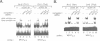Abstract
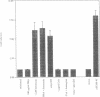
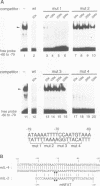
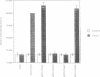
Murine T helper cell clones are classified into two distinct subsets, T helper 1 (Th1) and T helper 2 (Th2), on the basis of cytokine secretion patterns. Th1 clones produce interleukin-2 (IL-2), tumor necrosis factor-beta (TNF-beta) and interferon-gamma (IFN-gamma), while Th2 clones produce IL-4, IL-5, IL-6 and IL-10. These subsets differentially promote delayed-type hypersensitivity or antibody responses, respectively. The nuclear factor NF-AT is induced in Th1 clones stimulated through the T cell receptor-CD3 complex, and is required for IL-2 gene induction. The NF-AT complex consists of two components: NF-ATp, which pre-exists in the cytosol and whose appearance in the nucleus is induced by an increase of intracellular calcium, and a nuclear AP-1 component whose induction is dependent upon activation of protein kinase C (PKC). Here we report that the induction of the Th2-specific IL-4 gene in an activated Th2 clone involves an NF-AT complex that consists only of NF-ATp, and not the AP-1 component. On the basis of binding experiments we show that this 'AP-1-less' NF-AT complex is specific for the IL-4 promoter and does not reflect the inability of activated Th2 cells to induce the AP-1 component. We propose that NF-ATp is a common regulatory factor for both Th1 and Th2 cytokine genes, and that the involvement of PKC-dependent factors, such as AP-1, may help determine Th1-/Th2-specific patterns of gene expression.
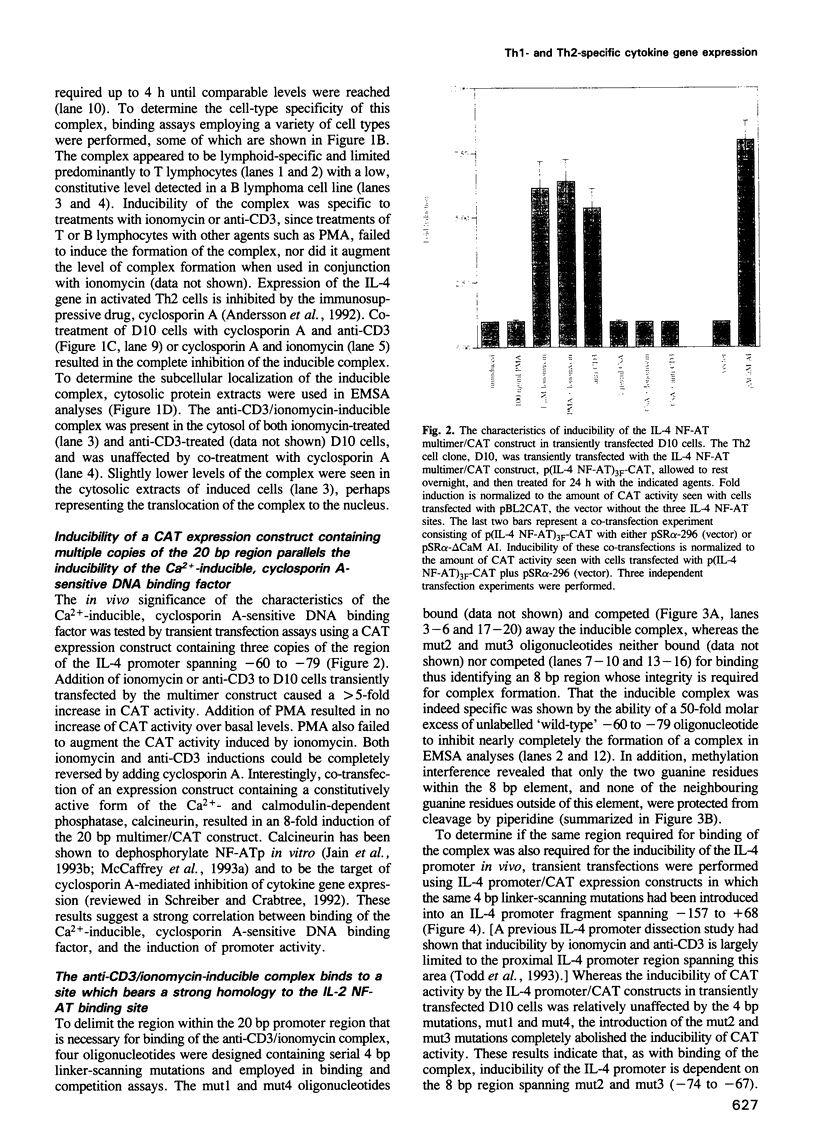
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbas A. K., Williams M. E., Burstein H. J., Chang T. L., Bossu P., Lichtman A. H. Activation and functions of CD4+ T-cell subsets. Immunol Rev. 1991 Oct;123:5–22. doi: 10.1111/j.1600-065x.1991.tb00603.x. [DOI] [PubMed] [Google Scholar]
- Abe E., De Waal Malefyt R., Matsuda I., Arai K., Arai N. An 11-base-pair DNA sequence motif apparently unique to the human interleukin 4 gene confers responsiveness to T-cell activation signals. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2864–2868. doi: 10.1073/pnas.89.7.2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J., Nagy S., Groth C. G., Andersson U. FK 506 and cyclosporine inhibit antigen- or mitogen-induced monokine and lymphokine production in vitro. Transplant Proc. 1992 Feb;24(1):321–325. [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Arai N., Naito Y., Watanabe M., Masuda E. S., Yamaguchi-Iwai Y., Tsuboi A., Heike T., Matsuda I., Yokota K., Koyano-Nakagawa N. Activation of lymphokine genes in T cells: role of cis-acting DNA elements that respond to T cell activation signals. Pharmacol Ther. 1992;55(3):303–318. doi: 10.1016/0163-7258(92)90054-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987 Jun 1;138(11):3688–3694. [PubMed] [Google Scholar]
- Coffman R. L., Carty J. A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-gamma. J Immunol. 1986 Feb 1;136(3):949–954. [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel R. J., Ro H. S., Rosen B. S., Groves D. L., Spiegelman B. M. Nucleoprotein complexes that regulate gene expression in adipocyte differentiation: direct participation of c-fos. Cell. 1987 Jun 19;49(6):835–844. doi: 10.1016/0092-8674(87)90621-0. [DOI] [PubMed] [Google Scholar]
- Downward J., Graves J. D., Warne P. H., Rayter S., Cantrell D. A. Stimulation of p21ras upon T-cell activation. Nature. 1990 Aug 23;346(6286):719–723. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- Fiering S., Northrop J. P., Nolan G. P., Mattila P. S., Crabtree G. R., Herzenberg L. A. Single cell assay of a transcription factor reveals a threshold in transcription activated by signals emanating from the T-cell antigen receptor. Genes Dev. 1990 Oct;4(10):1823–1834. doi: 10.1101/gad.4.10.1823. [DOI] [PubMed] [Google Scholar]
- Flanagan W. M., Corthésy B., Bram R. J., Crabtree G. R. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991 Aug 29;352(6338):803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- Gajewski T. F., Schell S. R., Fitch F. W. Evidence implicating utilization of different T cell receptor-associated signaling pathways by TH1 and TH2 clones. J Immunol. 1990 Jun 1;144(11):4110–4120. [PubMed] [Google Scholar]
- Ghosh D. A relational database of transcription factors. Nucleic Acids Res. 1990 Apr 11;18(7):1749–1756. doi: 10.1093/nar/18.7.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hivroz-Burgaud C., Clipstone N. A., Cantrell D. A. Signaling requirements for the expression of the transactivating factor NF-AT in human T lymphocytes. Eur J Immunol. 1991 Nov;21(11):2811–2819. doi: 10.1002/eji.1830211124. [DOI] [PubMed] [Google Scholar]
- Jain J., McCaffrey P. G., Miner Z., Kerppola T. K., Lambert J. N., Verdine G. L., Curran T., Rao A. The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature. 1993 Sep 23;365(6444):352–355. doi: 10.1038/365352a0. [DOI] [PubMed] [Google Scholar]
- Jain J., McCaffrey P. G., Valge-Archer V. E., Rao A. Nuclear factor of activated T cells contains Fos and Jun. Nature. 1992 Apr 30;356(6372):801–804. doi: 10.1038/356801a0. [DOI] [PubMed] [Google Scholar]
- Jain J., Miner Z., Rao A. Analysis of the preexisting and nuclear forms of nuclear factor of activated T cells. J Immunol. 1993 Jul 15;151(2):837–848. [PubMed] [Google Scholar]
- Jain J., Valge-Archer V. E., Rao A. Analysis of the AP-1 sites in the IL-2 promoter. J Immunol. 1992 Feb 15;148(4):1240–1250. [PubMed] [Google Scholar]
- Jamieson C., McCaffrey P. G., Rao A., Sen R. Physiologic activation of T cells via the T cell receptor induces NF-kappa B. J Immunol. 1991 Jul 15;147(2):416–420. [PubMed] [Google Scholar]
- Kang S. M., Beverly B., Tran A. C., Brorson K., Schwartz R. H., Lenardo M. J. Transactivation by AP-1 is a molecular target of T cell clonal anergy. Science. 1992 Aug 21;257(5073):1134–1138. doi: 10.1126/science.257.5073.1134. [DOI] [PubMed] [Google Scholar]
- Kaye J., Porcelli S., Tite J., Jones B., Janeway C. A., Jr Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells. J Exp Med. 1983 Sep 1;158(3):836–856. doi: 10.1084/jem.158.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey P. G., Jain J., Jamieson C., Sen R., Rao A. A T cell nuclear factor resembling NF-AT binds to an NF-kappa B site and to the conserved lymphokine promoter sequence "cytokine-1". J Biol Chem. 1992 Jan 25;267(3):1864–1871. [PubMed] [Google Scholar]
- McCaffrey P. G., Luo C., Kerppola T. K., Jain J., Badalian T. M., Ho A. M., Burgeon E., Lane W. S., Lambert J. N., Curran T. Isolation of the cyclosporin-sensitive T cell transcription factor NFATp. Science. 1993 Oct 29;262(5134):750–754. doi: 10.1126/science.8235597. [DOI] [PubMed] [Google Scholar]
- McCaffrey P. G., Perrino B. A., Soderling T. R., Rao A. NF-ATp, a T lymphocyte DNA-binding protein that is a target for calcineurin and immunosuppressive drugs. J Biol Chem. 1993 Feb 15;268(5):3747–3752. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Murray H. W. Gamma interferon, cytokine-induced macrophage activation, and antimicrobial host defense. In vitro, in animal models, and in humans. Diagn Microbiol Infect Dis. 1990 Sep-Oct;13(5):411–421. doi: 10.1016/0732-8893(90)90012-k. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Spitalny G. L., Nathan C. F. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon-gamma. J Immunol. 1985 Mar;134(3):1619–1622. [PubMed] [Google Scholar]
- Muñoz E., Zubiaga A. M., Muñoz J., Huber B. T. Regulation of IL-4 lymphokine gene expression and cellular proliferation in murine T helper type II cells. Cell Regul. 1990 Apr;1(5):425–434. doi: 10.1091/mbc.1.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe S. J., Tamura J., Kincaid R. L., Tocci M. J., O'Neill E. A. FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature. 1992 Jun 25;357(6380):692–694. doi: 10.1038/357692a0. [DOI] [PubMed] [Google Scholar]
- Rao A., Faas S. J., Cantor H. Activation specificity of arsonate-reactive T cell clones. Structural requirements for hapten recognition and comparison with monoclonal antibodies. J Exp Med. 1984 Feb 1;159(2):479–494. doi: 10.1084/jem.159.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A. Signaling mechanisms in T cells. Crit Rev Immunol. 1991;10(6):495–519. [PubMed] [Google Scholar]
- Rayter S. I., Woodrow M., Lucas S. C., Cantrell D. A., Downward J. p21ras mediates control of IL-2 gene promoter function in T cell activation. EMBO J. 1992 Dec;11(12):4549–4556. doi: 10.1002/j.1460-2075.1992.tb05556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Identification of a novel lymphoid specific octamer binding protein (OTF-2B) by proteolytic clipping bandshift assay (PCBA). EMBO J. 1988 Dec 20;7(13):4221–4229. doi: 10.1002/j.1460-2075.1988.tb03319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S. L., Crabtree G. R. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992 Apr;13(4):136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- Serfling E., Barthelmäs R., Pfeuffer I., Schenk B., Zarius S., Swoboda R., Mercurio F., Karin M. Ubiquitous and lymphocyte-specific factors are involved in the induction of the mouse interleukin 2 gene in T lymphocytes. EMBO J. 1989 Feb;8(2):465–473. doi: 10.1002/j.1460-2075.1989.tb03399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. P., Utz P. J., Durand D. B., Toole J. J., Emmel E. A., Crabtree G. R. Identification of a putative regulator of early T cell activation genes. Science. 1988 Jul 8;241(4862):202–205. doi: 10.1126/science.3260404. [DOI] [PubMed] [Google Scholar]
- Sleigh M. J. A nonchromatographic assay for expression of the chloramphenicol acetyltransferase gene in eucaryotic cells. Anal Biochem. 1986 Jul;156(1):251–256. doi: 10.1016/0003-2697(86)90180-6. [DOI] [PubMed] [Google Scholar]
- Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988 Jan;8(1):466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd M. D., Grusby M. J., Lederer J. A., Lacy E., Lichtman A. H., Glimcher L. H. Transcription of the interleukin 4 gene is regulated by multiple promoter elements. J Exp Med. 1993 Jun 1;177(6):1663–1674. doi: 10.1084/jem.177.6.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodrow M. A., Rayter S., Downward J., Cantrell D. A. p21ras function is important for T cell antigen receptor and protein kinase C regulation of nuclear factor of activated T cells. J Immunol. 1993 May 1;150(9):3853–3861. [PubMed] [Google Scholar]