Abstract
Itch is an aversive sensory experience and while systemic therapies, such as acupuncture, have shown promise in alleviating itch in patients suffering from chronic itch, their antipruritic mechanisms are unknown. As several lines of evidence implicate brain-focused mechanisms, we applied functional magnetic resonance imaging and our validated temperature-modulation itch model to evaluate the underlying brain circuitry supporting allergen-induced itch reduction in atopic dermatitis patients by acupuncture, antihistamine, and respective placebo treatments. Brain response to allergen itch demonstrated phase dependency. During an increasing itch phase, activation was localized in anterior insula and striatum, regions associated with salience/interoception and motivation processing. Once itch reached peak plateau, robust activation was noted in prefrontal cognitive and premotor areas. Acupuncture reduced itch and itch-evoked activation in the insula, putamen, and premotor and prefrontal cortical areas. Neither itch sensation nor itch-evoked brain response was altered following antihistamine or placebo acupuncture. Greater itch reduction following acupuncture was associated with greater reduction in putamen response, a region implicated in motivation and habitual behavior underlying the urge to scratch, specifically implicating this region in acupuncture's antipruritic effects. Understanding brain circuitry underlying itch reduction following acupuncture and related neuromodulatory therapies will significantly impact the development and applicability of novel therapies to reduce an itch.
Keywords: atopic dermatitis, counter irritation, counter stimulus, insula, itch, pruritus, putamen
Introduction
The sensation of itch is defined as a complex and unpleasant sensory experience that induces the urge to scratch (Hafenreffer 1660). While itch has evolutionarily protective functions (e.g. insect localization on the skin), chronic itch is a prevalent and highly debilitating symptom of many inflammatory skin disorders and allergies (Boguniewicz 2005). Placebo-controlled studies have shown that acupuncture can reduce itch in healthy adults (Belgrade et al. 1984; Lundeberg et al. 1987; Pfab et al. 2005) and chronic itch patients (Pfab, Huss-Marp, et al. 2010; Pfab, Valet, et al. 2010; Pfab, Kirchner, et al. 2012), potentially with greater efficacy (Pfab, Kirchner, et al. 2012) than antihistamines, the first-line preventive systemic therapy (Ikoma et al. 2006). Additionally, while central nervous system mechanisms may predominate for systemic, as opposed to topical, therapies, and recent neuroimaging studies have begun to unravel the brain circuitries mediating itch perception in humans (Pfab, Valet M, et al. 2012), the mechanisms by which therapeutic interventions such as acupuncture and antihistamines modulate these brain circuitries are not known.
Pruriceptive itch is commonly perceived when inflammatory mediators activate a specific subset of peripheral sensory nerve endings, which relay afference via the spinothalamic tract to the brain (Davidson et al. 2007). Neuroimaging studies, which have mainly focused on histamine-induced itch in healthy humans, have implicated a diffuse network of brain areas (Pfab, Valet M, et al. 2012) mediating somatosensory, cognitive/attention, affective/motivation, and salience/interoceptive components of the multidimensional itch sensation. Interestingly, compared with histamine itch, an itch produced by mediators better approximating chronic itch (e.g. cowhage) is stronger and induces greater activation of insula, thalamus, and putamen (Papoiu et al. 2012). Additionally, chronic itch patients with conditions such as atopic dermatitis (AD) demonstrate central sensitization for itch (Ikoma et al. 2004), wherein nociceptive stimuli of various modalities produce itch, not pain sensation. Moreover, AD itch is also known to be produced by specific allergens (Koblenzer 1999). A previous neuroimaging study applying allergen itch in patients sensitized to this allergen noted robust activation of striatum, thalamus, and ventral prefrontal cortices (Leknes et al. 2007). Interestingly, the striatum plays a critical integrative role in striato-thalamo-cortical circuits implicated in motivational processing and found to be dysregulated in generalized urge suppression pathology (Koob and Volkow 2010) such as obsessive/compulsive disorder, obesity, and addiction. In chronic itch, striato-thalamo-cortical circuits likely support the urge to scratch (Leknes et al. 2007). Additionally, itch perception includes temporally distinct phases including increasing itch sensation versus steady-state itch (Pfab et al. 2006; Pfab, Huss-Marp, et al. 2010; Pfab, Valet, et al. 2010). Ultimately, striato-thalamo-cortical involvement in these distinct phases of itch perception, and its role in supporting different antipruritic therapies is unknown.
In this study, we applied functional magnetic resonance imaging (fMRI) and our validated temperature-modulation itch model (Pfab et al. 2006) to evaluate the underlying brain circuitry supporting allergen-itch reduction in chronic itch (AD) patients by acupuncture, antihistamine, and respective placebo treatments. Different phases of itch experience were evaluated. Based on our previous behavioral study (Pfab, Kirchner, et al. 2012), we hypothesized that acupuncture will more readily down-regulate the itch, and via specific modulation of the striato-thalamo-cortical circuits thought to underlie the urge to scratch.
Materials and Methods
Subjects
Fourteen (14) subjects with a clinical diagnosis of AD were enrolled in the study. Inclusion criteria included 1) male or female adults aged 18–60, 2) AD diagnosis with a SCORAD (SCORing AD; European Task Force on Atopic Dermatitis 1993) score >18, 3) type-I sensitivity to grass pollen, birch pollen, and/or Dermatophagoides pteronyssinus or Dermatophagoides farinae with wheal and flare formation upon skin prick testing. Patients had to stop all immunosuppressive medications at least 10 days prior to the study to avoid potential suppression of itch perception. Patients had no prior experience with acupuncture. All patients gave informed consent and the protocol was approved by the Human Research Committee of Massachusetts General Hospital.
Patients were recruited by print and email advertisement, as well as physician colleagues in the Department of Dermatology at MGH. Patients were screened via phone for eligibility in the study prior to initial evaluation. Patients who met eligibility criteria by phone were then invited to a training session and evaluated with a focused history, physical exam, and allergen skin prick testing to confirm eligibility by a licensed dermatologist (F.P.).
Experimental Protocol and Itch Provocation Model
AD patients were evaluated using a cross-over design, wherein every patient experienced all 4 intervention MRI sessions. The therapeutic interventions included 1) verum, or real, electroacupuncture (VAC), 2) placebo acupuncture (PAC), 3) verum antihistamine solution (VSO), and 4) placebo solution (PSO). An intervention order was counterbalanced and fMRI scan sessions with different interventions were separated by at least 1 week.
Prior to any MRI sessions, patients were evaluated with a behavior training session where the most effective itch-inducing allergen was determined, and the subjects could experience the temperature-modulated itch provocation model. During the training session, patients also completed several questionnaires including an expectancy VAS questionnaire, within which expected itch intensity without any therapy could be contrasted (nonparametric Wilcoxon signed-ranks test) with the itch expected following both acupuncture and antihistamine therapies.
During each MRI visit (Fig. 1), brain response to itch was evaluated at baseline (itch fMRI scan 1) and following 1 of the 4 therapeutic interventions (itch fMRI scan 2). Each itch fMRI scan included a skin prick test performed just prior to imaging (see below). Following each itch fMRI scan, a numerical rating scale from 0 (no itch) to 100 (most intense itch imaginable) was used to rate the intensity of itch experienced separately during warm and cool temperature-modulation blocks. As in our previous studies (Pfab et al. 2005, 2006; Valet et al. 2008; Pfab, Huss-Marp, et al. 2010; Pfab, Valet, et al. 2010; Pfab et al. 2011), subjects were instructed that a rating of 33 corresponds to an “urge to scratch” threshold. Above this threshold, each individual feels the clear-cut desire to scratch, which, however, was not permitted (and confirmed by observation). Prior to any itch fMRI scan, a simple temperature-modulation fMRI scan was also performed as a control for the itch fMRI scans. This scan did not involve any allergen skin prick testing and controlled for any brain response to simply cooling and warming the skin. Following this control scan, subjects also rated any itch experienced during cool and warm blocks.
Figure 1.
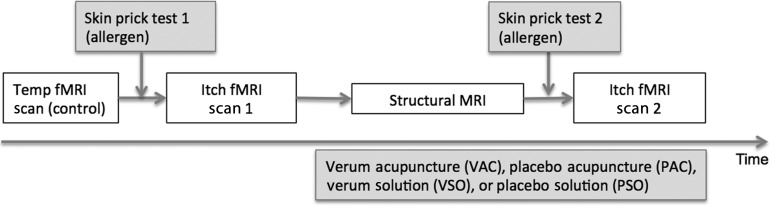
Schematic of scan session. Subjects experienced a temperature-modulated itch fMRI scan before (itch fMRI scan 1) and after (itch fMRI scan 2) 1 of the 4 different therapeutic interventions involving acupuncture or antihistamine solution (VAC, PAC, VSO, or PSO). Subjects also experienced a control fMRI scan where temperature was modulated, but without a preceding skin prick test. The therapeutic intervention extended into itch fMRI scan 2 for all interventions.
The temperature-modulated itch provocation model has been previously validated (Pfab et al. 2006) and applied during fMRI (Valet et al. 2008; Pfab, Huss-Marp, et al. 2010; Pfab, Valet, et al. 2010). Briefly, a nonlesion site on the left volar forearm was chosen for itch induction. A single drop of allergen was applied, followed by a superficial puncture of the skin with a plastic MR-compatible lancet (Duotip Test II, Lincoln Diagnostics, Decatur, IL, USA). The allergens used for itch provocation were individually determined to be most pruritogenic from the behavior testing session and included Timothy grass pollen (100 000 BAU/mL), D. pteronyssinus or European house dust mite (10 000 AU/mL), and D. farinae or American house dust mite (10 000 AU/mL, Allergy Laboratories, Oklahoma City, OK, USA). The skin puncture results in a deposition of allergen solution at the dermal–epidermal junction, where the terminals of itch-related C-fibers are located (Shelley and Arthur 1957). After a median latency of 35s, an itch sensation develops with peak itch intensity approximately 120s after application (Pfab, Huss-Marp, et al. 2010; Pfab, Valet, et al. 2010). Sustained pain is avoided by the delicate and shallow puncture, as previously reported (Pfab et al. 2006), thus the stimulus can be considered a pure itch, and not combined pain/itch, sensation. The drop of allergen solution was carefully removed with an absorbent gauze pad 120s after application. A 30 × 30-mm sized MRI-compatible thermode probe (TSA II NeuroSensory Analyzer thermode, Medoc Advanced Medical Systems, Rimat Yishai, Israel) was then placed over the allergen treated skin area. During the block-design itch fMRI scans (itch fMRI scans 1 and 2), skin temperature was modulated over successive cooling and warming blocks. Starting from a neutral skin temperature of 32°C, 16 equal duration (20s) blocks, alternating between 32°C (warm) and 25°C (cool), were applied. A transit time of 1.5s (ramp 5°C/s) was used between warm and cool blocks, and modeled accordingly in the fMRI general linear model (GLM, see below). Stimulation during the fMRI scan (see below for detail) included 8 cool/warm blocks over a 6-min scan run. This procedure has reliably been used to modulate itch, such that cooling the skin increases itch sensation (Pfab et al. 2006).
Therapeutic Interventions—VAC, PAC, VSO, PSO
The 4 MRI sessions included 1 of the 4 different therapeutic interventions: VAC, PAC, VSO, or PSO. MRI sessions were counterbalanced in order and separated in time by at least 1 week.
During each MRI session, approximately 30min elapsed between cessation of itch fMRI scan 1 and initiation of itch fMRI scan 2. During this time, all interventions were performed. This duration of time allowed for itch sensation experienced during baseline testing to wane, while also allowing for antihistamine pharmacotherapy to reach peak plasma concentration, at approximately 0.5h (Teva Pharmaceuticals Ltd., Sellersville, PA, United States of America). A solution was used instead of a tablet as the latter has been reported to reach mean peak plasma concentration in a much longer duration of 1.2h (Simons et al. 2005). Both VAC and PAC were initiated 15min before itch fMRI scan 2, as acupuncture has delayed effects in terms of both brain response (Napadow et al. 2009) and clinical efficacy. A similar 15-min prestimulation period was successfully used in a prior study investigating acupuncture alleviation of itch (Pfab et al. 2005). All interventions (VAC, PAC, VSO, and PSO) were “active” during itch fMRI scan 2, that is, subjects were experiencing electrostimulation, and antihistamine effects, during this temperature-modulated itch fMRI scan run.
VAC was performed for all subjects by the same investigator (V.N.), who has more than 10 years of clinical experience, and is very experienced in performing acupuncture in the fMRI setting. Electroacupuncture stimulation was performed between acupoints LI-11 (Quchi) and HT-3 (Shaohai), located around the right elbow. Specifically, LI-11 is located on the elbow at the midpoint of the line joining the lateral end of the transverse cubital crease and the lateral epicondyle of the humerus. This acupoint has been noted in clinical texts to have antipruritic effects and has been used in our previous studies (Pfab et al. 2005; Pfab, Huss-Marp, et al. 2010; Pfab, Valet, et al. 2010; Pfab et al. 2011; Pfab, Kirchner, et al. 2012) to evaluate acupuncture efficacy in itch reduction. HT-3 is located between the ulnar end of the cubital crease and the medial epicondyle of the humerus. Specially manufactured, pure titanium acupuncture needles (0.20 × 30 mm, DongBang Acupuncture, Inc., Boryeong, Korea) were used, as they are stiff, nonmagnetic, and MR-compatible. Needles were inserted at the above locations using plastic guidetubes. Electrical current was delivered with a modified current-constant HANS (Han's Acupoint Nerve Stimulator) LH202 (Neuroscience Research Center, Peking University, Beijing, China) device. Stimulus frequency was 100 Hz, with a bipolar pulse width of 200 µs. Stimulus intensity was adjusted until the perceived sensation was moderately strong but not painful. The current intensity used for VAC was 1.4 ± 0.8 mA (µ ± σ).
PAC was designed to mimic the ritual of VAC, but without any stimulation of real acupoints, or electrostimulation. We also used MR-compatible needles (pure-silver, nonferrous acupuncture needles, 0.25 mm diameter, 40 mm length, Maeda Corporation, Japan), modified to have blunt tips that would not penetrate skin. Nonacupoint skin locations were stimulated by sham insertions through plastic guidetubes, identical to VAC. The nonacupoints, SH-1 and SH-2, were located on the ulnar aspect of the forearm. Similar to VAC, electrodes were placed at the stimulation locations, but were not connected to an electrostimulation source. Subjects, whose heads were inside the MRI head coil, could not see the VAC or PAC procedures performed at their periphery. The consent form instructed subjects that they would receive “different forms of active or inactive acupuncture,” and that “stimulation may range from barely perceptible to moderately strong but not painful.” While these procedures allowed the subjects to be blinded as to verum or placebo intervention, our study was considered single blinded. A double-blinding procedure in electroacupuncture research has not been developed and, thus, the experimenter was not blinded.
For the oral VSO scan session, we used a common 3rd generation antihistamine, levocetirizine (Xyzal, 5 mg, Teva Pharmaceuticals Ltd., Sellersville, PA, United States of America). Following itch fMRI scan 1, subjects were removed from the MRI scanner bore, allowed to sit up, and asked to drink 10 mL of levocetirizine solution in a generic paper cup. Once subjects ingested the solution, they were placed back into the scanner. Procedures for the PSO MRI session were identical to the VSO session; however, subjects received 10 mL of sweetened water solution, which was both color- and flavor-matched to the VSO solution (Doc Johnson Enterprises, Inc., North Hollywood, CA, United States of America).
Psychophysical Analyses
Itch severity was rated for warm and cool blocks following the temperature-only control fMRI scan, itch fMRI scans 1 and 2. Itch intensities for the cool temperature block (more severe itch) were compared between the temperature-only control scan and itch fMRI scan 1 using a nonparametric Wilcoxon signed-ranks test, as itch rated during temperature-only scanning was not normally distributed (Shapiro-Wilk statistic (df = 14) = 0.854, P < 0.05, SPSS 18.0, Armonk, NY, USA). To test treatment effects, itch intensities for the cool temperature block were evaluated using a repeated-measures analysis of variance (ANOVA) model with a between-subjects factor THERAPY (levels: VAC, PAC, VSO, and PSO) and a within-subjects repeated-measures factor TIME (levels: Baseline and post-therapy). Post hoc testing comparing itch fMRI scan 1 versus 2 (the intervention effect) for each different intervention was also performed using Wilcoxon signed-ranks tests due to previously noted non-normality. Spearman's correlation analysis was used to evaluate any relationship between clinical severity (using the SCORAD scale) and itch ratings for the cool temperature block. As a secondary measure of treatment effects, we also evaluated how the residual itch from the first skin prick test decreased pre- versus postintervention, before the second skin prick test was administered. A repeated-measures 1-way ANOVA was performed for the VAS change score for each therapy (VAC, PAC, VSO, and PSO). Post hoc testing using the nonparametric Wilcoxon signed-ranks test compared the different interventions. The significance level for all tests was set at alpha = 0.05.
At the end of both the VAC and PAC scan session, subjects rated the intensity of acupuncture-induced sensations using the MGH Acupuncture Sensation Scale (MASS; Kong et al. 2007). The MASS index (MI), which is a summary metric that weights both the breadth and depth of sensations evoked by acupuncture, was calculated from the response data.
Following scanning, at the end of each session, patients also completed the d2 test of attention, as our recent studies have suggested that antihistamines may effect attentional processing differently from acupuncture (Pfab, Kirchner, et al. 2012). This instrument is considered to be the standard for measuring concentration speed and attention in both clinical and experimental settings (Brickenkamp and Zillmer. 1998). It was performed after all intervention procedures were completed.
MRI Scanning Protocol
Imaging was performed at 3T on a Siemens Trio MRI scanner (Siemens AG, Erlangen, Germany). fMRI data were collected with a gradient echo T2*-weighted pulse sequence [time repetition (TR)/time echo (TE) = 2 s/30 ms, 32 anterior-posterior commissure aligned slices, slice thickness = 3.6 mm, matrix = 64 × 64, field of view (FOV) = 200 mm, flip angle (FA) = 90°] and a 32-channel multiarray coil. All fMRI scans included 8 cool/warm blocks and a total of 180 timepoints over a 6-min scan run. High-resolution T1-weighted structural imaging was completed with an isotropic multiecho MPRAGE pulse sequence (TR/TE1/time to inversion = 2530/1.64/1200 mS, matrix = 256 × 256, FOV = 256 mm, FA = 7°).
fMRI Data Analysis
BOLD images were preprocessed using the FMRIB Software Library (FSL) and tools available through the FreeSurfer software package (Dale et al. 1999; Fischl et al. 1999). Data were skull stripped (brain extraction tool, Smith 2002), slice-timing corrected, motion-corrected (MCFLIRT) (Jenkinson et al. 2002), and spatially smoothed with a full-width at half-maximum 5-mm Gaussian kernel (Smith et al. 2004). Subject fMRI data were excluded if gross translational motion exceeded 3 mm on any axis, or if discrete motion spikes exceeded 1.5 mm. Data were high-pass filtered (fhigh = 0.017 Hz) and analyzed with a GLM.
For itch fMRI scans, which included 8 cool/warm blocks, 3 regressors were used. These regressors were based on the stereotyped dynamics of temperature-modulated itch sensation, as demonstrated in our previous studies using the identical model and continuous itch ratings (Pfab et al. 2006; Pfab, Huss-Marp, et al. 2010; Pfab, Valet, et al. 2010; Pfab, Kirchner, et al. 2012). The first regressor covered the first half of the cool temperature stimulus blocks and was aimed at capturing increasing itch sensation. The second regressor covered the second half of the cool temperature blocks, when itch sensation is known to plateau to its peak level. Finally, a regressor of no-interest was included that covered the first 12s of the warm blocks, when itch sensation is known to be decreasing. Hence, the baseline for all regressors of interest was the latter portion of warm blocks, when itch sensation had leveled off to its nadir. This analysis was performed on data acquired during the temperature control scan.
Parameter estimates and their variances from the subject level GLM were then passed up to group level by transformation to a standard space. Cortical surface reconstruction was performed using FreeSurfer to improve structural–functional coregistration through boundary-based registration (Greve and Fischl 2009). Functional data were then registered to standard Montreal Neurological Institute (MNI) space using FMRIB's Non-linear Image Registration Tool (FNIRT, FSL).
Brain response to itch was evaluated by first combining results for itch fMRI scan 1 from all 4 MRI sessions within-subjects, using a fixed effects model. A mixed effects model was then used between subjects to contrast brain response between temperature-modulated itch and brain response to temperature modulation alone—the control condition (FMRI Expert Analysis Tool, FEAT, FSL). Results for the increasing itch (INCR) and peak itch (PEAK) regressors were cluster corrected for multiple comparisons (z > 2.3, P < 0.05), and peristimulus plots from significant clusters were created to visualize the temporal evolution of fMRI signal response to temperature-modulated itch.
To investigate the influence of different therapeutic interventions on brain response to itch, we calculated difference maps between itch fMRI scans 1 and 2. These maps were calculated for each intervention using a paired t-test mixed effects model, also cluster corrected for multiple comparisons (z > 2.3, P < 0.05). In order to confirm that brain areas reported when contrasting itch fMRI scans 1 and 2 for VAC were also significantly different between VAC and PAC, we used an ANOVA to calculate the interaction VAC (itch2–itch1)–PAC (itch2–itch1). This analysis was performed using a whole-brain approach, cluster corrected for multiple comparisons (z > 2.3, P < 0.05), as well as a more focused regions of interest (ROI)-based approach guided by anatomical labels from the Harvard-Oxford Atlas corresponding to the regions reported when contrasting itch fMRI scans 1 and 2 for VAC (see Results, Table 1). Significant clusters for the ROI analysis were determined using an uncorrected threshold of P < 0.01 and a minimum cluster size of 50 mm3.
Table 1.
Phase-variant brain response to allergen itch in AD patients
| Side | Size (mm3) | Location (MNI) |
Z-score | fMRI %-Change |
|||||
|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||||
| Cool + itch | Cool only | ||||||||
| Increasing itch sensation | |||||||||
| Insula | R | 25 936 | 30 | 18 | 2 | 4.19 | 0.14 ± 0.09 | 0.07 ± 0.21 | |
| aMCC | R | 25 936 | 8 | 34 | 32 | 2.73 | 0.19 ± 0.24 | 0.08 ± 0.53 | |
| Putamen | R | 25 936 | 24 | 18 | 4 | 4.04 | 0.15 ± 0.11 | 0.02 ± 0.26 | |
| L | 7872 | −22 | 10 | 10 | 3.24 | 0.13 ± 0.11 | −0.01 ± 0.18 | ||
| Caudate | R | 25 936 | 20 | 18 | 8 | 3.65 | 0.12 ± 0.10 | 0.03 ± 0.24 | |
| L | 7872 | −20 | 10 | 16 | 3.40 | 0.12 ± 0.11 | 0.02 ± 0.14 | ||
| Glob. pallidus | R | 25 936 | 18 | 4 | 4 | 3.39 | 0.11 ± 0.09 | 0.02 ± 0.21 | |
| vlPFC | R | 25 936 | 34 | 40 | 6 | 3.52 | 0.16 ± 0.16 | 0.07 ± 0.47 | |
| Peak plateau itch sensation | |||||||||
| dlPFC | R | 9096 | 36 | 20 | 52 | 3.87 | 0.24 ± 0.25 | 0.14 ± 0.68 | |
| Premotor | R | 9096 | 22 | −4 | 62 | 3.06 | 0.13 ± 0.12 | −0.04 ± 0.36 | |
| L | 4960 | −20 | −20 | 62 | 3.55 | 0.10 ± 0.10 | 0.04 ± 0.23 | ||
| SPL | L | 4960 | −28 | −44 | 64 | 2.74 | 0.29 ± 0.31 | 0.09 ± 0.48 | |
We also evaluated the correlation between the change in brain response to itch (both INCR and PEAK) and the change in itch ratings. This mixed effects linear regression analysis (FEAT, FSL) was also cluster corrected for multiple comparisons (z > 2.3, P < 0.05). Correlation analyses were used to evaluate any relationship between clinical severity (using the SCORAD scale) and 1) significant brain responses to allergen itch and 2) significant changes in brain response following therapy.
Results
Fourteen patients (age: 25.4 ± 9.1 years old, SCORAD: 38.7 ± 14.9, mean ± standard deviation, except where noted) were enrolled in the study. All but 1 patient were right handed (Edinburgh Inventory; Oldfield 1971). The allergens used to elicit itch were concentrated solutions of grass pollen (N = 8), D. pteronyssinus (European house dust mite, N = 3), or D. farinae (American house dust mite, N = 3).
Psychophysics of Itch and Acupuncture
Itch was elicited by allergen prick testing and was significantly greater during cool blocks (59.3 ± 13.5) compared with warm blocks (32.2 ± 14.1, P < 0.001). Itch sensation elicited by allergen prick testing was also greater than itch perceived during the temperature-only control fMRI scan (cool: 14.4 ± 16.2; warm: 4.1 ± 9.9; both P < 0.001). No significant correlation (P > 0.1) was found between allergen-itch intensity and clinical severity (SCORAD score).
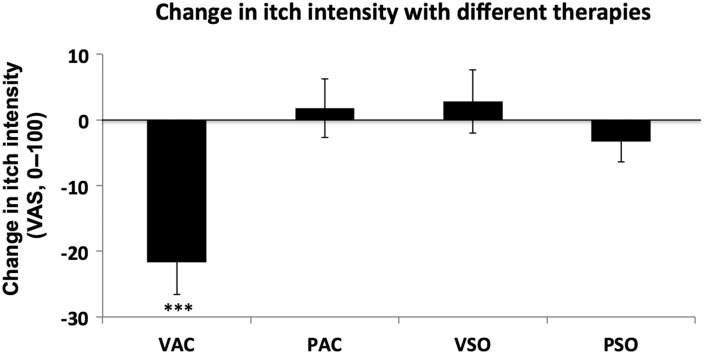
Treatment effects for itch during cool blocks (most severe itch) were evaluated using a repeated-measures ANOVA, which found a significant effect for TIME (F1,55 = 5.34, P = 0.025) and TIME × THERAPY interaction (F1,55 = 6.64, P < 0.001). Post hoc testing demonstrated that itch sensation was significantly (P = 0.004) decreased following VAC during cool temperature blocks (−21.6 ± 18.6; Fig. 2). There was no significant change following PAC, VSO, or PSO during cool temperature blocks (PAC: 1.8 ± 16.7; VSO: 2.8 ± 18.1; PSO: −3.3 ± 11.6). Expectancy for itch relief, assessed prior to fMRI, demonstrated that subjects expected greater relief following antihistamine (−35.8 ± 15.4) compared with acupuncture (−23.8 ± 14.9, P = 0.006). There was no correlation between expectancy of relief and actual itch relief (cool block ratings), for any intervention. There were also no correlations between changes in allergen-itch intensity and clinical severity (SCORAD score).
Figure 2.
Following VAC, itch intensity rated for cool blocks (itch fMRI scan 2–itch fMRI scan 1) was significantly diminished. Other therapies (PAC, VSO, and PSO) did not appreciably change itch intensity. ***P < 0.005. VAC, verum acupuncture; PAC, placebo acupuncture; VSO, verum antihistamine solution; PSO, placebo antihistamine solution.
A secondary measure of treatment effects evaluated how the residual itch from the first skin prick test decreased pre- versus postintervention, before the second skin prick test was administered. A repeated-measures 1-way ANOVA found a significant effect for THERAPY (F3,53 = 4.54, P = 0.008). Post hoc testing demonstrated that residual itch sensation from the first skin prick test was significantly more reduced following VAC (−25.1 ± 12.8) compared with PAC (−13.4± 16.7, P = 0.02), VSO (−7.4 ± 18.6, P = 0.01), or PSO (−10.6 ± 15.8, P = 0.003).
VAC was associated with greater acupuncture sensation intensity (MI: 5.5 ± 1.9), compared with PAC (MI: 1.4 ± 1.4, P < 0.001). The strongest sensations reported for VAC were tingling (6.3 ± 2.5, on a scale of 0–10) and deep pressure (3.5 ± 2.4), while for PAC they were tingling (1.3 ± 1.5) and warmth (1.0 ± 1.3).
Mean attention scores (correct hits minus errors) were assessed at the end of all sessions (∼30–45min after therapeutic interventions) with the d2 test of attention. We found no significant difference between interventions (VAC: 193.6 ± 29.3; PAC: 192.6 ± 35.5; VSO: 188.3 ± 23.9; PSO: 188.8 ± 30.1; 1-way ANOVA, P > 0.1).
fMRI Results
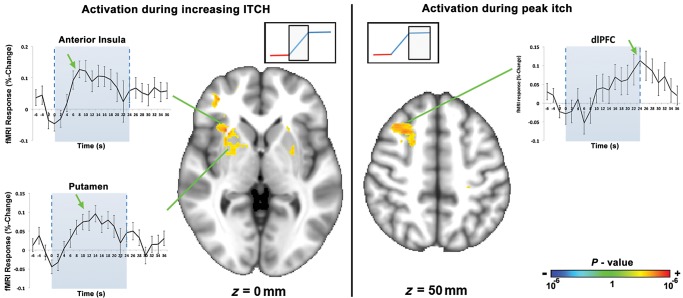
fMRI data were analyzed with separate regressors for the first half of the cool temperature block (when, from our previous studies, itch is known to be increasing; Pfab, Huss-Marp, et al. 2010; Pfab, Valet, et al. 2010) and for the second half of the cool temperature block (when itch is known to have reached a peak plateau; Pfab, Huss-Marp, et al. 2010; Pfab, Valet, et al. 2010). A separate scan, during which temperature was modulated but no skin prick testing was done to induce itch, was used as a control. We found that brain activation during increasing itch differed from the activation noted during peak itch (Fig. 3, Table 1). Controlling for brain response to temperature change, we found that increasing itch sensation was associated with activation in the right anterior insula, anterior middle cingulate cortex (aMCC), bilateral putamen and caudate, globus pallidus, and right ventrolateral prefrontal cortex (vlPFC). In contrast, peak itch sensation was associated with activation in the right dorsolateral prefrontal cortex (dlPFC), bilateral premotor, and left superior parietal lobule (SPL). fMRI timeseries extracted from regions such as right anterior insula and putamen, which were activated during increasing itch, clearly peaked prior to the dlPFC, which was activated during peak itch (Fig. 3). No regions demonstrated significant deactivation (decreased fMRI signal) in response to either increasing or peak itch. There were no correlations between brain response to itch and clinical severity (SCORAD score).
Figure 3.
Brain response to itch was phase dependent. During the first half of the cool temperature block, when itch is known to be increasing (Pfab, Huss-Marp, et al. 2010; Pfab, Valet, et al. 2010), activation was noted in several regions including the anterior insula, putamen, and vlPFC. During the second half of the cool temperature block, when itch is known to have reached its peak plateau, activation was noted in the dlPFC. dlPFC, dorsolateral prefrontal cortex.
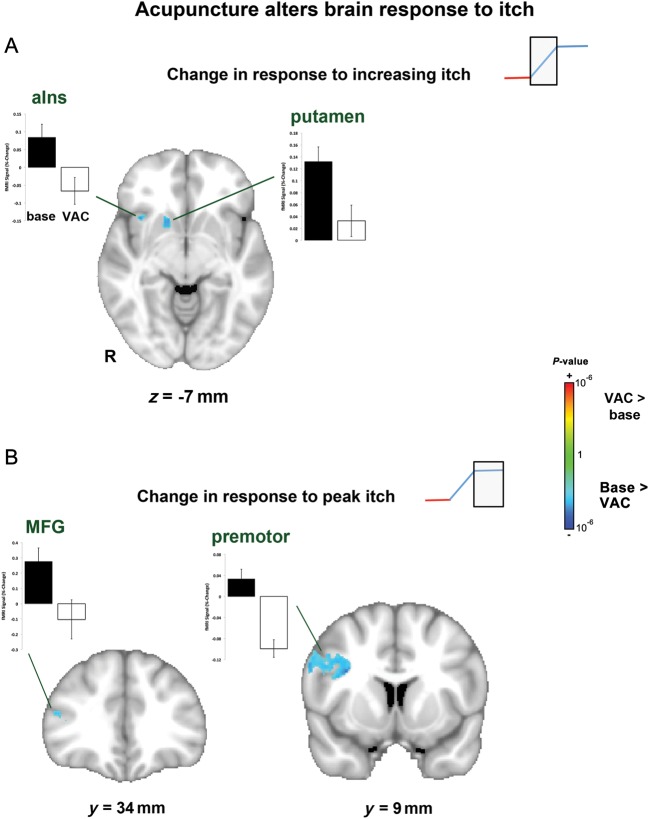
In concordance with diminished itch intensity following VAC, we also noted reduced brain activation, during both the increasing itch phase and peak plateau itch phase (Fig. 4, Table 2). During increasing itch, there was reduced activation in the right anterior insula, claustrum, putamen, globus pallidus, caudate, and nucleus accumbens. During the peak itch phase, there was reduced activation in bilateral primary somatosensory/motor cortex, right secondary somatosensory and premotor cortices, middle frontal gyrus, and cuneus, as well as left posterior cingulate cortex. There were no brain regions that demonstrated increased activation to itch following VAC. There were also no brain regions that demonstrated increased or decreased activation in response to itch sensation following PAC. A confirmatory whole-brain ANOVA analysis directly contrasts VAC with PAC by calculating the interaction: VAC (itch2–itch1)–PAC (itch2–itch1). This analysis found a significant interaction for the peak itch phase in regions showing a significant decrease following VAC: premotor cortex and middle frontal gyrus. An ROI analysis for regions previously noted to reduce itch-evoked activation following VAC noted a significant interaction in the right insula, putamen, caudate, and nucleus accumbens for an increasing itch phase and left S1/M1, right S2, premotor cortex, and middle frontal gyrus for the peak itch phase (Supplementary Table 1). While there were no changes following VSO or PSO during increasing itch, patients demonstrated greater deactivation in the precuneus and posterior cingulate cortex during the peak itch phase following PSO. There were no correlations between changes in brain response to itch and patients' clinical severity (SCORAD score).
Figure 4.
Brain response to itch was diminished following VAC. During the increasing itch phase, there was less activation in the insula and putamen. During the peak itch phase, there was less activation in prefrontal (MFG) and premotor cortices. aIns, anterior insula; MFG, middle frontal gyrus.
Table 2.
Therapy associated change in brain response to allergen itch in AD patients
| Side | Size (mm3) | Location (MNI) |
Z-score | fMRI %-Change |
|||||
|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||||
| Baseline | Post-VAC | ||||||||
| Verum acupuncture | |||||||||
| Change in response to increasing itch | |||||||||
| Anterior insula | R | 3720 | 36 | 20 | 14 | −3.42 | 0.09 ± 0.12 | −0.06 ± 0.14 | |
| Putamen | R | 3720 | 26 | 14 | 8 | −3.46 | 0.13 ± 0.09 | 0.03 ± 0.10 | |
| Glob. pallidus | R | 3720 | 12 | 4 | 2 | −3.52 | 0.11 ± 0.11 | −0.02 ± 0.15 | |
| Caudate | R | 3720 | 12 | 16 | −4 | −2.82 | 0.04 ± 0.15 | −0.15 ± 0.23 | |
| NAcc | R | 3720 | 14 | 14 | −6 | −3.07 | 0.01 ± 0.16 | −0.21 ± 0.20 | |
| Claustrum | R | 3720 | 28 | 18 | −2 | −3.17 | 0.12 ± 0.14 | −0.05 ± 0.20 | |
| Change in response to peak itch | |||||||||
| S1/M1 | R | 7312 | 50 | −2 | 54 | −3.84 | 0.15 ± 0.28 | −0.15 ± 0.25 | |
| L | 3624 | −42 | −14 | 64 | −3.13 | 0.45 ± 0.55 | −0.11 ± 0.37 | ||
| S2 | R | 5384 | 58 | −30 | 24 | −3.25 | 0.06 ± 0.29 | −0.23 ± 0.25 | |
| Premotor | R | 7312 | 36 | 0 | 32 | −4.04 | 0.03 ± 0.07 | −0.10 ± 0.06 | |
| MFG | R | 7312 | 42 | 28 | 16 | −2.89 | 0.09 ± 0.16 | −0.16 ± 0.31 | |
| PCC | L | 7744 | −10 | −48 | 8 | −3.13 | 0.07 ± 0.20 | −0.15 ± 0.23 | |
| Cuneus | R | 7744 | 22 | −66 | 12 | −3.50 | 0.13 ± 0.22 | −0.03 ± 0.15 | |
| Placebo acupuncture | |||||||||
| None | |||||||||
| Verum antihistamine solution | |||||||||
| None | |||||||||
| Placebo antihistamine solution | |||||||||
| Change in response to increasing itch | |||||||||
| None | |||||||||
| Change in response to peak itch | Baseline | post-PSO | |||||||
| PC | R | 7088 | 2 | −66 | 52 | −3.60 | 0.24 ± 0.31 | −0.35 ± 0.43 | |
| PCC | R | 7088 | 6 | −52 | 14 | −3.21 | 0.08 ± 0.19 | −0.13 ± 0.23 | |
To probe more specifically the brain circuitry underlying reduced itch in AD, we used an intersubject regression model to investigate changes in brain response to itch that correlated with reduced itch sensation. We found that diminished itch following VAC was correlated with the change in fMRI response (for the increasing itch phase) in the left putamen, and mid-insula cortex (Fig. 5, Table 3). There were no significant correlations for the peak itch phase nor for any other therapies (i.e. INCR, PEAK).
Figure 5.
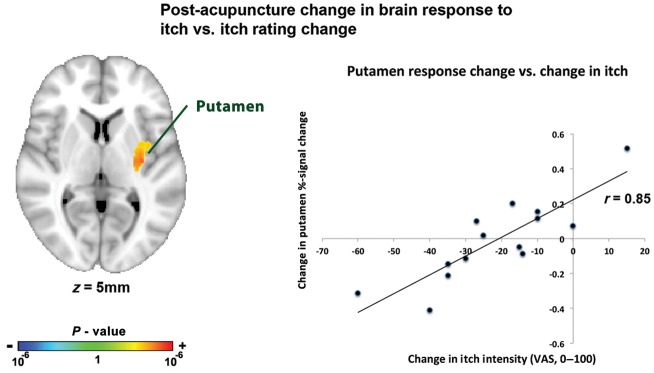
A whole-brain analysis found that following VAC, decrease in putamen response to itch (during the increasing itch phase) was correlated with reduced itch intensity following this intervention.
Table 3.
Post-VAC change in brain response versus change in itch sensation
| Side | Size (mm3) | Location (MNI) |
Z-score | |||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Δ response to increasing itch versus Δ itch sensation | ||||||
| Putamen | L | 4200 | −32 | −14 | 6 | 3.88 |
| Mid-insula | L | 4200 | −40 | −14 | 14 | 3.38 |
| Δ response to peak itch versus Δ itch sensation | ||||||
| None | ||||||
Discussion
Itch is an unpleasant, aversive sensation and is the cardinal symptom of several chronic dermatological and allergy-associated diseases, including AD. We applied our validated temperature-modulated itch model in conjunction with fMRI neuroimaging to noninvasively probe the brain circuitry supporting allergen-induced itch perception in AD patients. Brain activation to itch during the increasing itch phase was localized in the anterior insula and striatum, regions associated with salience/interoception and motivation processing. Robust activation was noted in dlPFC and premotor areas once itch reached a peak plateau. Acupuncture reduced itch and itch-evoked activation in the insula, putamen, and premotor and prefrontal cortical areas. Neither itch sensation nor itch-evoked brain response was altered following antihistamine or PAC. Greater itch reduction following acupuncture was associated with greater reduction in putamen response, a region implicated in motivation and habitual behavior underlying the urge to scratch, specifically implicating this region in acupuncture's antipruritic effects.
We found that clinically relevant itch elicited by allergen prick testing in AD patients was significantly greater during cool blocks compared with warm blocks, consistent with our previous studies (Pfab et al. 2006; Pfab, Kirchner, et al. 2012 Valet et al. 2008; Pfab, Huss-Marp, et al. 2010; Pfab, Valet, et al. 2010). Separate regressors modeled the first half of the cool block, when itch is increasing in magnitude (according to our prior studies (Pfab, Huss-Marp, et al. 2010; Pfab, Valet, et al. 2010), and the second half of the cool block, when itch has reached a peak plateau (Pfab, Huss-Marp, et al. 2010; Pfab, Valet, et al. 2010). We found that brain regions known to process salience (insula and aMCC) and affect/motivation (vlPFC, insula, and striatum) were activated during increasing itch, consistent with the onset of a highly salient and aversive stimulus. In contrast, as patients were instructed to refrain from movement, even with the urge to scratch, activation of dlPFC and premotor areas during steady-state peak itch likely relates to prefrontal cognitive control (dlPFC) over motor planning areas (premotor) in order to limit scratching-related movements. Regions such as the insula, cingulate, striatum, and lateral prefrontal cortices have all been previously implicated as components of the brain circuitry supporting itch perception (Leknes et al. 2007; Pfab, Valet M, et al. 2012; Ishiuji et al. 2009). While previous itch neuroimaging studies have not differentiated an increasing itch from a peak plateau itch phase, previous pain studies have reported that the brain circuitry supporting “increasing” pain differs from the circuitry supporting pain that has reached a peak plateau (Baliki et al. 2006). Itch has similarities to pain (Davidson and Giesler 2010), but differs in that it induces the urge to scratch. Thus, while Baliki et al. (2006) found that right anterior insula and MCC were activated during increasing pain, and prefrontal cortex was activated during a high pain plateau, the authors did not find putamen activation during increasing pain nor premotor activation at high pain plateau. While this null result could be due to thresholding, it does support the hypothesis that putamen activity likely mediates increasing itch, while premotor activation at high itch plateau likely supports motor planning underlying the urge to scratch.
The putamen, activated by increasing itch, is a critical component of the striato-thalamo-cortical circuitry implicated in motivational processing, habitual behavior, and action initiation (Graybiel 2008). Scratching is known to alleviate itch (Yosipovitch et al. 2007; Kosteletzky et al. 2009; Vierow et al. 2009) and putamen activation is greater when scratching is experienced during itch, than outside this itch-scratch context (Vierow et al. 2009). In fact, Vierow et al. report that putamen activity during itch preceded scratching, suggesting that this brain region also codes the urge to scratch (Vierow et al. 2009). Our study found that fMRI signal in the putamen increases during increasing itch. Following acupuncture, itch sensation was reduced and putamen response to evoked itch sensation was also reduced. Moreover, reduced putamen activation was found to correlate with reduced itch following acupuncture. Specifically, patients with greatest reduction in itch actually responded with putamen deactivation to evoked itch, consistent with a hypothesis that reduced itch resulted from an inhibitory influence on this brain region.
Interestingly, electroacupuncture is known to activate the putamen (Napadow et al. 2005), and in our study, electroacupuncture activated putamen bilaterally (Supplementary material). As acupuncture was applied concurrent to post-VAC itch stimuli, active putamen recruitment by tonic acupuncture stimulation may have limited further itch-locked phasic activation. This relationship may be mediated by dopamine, as increased tonic dopamine levels in the striatum are known to inhibit subsequent phasic dopaminergic activation (Wood 2006). As the putamen has also been implicated in salience detection, particularly for aversive stimuli such as pain (Downar et al. 2003), diminished phasic activation to experimental itch may lead to a reappraisal of perceived itch as less salient and demanding of behavioral (scratch) response. This mechanism may be similar to other counter stimuli, such as scratching, which also reduces itch and activates putamen (Vierow et al. 2009). However, while peripheral mechanisms for counter stimuli may also exist, in our study, acupuncture was applied on the arm “opposite” to the itch induction site (in contrast to most scratching studies), suggesting that the antipruritic effects of acupuncture are indeed mediated by the central nervous system.
Reduced itch following acupuncture was accompanied by altered itch-evoked response in other brain areas as well. The right anterior insula, which was activated during the increasing itch phase, showed reduced activity during this phase following acupuncture. Moreover, reduced itch sensation was correlated with reduced insula activation, suggesting a stronger coupling between acupuncture-mediated itch reduction and underlying brain activity. Previous studies have also noted that the insula is commonly activated by itch (Pfab, Valet M, et al. 2012), and that increasing experimentally induced itch intensity is correlated with increasing insula activation (Ishiuji et al. 2009). This region is also commonly activated by other aversive and broadly defined interoceptive sensations such as pain (Craig 2003; Apkarian et al. 2005) and nausea (Napadow et al. 2013). Insula is also commonly activated by electroacupuncture (Napadow et al. 2005) and was noted in our AD patients as well (Supplementary material). Robust anterior insula activation to itch may be associated with its high degree of salience, and reduced itch-evoked insula activity further supports the contention that acupuncture reduced the salience, as well as the magnitude of perceived itch.
For the peak itch phase, somatosensory, premotor, and prefrontal cortical activation was also reduced following acupuncture. Acupuncture, which was performed on the right arm, may have reduced right (ipsilateral) premotor and somatosensory activation due to active interhemispheric inhibition from left (contralateral) S1, a known cross-hemispheric interaction between sensorimotor-related regions (Ni et al. 2009). However, reduced premotor and prefrontal activities during the peak itch phase may have also resulted from diminished activation in salience and affective/motivation regions during the preceding increasing itch phase. This latter contention is supported by the fact that reduced itch was better correlated with reduced putamen and insula activation during increasing itch compared with brain response changes during peak itch.
Acupuncture is an interesting multidimensional intervention, which modulates anticipatory, somatosensory, and cognitive re-appraisal circuitries. While the specific effect of acupuncture in reducing aversive sensations such as itch or pain is not completely understood, the somatosensory aspect of this intervention may prove to be important for its antipruritic effects. We found that electroacupuncture, but not PAC (associated with significantly less somatosensory afference), reduced evoked itch intensity. Ultimately, acupuncture might be considered a counter stimulus (Ward et al. 1996) in its ability to regulate itch. Counter stimulation is thought to modulate itch by multiple central nervous pathways, in a pruritogen-dependant manner (Davidson and Giesler 2010). As we found no correlation between expectancy for itch relief evaluated at baseline and eventual itch reduction (in fact, patients expected greater itch relief from the antihistamine than for acupuncture), and as our electroacupuncture intervention was actively providing somatosensory afference “during” itch induction, it is likely that somatosensory processing was an important component in mediating the reduction of itch by acupuncture. Furthermore, as acupuncture's antipruritic effects were demonstrated to have a central component, future studies should explore synergistic effects of acupuncture and peripherally acting agents, such as topical corticosteroids.
Antihistamine therapy did not reduce itch sensation and brain response to itch was not altered following this intervention. Interestingly, our previous study found that antihistamine reduced allergen-associated itch using an identical temperature-modulated itch model (Pfab, Kirchner, et al. 2012). However, this previous study used a different, second generation antihistamine (cetirizine), and in addition to reduced itch, we also found decreased attention levels with the d2 test of attention. Our current neuroimaging study used a third generation antihistamine (levocetirizine), which is touted as being less likely to induce drowsiness as a side effect. In fact, we found no significant decrement on the d2 test of attention. In sum, our results support the contention that histamine may not be an important mediator of itch in AD, and other, perhaps brain-derived attentional mechanisms may support antihistamine relief of AD itch.
Several limitations should be noted. Our study design was limited to the evaluation of the brain circuitry subserving a direct abortive antipruritic effect of electroacupuncture, which may differ from the brain mechanisms underlying reduced AD itch following a longitudinal course of acupuncture therapy. Another potential limitation is that our confirmatory ANOVA interaction for VAC versus PAC and baseline versus post-therapy brain response did not find significant clusters corresponding to every brain region identified as showing reduced activity following VAC. This could have been due to the increased power necessitated by the ANOVA interaction analysis approach, or that reduced brain activity in some areas was comparable across conditions.
In conclusion, we have identified different brain regions that process clinically relevant allergen itch in AD patients during the increasing itch phase compared with a peak plateau itch phase. Acupuncture significantly reduced itch sensation in AD patients. Acupuncture also reduced insula and striatum activation during the increasing itch phase and premotor activation during the peak itch phase. Neither antihistamine nor sham acupuncture reduced itch or altered brain response to evoked itch. Greater itch reduction following acupuncture was associated with greater reduction in putamen response to increasing itch, specifically implicating this region in acupuncture's antipruritic effect. Our study investigated, for the first time, the brain circuitry underlying therapy-associated antipruritic effects in patients suffering from chronic itch. Novel therapies for AD patients are needed and understanding mechanisms for itch reduction will significantly impact the development and applicability of acupuncture and related neuromodulatory therapies for AD.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
This study was supported by NIH (V.N.: K01-AT002166, R01-AT004714, BRR: P01-AT002048; T.K.: K24-AT004095; E.L.: R01-AR057744, P41RR14075) CRC 1 UL1 RR025758, Harvard Clinical and Translational Science Center, Mental Illness and Neuroscience Discovery (MIND) Institute, and the International Foundation of Functional Gastrointestinal Disorders. This study was also partly funded by a grant of the German Research Foundation (pf 690/2-1), and the Christine Kühne Center of Allergy and Education (CK-Care). The content is solely the responsibility of the authors and does not necessarily represent the official views of our sponsors.
Supplementary Material
Notes
We thank Drs Randy Gollub and David Borsook for providing access to their laboratories' thermode stimulation system. Conflict of Interest: None declared.
References
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9(4):463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26(47):12165–12173. doi: 10.1523/JNEUROSCI.3576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgrade MJ, Solomon LM, Lichter EA. Effect of acupuncture on experimentally induced itch. Acta Derm Venereol. 1984;64(2):129–133. [PubMed] [Google Scholar]
- Boguniewicz M. Atopic dermatitis: beyond the itch that rashes. Immunol Allergy Clin North Am. 2005;25(2):333–351. doi: 10.1016/j.iac.2005.02.006. vii. [DOI] [PubMed] [Google Scholar]
- Brickenkamp R, Zillmer E. D2 test of attention. Hogrefe & Huber Publishing, Göttingen, Germany; 1998. [Google Scholar]
- Craig AD. A new view of pain as a homeostatic emotion. Trends Neurosci. 2003;26(6):303–307. doi: 10.1016/s0166-2236(03)00123-1. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010;33(12):550–558. doi: 10.1016/j.tins.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ., Jr The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27(37):10007–10014. doi: 10.1523/JNEUROSCI.2862-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, Mikulis DJ, Davis KD. Neural correlates of the prolonged salience of painful stimulation. Neuroimage. 2003;20(3):1540–1551. doi: 10.1016/s1053-8119(03)00407-5. [DOI] [PubMed] [Google Scholar]
- European Task Force on Atopic Dermatitis. Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology. 1993;186(1):23–31. doi: 10.1159/000247298. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Ann Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48(1):63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafenreffer S. De pruritu, in Nosodochium, in quo cutis, eique adaerentium partium, affectus omnes, singulari methodo, et cognoscendi et curandi fidelissime traduntur, Kuhnen B. Ulm, Germany. 1660. pp. 98–102. [Google Scholar]
- Ikoma A, Fartasch M, Heyer G, Miyachi Y, Handwerker H, Schmelz M. Painful stimuli evoke itch in patients with chronic pruritus: central sensitization for itch. Neurology. 2004;62(2):212–217. doi: 10.1212/wnl.62.2.212. [DOI] [PubMed] [Google Scholar]
- Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7(7):535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- Ishiuji Y, Coghill RC, Patel TS, Oshiro Y, Kraft RA, Yosipovitch G. Distinct patterns of brain activity evoked by histamine-induced itch reveal an association with itch intensity and disease severity in atopic dermatitis. Br J Dermatol. 2009;161(5):1072–1080. doi: 10.1111/j.1365-2133.2009.09308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Koblenzer CS. Itching and the atopic skin. J Allergy Clin Immunol. 1999;104(3 Pt 2):S109–113. doi: 10.1016/s0091-6749(99)70052-7. [DOI] [PubMed] [Google Scholar]
- Kong J, Gollub R, Huang T, Polich G, Napadow V, Hui K, Vangel M, Rosen B, Kaptchuk TJ. Acupuncture de qi, from qualitative history to quantitative measurement. J Altern Complement Med. 2007;13(10):1059–1070. doi: 10.1089/acm.2007.0524. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosteletzky F, Namer B, Forster C, Handwerker HO. Impact of scratching on itch and sympathetic reflexes induced by cowhage (Mucuna pruriens) and histamine. Acta Derm Venereol. 2009;89(3):271–277. doi: 10.2340/00015555-0624. [DOI] [PubMed] [Google Scholar]
- Leknes SG, Bantick S, Willis CM, Wilkinson JD, Wise RG, Tracey I. Itch and motivation to scratch: an investigation of the central and peripheral correlates of allergen- and histamine-induced itch in humans. J Neurophysiol. 2007;97(1):415–422. doi: 10.1152/jn.00070.2006. [DOI] [PubMed] [Google Scholar]
- Lundeberg T, Bondesson L, Thomas M. Effect of acupuncture on experimentally induced itch. Br J Dermatol. 1987;117(6):771–777. doi: 10.1111/j.1365-2133.1987.tb07359.x. [DOI] [PubMed] [Google Scholar]
- Napadow V, Dhond R, Park K, Kim J, Makris N, Kwong KK, Harris RE, Purdon PL, Kettner N, Hui KK. Time-variant fMRI activity in the brainstem and higher structures in response to acupuncture. Neuroimage. 2009;47(1):289–301. doi: 10.1016/j.neuroimage.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, Makris N, Liu J, Kettner NW, Kwong KK, Hui KK. Effects of electroacupuncture versus manual acupuncture on the human brain as measured by fMRI. Hum Brain Mapp. 2005;24(3):193–205. doi: 10.1002/hbm.20081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, Sheehan J, Kim J, LaCount L, Park K, Kaptchuk T, Rosen B, Kuo B. The brain circuitry underlying the temporal evolution of nausea in humans. Cereb Cortex. 2013;23(4):806–813. doi: 10.1093/cercor/bhs073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Gunraj C, Nelson AJ, Yeh IJ, Castillo G, Hoque T, Chen R. Two phases of interhemispheric inhibition between motor related cortical areas and the primary motor cortex in human. Cereb Cortex. 2009;19(7):1654–1665. doi: 10.1093/cercor/bhn201. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Papoiu AD, Coghill RC, Kraft RA, Wang H, Yosipovitch G. A tale of two itches. Common features and notable differences in brain activation evoked by cowhage and histamine induced itch. Neuroimage. 2012;59(4):3611–3623. doi: 10.1016/j.neuroimage.2011.10.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfab F, Athanasiadis GI, Huss-Marp J, Fuqin J, Heuser B, Cifuentes L, Brockow K, Schober W, Konstantinow A, Irnich D, et al. Effect of acupuncture on allergen-induced basophil activation in patients with atopic eczema: a pilot trial. J Altern Complement Med. 2011;17(4):309–314. doi: 10.1089/acm.2009.0684. [DOI] [PubMed] [Google Scholar]
- Pfab F, Hammes M, Backer M, Huss-Marp J, Athanasiadis GI, Tolle TR, Behrendt H, Ring J, Darsow U. Preventive effect of acupuncture on histamine-induced itch: a blinded, randomized, placebo-controlled, crossover trial. J Allergy Clin Immunol. 2005;116(6):1386–1388. doi: 10.1016/j.jaci.2005.08.055. [DOI] [PubMed] [Google Scholar]
- Pfab F, Huss-Marp J, Gatti A, Fuqin J, Athanasiadis GI, Irnich D, Raap U, Schober W, Behrendt H, Ring J, et al. Influence of acupuncture on type I hypersensitivity itch and the wheal and flare response in adults with atopic eczema—a blinded, randomized, placebo-controlled, crossover trial. Allergy. 2010;65(7):903–910. doi: 10.1111/j.1398-9995.2009.02284.x. [DOI] [PubMed] [Google Scholar]
- Pfab F, Kirchner MT, Huss-Marp J, Schuster T, Schalock PC, Fuqin J, Athanasiadis GI, Behrendt H, Ring J, Darsow U, et al. Acupuncture compared with oral antihistamine for type I hypersensitivity itch and skin response in adults with atopic dermatitis—a patient- and examiner-blinded, randomized, placebo-controlled, crossover trial. Allergy. 2012;67(4):566–573. doi: 10.1111/j.1398-9995.2012.02789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfab F, Valet M, Sprenger T, Huss-Marp J, Athanasiadis GI, Baurecht HJ, Konstantinow A, Zimmer C, Behrendt H, Ring J, et al. Temperature modulated histamine-itch in lesional and nonlesional skin in atopic eczema–a combined psychophysical and neuroimaging study. Allergy. 2010;65(1):84–94. doi: 10.1111/j.1398-9995.2009.02163.x. [DOI] [PubMed] [Google Scholar]
- Pfab F, Valet M, Sprenger T, Toelle TR, Athanasiadis GI, Behrendt H, Ring J, Darsow U. Short-term alternating temperature enhances histamine-induced itch: a biphasic stimulus model. J Invest Dermatol. 2006;126(12):2673–2678. doi: 10.1038/sj.jid.5700577. [DOI] [PubMed] [Google Scholar]
- Pfab F, Valet M, Napadow V, Tölle TR, Behrendt H, Ring J, Darsow U. Itch and the brain. Chem Immunol Allergy. 2012;98:253–265. doi: 10.1159/000336529. [DOI] [PubMed] [Google Scholar]
- Shelley WB, Arthur RP. The neurohistology and neurophysiology of the itch sensation in man. AMA Arch Derm. 1957;76(3):296–323. doi: 10.1001/archderm.1957.01550210020004. [DOI] [PubMed] [Google Scholar]
- Simons SB, Tannan V, Chiu J, Favorov OV, Whitsel BL, Tommerdahl M. Amplitude-dependency of response of SI cortex to flutter stimulation. BMC Neurosci. 2005;6:43. doi: 10.1186/1471-2202-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Valet M, Pfab F, Sprenger T, Woller A, Zimmer C, Behrendt H, Ring J, Darsow U, Tolle TR. Cerebral processing of histamine-induced itch using short-term alternating temperature modulation–an fMRI study. J Invest Dermatol. 2008;128(2):426–433. doi: 10.1038/sj.jid.5701002. [DOI] [PubMed] [Google Scholar]
- Vierow V, Fukuoka M, Ikoma A, Dorfler A, Handwerker HO, Forster C. Cerebral representation of the relief of itch by scratching. J Neurophysiol. 2009;102(6):3216–3224. doi: 10.1152/jn.00207.2009. [DOI] [PubMed] [Google Scholar]
- Ward L, Wright E, McMahon SB. A comparison of the effects of noxious and innocuous counterstimuli on experimentally induced itch and pain. Pain. 1996;64(1):129–138. doi: 10.1016/0304-3959(95)00080-1. [DOI] [PubMed] [Google Scholar]
- Wood PB. Mesolimbic dopaminergic mechanisms and pain control. Pain. 2006;120(3):230–234. doi: 10.1016/j.pain.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Duque MI, Fast K, Dawn AG, Coghill RC. Scratching and noxious heat stimuli inhibit itch in humans: a psychophysical study. Br J Dermatol. 2007;156(4):629–634. doi: 10.1111/j.1365-2133.2006.07711.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.