- 45.Gajewski PD, Hengslter JG, Golka K, et al. The met-allele of the BDNF Val66Met polymorphism enhances task switching in elderly. Neurobiol Aging 2011;32:2327. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka H, DeSouza CA, Jones PP, et al. Greater rate of decline in maximal aerobic capacity with age in physically active vs. sedentary healthy women. J Appl Physiol 1997;83:1947–1953 [DOI] [PubMed] [Google Scholar]