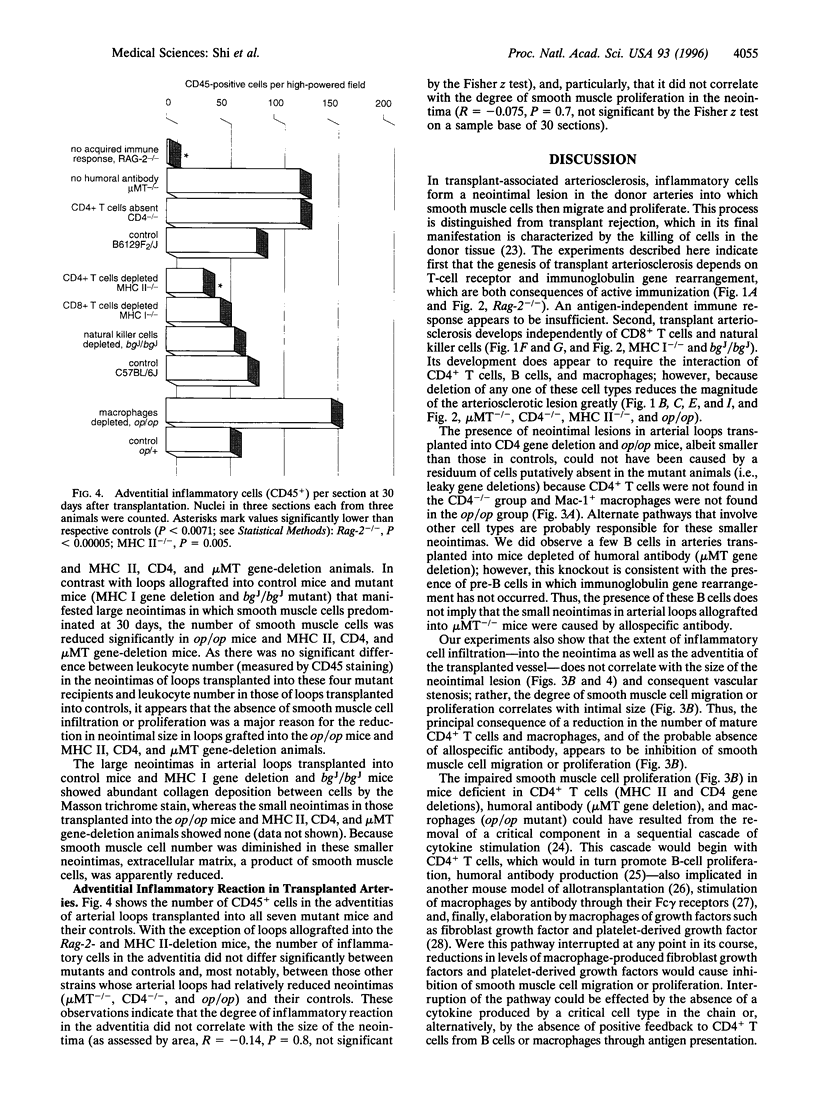
Abstract
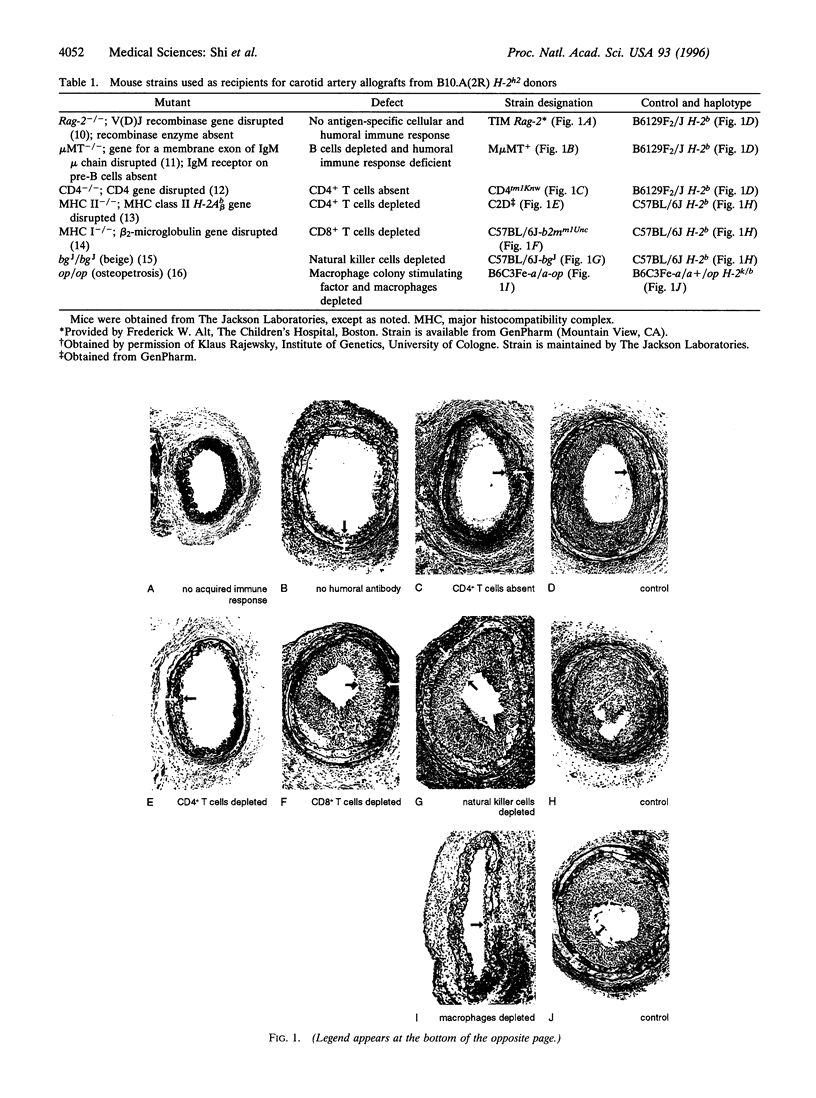
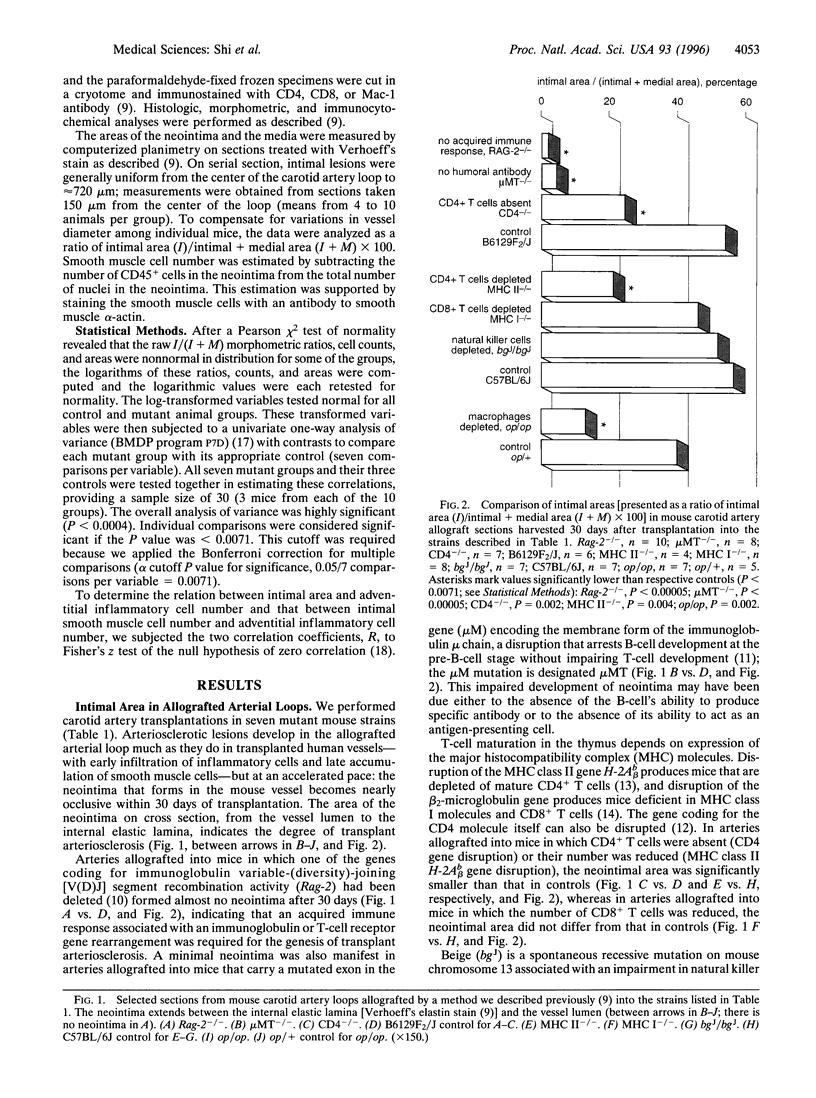
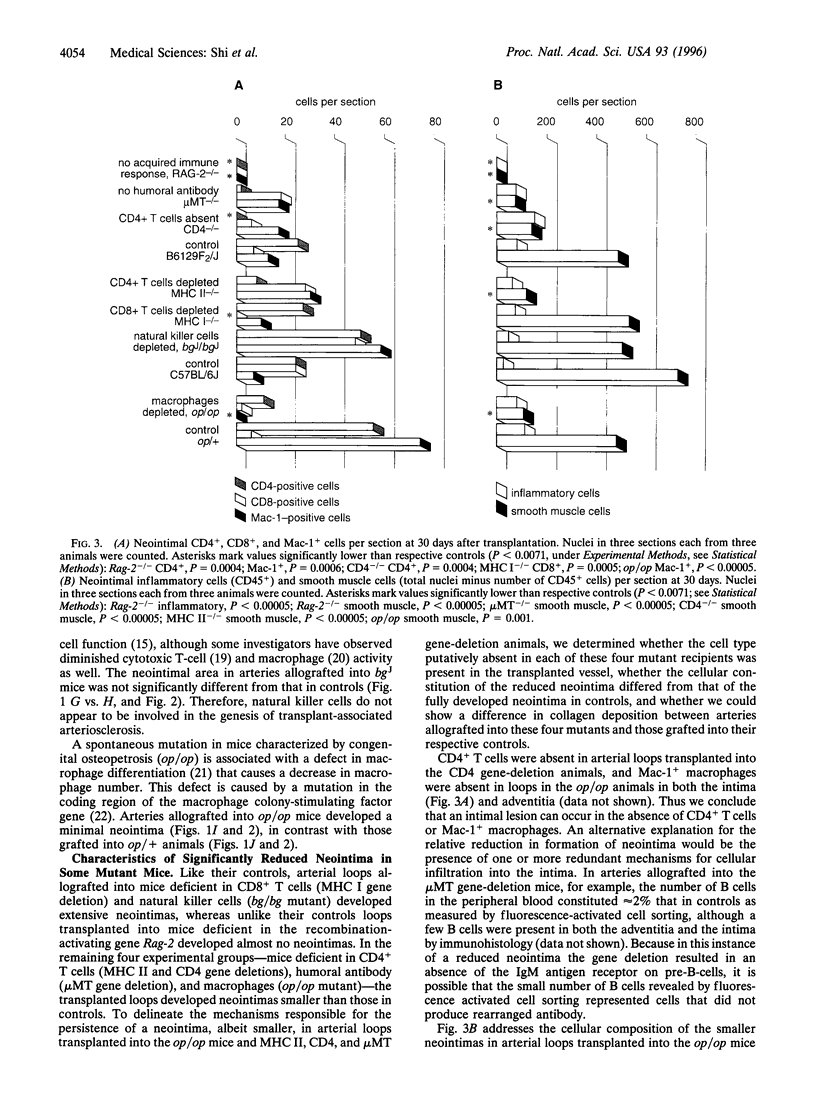
Although immunosuppressive therapy minimizes the risk of graft failure due to acute rejection, transplant-associated arteriosclerosis of the coronary arteries remains a significant obstacle to the long-term survival of heart transplant recipients. The participation of specific inflammatory cell types in the genesis of this lesion was examined in a mouse model in which carotid arteries were transplanted across multiple histocompatibility barriers into seven mutant strains with immunologic defects. An acquired immune response--with the participation of CD4+ (helper) T cells, humoral antibody, and macrophages--was essential to the development of the concentric neointimal proliferation and luminal narrowing characteristic of transplant arteriosclerosis. CD8+ (cytotoxic) T cells and natural killer cells were not involved in the process. Arteries allografted into mice deficient in both T-cell receptors and humoral antibody showed almost no neointimal proliferation, whereas those grafted into mice deficient only in helper T cells, humoral antibody, or macrophages developed small neointimas. These small neointimas and the large neointimas of arteries grafted into control animals contained a similar number of inflammatory cells; however, smooth muscle cell number and collagen deposition were diminished in the small neointimas. Also, the degree of inflammatory reaction in the adventitia did not correlate with the size of the neointima. Thus, the reduction in neointimal size in arteries allografted into mice deficient in helper T cells, humoral antibody, or macrophages may be accounted for by a decrease in smooth muscle cell migration or proliferation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billingham M. E. Graft coronary disease: the lesions and the patients. Transplant Proc. 1989 Aug;21(4):3665–3666. [PubMed] [Google Scholar]
- Cramer D. V., Wu G. D., Chapman F. A., Cajulis E., Wang H. K., Makowka L. Lymphocytic subsets and histopathologic changes associated with the development of heart transplant arteriosclerosis. J Heart Lung Transplant. 1992 May-Jun;11(3 Pt 1):458–466. [PubMed] [Google Scholar]
- Grusby M. J., Johnson R. S., Papaioannou V. E., Glimcher L. H. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science. 1991 Sep 20;253(5026):1417–1420. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- Hruban R. H., Beschorner W. E., Baumgartner W. A., Augustine S. M., Ren H., Reitz B. A., Hutchins G. M. Accelerated arteriosclerosis in heart transplant recipients is associated with a T-lymphocyte-mediated endothelialitis. Am J Pathol. 1990 Oct;137(4):871–882. [PMC free article] [PubMed] [Google Scholar]
- Kitamura D., Roes J., Kühn R., Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991 Apr 4;350(6317):423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Koller B. H., Marrack P., Kappler J. W., Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science. 1990 Jun 8;248(4960):1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A. Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu Rev Immunol. 1990;8:773–793. doi: 10.1146/annurev.iy.08.040190.004013. [DOI] [PubMed] [Google Scholar]
- Mahoney K. H., Morse S. S., Morahan P. S. Macrophage functions in beige (Chédiak-Higashi syndrome) mice. Cancer Res. 1980 Nov;40(11):3934–3939. [PubMed] [Google Scholar]
- Mandel T. E. Basic immunology of transplantation. Med J Aust. 1992 Jul 20;157(2):126–131. doi: 10.5694/j.1326-5377.1992.tb137047.x. [DOI] [PubMed] [Google Scholar]
- McCarrick J. W., 3rd, Parnes J. R., Seong R. H., Solter D., Knowles B. B. Positive-negative selection gene targeting with the diphtheria toxin A-chain gene in mouse embryonic stem cells. Transgenic Res. 1993 Jul;2(4):183–190. doi: 10.1007/BF01977348. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Paul L. C. Chronic rejection of organ allografts: magnitude of the problem. Transplant Proc. 1993 Apr;25(2):2024–2025. [PubMed] [Google Scholar]
- Pucci A. M., Forbes R. D., Billingham M. E. Pathologic features in long-term cardiac allografts. J Heart Transplant. 1990 Jul-Aug;9(4):339–345. [PubMed] [Google Scholar]
- Roder J., Duwe A. The beige mutation in the mouse selectively impairs natural killer cell function. Nature. 1979 Mar 29;278(5703):451–453. doi: 10.1038/278451a0. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Russell M. E., Adams D. H., Wyner L. R., Yamashita Y., Halnon N. J., Karnovsky M. J. Early and persistent induction of monocyte chemoattractant protein 1 in rat cardiac allografts. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6086–6090. doi: 10.1073/pnas.90.13.6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. E., Wallace A. F., Hancock W. W., Sayegh M. H., Adams D. H., Sibinga N. E., Wyner L. R., Karnovsky M. J. Upregulation of cytokines associated with macrophage activation in the Lewis-to-F344 rat transplantation model of chronic cardiac rejection. Transplantation. 1995 Feb 27;59(4):572–578. [PubMed] [Google Scholar]
- Russell P. S., Chase C. M., Winn H. J., Colvin R. B. Coronary atherosclerosis in transplanted mouse hearts. I. Time course and immunogenetic and immunopathological considerations. Am J Pathol. 1994 Feb;144(2):260–274. [PMC free article] [PubMed] [Google Scholar]
- Russell P. S., Chase C. M., Winn H. J., Colvin R. B. Coronary atherosclerosis in transplanted mouse hearts. II. Importance of humoral immunity. J Immunol. 1994 May 15;152(10):5135–5141. [PubMed] [Google Scholar]
- Saxena R. K., Saxena Q. B., Adler W. H. Defective T-cell response in beige mutant mice. Nature. 1982 Jan 21;295(5846):240–241. doi: 10.1038/295240a0. [DOI] [PubMed] [Google Scholar]
- Sharples L. D., Caine N., Mullins P., Scott J. P., Solis E., English T. A., Large S. R., Schofield P. M., Wallwork J. Risk factor analysis for the major hazards following heart transplantation--rejection, infection, and coronary occlusive disease. Transplantation. 1991 Aug;52(2):244–252. doi: 10.1097/00007890-199108000-00012. [DOI] [PubMed] [Google Scholar]
- Shi C., Russell M. E., Bianchi C., Newell J. B., Haber E. Murine model of accelerated transplant arteriosclerosis. Circ Res. 1994 Aug;75(2):199–207. doi: 10.1161/01.res.75.2.199. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Rathbun G., Lam K. P., Oltz E. M., Stewart V., Mendelsohn M., Charron J., Datta M., Young F., Stall A. M. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992 Mar 6;68(5):855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Takai T., Li M., Sylvestre D., Clynes R., Ravetch J. V. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994 Feb 11;76(3):519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W. W., Ahmed A., Szczylik C., Skelly R. R. Hematological characterization of congenital osteopetrosis in op/op mouse. Possible mechanism for abnormal macrophage differentiation. J Exp Med. 1982 Nov 1;156(5):1516–1527. doi: 10.1084/jem.156.5.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W., Bartocci A., Ferrante A. W., Jr, Ahmed-Ansari A., Sell K. W., Pollard J. W., Stanley E. R. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4828–4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Hayashi S., Kunisada T., Ogawa M., Nishikawa S., Okamura H., Sudo T., Shultz L. D., Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990 May 31;345(6274):442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]