Abstract
Dopamine (DA) plays an essential role in the enablement of cognition. It adds color to experience-dependent information storage, conferring salience to the memories that result. At the synaptic level, experience-dependent information storage is enabled by synaptic plasticity, and given its importance for memory formation, it is not surprising that DA comprises a key neuromodulator in the enablement of synaptic plasticity, and particularly of plasticity that persists for longer periods of time: Analogous to long-term memory. The hippocampus, that is a critical structure for the synaptic processing of semantic, episodic, spatial, and declarative memories, is specifically affected by DA, with the D1/D5 receptor proving crucial for hippocampus-dependent memory. Furthermore, D1/D5 receptors are pivotal in conferring the properties of novelty and reward to information being processed by the hippocampus. They also facilitate the expression of persistent forms of synaptic plasticity, and given reports that both long-term potentiation and long-term depression encode different aspects of spatial representations, this suggests that D1/D5 receptors can drive the nature and qualitative content of stored information in the hippocampus. In light of these observations, we propose that D1/D5 receptors gate hippocampal long-term plasticity and memory and are pivotal in conferring the properties of novelty and reward to information being processed by the hippocampus.
Keywords: cognition, hippocampus, learning and memory, review, synaptic plasticity
Introduction
Dopamine (DA) is a neurotransmitter in the central nervous system that belongs to the catecholamines (Carlsson et al. 1962). DA neurons are categorized in dopaminergic systems based on their innervation territories. Four axonal dopaminergic pathways are described: 1) nigrostriatal, 2) mesolimbic, 3) mesocortical, and 4) tuberoinfundibular (Vallone et al. 2000). DA subserves a multitude of roles in cognition-related brain functions: It regulates memory, motivation, mood, motor activity, and neuroendocrine integration (Horn et al. 1979; Fluckiger et al. 1987) and is released after novel (Ljungberg et al. 1992), salient sensory (Ungless 2004), aversive (Bromberg-Martin et al. 2010), or reinforcement-relevant (reward) stimuli (Schultz et al. 1993). For many decades, its role in cognitive disorders and brain disease has been intensely studied. This derived from observations that a strikingly low DA concentration occurs in the basal ganglia of patients with Parkinson's disease (Ehringer and Hornykiewicz 1960) and that DA dysfunctions contribute to cognitive disorders such as schizophrenia (Goto and Grace 2007; Lodge and Grace 2011), drug addiction (Robinson and Berridge 1993), attention deficit hyperactivity disorder (Del Campo et al. 2011), and possibly Alzheimer's disease (Kumar and Patel 2007; Jürgensen et al. 2011).
Experimental evidence suggests that DA is highly relevant for the modulation of hippocampus-dependent synaptic plasticity and memory (Jay 2003; Lisman and Grace 2005; Lisman et al. 2011). These effects are mediated by 2 distinct groups of DA receptors: The D1/D5 (D1-like) receptors and the D2-like receptors (Tiberi et al. 1991; Vallone et al. 2000; Beaulieu and Gainetdinov 2011) (Fig. 1), whereby, in recent decades, the D1/D5 receptors have received increasing attention. This is because of the significant role that they play in the regulation of both hippocampus-dependent synaptic plasticity (the mechanisms believed to underlie learning) and hippocampus-dependent memory (Huang and Kandel 1995; Lemon and Manahan-Vaughan 2006; Bethus et al. 2010; Clausen et al. 2011; Da Silva et al. 2012). Intriguingly, D1/D5 receptors regulate both forms of persistent (>24 h) synaptic plasticity and appear to contribute importantly to the earmarking of information as novel or salient (Davis et al. 2004; Ungless 2004; Lemon and Manahan-Vaughan 2006, 2011), which in turn strongly influences hippocampus-dependent memory encoding and retention (Adcock et al. 2006). The D2-like receptors, in contrast, seem less significant for hippocampus-dependent information processing, be it at the levels of synaptic plasticity or memory formation (Kulla and Manahan-Vaughan 2003; Xing et al. 2010). Activation of D1/D5 receptors alter excitability in the hippocampus (Ito and Schumann 2007; Hamilton et al. 2010) and therefore influence the thresholds for the induction of synaptic plasticity or memory encoding. Different hippocampal subregions such as the dentate gyrus (DG), cornus ammonis 1 (CA1) and subiculum that exercise distinct functions in information processing within the hippocampus are also modulated by the activation of D1/D5 receptors (Kulla and Manahan-Vaughan 2000; Lemon and Manahan-Vaughan 2006; Othmakhova and Lisman 1996; Roggenhofer et al. 2010).
Figure 1.
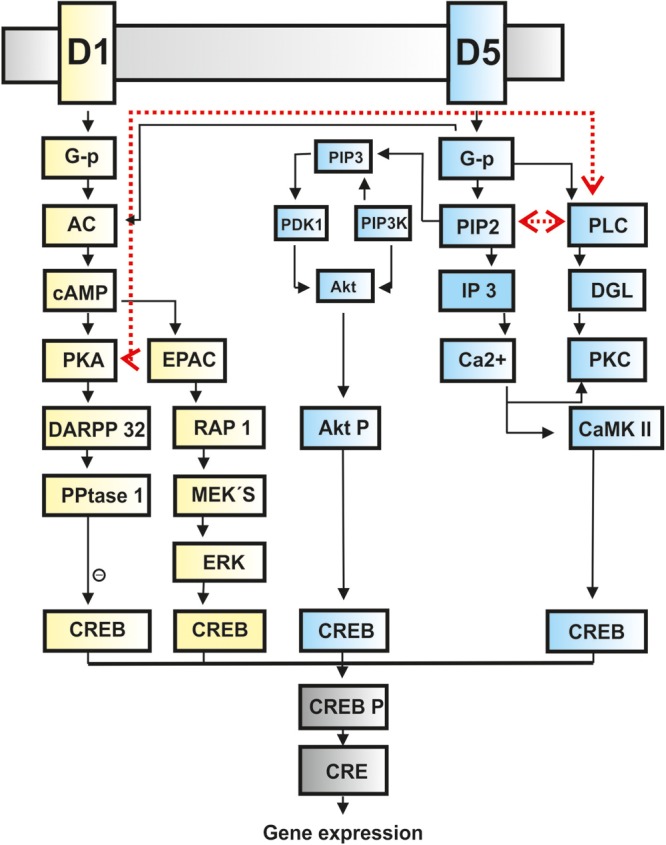
Signal cascades of D1 and D5 receptors. Schematic demonstration of the different molecular pathways of D1 (yellow boxes) and D5 receptors (blue boxes) ending in a common CREB activation (gray boxes). Crosstalk between the D1/D5 system is indicated by red dashed lines. An inhibitory effect is signified by a circle containing a minus symbol. Abbreviations: AC: adenylcyclase; AktP: Akt phosphorylated; CaMKII: calcium–calmodulin-dependent protein kinase type II; cAMP: cyclic 3′5′ adenosine monophosphate; CRE: cAMP response element; CREB: cAMP response element-binding protein; CREB P: CREB phosphorylated; DARPP-32: phosphoprotein of 32 kDa; DGL: diacylglycerol; D1: dopamine receptor 1; D5: dopamine receptor 5; EPAC: exchange protein activated by cAMP; ERK: extracellular signal-regulated kinase; G-p: G protein; IP3: inositol trisphosphate (IP3); by cAMP; ERK: extracellular signal-regulated kinase; MEK's: mitogen-activated kinases; PDK1: phosphoinositide-dependent kinase-1; PIP 2: phosphatidylinositol-4;5-bisphosphate; PIP3: phosphatidylinositol-3;4;5-triphosphate; PI3K: phosphatidylinositol-3-kinase; PKA: protein kinase A; PKC: protein kinase C; PLC: phospholipase C; PPtase 1: protein phosphatase 1; RAP 1: member of the RAS family of small GTP-binding proteins (Undieh 2010; Beaulieu and Gainetdinov 2011).
Activity-dependent alterations in synaptic strength encode new information in the brain. Two major forms can be distinguished: 1) long-term potentiation (LTP; Bliss and Lomo 1973; Bliss and Collingridge 1993) and 2) long-term depression (LTD) of synaptic strength (Dudek and Bear 1992; Manahan-Vaughan 1997). LTP that is induced solely by electrical afferent stimulation (electrically induced plasticity) was first reported roughly 40 years ago in the DG of the rabbit after high-frequency stimulation (HFS) of the perforant path (Bliss and Lomo 1973). Hippocampal LTD was described for the first time in Schaffer collateral (SC)–CA1 synapses (Dunwiddie and Lynch 1978) and is electrically induced by low-frequency stimulation (LFS: 1–3 Hz for 5–15 min). Both phenomena are believed to underlie hippocampal learning and memory (Bliss and Collingridge 1993; Bear 1996; Kemp and Manahan-Vaughan 2007). This likelihood is supported by more recent studies that address a phenomenon known as learning-facilitated plasticity. Here, weak electrical afferent stimulation that under control conditions elicits either no change in basal synaptic strength, or elicits short-term plasticity, leads to persistent plasticity if coupled with a novel learning experience (Manahan-Vaughan and Braunewell 1999; Goh and Manahan-Vaughan 2012).
Studies of learning-facilitated plasticity suggest that LTP and LTD are responsible for the encoding of different elements of a memory representation. Thus, LTP is associated with the encoding of global space, spatial change, or contextual fear (Straube et al. 2003; Kemp and Manahan-Vaughan 2004; Whitlock et al. 2006), whereas LTD is associated with the encoding of spatial context (Manahan-Vaughan and Braunewell 1999; Etkin et al. 2006; Kemp and Manahan-Vaughan 2004, 2007, 2008a; Goh and Manahan-Vaughan 2012). The precise contributions of LTP and LTD to spatial representation are tightly linked to the respective hippocampal subregions (Kemp and Manahan-Vaughan 2008a; Hagena and Manahan- Vaughan 2011). What is striking, however, is that D1/D5 receptors regulate both persistent LTP (Huang and Kandel 1995; Lemon and Manahan-Vaughan 2006) and persistent LTD (Lemon and Manahan-Vaughan 2006), suggesting that these receptors exert control over the kind of information contributed by the different forms of synaptic plasticity to memory representations.
LTP has been subdivided into temporal categories referred as to 1) short-term potentiation that typically requires calcium entry through, for example, N-methyl-d-aspartate (NMDA) receptors, 2) early (E)-LTP requiring both NMDA receptors and the activation of metabotropic glutamate (mGlu) receptors (Bashir et al. 1993) and protein kinases (Malenka et al. 1989), and to a lesser extent, phosphatases; 3) late (L)-LTP that is based on the expression of early immediate genes (Jones et al. 2001) requires protein translation, and (4) late late (LL)-LTP that requires protein transcription (Nguyen et al. 1994; Villers et al. 2010) and facilitates LTP consolidation (Ryan et al. 2012). Similar delineations are evident for LTD: An early LTD (E)-LTD reliant on the activation of NMDA receptors (at least in the CA1 region; Dudek and Bear 1992; Manahan-Vaughan 1997), mGlu receptors (Manahan-Vaughan 1997), and protein phosphatases (Mulkey et al. 1993), late LTD (L)-LTD that is dependent on gene expression (Abraham et al. 1994) and protein translation (Manahan-Vaughan et al. 2000; Parvez et al. 2010), and late late (LL)-LTD that requires protein transcription (Kauderer and Kandel 2000). Although electrically induced and learning-facilitated plasticity share similarities in their underlying mechanisms (Manahan-Vaughan 1997; Popkirov and Manahan-Vaughan 2011), they also display quite distinct properties. For example, learning-facilitated and not electrically induced persistent plasticity require beta-adrenoreceptors (Kemp and Manahan-Vaughan 2008b), and Madronal et al. (2009) showed that paired-pulse facilitation is differently modulated by electrically induced LTP or changes in synaptic strength evoked by classic eyeblink conditioning (i.e. learning-facilitated plasticity). It is however quite possible that learning-facilitated plasticity is more sensitive to neuromodulation, and more physiological than plasticity elicited by electrical stimulation alone, which could explain the abovementioned data.
The hippocampus contributes to many behaviors such as anxiety (Engin and Treit 2007), goal-directed behavior (Pennartz et al. 2011), informational processing, olfactorial identification, and spatial navigation and orientation (Hölscher 2003). But most strikingly, the different hippocampal subregions are believed to engage in different aspects of creation of a memory trace. Whereas the DG is postulated to engage in pattern separation, whereby similar information is recognized as not being the same, the CA3 region engages in pattern completion, whereby incoming information leads to the complete retrieval of a stored representation, should that information have contributed previously to the creation of a memory (Lee et al. 2004; Goodrich-Hunsaker et al. 2008). The CA1 region is believed to integrate information coming from the other subregions and also participates in mismatch detection (Lismann and Otmakhova 2001). Given this division of labor, it is perhaps not so surprising that the D1/D5 receptors exert a differential influence on synaptic plasticity in these structures. Here, it must be however emphasized that the role of these receptors in the CA3 region has not yet been examined.
DA Release in the Hippocampus
DA is released from axon terminals residing in the hippocampus (Frey et al. 1990), which originates from midbrain sources with the ventral tegmental area (VTA, A10 cell group in the rat nomenclature), comprising the main source. Release occurs in the hippocampus some minutes after novelty exposure in the hippocampus (Ihalainen et al. 1999). This implies that DA is a key component in enabling processing of novel information in the hippocampus. In temporoammonic (TA) synapses, DA acts over a range of stimulus frequencies (5–100 Hz) as a high-pass filter that enhances responses to high-frequency inputs, while reducing the influence of low-frequency inputs (Ito and Schumann, 2007). Strikingly, LTP at SC–CA1 synapses is unaffected, whereas LTP at TA synapses is enhanced by DA. This suggests that DA increases the relevance of information being passed from the entorhinal cortex via the TA synapses directly to the hippocampus, compared with information being processed “internally” at SC–CA1 synapses, which in turn alters informational content and the nature of information storage by influencing the direction of change in synaptic weights. This may subserve the integration of new information with previously encoded information as it exits the hippocampus.
The VTA is not the only source of DA for the hippocampus. Aside from the VTA, the hippocampus receives inputs from the retrorubral area A8 and the substantia nigra pars compacta A9 (Beckstead et al. 1979) and interacts with other dopaminergic nuclei such as the nucleus accumbens (NAcc; Figs 2 and 3). For example, mesocortical DA projections from the VTA to the prefrontal cortex (PFC) may play a critical role in modulating information processing by hippocampus–PFC interactions (Seamans et al. 1998; Goto and Grace 2008a). In addition, although it does not project directly to the hippocampus, the NAcc (together with the ventral pallidum, VP) serves as the downward arm of the hippocampal–VTA loop, serving to help combine the novelty signal with salience and goal information (Lisman and Grace 2005; Figs 2 and 3). In addition to its main role in gating limbic and cortical inputs, the NAcc is involved in improving goal-directed behavior (Gruber et al. 2009) and in enabling hippocampal-dependent spatial information to gain control over appetitive learning (Ito and Hayen 2011). The role of the NAcc in informational gating has been reviewed elsewhere in great detail (Grace et al. 2007; Goto and Grace 2008b; Yin et al. 2008).
Figure 2.
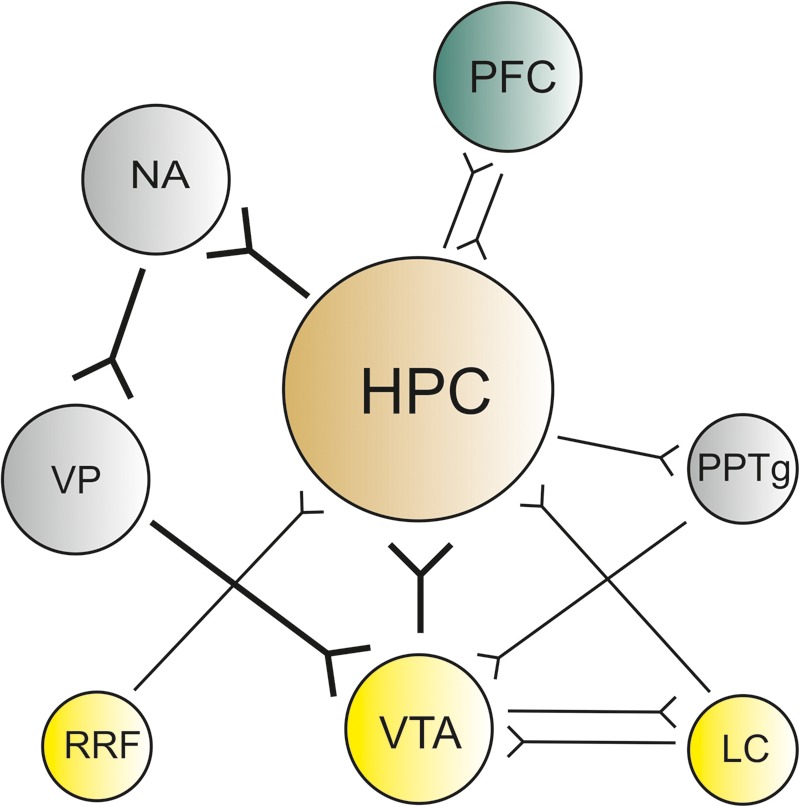
Anatomical connections between the hippocampus and dopaminergic nuclei. The VTA, retrorubral field (RRF), and LC all send projections to the hippocampus (HPC). The hippocampus in turn projects, on the one hand, to the NAcc that is connected with the VTA (hippocampal–VTA loop) and substantia nigra (SN) by the VP. On the other hand, the hippocampus projects to the peduncolopontine tegmentum (PPTg) that sends projections to the VTA and SN. Additionally, the PFC sends and receives projections from the hippocampus.
Figure 3.
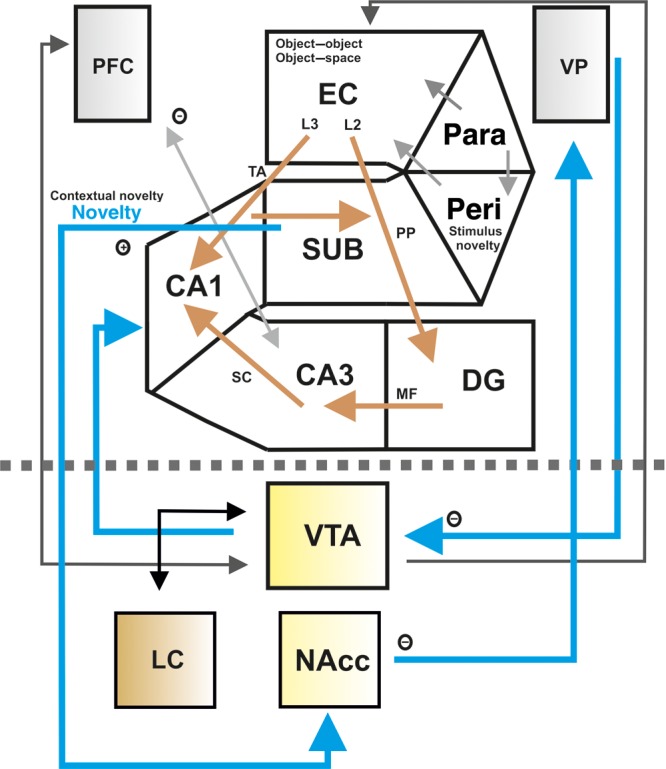
Regulation of hippocampal synaptic plasticity by the VTA and other dopaminergic nuclei. The dopaminergic regulation of hippocampal synaptic plasticity and the underlying network is depicted here. Blue arrows indicate the novelty activated VTA–hippocampal loop. The novelty signal is mediated from the perirhinal cortex (PERI) to the enthorinal cortex (EC). Additionally, the EC conveys object and space information to the DG and CA1. Here, the information is integrated to “object-in-context” representations and then processed via the NAcc and VP as the descending arc of the hippocampal–VTA loop to the VTA. The gray and black arrows show the different projections between the dopaminergic brainstem nuclei and the brain structures referring to the hippocampus and VTA involved in the dopaminergic modulation of hippocampal synaptic transmission. The gray dashed line indicates the separation of the cerebrum and brainstem. An inhibitory projection is indicated by a circle containing a minus symbol and an excitatory projection is indicated by a circle combined with a plus symbol. In this figure not all, but rather the main connections are shown. Abbreviations: EC: entorhinal cortex, LC: locus coeruleus, L2/3: layer 2/3 of the EC, MF: mossy fibers, NAcc: nucleus accumbens, PARA: parahippocampus, PERI: perirhinal cortex, PP: perforant path, SC: Schaffer collaterals, SUB: subiculum, TA: temporoammonic pathway, VP: ventral palladium, VTA: ventral tegmental area. (Simon et al. 1979; Grace 1991; Howland et al. 2002; Lisman and Grace 2005; Yin et al. 2008; Sara 2009; Lisman et al. 2011).
Taken together, the known role of the dopaminergic nuclei that impinge on the hippocampus supports that DA is released by novelty and reward-related experience and that this information enables the hippocampus to add meaning to the information it processes. By this means, salience is conferred to information stored. The relative regulation of LTP and LTD in the different hippocampal subfields is one possible means through which the hippocampus then integrates and encodes this information into a memory engram or spatial representation.
Influence of D1/D5 Receptors on LTP in the DG
The DG is the functional “gateway” to the hippocampus. Mixed effects on electrically induced LTP have been reported after activation of D1/D5 receptors in this structure (Table 1A), but predominantly an inhibition of LTP has been reported following D1/D5 receptor antagonism (Yanagihashi and Ishikawa 1992; Kusuki et al. 1997; Swanson-Park et al. 1999). This suggests that these receptors play a pivotal role in determining whether LTP occurs in the DG in response to incoming stimuli. D1/D5 receptor activation during a “reward” or “novelty” signal has been proposed to increase DG excitability (Hamilton et al. 2010) such that novel sensory information passes the informational gateway and filter of the DG to enter the circuitry of the hippocampal CA3–CA1 region (Heinemann et al. 1992; Hamilton et al. 2010). This in turn may relate to the function of the DG in pattern completion (Kesner et al. 2000).
Table 1.
D1/D5 receptors and hippocampal synaptic plasticity
| Drug/knock out | First application time | Hippocampal region | Plasticity protocol | Effect on plasticity | Duration of effect | References |
|---|---|---|---|---|---|---|
| (A) Effects of DA on hippocampal LTP via D1/D5 receptors | ||||||
| In vitro electrophysiology | ||||||
| D1/D5 agonist | Before HFS | CA1 | HFS (10 × 100 Hz) | E-LTP↑ | 40 min | Otmakhova and Lisman (1996) |
| D1/D5 agonist | Before HFS | CA1 | HFS (3 × 100 Hz) | LTP↑ | 2 h | Swanson-Park et al. (1999) |
| D1/D5 agonist | Before HFS | SUB | HFS (4 × 100 Hz) | LTP↑ | 40 min | Roggenhofer et al. (2010) |
| D1/D5 agonist | After 1 h of baseline | CA1 | Test pulses (0.2 Hz) | Slow-onset late-LTP | 5 h | Navakodde et al. (2007) |
| D1/D5 agonist | ||||||
| D1/D5 antagonist | After HFS | CA1 | HFS (3 × 100 Hz) | No effect | 3 h | Frey et al. (1991) |
| D1/D5 antagonist | Before HFS | DG | HFS (3 × 100 Hz) | No effect | >3 h | Swanson-Park et al. (1999) |
| D1/D5 antagonist | Before HFS | CA1 | HFS (10 × 100 Hz) | Early LTP↓ | 40 min | Otmakhova and Lisman (1996) |
| D1/D5 antagonist | Before HFS | CA1 | HFS (3 × 100 Hz) | LTP↓ | >3 h | Swanson-Park et al. (1999) |
| D1/D5 antagonist | During HFS | CA1 | HFS (3 × 100 Hz) | L-LTP ↓ | >4 h | Frey et al. (1990) |
| D1/D5 antagonist | During HFS | CA1 | HFS (3 × 100 Hz) | L-LTP ↓ | >6 h | Huang and Kandel (1995) |
| D1/D5 antagonist | During HFS | CA1 | HFS (3 × 100 Hz) | L- LTP ↓↓ | >2 h | Frey et al. (1991) |
| D1/D5 antagonist | During HFS | CA1 | HFS (21 × 100 Hz) | S1:LTP↓↓ | 6 h | Navakodde et al. (2010) |
| S2: LTP↓↓ | 6 h | |||||
| D (1)−/− mice | – | CA1 | HFS (3 × 100 Hz) | E-, L-LTP ↓ | 6 h | Granado et al. (2008) |
| D (1) −/− mice | – | CA1 | HFS (3 × 100 Hz) | LTP↓ | 2 h | Matthies et al. (1997) |
| In vivo electrophysiology | ||||||
| D1/D5 agonist | Before HFS | DG | HFS (100 p × 100 Hz) | LTP↑ | 1 h | Kusuki et al. (1997) |
| D1/D5 agonist | Before HFS | CA1 | Spatial novelty | LTP↑ | 3 h | Li et al. (2003) |
| D1/D5 agonist | Before HFS | CA1 | HFS (4 × 100 Hz) | LTP↑ | 4 h | Lemon and Manahan-Vaughan (2006) |
| D1/D5 agonist | Before HFS | DG | HFS (10 × 200 Hz) | No effect | 24 h | Kulla and Manahan-Vaughan (2000) |
| D1/D5 agonist | Before HFS | DG | HFS (10 × 400 Hz) | LTP ↓↓ | 2 h | Yanagihashi and Ishikawa (1992) |
| D1/D5 antagonist | Before HFS | DG | HFS (10 × 200 Hz) | No effect | 24 h | Kulla and Manahan-Vaughan (2000) |
| D1/D5 antagonist | Before HFS | DG | HFS (3 × 100 Hz) | No effect | >3 h | Swanson-Park et al. (1999) |
| D1/D5 antagonist | Before HFS | CA1 | HFS (3 × 100 Hz) | LTP↓ | >3 h | Swanson-Park et al. (1999) |
| D1/D5 antagonist | Before HFS | DG | HFS (10 × 100 Hz) | LTP ↓↓ | 1 h | Kusuki et al. (1997) |
| D1/D5 antagonist | Before HFS | CA1 | HFS (4 × 100 Hz) | L-LTP↓↓ | >2 h | Lemon and Manahan-Vaughan (2006) |
| D1/D5 antagonist | Before HFS | CA1 | Empty holeboard | L-LTP↓↓ | 4 h | Lemon and Manahan-Vaughan (2006) |
| D1/D5 antagonist | Before HFS | DG | HFS (10 × 400 Hz) | Reversal of inhibited LTP | 2 h | Yanagihashi and Ishikawa (1992) |
| (B) Effects of DA on hippocampal LTD by D1/D5 receptors | ||||||
| In vitro electrophysiology | ||||||
| D1/D5 agonist | After LFS | CA1 | DHPG | No effect on LTD | 2 h | Mocket et al. (2007) |
| D1/D5 agonist | Before LFS | CA1 | LFS (450 × 1 Hz) | LTD↑ | 40 min | Chen et al. (1995) |
| D1/D5 agonist | Before LFS | CA1 | LFS (450 × 1 Hz) | LTD↑ | 1 h | Liu et al. (2009) |
| D1/D5 agonist | After LFS | CA1 | LFS (1200 × 3 Hz) | LTD↓ | 2 h | Mocket et al. (2007) |
| D1/D5 agonist | After LFS | CA1 | NMDA | LTD↓ | 2 h | Mocket et al. (2007) |
| D1/D5 antagonist | Before LFS | CA1 | LFS (450 × 1 Hz) | LTD↓↓ | 40 min | Chen et al. (1995) |
| D1/D5 antagonist | During sLFS | CA1 | SLFS (2700 × 1 Hz) | L-LTD↓ (S1 + S2) | 7 h | Saijkumar and Frey (2004) |
| D1/D5 antagonist | 30 min after LTD in S1 | CA1 | SLFS (2700 × 1 Hz) | No effect L-LTD (S1 + S2) | 7 h | Saijkumar and Frey (2004) |
| In vivo electrophysiology | ||||||
| D1/D5 agonist | Before novel spatial exploration | CA1 | Novel spatial exploration, afferent stimulation | LTD | 4 h | Lemon and Manahan-Vaughan (2011) |
| D1/D5 agonist | Before HFS | CA1 | HFS (2 × 100 Hz) of LC | E- into L-LTD↑ | 24 h | Lemon and Manahan-Vaughan (2011) |
| D1/D5 agonist | Before HFS | CA1 | LFS (600 × 1 Hz) | LTD↑ | 4 h | Lemon and Manahan-Vaughan (2006) |
| D1/D5 antagonist | Before HFS | CA1 | LFS (900 × 1 Hz) | LTD↓ | 4 h | Lemon and Manahan-Vaughan (2006) |
| D1/D5 antagonist | Before HFS | CA1 | Novel spatial exploration | L-LTD↓ | 4 h | Lemon and Manahan-Vaughan (2006) |
| D1/D5 antagonist | Before HFS | CA1 | HFS (2 × 100 Hz) of LC | LTD ↓↓ | >4 h | Lemon and Manahan-Vaughan (2011) |
| (C) Effects of DA on hippocampal depotentiation by D1/D5 receptors | ||||||
| In vitro electrophysiology | ||||||
| D1/D5 agonist | Before LFS | CA1 | LFS (2 Hz, 10 min) | Depotentiation↓ | 40 min | Otmakhova and Lisman (1998) |
| In vivo electrophysiology | ||||||
| D1/D5 agonist | Before LFS | CA1 | LFS (600 × 5 Hz) | Depotentiation↓ | 25 h | Kulla and Manahan-Vaughan (2000) |
| D1/D5 agonist | Before LFS | DG | LFS (600 × 5 Hz) | Depotentiation↓ | 25 h | Kulla and Manahan-Vaughan (2000) |
| D1/D5 antagonist | Before LFS | CA1 | LFS (600 × 5 Hz) | Prevented inhibition of depotentiation | 25 h | Kulla and Manahan-Vaughan (2000) |
| D1/D5 antagonist | Before LFS | DG | LFS (600 × 5 Hz) | Prevented inhibition of depotentiation | 25 h | Kulla and Manahan-Vaughan (2000) |
D: dopamine; DG: dentate gyrus; E-LTD/LTP: early long-term depression/potentiation; HFS: high-frequency stimulation; LC: locus coeruleus; LFS: low-frequency stimulation; L-LTD/LTP: late long-term depression/potentiation; LTD: long-term depression; LTP: long-term potentiation; p: pulses; PP: perforant path; SC: Schaffer collaterals; SUB: subiculum; S1 or 2: synaptic input 1 or 2.
Information gating in a more global sense is also supported by the DA. This type of gating may enable oscillatory network activity between different brain areas involved in learning (Buzsaki and Draguhn 2004). D1/D5 receptor activation can, for example, modulate theta burst firing in the medial septal/vertical limb of diagonal band neurons that project to the hippocampus (Fitch et al. 2006). Furthermore, DA suppresses cholinergic gamma oscillations in area CA3 via D1 receptor activation (Weiss et al. 2003). It has been suggested that DA in particular alters the frequency firing pattern of neurons (theta and gamma frequency volleys; Ito and Schumann 2007) that have been observed in the enthorinal cortex during exploratory behavior of rodents (Chrobak et al. 2000) thereby changing informational content.
Influence of D1/D5 Receptors on LTP in the CA1 Region
In contrast to the DG, where only L-LTP is affected, in vitro studies of SC–CA1 synapses showed that both E-LTP (Otmakhova and Lisman 1996) and L-LTP (Frey et al. 1991; Huang and Kandel 1995) are prevented or reduced (Swanson-Park et al. 1999) by a D1/D5 antagonist, whereas agonists of D1/D5 receptors lead to enhanced E-LTP (Otmakhova and Lisman 1996). The magnitude of both E- and L-LTP is also markedly reduced in hippocampal slices from D1 receptor−/− mice compared with wild-type mice (Granado et al. 2008). In line with these findings, in vivo studies showed that HFS-induced LTP at SC–CA1 synapses is facilitated by D1/D5 receptor activation in freely behaving rats (Lemon and Manahan-Vaughan 2006; Table 1A). However, D1/D5 receptor antagonism prevents only L-LTP in SC–CA1 synapses (Lemon and Manahan-Vaughan 2006). The differences between the in vitro and in vivo studies may relate to the different kinds of stimulation protocols that were used to elicit LTP of differing robustness and durations.
Effect of D1/D5 Receptor Activity on Depotentiation of LTP
Although it is not a persistent form of synaptic plasticity, depotentiation bears a mention in the context of D1/D5 regulation of hippocampal synaptic plasticity (Table 1C). Depotentiation is an interesting phenomenon that occurs when LFS is applied within a very short time-window (maximum 30 min) of inducing LTP (Staubli and Lynch 1990; Kulla et al. 1999) and has been proposed to comprise a functional correlate of active forgetting or perhaps of learning interference. This form of synaptic plasticity is distinct to LTD, as it does not engage the same phosphorylation/dephosphorylation profile of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor receptor (Lee et al. 1998) and has different sensitivities to, for example, mGlu receptor ligands (Manahan-Vaughan 1997; Fitzjohn et al 1998; Kulla et al. 1999; Kulla and Manahan-Vaughan 2008). Another aspect of depotentiation is the associative regulation of this phenomenon, as exemplified by an vitro study in rats showing that the synthesis of plasticity-related proteins (PRPs) by L-LTP in one input facilitated E- into L-LTD in another input. Thus, long-term plasticity in one synaptic input is associatively induced by PRPs of another synaptic input, in a process referred to “cross tagging” (Sajikumar and Frey 2004). Associative long-term plasticity and synaptic tagging also seem to be dependent on D1/D5 receptor activation (Sajikumar and Frey 2004).
Interestingly, D1/D5 receptor manipulation affects LFS-induced depotentiation of LTP both in vitro and in vivo (Otmakhova and Lisman 1998; Kulla and Manahan-Vaughan 2000). D1/D5 receptor agonists reduce the depotentiation of LTP by LFS in CA1 and in DG, whereas D1/D5 receptor antagonists inhibit this effect (Kulla and Manahan-Vaughan 2000) presumably via a cyclic 3′5′ adenosine monophosphate (cAMP)-dependent mechanism (Otmakhova and Lisman 1998).
If depotentiation comprises forgetting, it suggests that D1/D5 receptor activation can hinder this process. As depotentiation of LTP is a successive process—first LTP is induced and afterwards depotentiation is initiated, it implies that D1/D5 receptor activation can veto a “decision” to forget information that was initially earmarked for long-term storage. Again, this possibility fits well into a role for these receptors in mediating informational salience.
Effect of D1/D5 Receptor Activity on Hippocampal LTD
LTD to some extent is a mirror image of LTP, comprising persistent decreases in synaptic strength that occur following patterned afferent stimulation to the hippocampus. In recent years, it has become apparent that this phenomenon is an information storage mechanism that likely cooperates with LTP to generate spatial and/or memory representations (Kemp and Manahan-Vaughan 2007). For this phenomenon, like LTP, D1/D5 receptors also appear to play a pivotal role. In contrast to LTP, where the CA1 region and the DG have been intensively studied, so far, information only exists with regard to D1/D5 receptor effects on LTD in the CA1 region (Table 1B).
E-LTD, induced by LFS of CA1 synapses, is facilitated by D1/D5 receptor agonism in vitro (Chen et al. 1995; Liu et al. 2009). By contrast, E-LTD is blocked by a D1/D5 receptor antagonist in CA1 synapses in vitro (Chen et al. 1995). Furthermore, in vitro studies showed that both E-and L-LTD in CA1 synapses are dependent on D1/D5 receptor activation (Mockett et al. 2007; Liu et al. 2009). In vivo data agree with these results, as D1/D5 receptor agonism facilitates LFS-induced E-LTD and L-LTD, whereas D1/D5 receptor antagonism prevents LFS-induced E-LTD and L-LTD (Lemon and Manahan-Vaughan 2006). However, in one in vitro study, D1/D5 receptor agonism partially reversed LFS-induced LTD (Mockett et al. 2007). These different in vitro effects could be due to the use of different LFS protocols [1200 × 3 Hz (Mockett et al. 2007) vs. 450 × 1 Hz (Chen et al. 1995)] that evoke LTD of differing magnitudes and durations. In vivo, 1-Hz LFS using <600 pulses elicits very short-term depression (STD) at CA1 synapses (Popkirov and Manahan-Vaughan 2011), whereas 3-Hz stimulation elicits more prolonged effects (Manahan-Vaughan 2000). Differences in the regulation of D1/D5 receptor agonists of synaptic depression of different strengths and durations may functionally relate to the relevance of these forms of plasticity for information processing: Weak responses may be strengthened and strong responses could conceivably become weakened, so that information processing is optimized.
D1/D5 Receptors and Learning-Facilitated Plasticity
In vivo studies showed that learning-facilitated E-LTP and L-LTP by exploration of novel empty space can be prevented by D1/D5 receptor antagonism in CA1 synapses (Li et al. 2003; Lemon and Manahan-Vaughan 2006). Furthermore, the pharmacological activation of D1/D5 receptors mimics the spatial novelty-induced facilitation of LTP (Li et al. 2003). D1/D5 receptor agonism facilitates STD into LTD in CA1 synapses in vivo (Lemon and Manahan-Vaughan 2006). This supports the possibility that D1/D5 receptor activation lowers the threshold for CA1 LTD. In addition, a role for D1/D5 receptors in learning-facilitated LTD has been reported. Here, L-LTD that was facilitated by novel spatial exploration was prevented by D1/D5 receptor antagonism (Lemon and Manahan-Vaughan 2006). Novel spatial exploration concomitant with afferent stimulation combined with D1/D5 receptor activation also enables a slow-onset depression in CA1 synapses, thereby also supporting that D1/D5 receptor activation might lower the threshold for information storage by LTD in hippocampal synapses (Lemon and Manahan-Vaughan 2011; Table 1B). Thus, learning-facilitated E- and L-LTP can be modulated by activating D1/D5 receptors (Table 1A). Again, this finding links the D1/D5 receptors strongly to novel experience and suggests that these receptors may be one of the factors that confer salience and relevance to incoming sensory information reaching the hippocampus.
What Enables the Differences in D1/D5 Regulation of Hippocampal Synaptic Plasticity?
Taken together, these studies support that D1/D5 receptors do not have identical effects on LTP in the CA1 region and DG. The CA1 region appears to be more sensitive, with both E-LTP and L-LTP being regulated by D1/D5 receptors. In the DG, by contrast, only L-LTP is affected. Adding to this functional spectrum is the regulation by D1/D5 receptors of LTD, depotentiation and learning-facilitated plasticity. This targeted regulation by D1/D5 receptors of so many different facets of synaptic plasticity may relate to the relative expression of D1/D5 receptors in the hippocampus, and the relative coupling of these receptors to signaling cascades. Both D1 and D5 receptors are both prominent in pyramidal cells of the hippocampus in monkeys (Bergson et al. 1995) and pyramidal neurons in the CA1–3, including cells in the stratum oriens and radiatum express D1/D5 receptors in rats (Fremeau et al. 1991). D1/D5 receptor mRNA is also localized dorsally in granule cells of the DG and ventrally in most neurons of the subiculum complex (Fremeau et al. 1991). Furthermore, D5 receptors are expressed in the hilus and granule cells of the DG, in pyramidal cells of the subiculum, and in the CA1–CA3 region of rats, humans, and monkeys (Ciliax et al. 2000; Khan et al. 2000). Thus, a relatively even distribution of D1/D5 receptors occurs in the hippocampus. However, some differences in the neuronal localization of the D1 and D5 receptors appear to exist: D1 receptors in the cerebral cortex are found mainly on dendritic spines, whereas D5 receptors occur predominantly on dendritic shafts in the PFC (Bergson et al. 1995). These subcellular differences in the localization of D1/D5 receptors may have functional implications (Bergson et al. 1995). As the pyramidal dendritic spines receive excitatory glutamatergic (Harris and Kater 1994) and the dendritic shafts inhibitory gamma-aminobutyric acid (GABA)ergic input (Jones 1993), it is possible that D1 receptors are mainly involved in excitatory, and the D5 receptors in inhibitory, neuromodulation (Bergson et al. 1995).
Immunohistochemical investigations localized the D1 receptor to glutamatergic excitatory projection neurons of the granule cell layer of the DG and to multiple types of inhibitory GABAergic interneurons of the hilus and CA3/CA1 fields in the mouse hippocampus (Gangarossa et al. 2012). These GABAergic interneurons may regulate the synchronized output of the granule cells (Miles et al. 1996), indicating that DA acting on these interneurons may influence information processing in the hippocampal circuit. In the CA1 region of the hippocampus and PFC in the monkey, D1/D5 receptors are pre- and postsynaptically localized (Bergson et al. 1995), indicating pre- and postsynaptic DA-mediated mechanisms induce the modulation of synaptic strength. A tight regulation of excitability through the GABAergic system is an important factor not only in preventing LTP phenomena from escalating into epileptiform events, but also for LTD and the maintenance of synaptic excitability within a functional range (Baudry 1986; Wagner and Alger 1995; Kullmann et al. 2000).
Paradoxically, Gangarossa et al. (2012) showed that there are no D1 receptors in CA1 stratum radiatum of the mouse, although in this subregion D1/D5 receptor activation is necessary for hippocampal-dependent learning, memory (O'Carroll et al. 2006; Bethus et al. 2010), and persistent plasticity at SC–CA1 synapses (Lemon and Manahan-Vaughan 2006). This suggests that D5 receptors may be the prime mediators of effects on plasticity at SC–CA1 synapses. D1 receptors were found on TA–CA1 synapses, however (Gangarossa et al. 2012), suggesting that in contrast to SC–CA1 synapses, plasticity at TA–CA1 synapses may be regulated by D1 receptors.
It is also important to point out that a mismatch between dopaminergic D1/D5 receptor distribution and dopaminergic fiber innervations of the hippocampus exists. Studies in rats demonstrated that the dorsal hippocampus receives dense noradrenergic innervations, but seldom dopaminergic innervation from the VTA (Swanson and Hartman 1975; Scatton et al. 1980). Furthermore, a discrepancy was observed between the robust immunostaining of D1/D5 receptors in the hippocampus and nearly-absent dopaminergic fibers (Smith and Greene 2012). dopaminergic fibers project from the VTA to the hippocampus (Scatton et al. 1980; Gasbarri et al. 1994, 1997), but this dopaminergic input from the VTA primarily targets the ventral hippocampus, and does not innervate structures such as the stratum radiatum of the dorsal hippocampus (Swanson 1982; Gasbarri et al. 1994, 1997). This provokes the question as to how DA can influence dorsal hippocampal function at all. However, DA levels in the hippocampus do not just depend on dopaminergic innervations, as lesions in, for example, hippocampal noradrenergic neurons significantly reduce DA levels (Bischoff et al. 1979). Moreover, locus coeruleus (LC) fibers densely innervate the hippocampal formation including the stratum radiatum (Moudy et al. 1993) and enable a direct release of DA from noradrenergic LC fibers in the CA1 region (Smith and Greene 2012). It is thus possible that DA might be released from noradrenergic fiber terminals to “compensate” for the limited, or absent, VTA-mediated release of DA to the stratum radiatum and other dorsal hippocampal subregions, thus enabling DA to regulate synaptic plasticity and learning processes that are mediated by dorsal hippocampal structures.
D1/D5 receptors differentially regulate E-LTP and -LTP depending on the hippocampal subregions concerned (Huang and Kandel 1995; Otmakhova and Lisman 1996; Kulla and Manahan-Vaughan 2000; Lemon and Manahan-Vaughan 2006; Granado et al. 2008). A relatively different expression of D1 and D5 receptors could mediate this effect, in part, by influencing the different phases of LTP, due to the fact that the receptors engage different signal cascades. D1 receptor signaling is enabled via positive coupling to adenyl cyclase (AC), whereas D5 receptor responses are predominantly mediated through positive coupling to phosphoinositide (Undieh 2010; Fig. 1). Thus, activation of either receptor will inevitably lead to phosphorylation processes, albeit of possibly different proteins. Both signal cascades (D1 and D5 receptors) converge ultimately on a common pathway converging on cAMP response element-binding protein (CREB) that supports long-term synaptic plasticity in the hippocampus (Barco et al. 2002).
Activation of AC via D1 receptors catalyzes the conversion of adenosine triphosphate to the intracellular second messenger cAMP. As a result, protein kinase A (PKA) activity, a target of cAMP, increases (Vallone et al. 2000; Undieh 2010). A target of PKA phosphorylation is the DA and cAMP-regulated 32-kDa phosphoprotein (DARPP-32) expressed in the DG of the hippocampus (DARPP-32; Undieh 2010), activation of which leads to potentiation of NMDA receptor function (Cepeda and Levine 2006). DA-sensitive PKA activation also regulates T-type Ca2+ currents (Drolet et al. 1997) and activation of the nuclear transcription factor calcium-response element-binding and CREB proteins leading to CREB protein expression (Undieh 2010; Fig. 1).
In contrast to D1 receptors, signaling via the phosphoinositide pathway of the D5 receptors activates phospholipase C (PLC), which induces hydrolysis of the phosphotidylinositol-4,5-biphosphonate to produce the second messengers diacylglycerol and inositol-1,4,5-trisphosphate (Berridge and Irvine 1984). However, the activation of D5 receptors can also stimulate cAMP and the PKA pathway (Beaulieu and Gainetdinov 2011; Fig. 1). The formation of inositol phosphates causes a mobilization of intracellular calcium stores (Undieh 2010), that in turn, is critical step in the enablement of synaptic plasticity. Increased intracellular calcium activates calcium–calmodulin-dependent protein kinase type II leading to CREB activation (Fig. 1). Thus, activation of D1 and D5 receptors can lead to CREB activation via 2 distinct signaling pathways (Undieh 2010). Several crosstalks between the AC and PLC systems exist (Undieh 2010, Fig. 1). The coupling of different signal cascades of D1/D5 receptor activation may thus not only support different functions with regard to the regulation of phases of LTP, but also of LTD (Centonze et al. 2003) along with interactions with other receptors or neuromodulators (Liu et al. 2000) that in turn can influence the longevity of these plasticity phenomena.
As D1/D5 Receptors Stimulate Local Protein Synthesis in the Dendrites of Hippocampal Neurons (Smith et al. 2005), it is likely that D1/D5 receptors are involved in the protein translation that is required for L-LTP. In line with this, the blockade of hippocampal D1/D5 receptors (within 15 min of novelty exploration) blocks L-LTP and prevents place memory (Wang et al. 2010). Novelty exploration induces DA release, triggering an up-regulation of the immediate early gene Arc in the CA1 region (Guzowski et al. 1999). D1 receptor activation may also cause increases in Zif268 and Arc/Arg3.1 expression in the DG and both genes are involved in transcriptional regulation and synaptic plasticity (Gangarossa et al. 2011). This suggests that DA, via D1/D5 receptors, stimulates transcriptional processes leading to long-term plasticity. Hippocampal D1/D5 receptors are specifically required to induce the synthesis of plasticity-related proteins necessary to consolidate long-term plasticity and memory (Moncada et al. 2011). The setting of a “synaptic tag” at a particular synapse for subsequent PRPs such as protein kinase M zeta (Navakkode et al. 2010) is essential for sustained LTP (Frey and Morris 1997). In vitro experiments suggest that D1/D5 receptor activation may be involved in this process (Sajikumar and Frey 2004; see Table 1). L-LTP inhibition by D1/D5 receptor antagonism can thus be explained on a molecular level by inhibited protein synthesis induced by the antagonism of these receptors.
The dual action of DA in inducing either LTD or LTP may be due to a concentration-dependent effect on different phosphorylation processes leading either in LTD or in LTP (Saijkumar and Frey 2004). The modulation of an NMDA receptor dependent form of both E-LTP and E-LTD via D1/D5 receptor activation in the CA1 region may be due to the fact that the DA signal converges on the NMDA receptor to induce ERK2 activation in this hippocampal subregion (Kaphzan et al. 2006). D1/D5 receptors also regulate the NMDA receptor directly (Cepeda et al. 1998; Stramiello and Wagner 2008; Varela et al. 2009) and could affect both LTP and LTD induction thresholds (Cummings et al. 1996), and signaling cascades activated by the D1/D5 receptors that lead to the activation of CREB and protein synthesis (Smith et al. 2005; Moncada et al. 2011; Sarantis et al. 2012). LTD is protein synthesis dependent (Manahan-Vaughan et al. 2000). Due to the fact that antagonism of D1/D5 receptors prevents LTD maintenance (Sajikumar and Frey 2004) in a manner similar to protein synthesis inhibitors (Sajikumar and Frey 2003), it is tempting to postulate that DA might be directly be involved in processes required for the synthesis of plasticity-related proteins that not only relate to LTP, but also to LTD (Sajikumar and Frey 2004).
Effect of D1/D5 Receptor Activity on Hippocampus-Dependent Learning
The aforementioned findings suggest that a very tight link exists between the regulation of synaptic plasticity by D1/D5 receptors and their role in hippocampus-dependent learning. The hippocampus plays a crucial role in learning and memory (Eichenbaum et al. 1990; Mishkin et al. 1998) and is involved in spatial and episodic memory (Burgess et al. 2002). The dopaminergic midbrain participates in human episodic memory formation (Schott et al. 2006). Furthermore, in rodents, long-term memory of hippocampus-mediated acquisition of new paired associates (episodic-like memory task) requires the activation of D1/D5 receptors. In contrast, early memory is unaffected by D1/D5 receptor antagonism (Bethus et al. 2010), and DA has no effect on already-established memories or on retrieval (O'Caroll et al. 2006; Table 2).
Table 2.
D1/D5 receptors and hippocampus-dependent learning
| Drug/knock out | Application route | Learning apparatus and/or learning task | Effect on learning | Reference |
|---|---|---|---|---|
| D1/D5 agonist | Intraperitoneal infusion | Barnes maze | Spatial memory↑ | Bach et al. (1999) |
| D1/D5 agonist | Intra CA1 infusion | Water maze | Spatial memory↑ | daSilva et al. (2012) |
| No effect on nonspatial | ||||
| D1/D5 agonist | Intrahippocampal infusion | Inhibitory avoidance task | Fear long-term memory↑ | Rossato et al. (2009) |
| L-LTP↑ | ||||
| D1/D5 antagonist | Intrahippocampal infusion | Special event arena | Spatial memory↑ | Bethus et al. (2010) |
| Infusion | ||||
| D1/D5 antagonist | Intra CA1 infusion | Water maze | Spatial memory formation↓ | da Silva et al. (2012) |
| D1/D5 antagonist | Systemic/prelimbic infusion | Cross maze, open field | Spatial short-term memory↓ | Clausen et al. (2011) |
| D1/D5 antagonist | Intrahippocampal infusion | Inhibitory avoidance task | Retrograde amnesia | Bernabeu et al. (1997) |
| D1/D5 antagonist | Intrahippocampal infusion | Fear long-term memory↓ | Rossato et al. (2009) | |
| D1/D5 antagonist | Intraventral subiculum infusion | Instrumental learning task | Instrumental learning↓ | Andrejewski et al. (2006) |
| D1/D5 antagonist | Intradorsal subiculum infusion | Instrumental learning task | No effect on instrumental learning | Andrejewski et al. (2006) |
| D1/D5 antagonist | Intrahippocampal infusion | Open-field water maze | Long-term memory↓ | O'Carroll et al. (2006) |
| D1/D5 antagonist | Systemic/prelimbic infusion | Open field | Short-term object retention↓ | Clausen et al. (2011) |
| D1 knock out | – | Morris water maze | Spatial learning↑ | Xing et al. (2010) |
| D1 knock out | – | Morris water maze | Spatial learning↓ | El Ghundi et al.(1999) |
| D1 knock out | – | Open field (square/circular) | Spatial learning↓ | Tran et al. (2008) |
| Spatial learning↓ | ||||
| D1 knock out | – | Morris water maze | Spatial learning↓ | Granado et al. (2008) |
| E- and L-LTP↓ | ||||
| D1 knock out | – | Barnes maze | Spatial and fear learning↓ | Ortiz et al. (2010) |
| Elevated plus maze | ||||
| Passive avoidance task | ||||
| Fear conditioning/extinction | ||||
| 6-Hydroxydopamine-lesion | – | Morris water maze | Spatial navigation↓ | Gasbarri et al. (1996) |
| Memory for place navigation↓ |
E-LTP and L-LTP: early and late long-term potentiation, respectively.
D1 agonist treatment in rats enhances hippocampus-dependent spatial memory (Bach et al. 1999; da Silva et al. 2012) without affecting nonspatial memory (da Silva et al. 2012). By contrast, D1/D5 receptor antagonists impair short- and long-term spatial memory (Clausen et al. 2011; da Silva et al. 2012). Studies in transgenic mice suggest that the D1 receptor (El-Gundi et al. 1999) and not the D3 or D5 receptor are essential for spatial learning (Granado et al. 2008; Xing et al. 2010). The D1 receptor is also crucial for the encoding of novel environments and hippocampal representations of plasticity (Tran et al. 2008). The D1 receptor is critical for the induction of Zif268 and arc, proteins required for the transition of E-LTP into L-LTP and memory consolidation in mammals (Granado et al. 2008), and the activation of D1/D5 receptors is required during memory encoding to generate a persistent memory trace in the hippocampus (O'Carroll et al. 2006). Learning-dependent changes in synaptic strength of other forms of hippocampal-dependent learning, such as classic eyeblink conditioning (Kuo et al. 2006, Suzuki 2007; Madronal et al. 2009), are also modulated by D1 receptor activation (Ortiz et al. 2010). These findings suggest that D1/D5 receptor activation is a crucial factor in the formation of spatial long-term memory in the mammalian brain.
The Role of the Novelty Signal
These observations raise the question as to what drives changes in DA levels in the hippocampus and the relative contribution of D1/D5 receptors to synaptic plasticity and memory formation. One major factor is the response to novelty. A highly significant source of DA release in the hippocampus derives from the VTA, the dopaminergic neurons of which discharge in response to novel stimuli (Ljunberg et al. 1992; Grenhoff et al. 1993) with a phasic burst pattern (Ljunberg et al. 1992). As the latencies of the response to a novel stimulus are quite similar between the VTA and hippocampus (50–200 ms), Lisman and Grace proposed a theoretical model illustrating how novel information is first processed by the hippocampus and secondly, leads to indirect activation of the VTA via the NAcc and VP. The indirect activation of the VTA occurs through an excitatory glutamatergic projection from the subiculum to the NAcc, an inhibitory GABAergic projection of the NAcc to the VP, and finally, an inhibitory GABAergic projection of the VP to the VTA (Legault et al. 2000; Floresco et al. 2001, Legault and Wise 2001; Figs 2 and 3).
It has been suggested that stored sensory information in the DG–CA3 system sends “predictive” information to the CA1 via the SC that “compares” the actual novel sensory data from the perforant path. This resulting “mismatch” signal activates the VTA via the indirect pathway (NAcc and VP) of the hippocampal–VTA loop (Lisman and Grace 2005). Neuroimaging studies in humans support the hippocampal–VTA-dependent encoding of novel stimuli (Wittmann et al. 2005; Adcock et al. 2006). Further neuroimaging data in humans highlighted the coactivation of the VTA, hippocampus, and VP by stimulus novelty (Bunzeck and Düzel 2006), and an in vivo study in rats showed that novel stimuli induced a rise in DA in the NAc dependent on information processing from the ventral subiculum of the hippocampus (Legault and Wise 2001).
Taken together, these findings support that novel information may first be registered by the hippocampus that in turn activates the VTA to generate the novelty signal that subsequently influences qualitative hippocampal information encoding. In line with this, an enhancement of long-term plasticity in the DG induced by HFS is observed when a rat is placed in a novel environment (Davis et al. 2004), suggesting that novelty has a marked influence on hippocampal excitability. Accordingly, novelty induces a rise in hippocampal activity in rabbits (Vinogradova 2001), rats (Jenkins et al. 2004), and humans (Tulving et al. 1996; Strange and Dolan 2001). Furthermore, it is the hippocampus and not the VTA that appears to initiate the novelty response: Event-related potentials in the hippocampus of the cat (Ruusurvita et al. 1995) and rat (Brankack et al. 1996) indicate that the hippocampus triggers novelty-related firing of the VTA. Correspondingly, DA release occurs after novel stimuli in the mouse hippocampus (Ihalainen et al. 1999) and hippocampal novelty signals increase the number of tonically activated DA VTA neurons (Floresco et al. 2003; Figs 2 and 3). The dialog between hippocampus and VTA appears essential for long-term information storage. Thus, a reciprocal interaction of the VTA/hippocampus circuit enables the encoding of novel information into long-term memory by VTA DA release (Mizumori et al. 2004; Lisman and Grace 2005; Wittmann et al. 2005; Adcock et al. 2006).
Processing of novelty by the hippocampus, however, may be supported by structures other than the VTA. For example, the noradrenergic LC fires rhythmically in response to novel experience (Sara et al. 1994). Activation of this structure changes hippocampal excitability (Kitchigina et al. 1997) and facilitates synaptic plasticity (Lemon et al. 2009). But the LC and the VTA are interlinked on both functional and anatomical levels. A study using anterograde and retrograde tracing techniques showed that the LC and VTA have anatomical connections (Simon et al. 1979). The VTA projects directly to the LC and is likely to release DA there, indicating a feedforward connection between the VTA and LC (Ornstein et al. 1987; Sara 2009). Moreover, the VTA can induce PFC activation via DA release that, in turn, alters LC neuronal activity via glutamate release (Sara 2009), and VTA DA neurons are modulated via noradrenaline that is released as a consequence of electrical stimulation of the LC (Grenhoff et al. 1993). Lesions studies of LC noradrenaline neurons and VTA DA neurons suggest that LC noradrenaline neurons and VTA DA neurons exert an inhibitory influence on DA neurons firing in the VTA and noradrenaline neurons in the LC, respectively (Guiard et al. 2008). However, α1-receptor antagonism by prazosine in LC revealed a decrease in DA neuron firing in the VTA, suggesting an excitatory effect of LC noradrenaline neurons to the VTA DA neurons as well (Grenhoff and Svensson 1993). Thus, the LC engages in a complex functional dialog with the VTA.
Both the LC (Vankov et al. 1995) and VTA (Schultz et al. 1993) neurons are activated by novelty, acting as learning signals in a complementary fashion (Harley 2004). Whereas the LC becomes immediate active when novel experience commences (Aston-Jones and Bloom 1981; Sara et al. 1994), the VTA becomes active within hundreds of milliseconds later (Ljungberg et al. 1992). This suggest that the LC, either via direct communication with the VTA, or via the hippocampus–VTA loop, may regulate DA release from the VTA to the hippocampus. In line with this possibility, D1/D5 receptors are involved in regulating hippocampal LTD that is induced by LC stimulation (Lemon et al. 2009; Lemon and Manahan-Vaughan 2011). Here, D1/D5 receptor antagonism prevents LC–CA1 LTD. Furthermore, application of a D1/D5 receptor agonist facilitates LC-induced CA1 E-LTD into L-LTD that lasts for over 24 h (Lemon and Manahan-Vaughan 2011; Table 1B). These findings suggest that the D1/D5 receptor system serves to lower the threshold required for persistent storage of information under conditions of novelty or increased arousal regardless of the source of the novelty signal (Lemon and Manahan-Vaughan 2011).
D1/D5 Receptors are Pivotal for Hippocampal Information Storage
Based on current knowledge, it is clear that D1/D5 receptors play an intriguing and decisive role in the enablement of information encoding and storage in the hippocampus. They can facilitate the expression of both LTP and LTD, and taking into account the accumulating evidence that LTP encodes different aspects of spatial representations (Kemp and Manahan-Vaughan 2007, 2008a; Goh and Manahan-Vaughan 2012), this suggests that D1/D5 receptors can drive the nature and qualitative content of stored information in the hippocampus. Strikingly, on a functional level and in line with this postulate, D1/D5 receptor activation leads to increased processing within the trisynaptic DG–CA3–CA1 circuit, to the disadvantage of the direct entorhinal–CA1 input (Varela et al. 2009), thereby minimizing the influence of mismatch detection (Lismann and Otmakhova 2001) in favor of prioritizing information storage. This in turn is likely to be highly relevant in the coupling of information storage and memory with reward experiences.
Taken together with the observations that D1/D5 receptor activation modulates hippocampus-dependent episodic and spatial long-term memory, these data indicate that D1/D5 receptors gate hippocampal long-term plasticity and memory in the mammalian brain, and are pivotal in conferring the properties of novelty and reward to information being processed by the hippocampus.
Funding
This work is supported by a grant from the German research foundation (Deutsche Forschungsgemeinscaft, www.dfg.de) to Denise Manahan-Vaughan (Ma1843/6-2).
Notes
Conflict of Interest: None declared.
References
- Abraham WC, Christie BR, Logan B, Lawlor P, Dragunow M. Immediate early gene expression associated with the persistence of heterosynaptic long-term depression in the hippocampus. Proc Natl Acad Sci U S A. 1994;91:10049–10053. doi: 10.1073/pnas.91.21.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JD. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50:507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Andrzejewski ME, Spencer RC, Kelley AE. Dissociating ventral and dorsal subicular dopamine D1 receptor involvement in instrumental learning, spontaneous motor behavior, and motivation. Behav Neurosci. 2006;120:542–553. doi: 10.1037/0735-7044.120.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci. 1981;1:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci U S A. 1999;96:5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir ZI, Bortolotto ZA, Davies CH, Berretta N, Irving AJ, Seal AJ, Henley JM, Jane DE, Watkins JC, Collingridge GL. Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature. 1993;363:347–350. doi: 10.1038/363347a0. [DOI] [PubMed] [Google Scholar]
- Barco A, Alarcon JM, Kandel ER. Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell. 2002;108:689–703. doi: 10.1016/s0092-8674(02)00657-8. [DOI] [PubMed] [Google Scholar]
- Baudry M. Long-term potentiation and kindling: similar biochemical mechanisms? Adv Neurol. 1986;44:401–410. [PubMed] [Google Scholar]
- Bear MF. A synaptic basis for memory storage in the cerebral cortex. Proc Natl Acad Sci U S A. 1996;93:13453–13459. doi: 10.1073/pnas.93.24.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Beckstead RM, Domesick VB, Nauta WJ. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res. 1979;175:191–217. doi: 10.1016/0006-8993(79)91001-1. [DOI] [PubMed] [Google Scholar]
- Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS. Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J Neurosci. 1995;15:7821–7836. doi: 10.1523/JNEUROSCI.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabeu R, Bevilaqua L, Ardenghi P, Bromberg E, Schmitz P, Bianchin M, Izquierdo I, Medina JH. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc Natl Acad Sci U S A. 1997;94:7041–7046. doi: 10.1073/pnas.94.13.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;312:315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bethus I, Tse D, Morris RG. Dopamine and memory: modulation of the persistence of memory for novel hippocampal NMDA receptor-dependent paired associates. J Neurosci. 2010;30:1610–1618. doi: 10.1523/JNEUROSCI.2721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff S, Scatton B, Korf J. Biochemical evidence for a transmitter role of dopamine in the rat hippocampus. Brain Res. 1979;165:161–165. doi: 10.1016/0006-8993(79)90056-8. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brankack J, Seidenbecher T, Müller-GaÅNrtner HW. Task-relevant late positive component in rats: is it related to hippocampal theta rhythm? Hippocampus. 1996;6:475–482. doi: 10.1002/(SICI)1098-1063(1996)6:5<475::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunzeck N, Düzel E. Absolute coding of stimulus novelty in the human substantia nigra/VTA. Neuron. 2006;51:369–379. doi: 10.1016/j.neuron.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O'Keefe J. The human hippocampus and spatial and episodicmemory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Falck B, Hillarp NA. Cellular localization of brain monoamines. Acta Physiol Scand Suppl. 1962;56:1–28. [PubMed] [Google Scholar]
- Centonze D, Grande C, Saulle E, Martin AB, Gubellini P, PavoÅLn N, Pisani A, Bernardi G, Moratalla R, Calabresi P. Distinct roles of D1 and D5 dopamine receptors in motor activity and striatal synaptic plasticity. J Neurosci. 2003;23:8506–8512. doi: 10.1523/JNEUROSCI.23-24-08506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Colwell CS, Itri JN, Chandler SH, Levine MS. Dopaminergic modulation of NMDA-induced whole cell currents in neostriatal neurons in slices: contribution of calcium conductances. J Neurophysiol. 1998;79:82–94. doi: 10.1152/jn.1998.79.1.82. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Levine MS. Where do you think you are going? The NMDA-D1 receptor trap. Sci STKE. 2006;333:20. doi: 10.1126/stke.3332006pe20. [DOI] [PubMed] [Google Scholar]
- Chen Z, Fujii S, Ito K, Kato H, Kaneko K, Miyakawa H. Activation of dopamine D1 receptors enhances long-term depression of synaptic transmission induced by low frequency stimulation in rat hippocampal CA1 neurons. Neurosci Lett. 1995;188:195–198. doi: 10.1016/0304-3940(95)11430-5. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Lórincz A, Busaki G. Physiological patterns in the hippocampo-enthorinal cortex system. Hippocampus. 2000;10:457–465. doi: 10.1002/1098-1063(2000)10:4<457::AID-HIPO12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Nash N, Heilman C, Sunahara R, Hartney A, Tiberi M, Rye DB, Caron MG, Niznik HB, Levey AI. Dopamine D(5) receptor immunolocalization in rat and monkey brain. Synapse. 2000;37:125–145. doi: 10.1002/1098-2396(200008)37:2<125::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Clausen B, Schachtman TR, Mark LT, Reinholdt M, Christoffersen GR. Impairments of exploration and memory after systemic or prelimbic D1-receptor antagonism in rats. Behav Brain Res. 2011;223:241–254. doi: 10.1016/j.bbr.2011.03.069. [DOI] [PubMed] [Google Scholar]
- Cummings JA, Mulkey RM, Nicoll RA, Malenka RC. Ca2+ signaling requirements for long-term depression in the hippocampus. Neuron. 1996;16:825–833. doi: 10.1016/s0896-6273(00)80102-6. [DOI] [PubMed] [Google Scholar]
- da Silva WC, Köhler CC, Radiske A, Cammarota M. D1/D5 dopamine receptors modulate spatial memory formation. Neurobiol Learn Mem. 2012;97:271–275. doi: 10.1016/j.nlm.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Davis CD, Jones FL, Derrick BE. Novel environments enhance the induction and maintenance of long-term potentiation in the dentate gyrus. J Neurosci. 2004;24:6497–6506. doi: 10.1523/JNEUROSCI.4970-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69:145–157. doi: 10.1016/j.biopsych.2011.02.036. [DOI] [PubMed] [Google Scholar]
- Drolet P, Bilodeau L, Chorvatova A, Laflamme L, Gallo-Payet N, Payet MD. Inhibition of the T-type Ca2+ current by the dopamine D1 receptor in rat adrenal glomerulosa cells: requirement of the combined action of the G betagamma protein subunit and cyclic adenosine 3′,5′-monophosphate. Mol Endocrinol. 1997;11:503–514. doi: 10.1210/mend.11.4.9910. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci U S A. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie T, Lynch G. Long-term potentiation and depression of synaptic responses in the rat hippocampus: localization and frequency dependency. J Physiol. 1978;276:353–367. doi: 10.1113/jphysiol.1978.sp012239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehringer H, Hornykiewicz O. Verteilung von Noradrenalin und Dopamin (3- Hydroxytryamin) im Gehirn des Menschen und ihr Verhalten bei Erkrankungen des Extrapyramidalen Systems. Klin Wochenschrift. 1960;38:1236–1239. doi: 10.1007/BF01485901. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Stewart C, Morris RG. Hippocampal representation in place learning. J Neurosci. 1990;10:3531–3542. doi: 10.1523/JNEUROSCI.10-11-03531.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ghundi M, Fletcher PJ, Drago J, Sibley DR, O'Dowd BF, George SR. Spatial learning deficit in dopamine D(1) receptor knockout mice. Eur J Pharmacol. 1999;383:95–106. doi: 10.1016/s0014-2999(99)00573-7. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D. The role of hippocampus in anxiety: intracerebral infusion studies. Behav Pharmacol. 2007;18:365–374. doi: 10.1097/FBP.0b013e3282de7929. [DOI] [PubMed] [Google Scholar]
- Etkin A, Alarcón JM, Weisberg SP, Touzani K, Huang YY, Nordheim A, Kandel ER. A role in learning for SRF: deletion in the adult forebrain disrupts LTD and the formation of an immediate memory of a novel context. Neuron. 2006;50:127–143. doi: 10.1016/j.neuron.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Fitch TE, Sahr RN, Eastwood BJ, Zhou FC, Yang CR. Dopamine D1/D5 receptor modulation of firing rate and bidirectional theta burst firing in medial septal/vertical limb of diagonal band neurons in vivo. J Neurophysiol. 2006;95:2808–2820. doi: 10.1152/jn.01210.2005. [DOI] [PubMed] [Google Scholar]
- Fitzjohn SM, Bortolotto ZA, Palmer MJ, Doherty AJ, Ornstein PL, Schoepp DD, Kingston AE, Lodge D, Collingridge GL. The potent mGlu receptor antagonist LY341495 identifies roles for both cloned and novel mGlu receptors in hippocampal synaptic plasticity. Neuropharmacology. 1998;37:1445–1458. doi: 10.1016/s0028-3908(98)00145-2. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21:4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Fluckiger E, Muller EE, Thorner MO. Basic and clinical aspects of neuroscience. New York: Springer-Verlag; 1987. [Google Scholar]
- Fremeau RT, Jr, Duncan GE, Fornaretto MG, Dearry A, Gingrich JA, Breese GR, Caron MG. Localization of D1 dopamine receptor mRNA in brain supports a role in cognitive, affective, and neuroendocrine aspects of dopaminergic neurotransmission. Proc Natl Acad Sci U S A. 1991;88:3772–3776. doi: 10.1073/pnas.88.9.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Matthies H, Reymann KG, Matthies H. The effect of dopaminergic D1 receptor blockade during tetanization on the expression of long-term potentiation in the rat CA1 region in vitro. Neurosci Lett. 1991;129:111–114. doi: 10.1016/0304-3940(91)90732-9. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Frey U, Schroeder H, Matthies H. Dopaminergic antagonists prevent long-term maintenance of posttetanic LTP in the CA1 region of rat hippocampal slices. Brain Res. 1990;522:69–75. doi: 10.1016/0006-8993(90)91578-5. [DOI] [PubMed] [Google Scholar]
- Gangarossa G, Di Benedetto M, O`Sullivan GJ, Dunleavy M, Alcacer C, Bonito-Oliva A, Henshall DC, Waddingtion JL, Valjent E, Fisone G. Convulsant doses of dopamine D1 receptor agonist result in Erk-dependent increases in Zif268 and Arc/Arg3.1 expression in mouse dentate gyrus. PLoS One. 2011;3:e19415. doi: 10.1371/journal.pone.0019415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangarossa G, Longueville S, De Bundel D, Perroy J, HerveÅL D, Girault JA, Valjent E. Characterization of dopamine D1 and D2 receptor-expressing neurons in the mouse hippocampus. Hippocampus. 2012 doi: 10.1002/hipo.22044. doi:10.1002/hipo.22044. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Packard MG, Campana E, Pacitti C. Anterograde and retrograde tracing of projections from the ventral tegmental area to the hippocampal formation in the rat. Brain Res Bull. 1994;33:445–452. doi: 10.1016/0361-9230(94)90288-7. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Sulli A, Innocenzi R, Pacitti C, Brioni JD. Spatial memory impairment induced by lesion of the mesohippocampal dopaminergic system in the rat. Neuroscience. 1996;74:1037–1044. doi: 10.1016/0306-4522(96)00202-3. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Sulli A, Packard MG. The dopaminergic mesencephalic projections to the hippocampal formation in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1–22. doi: 10.1016/s0278-5846(96)00157-1. [DOI] [PubMed] [Google Scholar]
- Goh JJ, Manahan-Vaughan D. Spatial object recognition enables endogenous LTD that curtails LTP in the mouse hippocampus. Cereb Cortex. 2012;23:1118–1125. doi: 10.1093/cercor/bhs089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. The interactions and dissociations of the dorsal hippocampus subregions: how the dentate gyrus, CA3, and CA1 process spatial information. Behav Neurosci. 2008;122:16–26. doi: 10.1037/0735-7044.122.1.16. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopamine modulation of hippocampal-prefrontal cortical interaction drives memory-guided behavior. Cereb Cortex. 2008a;18:1407–1414. doi: 10.1093/cercor/bhm172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA. The dopamine system and the pathophysiology of schizophrenia: a basic science perspective. Int Rev Neurobiol. 2007;78:41–68. doi: 10.1016/S0074-7742(06)78002-3. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Limbic and cortical information processing in the nucleus accumbens. Trends Neurosci. 2008b;31:552–558. doi: 10.1016/j.tins.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Granado N, Ortiz O, Suárez LM, Martín ED, Ceña V, Solís JM, Moratalla R. D1 but not D5 dopamine receptors are critical for LTP, spatial learning, and LTP-induced arc and zif268 expression in the hippocampus. Cereb Cortex. 2008;18:1–12. doi: 10.1093/cercor/bhm026. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Nisell M, Ferré S, Aston-Jones G, Svensson TH. Noradrenergic modulation of midbrain dopamine cell firing elicited by stimulation of the locus coeruleus in the rat. J Neural Transm Gen Sect. 1993;93:11–25. doi: 10.1007/BF01244934. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Svensson TH. Prazosin modulates the firing pattern of dopamine neurons in rat ventral tegmental area. Eur J Pharmacol. 1993;233:79–84. doi: 10.1016/0014-2999(93)90351-h. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Hussain RJ, O'Donnell P. The nucleus accumbens: a switchboard for goal-directed behaviors. PLoS One. 2009;4:e5062. doi: 10.1371/journal.pone.0005062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiard BP, El Mansari M, Merali Z, Blier P. Functional interactions between dopamine, serotonin and norepinephrine neurons: an in-vivo electrophysiological study in rats with monoaminergic lesions. Int J Neuropsychopharmacol. 2008;11:625–639. doi: 10.1017/S1461145707008383. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Hagena H, Manahan-Vaughan D. Learning-facilitated synaptic plasticity at CA3 mossy fiber and commissural-associational synapses reveals different roles in information processing. Cereb Cortex. 2011;21:2442–2449. doi: 10.1093/cercor/bhq271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton TJ, Wheatley BM, Sinclair DB, Bachmann M, Larkum ME, Colmers WF. Dopamine modulates synaptic plasticity in dendrites of rat and human dentate granule cells. Proc Natl Acad Sci U S A. 2010;107:18185–18190. doi: 10.1073/pnas.1011558107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CW. Norepinephrine and dopamine as learning signals. Neural Plast. 2004;11:191–204. doi: 10.1155/NP.2004.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Kater SB. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Beck H, Dreier JP, Ficker E, Stabel J, Zhang CL. The dentate gyrus as a regulated gate for the propagation of epileptiform activity. Epilepsy Res Suppl. 1992;7:273–280. [PubMed] [Google Scholar]
- Hölscher C. Time, space and hippocampal functions. Rev Neurosci. 2003;14:253–284. doi: 10.1515/revneuro.2003.14.3.253. [DOI] [PubMed] [Google Scholar]
- Horn AS, Korf J, Westerrink BHC. The neurobiology of dopamine. London: Academic Press; 1979. [Google Scholar]
- Howland JG, Taepavarapruk P, Phillips AG. Glutamate receptor-dependent modulation of dopamine efflux in the nucleus accumbens by basolateral, but not central, nucleus of the amygdala in rats. J Neurosci. 2002;22:1137–1145. doi: 10.1523/JNEUROSCI.22-03-01137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proc Natl Acad Sci U S A. 1995;92:2446–2450. doi: 10.1073/pnas.92.7.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihalainen JA, Riekkinen P, Jr, Feenstra MG. Comparison of dopamine and noradrenaline release in mouse prefrontal cortex, striatum and hippocampus using microdialysis. Neurosci Lett. 1999;277:71–74. doi: 10.1016/s0304-3940(99)00840-x. [DOI] [PubMed] [Google Scholar]
- Ito HT, Schuman EM. Frequency-dependent gating of synaptic transmission and plasticity by dopamine. Front Neural Circuits. 2007;1:1. doi: 10.3389/neuro.04.001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Hayen A. Opposing roles of nucleus accumbens core and shell dopamine in the modulation of limbic information processing. J Neurosci. 2011;31:6001–6007. doi: 10.1523/JNEUROSCI.6588-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Prog Neurobiol. 2003;69:375–390. doi: 10.1016/s0301-0082(03)00085-6. [DOI] [PubMed] [Google Scholar]
- Jenkins TA, Amin E, Pearce JM, Brown MW, Aggleton JP. Novel spatial arrangements of familiar visual stimuli promote activity in the rat hippocampal formation but not the parahippocampal cortices: a c-fos expression study. Neuroscience. 2004;124:43–52. doi: 10.1016/j.neuroscience.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Jones EG. GABAergic neurons and their role in cortical plasticity in primates. Cereb Cortex. 1993;3:361–372. doi: 10.1093/cercor/3.5.361-a. [DOI] [PubMed] [Google Scholar]
- Jones MW, Errington ML, French PJ, Fine A, Bliss TV, Garel S, Charnay P, Bozon B, Laroche S, Davis S. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat Neurosci. 2001;4:289–296. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- Jürgensen S, Antonio LL, Mussi GE, Brito-Moreira J, Bomfim TR, De Felice FG, Garrido-Sanabria ER, Cavalheiro ÉA, Ferreira ST. Activation of D1/D5 dopamine receptors protects neurons from synapse dysfunction induced by amyloid-beta oligomers. J Biol Chem. 2011;286:3270–3276. doi: 10.1074/jbc.M110.177790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaphzan H, O'Riordan KJ, Mangan KP, Levenson JM, Rosenblum K. NMDA and dopamine converge on the NMDA-receptor to induce ERK activation and synaptic depression in mature hippocampus. PLoS One. 2006;1:e138. doi: 10.1371/journal.pone.0000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauderer BS, Eric R. Kandel. Capture of a protein synthesis-dependent component of long-term depression. Proc Natl Acad Sci U S A. 2000;97:13342–13347. doi: 10.1073/pnas.97.24.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Beta-adrenoreceptors comprise a critical element in learning-facilitated long-term plasticity. Cereb Cortex. 2008b;18:1326–1334. doi: 10.1093/cercor/bhm164. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. The hippocampal CA1 region and dentate gyrus differentiate between environmental and spatial feature encoding through long-term depression. Cereb Cortex. 2008a;18:968–977. doi: 10.1093/cercor/bhm136. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc Natl Acad Sci U S A. 2004;101:8192–8197. doi: 10.1073/pnas.0402650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression: master or minion in declarative memory processes. Trends Neurosci. 2007;30:111–118. doi: 10.1016/j.tins.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Gilbert PE, Wallenstein GV. Testing neural network models of memory with behavioral experiments. Curr Opin Neurobiol. 2000;10:260–265. doi: 10.1016/s0959-4388(00)00067-2. [DOI] [PubMed] [Google Scholar]
- Khan ZU, Gutiérrez A, Martín R, Peñafiel A, Rivera A, de la Calle A. Dopamine D5 receptors of rat and human brain. Neuroscience. 2000;100:689–699. doi: 10.1016/s0306-4522(00)00274-8. [DOI] [PubMed] [Google Scholar]
- Kitchigina V, Vankov A, Harley C, Sara SJ. Novelty-elicited, noradrenaline-dependent enhancement of excitability in the dentate gyrus. Eur J Neurosci. 1997;9:41–47. doi: 10.1111/j.1460-9568.1997.tb01351.x. [DOI] [PubMed] [Google Scholar]
- Kulla A, Manahan-Vaughan D. Depotentiation in the dentate gyrus of freely moving rats is modulated by D1/D5 dopamine receptors. Cereb Cortex. 2000;10:614–620. doi: 10.1093/cercor/10.6.614. [DOI] [PubMed] [Google Scholar]
- Kulla A, Manahan-Vaughan D. Modulation by group 1 metabotropic glutamate receptors of depotentiation in the dentate gyrus of freely moving rats. Hippocampus. 2008;18:48–54. doi: 10.1002/hipo.20366. [DOI] [PubMed] [Google Scholar]
- Kulla A, Manahan-Vaughan D. Regulation of depotentiation and long-term potentiation in the dentate gyrus of freely moving rats by dopamine D2-like receptors. Cereb Cortex. 2003;13:123–135. doi: 10.1093/cercor/13.2.123. [DOI] [PubMed] [Google Scholar]
- Kulla A, Reymann KG, Manahan-Vaughan D. Time-dependent induction of depotentiation in the dentate gyrus of freely moving rats: involvement of group 2 metabotropic glutamate receptors. Eur J Neurosci. 1999;11:3864–3872. doi: 10.1046/j.1460-9568.1999.00807.x. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Asztely F, Walker MC. The role of mammalian ionotropic receptors in synaptic plasticity: LTP, LTD and epilepsy. Cell Mol Life Sci. 2000;57:1551–1561. doi: 10.1007/PL00000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar U, Patel SC. Immunohistochemical localization of dopamine receptor subtypes (D1R-D5R) in Alzheimer's disease brain. Brain Res. 2007;1131:187–196. doi: 10.1016/j.brainres.2006.10.049. [DOI] [PubMed] [Google Scholar]
- Kuo AG, Lee G, Disterhoft JF. Simultaneous training on two hippocampus-dependent tasks facilitates acquisition of trace eyeblink conditioning. Learn Mem. 2006;13:201–207. doi: 10.1101/lm.98406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuki T, Imahori Y, Ueda S, Inokuchi K. Dopaminergic modulation of LTP induction in the dentate gyrus of intact brain. Neuroreport. 1997;8:2037–2040. doi: 10.1097/00001756-199705260-00046. [DOI] [PubMed] [Google Scholar]
- Lee HK, Kameyama K, Huganir RL, Bear MF. NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron. 1998;21:1151–1162. doi: 10.1016/s0896-6273(00)80632-7. [DOI] [PubMed] [Google Scholar]
- Lee I, Rao G, Knierim JJ. A double dissociation between hippocampal subfields: differential time course of CA3 and CA1 place cells for processing changed environments. Neuron. 2004;42:803–815. doi: 10.1016/j.neuron.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Legault M, Rompré PP, Wise RA. Chemical stimulation of the ventral hippocampus elevates nucleus accumbens dopamine by activating dopaminergic neurons of the ventral tegmental area. J Neurosci. 2000;20:1635–1642. doi: 10.1523/JNEUROSCI.20-04-01635.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legault M, Wise RA. Novelty-evoked elevations of nucleus accumbens dopamine: dependence on impulse flow from the ventralsubiculum and glutamatergic neurotransmission in the ventral tegmental area. Eur J Neurosci. 2001;13:819–828. doi: 10.1046/j.0953-816x.2000.01448.x. [DOI] [PubMed] [Google Scholar]
- Lemon N, Aydin-Abidin S, Funke K, Manahan-Vaughan D. Locus coeruleus activation facilitates memory encoding and induces hippocampal LTD that depends on beta-adrenergic receptor activation. Cereb Cortex. 2009;19:2827–2837. doi: 10.1093/cercor/bhp065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon N, Manahan-Vaughan D. Dopamine D1/D5 receptors contribute to de novo hippocampal LTD mediated by novel spatial exploration or locus coeruleus activity. Cereb Cortex. 2011;22:2131–2138. doi: 10.1093/cercor/bhr297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon N, Manahan-Vaughan D. Dopamine D1/D5 receptors gate the acquisition of novel information through hippocampal long-term potentiation and long-term depression. J Neurosci. 2006;26:7723–7729. doi: 10.1523/JNEUROSCI.1454-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6:526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- Lisman J, Grace AA, Duzel E. A neo Hebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends Neurosci. 2011;34:536–547. doi: 10.1016/j.tins.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Otmakhova NA. Storage, recall, and novelty detection of sequences by the hippocampus: elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus. 2001;11:551–568. doi: 10.1002/hipo.1071. [DOI] [PubMed] [Google Scholar]
- Liu F, Wan Q, Pristupa ZB, Yu XM, Wang YT, Niznik HB. Direct protein-protein coupling enables cross-talk between dopamine D5 and gamma-aminobutyric acid A receptors. Nature. 2000;403:274–280. doi: 10.1038/35002014. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang W, Wang F, Cai F, Hu ZL, Yang YJ, Chen J, Chen JG. Phosphatidylinositol-linked novel D(1) dopamine receptor facilitates long-term depression in rat hippocampal CA1 synapses. Neuropharmacology. 2009;57:164–171. doi: 10.1016/j.neuropharm.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Ljungberg T, Apicella P, Schultz W. Responses of monkey dopamine neurons during learning of behavioral reactions. J Neurophysiol. 1992;67:145–163. doi: 10.1152/jn.1992.67.1.145. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci. 2011;32:507–513. doi: 10.1016/j.tips.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madronal N, Gruart A, Delgado-GarciÅLa JM. Differing presynaptic contributions to LTP and associative learning in behaving mice. Front Behav Neurosci. 2009;3:7. doi: 10.3389/neuro.08.007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Kauer JA, Perkel DJ, Mauk MD, Kelly PT, Nicoll RA, Waxham MN. An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. Nature. 1989;340:554–557. doi: 10.1038/340554a0. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D. Group 1 and 2 metabotropic glutamate receptors play differential roles in hippocampal long-term depression and long-term potentiation in freely moving rats. J Neurosci. 1997;17:3303–3311. doi: 10.1523/JNEUROSCI.17-09-03303.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D. Long-term depression in freely moving rats is dependent upon strain variation, induction protocol and behavioral state. Cereb Cortex. 2000;10:482–487. doi: 10.1093/cercor/10.5.482. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell KH. Novelty acquisition is associated with induction of hippocampal long-term depression. Proc Natl Acad Sci U S A. 1999;96:8739–8744. doi: 10.1073/pnas.96.15.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthies H, Becker A, Schröder H, Kraus J, Höllt V, Krug M. Dopamine D1-deficient mutant mice do not express the late phase of hippocampal long-term potentiation. Neuroreport. 1997;8:3533–3535. doi: 10.1097/00001756-199711100-00023. [DOI] [PubMed] [Google Scholar]
- Miles R, Tóth K, Gulyás AI, Hájos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16:815–823. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Vargha-Khadem F, Gadian DG. Amnesia and the organization of the hippocampal system. Hippocampus. 1998;8:212–216. doi: 10.1002/(SICI)1098-1063(1998)8:3<212::AID-HIPO4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Mizumori SJ, Yeshenko O, Gill KM, Davis DM. Parallel processing across neural systems: implications for a multiple memory system hypothesis. Neurobiol Learn Mem. 2004;82:278–298. doi: 10.1016/j.nlm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Mockett BG, Guévremont D, Williams JM, Abraham WC. Dopamine D1/D5 receptor activation reverses NMDA receptor-dependent long-term depression in rat hippocampus. J Neurosci. 2007;27:2918–2926. doi: 10.1523/JNEUROSCI.0838-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada D, Ballarini F, Martinez MC, Frey JU, Viola H. Identification of transmitter systems and learning tag molecules involved in behavioral tagging during memory formation. Proc Natl Acad Sci U S A. 2011;108:12931–12936. doi: 10.1073/pnas.1104495108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moudy AM, Kunkel DD, Schwartzkroin PA. Development of dopamine-betahydroxylase-positive fiber innervation of the rat hippocampus. Synapse. 1993;15:307–318. doi: 10.1002/syn.890150407. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Herron CE, Malenka RC. An essential role for protein phosphatases in hippocampal long-term depression. Science. 1993;261:1051–1055. doi: 10.1126/science.8394601. [DOI] [PubMed] [Google Scholar]
- Navakkode S, Sajikumar S, Frey JU. Synergistic requirements for the induction of dopaminergic D1/D5-receptor-mediated LTP in hippocampal slices of rat CA1 in vitro. Neuropharmacology. 2007;52:1547–1554. doi: 10.1016/j.neuropharm.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Navakkode S, Sajikumar S, Sacktor TC, Frey JU. Protein kinase Mzeta is essential for the induction and maintenance of dopamine-induced long-term potentiation in apical CA1 dendrites. Learn Mem. 2010;17:605–611. doi: 10.1101/lm.1991910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PV, Abel T, Kandel ER. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- O'Carroll CM, Martin SJ, Sandin J, Frenguelli B, Morris RG. Dopaminergic modulation of the persistence of one-trial hippocampus-dependent memory. Learn Mem. 2006;13:760–769. doi: 10.1101/lm.321006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornstein K, Milon H, McRae-Degueurce A, Alvarez C, Berger B, Würzner HP. Biochemical and radioautographic evidence for dopaminergic afferents of the locus coeruleus originating in the ventral tegmental area. J Neural Transm. 1987;70:183–191. doi: 10.1007/BF01253597. [DOI] [PubMed] [Google Scholar]
- Ortiz O, Delgado-García JM, Espadas I, Bahí A, Trullas R, Dreyer JL, Gruart A, Moratalla R. Associative learning and CA3-CA1 synaptic plasticity are impaired in D1R null, Drd1a−/− mice and in hippocampal siRNA silenced Drd1a mice. J Neurosci. 2010;30:12288–12300. doi: 10.1523/JNEUROSCI.2655-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhova NA, Lisman JE. D1/D5 dopamine receptor activation increases the magnitude of early long-term potentiation at CA1 hippocampal synapses. J Neurosci. 1996;16:7478–7486. doi: 10.1523/JNEUROSCI.16-23-07478.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhova NA, Lisman JE. D1/D5 dopamine receptors inhibit depotentiation at CA1 synapses via cAMP-dependent mechanism. J Neurosci. 1998;18:1270–1279. doi: 10.1523/JNEUROSCI.18-04-01270.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvez S, Ramachandran B, Frey JU. Properties of subsequent induction of long-term potentiation and/or depression in one synaptic input in apical dendrites of hippocampal CA1 neurons in vitro. Neuroscience. 2010;171:712–720. doi: 10.1016/j.neuroscience.2010.09.018. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Ito R, Verschure PF, Battaglia FP, Robbins TW. The hippocampal striatal axis in learning, prediction and goal-directed behavior. Trends Neurosci. 2011;34:548–559. doi: 10.1016/j.tins.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Popkirov SG, Manahan-Vaughan D. Involvement of the metabotropic glutamate receptor mGluR5 in NMDA receptor-dependent, learning-facilitated long-term depression in CA1 synapses. Cereb Cortex. 2011;21:501–509. doi: 10.1093/cercor/bhq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Roggenhofer E, Fidzinski P, Bartsch J, Kurz F, Shor O, Behr J. Activation of dopamine D1/D5 receptors facilitates the induction of presynaptic long-term potentiation at hippocampal output synapses. Eur J Neurosci. 2010;32:598–605. doi: 10.1111/j.1460-9568.2010.07312.x. [DOI] [PubMed] [Google Scholar]
- Rossato JI, Bevilaqua LR, Izquierdo I, Medina JH, Cammarota M. Dopamine controls persistence of long-term memory storage. Science. 2009;325:1017–1020. doi: 10.1126/science.1172545. [DOI] [PubMed] [Google Scholar]
- Ryan MM, Ryan B, Kyrke-Smith M, Logan B, Tate WP, Abraham WC, Williams JM. Temporal profiling of gene networks associated with the late phase of long-term potentiation in vivo. PLoS One. 2012;7:e40538. doi: 10.1371/journal.pone.0040538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruusuvirta T, Korhonen T, Penttonen M, Arikoski J, Kivirikko K. Behavioral and hippocampal evoked responses in an auditory oddball situation when an unconditioned stimulus is paired with deviant tones in the cat: experiment II. Int J Psychophysiol. 1995;20:41–47. doi: 10.1016/0167-8760(95)00025-n. [DOI] [PubMed] [Google Scholar]
- Sajikumar S, Frey JU. Anisomycin inhibits the late maintenance of long-term depression in rat hippocampal slices in vitro. Neurosci Lett. 2003;338:147–150. doi: 10.1016/s0304-3940(02)01400-3. [DOI] [PubMed] [Google Scholar]
- Sajikumar S, Frey JU. Late-associativity, synaptic tagging, and the role of dopamine during LTP and LTD. Neurobiol Learn Mem. 2004;82:12–25. doi: 10.1016/j.nlm.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Vankov A, HerveÅL A. Locus coeruleus-evoked responses in behaving rats: a clue to the role of noradrenaline in memory. Brain Res Bull. 1994;35:457–465. doi: 10.1016/0361-9230(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Sarantis K, Antoniou K, Matsokis N, Angelatou F. Exposure to novel environment is characterized by an interaction of D1/NMDA receptors underlined by phosphorylation oft he NMDA and AMPA receptor subunits and activation of ERK1/2 signaling, leading to epigenetic changes and gene expression in rat hippocampus. Neurochem Int. 2012;60:57–67. doi: 10.1016/j.neuint.2011.10.018. [DOI] [PubMed] [Google Scholar]
- Scatton B, Simon H, Le Moal M, Bischoff S. Origin of dopaminergic innervation of the rat hippocampal formation. Neurosci Lett. 1980;18:125–131. doi: 10.1016/0304-3940(80)90314-6. [DOI] [PubMed] [Google Scholar]
- Schott BH, Seidenbecher CI, Fenker DB, Lauer CJ, Bunzeck N, Bernstein HG, Tischmeyer W, Gundelfinger ED, Heinze HJ, Düzel E. The dopaminergic midbrain participates in human episodic memory formation: evidence from genetic imaging. J Neurosci. 2006;26:1407–1417. doi: 10.1523/JNEUROSCI.3463-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J Neurosci. 1993;13:900–913. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci. 1998;18:1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon H, Le Moal M, Stinus L, Calas A. Anatomical relationships between the ventral mesencephalic tegmentum–a 10 region and the locus coeruleus as demonstrated by anterograde and retrograde tracing techniques. J Neural Transm. 1979;44:77–86. doi: 10.1007/BF01252703. [DOI] [PubMed] [Google Scholar]
- Smith CC, Greene RW. CNS dopamine transmission mediated by noradrenergic innervation. J Neurosci. 2012;32:6072–6080. doi: 10.1523/JNEUROSCI.6486-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WB, Starck SR, Roberts RW, Schuman EM. Dopaminergic stimulation of local protein synthesis enhances surface expression of GluR1 and synaptic transmission in hippocampal neurons. Neuron. 2005;45:765–779. doi: 10.1016/j.neuron.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Staubli U, Lynch G. Stable depression of potentiated synaptic responses in the hippocampus with 1–5 Hz stimulation. Brain Res. 1990;513:113–118. doi: 10.1016/0006-8993(90)91096-y. [DOI] [PubMed] [Google Scholar]
- Stramiello M, Wagner JJ. D1/D5 receptor-mediated enhancement of LTP requires PKA, Src family kinases, and NR2B-containing NMDARs. Neuropharmacology. 2008;55:871–877. doi: 10.1016/j.neuropharm.2008.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Dolan RJ. Adaptive anterior hippocampal responses to oddball stimuli. Hippocampus. 2001;11:690–698. doi: 10.1002/hipo.1084. [DOI] [PubMed] [Google Scholar]
- Straube T, Korz V, Frey JU. Bidirectional modulation of long-term potentiation by novelty-exploration in rat dentate gyrus. Neurosci Lett. 2003;344:5–8. doi: 10.1016/s0304-3940(03)00349-5. [DOI] [PubMed] [Google Scholar]
- Suzuki WA. Making new memories: the role of the hippocampus in new associative learning. Ann N Y Acad Sci. 2007;1097:1–11. doi: 10.1196/annals.1379.007. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Hartman BK. The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine-beta-hydroxylase as a marker. J Comp Neurol. 1975;163:467–505. doi: 10.1002/cne.901630406. [DOI] [PubMed] [Google Scholar]
- Swanson-Park JL, Coussens CM, Mason-Parker SE, Raymond CR, Hargreaves EL, Dragunow M, Cohen AS, Abraham WC. A double dissociation within the hippocampus of dopamine D1/D5 receptor and beta-adrenergic receptor contributions to the persistence of long-term potentiation. Neuroscience. 1999;92:485–497. doi: 10.1016/s0306-4522(99)00010-x. [DOI] [PubMed] [Google Scholar]
- Tiberi M, Jarvie KR, Silvia C, Falardeau P, Gingrich JA, Godinot N, Bertrand L, Yang-Feng TL, Fremeau RT, Jr, Caron MG. Cloning, molecular characterization, and chromosomal assignment of a gene encoding a second D1 dopamine receptor subtype: differential expression pattern in rat brain compared with the D1A receptor. Proc Natl Acad Sci U S A. 1991;88:7491–7495. doi: 10.1073/pnas.88.17.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran AH, Uwano T, Kimura T, Hori E, Katsuki M, Nishijo H, Ono T. Dopamine D1 receptor modulates hippocampal representation plasticity to spatial novelty. J Neurosci. 2008;28:13390–13400. doi: 10.1523/JNEUROSCI.2680-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ, Craik FE, Habib R, Houle S. Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cereb Cortex. 1996;6:71–79. doi: 10.1093/cercor/6.1.71. [DOI] [PubMed] [Google Scholar]
- Undieh AS. Pharmacology of signaling induced by dopamine D(1)-like receptor activation. Pharmacol Ther. 2010;128:37–60. doi: 10.1016/j.pharmthera.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA. Dopamine: the salient issue. Trends Neurosci. 2004;27:702–706. doi: 10.1016/j.tins.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Vallone D, Picetti R, Borrelli E. Structure and function of dopamine receptors. Neurosci Biobehav Rev. 2000;24:125–132. doi: 10.1016/s0149-7634(99)00063-9. [DOI] [PubMed] [Google Scholar]
- Vankov A, Hervé-Minvielle A, Sara SJ. Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. Eur J Neurosci. 1995;7:1180–1187. doi: 10.1111/j.1460-9568.1995.tb01108.x. [DOI] [PubMed] [Google Scholar]
- Varela JA, Hirsch SJ, Chapman D, Leverich LS, Greene RW. D1/D5 modulation of synaptic NMDA receptor currents. J Neurosci. 2009;29:3109–3119. doi: 10.1523/JNEUROSCI.4746-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villers A, Godaux E, Ris L. Latephase of L-LTP elicited in isolated CA1 dendrites cannot be transferred by synaptic capture. Neuroreport. 2010;21:210–215. doi: 10.1097/WNR.0b013e328335c311. [DOI] [PubMed] [Google Scholar]
- Vinogradova OS. Hippocampus as comparator: role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus. 2001;11:578–598. doi: 10.1002/hipo.1073. [DOI] [PubMed] [Google Scholar]
- Wagner JJ, Alger BE. GABAergic and developmental influences on homosynaptic LTD and depotentiation in rat hippocampus. J Neurosci. 1995;15:1577–1586. doi: 10.1523/JNEUROSCI.15-02-01577.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SH, Redondo RL, Morris RG. Relevance of synaptic tagging and capture to the persistence of long-term potentiation and everyday spatial memory. Proc Natl Acad Sci U S A. 2010;107:19537–19542. doi: 10.1073/pnas.1008638107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss T, Veh RW, Heinemann U. Dopamine depresses cholinergic oscillatory network activity in rat hippocampus. Eur J Neurosci. 2003;18:2573–2580. doi: 10.1046/j.1460-9568.2003.02970.x. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze HJ, Düzel E. Reward-related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus-dependent long-term memory formation. Neuron. 2005;45:459–467. doi: 10.1016/j.neuron.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Xing B, Kong H, Meng X, Wei SG, Xu M, Li SB. Dopamine D1 but not D3 receptor is critical for spatial learning and related signaling in the hippocampus. Neuroscience. 2010;169:1511–1519. doi: 10.1016/j.neuroscience.2010.06.034. [DOI] [PubMed] [Google Scholar]
- Yanagihashi R, Ishikawa T. Studies on long-term potentiation of the population spike component of hippocampal field potential by the tetanic stimulation of the perforant path rats: effects of a dopamine agonist, SKF-38393. Brain Res. 1992;579:79–86. doi: 10.1016/0006-8993(92)90744-t. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Balleine BW. Reward-guided learning beyond dopamine in the nucleus accumbens: the integrative functions of cortico-basal ganglia networks. Eur J Neurosci. 2008;28:1437–1448. doi: 10.1111/j.1460-9568.2008.06422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


