Abstract
Recently people often suffer from unhealthy energy metabolism balance as they tend to take more energy than required. Normally, excess energy taken in is converted into triglyceride and stored in adipocyte as lipid droplets. Recent studies have suggested that irregular accumulation of triglyceride in adipocyte might be a cause of many metabolic diseases. Thus, the awareness of the detrimental effects on health of excessive lipid droplets accumulation (LDA) has urged the development or finding of drugs to counter this effect, including those from botanical origins. This review summarized recent progress in this field from the viewpoint of crude drug studies with references to their anti-LDA activity. Possible mechanisms involved in their anti-LDA effect and isolations of the relevant bioactive compounds were also discussed.
Keywords: Adipocyte, Anti-lipid droplets accumulation, Anti-adipogenesis, Plant natural products, Drug lead
Introduction
Excess energy taken into the body is to be stored in an organelle, lipid droplets in the cells called adipocytes.Their major physiological role is to function as reservoir of energy [1]. Lipid droplets in adipocyte are always in a state of flux, when the energy excess is in the surplus, energy is converted into triglyceride, and when the energy is in short supply, the stored triglyceride is re-converted into energy [2]. Biologically, body lipid serves as body energy storage and insulates us from low temperature. But, it can act as a double edged sword [3]. This is because excessive storage of lipid has been recently shown to be a cause of various diseases including type-2 diabetes mellitus, cardiovascular disease, and atherosclerosis [4, 5].
The possible detrimental effects on health of excessive lipid droplets accumulation (LDA) have prompted the search for counter measures, including studies of drugs which reduce LDA. A natural product, berberine, has so far been reported to reduce LDA in vivo via down-regulation of peroxisome proliferator-activated receptor protein-γ (PPARγ) [6, 7]. Apart from berberine, other examples of plant-derived compounds with well-known anti-LDA effect includes, genistein, curcumin, and (-)-epigallocatechin-3-gallate (EGCG) [8–10]. A long history of the use of plant origin drugs by many nations resulted in the widespread consumers’ perception that plant natural origin drugs are safe or had fewer side effects.
Because of the knowledge of the importance of LDA in adipocyte and that a few compounds isolated from plants actually possess such an anti-LDA effect, researchers studying plant natural products with anti-LDA are gaining momentum recently. This review compiles information of botanicals that have been or are being investigated for their anti-LDA activity. Possible mechanisms involved in the anti-LDA process and possible active compounds are also included in this review.
Possible targets of anti-lipid droplets accumulation agents
Extra energy is stored in specialized cells, adipocytes. Adipocytes are grouped into two types, brown adipocytes and white adipocytes. Brown ones are mitochondria rich and use energy whereas white ones store energy [3, 11, 12]. The differentiation process of white adipocytes from the precursor cells and its LDA system could be a target in the study to find LDA inhibition (Fig. 1).
Fig. 1.
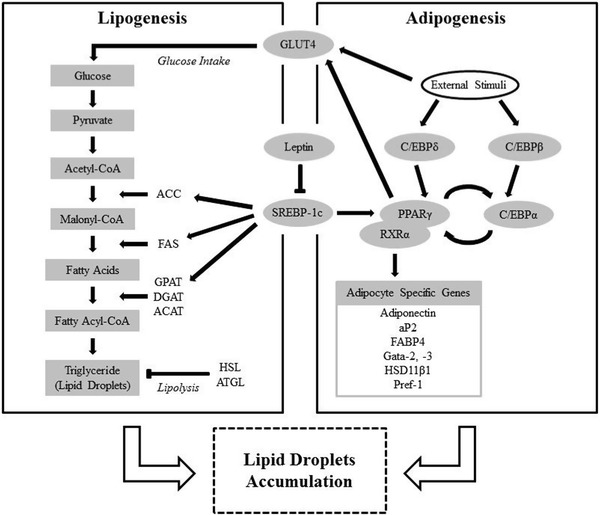
Simplified signaling cascade of lipogenesis and adipogenesis
Adipocytes originate from the same precursor stem cells as chondrocytes, osteocytes and myocytes, known as mesenchymal stem cells (MSCs) [13]. Differentiation initiates when the pluripotent MSCs receive signals from extracellular stimulating factors such as bone morphogenetic proteins (BMPs), transforming growth factor-β (TGF-β), activin, insulin-like growth factor 1 (IGF-1), interleukin-17 (IL-17), and fibroblast growth factors (FGF) 1 and 2 [14–22]. It is also been well documented that a mixture of 3-isobutyl-1-methylxanthine (IBMX), dexamethasone (DEX), and insulin (MDI inducer) stimulates the mouse embryonic fibroblast cell line, 3T3–L1, to differentiate into adipocytes in vitro [21]. Intensive studies on 3T3–L1 established that the presence of extracellular stimulating factors rapidly caused expression of early adipogenic regulators, and CCAAT/enhancer-binding proteins (C/EBPs) -β and -δ. The expressions of C/EBPβ and C/EBPδ convey the message from these extracellular stimulating factors to activate the master regulators of adipogenesis, PPARγ, and C/EBPα. C/EBPβ and -δ were proved to be essential for the expression of PPARγ and C/EBPα, because the cells with C/EBPβ and -δ knock out have down-regulated in PPARγ and C/EBPα expression. PPARγ and C/EBPα operates in a self-regulating positive feedback loop system. This feedback loop system increased expression of adipocyte-specific genes, which are important for the proper functioning of adipocytes [22–31]. Such specific genes includes glucose transporter type 4 (GLUT4), an insulin-regulated glucose transporter protein that enables translocation to plasma membrane to facilitate the uptake of glucose into cells. This protein is expressed primarily in muscles and fat tissues, which makes it an ideal marker for the determination of adipocyte differentiation [23]. In addition to GLUT4, another lipid homeostasis-related gene, lipoprotein lipase (LPL) is also studied. LPL is an enzyme that hydrolyzes triglycerides including very low-density lipoproteins (VLDL) into two free fatty acids and one monoacylglycerol molecule. It is mostly distributed in adipose, heart, and skeletal muscle tissues [24]. The 11β-hydroxysteroid dehydrogenase type 1 (HSD11β1) is another enzyme that is related to the metabolism, which is highly expressed in adipose tissues. This family of enzymes catalyzes the conversion of inactive cortisone to active cortisol, and vice versa [25–27]. Fatty acid binding protein 4 (FABP4) also known as adipocyte protein 2 (aP2), is another adipocyte specific protein expressed primarily in adipocyte that functions as a carrier protein [28]. The lengthy process leading to adipogenesis might provide numerous targets to be attacked for the inhibition of LDA.
All mammalian cells are known to contain some lipid droplets [29], but the differentiated cells called white and brown adipocytes possess larger sized lipid droplets in higher numbers [30]. These lipid droplets consist of neutral lipids, triacylglycerols and cholesterol esters encased in a monolayer of phospholipid [1, 32]. The esterified fat is on energy demand hydrolyzed to be used as an energy source [3, 30]. Of the two types of adipocytes, white ones generally contain larger lipid droplets occupying a major part of the cytosol, whereas the brown ones contain numerous and smaller lipid droplets. The formations of lipids are catalyzed by the enzymes known as acyl-CoA diglycerol acyltranferase (DGAT) and acyl-CoA cholesterol acyltranferase (ACAT) [31–34]. Although the enzymes responsible for the lipid synthesis are well-elucidated, the formation process of lipid droplets is not clearly established. Two of the most likely models are: (1) formation of tiny lipid droplets by DGAT or AGAT, which are deposited between the membrane leaflets of endoplasmic reticulum (ER), where the lipid droplets gradually increase in size to finally ‘bud off’ the ER, and (2) lipids accumulate between the luminal and cytoplasmic membranes, which subsequently are encapsulated by the membranes bilayer to formed lipid droplets [31–33]. Apart from inhibiting the adipogenesis process, anti-LDA effect may also be achieved by preventing the adipocyte from accumulating lipid droplets. As discussed before, inhibition of enzymes responsible for the lipid formation may provide a tantalizing target for inhibition of LDA. At present, since the exact mechanisms of lipid droplets formation is still under debate, future identification and understanding of the mechanisms involved may provide new anti-LDA targets.
During energy deprivation, lipid droplets stored in adipocyte hydrolyze their content, triacylglycerols to fatty acids and glycerol, under a process named lipolysis [35]. In addition to the inhibition of lipid droplets synthesis, up-regulation of the lipid metabolism or lipolysis process may also be effective in lowering the adipocyte lipid droplets. Lipolysis is known to be catalyzed by many enzymes generally called lipases, of which adipose triglyceride lipase (ATGL or desnutrin) is known to play a major role. The enzyme is responsible for some 75 % adipocyte lipolysis [30, 34–36]. A study indicated that the first step of lipolysis catalyzed by ATGL is the rate-limiting step, a fact which emphasized the importance of ATGL in the lipolysis [37]. Thus, inhibition of lipid droplets synthesis and promotions of lipolysis of adipocyte lipid droplets equally function to reduce LDA in adipocyte.
Plants investigated for anti-lipid droplets accumulation activity
Inhibitors of adipogenesis or expression of adipogenic factors
Aristolochia manshuriensis Kom—Aristolochiaceae
Aristolochia manshuriensis, a Korean traditional medicinal herb is distributed in Japan, China, and Korea. Preliminary studies showed that a stem extract of A. manshuriensis down-regulates the gene expression of C/EBPβ, PPARγ, and C/EBPα of 3T3–L1 cells. Further studies on the upstream regulators of C/EBPβ, PPARγ, and C/EBPα led to the conclusion that the extract disrupts the extracellular signal-regulated protein kinase 1/2 (ERK1/2) and Akt pathway leading to the inhibition of C/EBPβ, PPARγ, and C/EBPα expression, which eventually leads to the inhibition of the adipocyte differentiation. In addition, gene expression of fatty acid synthase (FAS), adiponectin, LPL, and aP2 is also significantly down-regulated. In an in vitro study, aristolochic acid (Fig. 2) isolated from the plant is shown to be responsible for the inhibition of triglyceride (TG) accumulation. Oral administration of a stem extract of A. manshuriensis leaves at 62.5 mg/kg/day is reported to significantly decrease the fat tissue weight, total cholesterol (TC) level, and low density lipoprotein-cholesterol (LDL-c) level of high-fat diet (HFD)-induced obesity mouse [38], though it is yet not verified if such decreases are due to the effect of aristolochic acid.
Fig. 2.
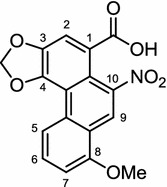
Structure of aristolochic acid
Brassica rapa (L.)—Brassicaceae
The roots of Brassica Rapa or commonly known as the turnip are reported to contain licochalcone A (Fig. 3), a major phenolic compound from the root of the Glycyrrhiza plant, commonly known as licorice [39]. This compound was found to suppress the differentiation of 3T3–L1 pre-adipocytes. Further investigation showed that licochalcone A significantly down-regulates the expression of PPARγ, C/EBPα, the sterol regulatory element-binding protein 1c (SREBP-1c), and their target genes, FABP4, FAS, stearoyl-CoA desaturase 1 (SCD1), and glycerol-3-phosphate acyltransferase (GAPH). An in vivo study using ICR mice fed with a high fat diet (HFD) showed that by administration of licochalcone A at 10 mg/kg, the bodyweight and the TG, TC, and non-esterified fatty acid (NEFA) levels were significantly decreased by 14.0, 48.2, 58.9, and 73.5 %, respectively [40].
Fig. 3.
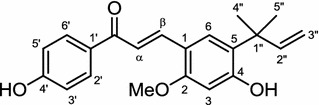
Structure of licochalcone A
Camelia sinensis (L.) Kuntze—Theaceae
Leaves of Camelia sinensis, or the tea plant are most commonly consumed in eastern Asia and Europe. Many compounds have been isolated from tea leaves including caffeine, (-)-epigallocatechin-3-gallate (EGCG) (Fig. 4), and (-)-epi-catechin-3-gallate [9]. EGCG was demonstrated to prevent adipogenesis and to cause adipocytes apoptosis, though the details of its mechanism of action are not known yet [41, 42]. In another study, the possible mechanism of anti-adipogenesis by EGCG was reported, thus, by the real-time PCR (RT-PCR) analysis of 3T3–L1 cells treated with EGCG it was shown to decrease PPARγ and C/EBPα mRNA. Significant decrease in the forkhead transcription factor class O1 (FoxO1) mRNA was also reported, which suggested it to be via PI3-K (phosphoinositide 3-kinase)/Akt and MEK [MAPK (mitogen-activated protein kinase)/ERK (extracellular-signal-regulated kinase) kinase] pathways [43]. Many other papers also refer to the anti-obesity effect of tea extract in in vivo studies [44–46].
Fig. 4.
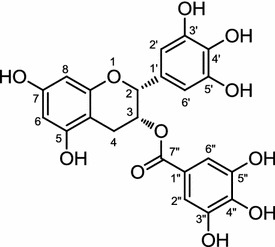
Structure of (-)-epigallocatechin-3-gallate (EGCG)
Chisocheton ceramicus (Miq.) C. DC.—Meliaceae
Chisocheton ceramicus, known to be a source of hardwood timber, is distributed in the tropical countries including Malaysia, Indonesia, Brunei, Papua New Guinea, Philippines and Vietnam. Like other plants of the Meliaceae, the plant is rich in limonoids and a series of limonoids have been isolated from the barks of this plant including the ceramicine and chisomicine series, and 14-deoxyxyloccensin K [47–53]. A bark extract of this plant was shown to possess an anti-LDA activity on the mouse pre-adipocyte cell line, MC3T3-G2/PA6 cells. Bioassay-guided separation of its hexane soluble fraction led to the isolation of 12 limonoids, ceramicines A–L, of which ceramicine B (Fig. 5) had the most potent anti-LDA activity (IC50 = 1.8 μM). Structure–activity relationship studies on ceramicines A–L and nine ceramicine B derivatives indicated that the C-17 furan moiety, C2–C3 double bond, and C14–C15 double bond play important roles in eliciting the anti-LDA activity [54]. Subsequent studies on the protein and mRNA expression suggested that the role ceramicine B in the anti-LDA activity was to inhibit adipogenesis via suppression of PPARγ expression. Detailed studies on the upstream regulator of PPARγ indicated that ceramicine B interrupts phosphorylations of FoxO1 [55]. Phosphorylations of FoxO1 at Thr 24, Ser 256, and Ser 319 are essential for advancement of adipogenesis and unphosphorylated FoxO1 plays a role in the repression of PPARγ mRNA transcription [56, 57]. Thus ceramicine B may be said to play an essential role in inhibiting LDA by interrupting phosphorylations of FoxO1 which then leads to suppression of PPARγ expression.
Fig. 5.
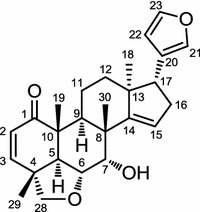
Structure of ceramicine B
Lysimachia foenum-graecum Hance.—Primulaceae
Lysimachia foenum-graecum has been used traditionally as an anti-inflammatory agent and also as a remedy for cold, headache, and toothache. From the whole plant extract of L. foenum-graecum, a series of triterpene saponin, foenumoside A-E and lysimachiagenoside A, C–F have been isolated [58–61]. By a high-through put screening this plant was demonstrated to suppress adipogenesis and this anti-adipogenesis activity was shown to be due to suppression of PPARγ, and C/EBPα protein expression in a concentration-dependent manner, with IC50 at 2.5 μg/ml, which down-regulate the downstream targets of PPARγ, and C/EBPα, aP2 and adiponectin [58]. Apart from the down-regulation of the adipogenic marker gene, the whole plant extract of this plant was also shown to suppress induction of gene expression of lipogenesis related genes, FAS, SREBP-1c, acetyl-CoA carboxylase (ACC), and SCD1. It is also to be noted that the lipolytic genes were up-regulated, such as acyl-CoA oxidase (ACO) and carnitine palmitoyltransferase 1 (CPT1). An in vivo study showed also that the body weight of C57BL/6 mice fed with HFD decreased when treated with L. foenum-graecum whole plant extract through oral-gavage at 100 mg/kg. Foenumoside B (Fig. 6) was identified to be responsible for the effect in both in vitro and in vivo studies. It inhibited the differentiation of 3T3-L1 preadipocytes in a dose-dependent manner with an IC50 of 0.2 μg/ml in the nile red staining assay. In an in vivo assay, foenumoside B was shown to suppress lipid accumulation in white adipose tissues and in the liver, and to lower the blood levels of glucose, triglycerides, alanine aminotransferase (ALT), and aspartate aminotransferase (AST), in HFD-induced mice [62].
Fig. 6.
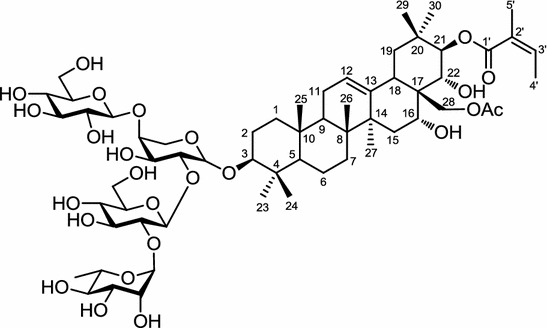
Structure of foenumoside B
Magnolia denudata Desr.—Magnoliaceae
The hexane soluble fraction of an extract of Magnolia denudata flowers was shown to inhibit gene expression of PPARγ and C/EBPα in 3T3–L1 cells without any observed cytotoxicity. Four known lignans from the M. denudata flower hexane soluble fraction, (+)-fargesin, (+)-eudesmin, (+)-epimagnolin A, and (+)-magnolin (Fig. 7), were examined for their anti-adipogenic property. At 50 μM, there was an inhibitory effect of these lignans on the protein expression of PPARγ, SREBP-1c, and C/EBPα. The order of potency is (+)-epimagnolin A > (+)-magnolin > (+)-eudesmin > (+)-fargesin [63].
Fig. 7.
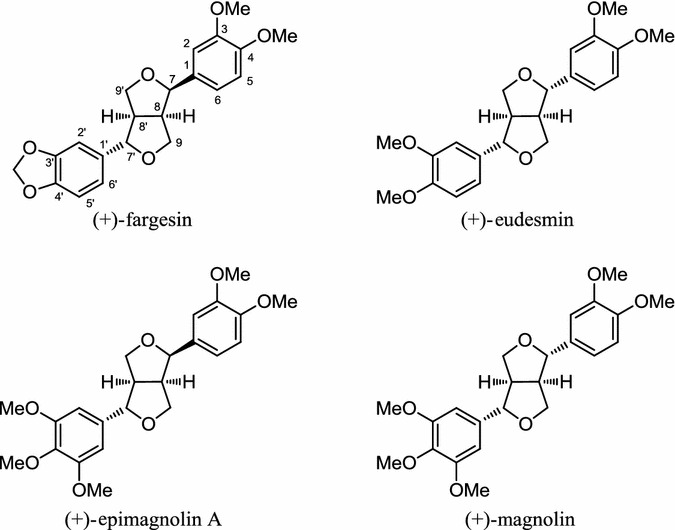
Structures of (+)-fargesin, (+)-eudesmin, (+)-epimagnolin A, and (+)-magnolin
Populus balsamifera (L.)—Salicaceae
Populus balsamifera or Balsam poplar is a medicinal plant used by the natives of Canada as a possible anti-diabetic remedy. Studies showed that a bark extract of this tree showed that it possesses the ability to inhibit adipogenesis and inhibits LDA in 3T3–L1 induced by MDI inducer. More detailed studies using the PPARγ reporter gene assay indicated that its extract functions as an antagonist to PPARγ activity giving the max PPARγ inhibition activity of 87 %. Several compounds were identified in the P. balsamifera, such as salicin and salicortin (Fig. 8), both salicortin isomers showing complete inhibition of PPARγ activity [64]. Another study showed that both ethanolic, an extract of P. balsamifera (250 or 125 mg/kg), and salicortin (12.5 mg/kg) effectively and equally reduced the accumulations of fat and liver TG in diet-induced obese (DIO) C57BL/6 mice [65]. Other salicortin-derivatives obtained from twigs of another plant of the same family were shown to inhibit adipogenesis via modulation of the C/EBPα and SREBP-1c dependent pathway (Table 1) [66].
Fig. 8.
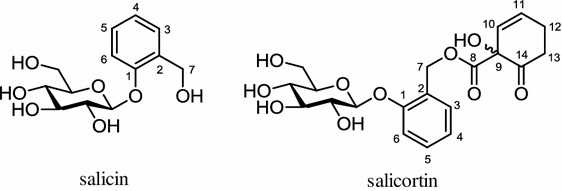
Structures of salicin and salicortin
Table 1.
List of plants having inhibitory effect on adipogenesis or expression of adipogenic factors
| Species name (family name) |
Plant parts used | Bioactive anti-LDA fraction(s) or compound(s) | Mechanism of action | Ref. no. |
|---|---|---|---|---|
| Alpinia officinarum Hance (Zingiberaceae) | Whole | Galangin | Inhibition of expression of PPARγ, and C/EBPα, subsequently SREBP1c and FAS | [67] |
| Codonopsis lanceolata Siebold. & Zucc. Trautv. (Campanulaceae) | Whole | Aqueous extract | Inhibition of expression of PPARγ, and C/EBPα | [68–70] |
| Clerodendron glandulosum Coleb. (Verbenaceae) | Leaf | Aqueous extract | Inhibition of expression of PPARγ, and subsequently SREBP1c and FAS | [71, 72] |
| Cucurbita moschata Duchesne ex Poir. (Cucurbitaceae) | Stem | Dihydroconiferyl alcohol | Inhibition of expression of PPARγ, and C/EBPα, and subsequently SREBP-1c, FABP4, FAS, SCD1, and Pref-1 | [73] |
| Dioscorea nipponica Makino. (Dioscoreaceae) | Rhizome | Pseudoprotodioscin | Inhibition of expression of PPARγ, and C/EBPα, subsequently LPL and leptin | [74, 75] |
| Ecklonia stolonifera Okamura. (Laminariaceae) | Whole | Fucosterol | Inhibition of expression of PPARγ and C/EBPα | [76] |
| Evodia rutaecarpa (Juss.) Benth. (Rutaceae) | Fruit | Evodiamine | Inhibition of adipogenesis via suppression of epidermal growth factor receptor (EGFR) | [77, 78] |
| Hibiscus sabdariffa (L.) (Malvaceae) | Calyx | Aqueous extract | Inhibition of expression of PPARγ, and C/EBPα via PI3-K and MAPK pathway | [79, 80] |
| Irvingia gabonensis (Aubry-Lecomte ex O’Rorke) Baill. (Irvingiaceae) | Seed | Extract | Inhibition of expression of PPARγ and reduction in glyceraldehyde 3-phosphate dehydrogenase (G3PDH), serum leptin, and increase in adiponectin | [81] |
| Lagerstroemia speciosa (L.) Per. (Lythraceae) | Leaf | Tannic acid | Inhibit expression of PPARγ | [82–84] |
| Lindera erythrocarpa Makino (Lauraceae) | Fruit | Lucidone | Inhibition of expression of PPARγ and C/EBPα, and subsequently LXR-α, LPL, aP2, GLUT4 and adiponectin | [85] |
| Momordica charantia (L.) (Cucurbitaceae) | Fruit | Fruit juice | Inhibition of expression of PPARγ, SREBP-1c, and perillipin | [86] |
| Panax ginseng C.A. Mey. (Araliaceae) | Root | Ginsenosides Rg3, Rh1, and Rh2. | Inhibition of expression of PPARγ, and C/EBPα, subsequently FABP4 and FAS | [87–89] |
| Petasites japonicus (Siebold & Zucc.) Maxim. (Asteraceae) | Flower Bud | Ethanol extract | Inhibition of expression of PPARγ, C/EBPα, and SREBP-1c | [90] |
| Salacia reticulata Wight (Celastraceae) | Whole | Aqueous extract | Inhibition of expression of PPARγ, C/EBPα, and GPDH | [91, 92] |
| Vigna nakashimae (Fabaceae) | Seed | Ethanol extract | Inhibition of expression of PPARγ via activation of adenosine monophosphate (AMP)-activated protein kinase (AMPK) | [93] |
| Vitis vinifera (L.) (Vitaceae) | Seed | Vitisin A | Inhibition of expression of PPARγ, and C/EBPα | [94] |
| Wasabia japonica (Miq.) Matsum. (Brassicaceae) | Leaf | Aqueous extract | Inhibition of PPARγ, C/EBPα, SREBP-1c, and adiponectin | [95, 96] |
| Zanthoxylum piperitum (L.) DC (Rutaceae) | Fruit | Ethanol extract | Inhibition of expression of PPARγ, C/EBPα, and SREBP-1c | [97] |
Inhibitor of lipid droplets production or promoter of lypolysis
Albizia julibrissin Durazz.—Fabaceae
Albizia julibrissin, used as a remedy for insomnia, amnesia, sore throat, and contusions, is a native plant in Japan, China, and Korea. Studies showed that a 90 % aqueous ethanol extract of A. julibrissin flowers inhibited TG accumulation in the mouse fibroblastic cell line, 3T3–L1. Bioassay-guided separation led to isolation of four flavanol acylglycosides, 3″-(E)-p-coumaroylquercitrin, 3″-(E)-p-feruloylquercitrin, 3″-(E)-p-cinnamoylquercitrin, and 2″-(E)-p-cinnamoylquercitrin (Fig. 9). The bioactivity assay of these four compounds was tested for the inhibition of GPDH that converts glycerol into TG, and showed that 3″-(E)-p-coumaroylquercitrin was the most potent of the four compounds to give 38.4 % inhibition of TG accumulation in 3T3–L1 cells [98].
Fig. 9.
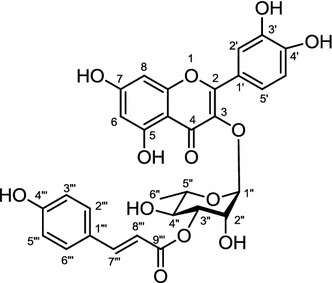
Stucture of 3″-(E)-p-coumaroylquercitrin
Citrus unshu Marcovitch—Rutaceae
Fruits of Citrus unshu or Citrus unshiu (Satsuma mandarin orange)are known to contain an active ingredient, p-synephrine (Fig. 10) [99]. Juice from unripen fruits of C. unshu was reported to induce lipolysis in fat cells isolated from male Wistar rats in a concentration-dependent manner. To elucidate the mechanism involved in inducing lipolysis by unripen C. unshu fruit juice, an aqueous extract of lyophilized C. unshu fruit juice was added to the isolated fat cells in the presence of β-blocker (inhibitor of lipolysis). Incubation with selective β1-antagonist (atenolol), β2-antagonist (IC118551), and β3-antagonist (SR59230A) and non-selective β-blocker (propranolol), indicated that an aqueous extract of C. unshu acts as a non-selective β-agonist. Another in vivo study, also showed that its aqueous extract increased lipolysis in rat visceral (epididymal and omental) and subcutaneous (abdominal and femoral) fat cells. Although p-synephrine is reported to be the bio-active ingredient that induces lipolysis, further studies are required to confirm this claim [100]. In another study, C. unshu peel extract was found to reduce as much as 50 % of perilipin, a lipid-associated protein known to be secreted only in adipocytes, and to be responsible for stabilizing lipid droplets. This fact may indicate that C. unshu peel extract is related to the inhibition of the formation of lipid droplets [31, 101]. Another in vivo investigation reported that oral administration of a major carotenoid compound from C. unshu peel, β-cryptoxanthin (Fig. 10), effectively reduces visceral adipose tissue, body weight, and abdominal circumference of Tsumura Suzuki Obese Diabetes (TSOD) mice [102].
Fig. 10.
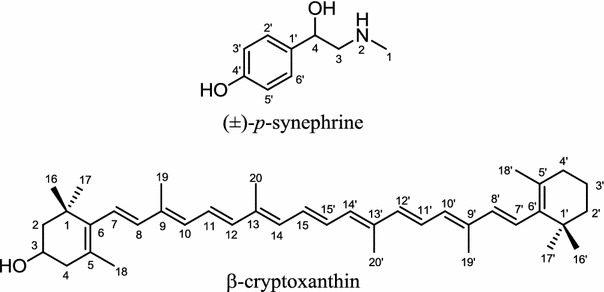
Structures of (±)-p-synephrine and β-cryptoxanthin
Capsicum annuum (L.)—Solanaceae
Capsicum annuum, more commonly known as paprika or red pepper, contains a well-known compound capsaisin (Fig. 11). Water extract from C. annuum fruits was recently shown to inhibit the action of LPL [103], a major enzyme which reduces lipoprotein into monoacylglycerol and free fatty acid [22]. Capsaisin is known to have an anti-LDA effect via several suggested routes, including promotion of thermogenesis and activation of AMPK [104, 105]. Another less well-known related compound of capsaisin, capsiate (Fig. 11), is also reported to increase thermogenesis [106–108]. In addition to capsaisin and capsiate, from a methanol extract of the fruit of C. annuum, was isolated 9-oxooctadeca-10,12-dienoic acid (Fig. 11), an inhibitor of ACC, an enzyme that carboxylates acetyl-CoA into malonyl-CoA, an important precursor in the fatty acid biosynthesis [109]. In addition to impairing the biosynthesis of lipid droplets, several studies also indicated that a fruit extract of this plant possesses an anti-adipogenic activity when tested by using 3T3-L1 cells [110, 111].
Fig. 11.
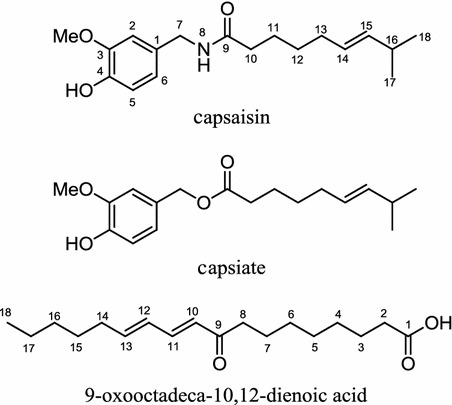
Structures of capsaisin, capsiate, and 9-oxooctadeca-10,12-dienoic acid
Coptis chinensis Franch.—Ranunculaceae
Coptis chinensis or Coptis japonica, is commonly known as Huanglian in China, Ouren in Japan or Hwangryunhaedok-tang in Korea. C. chinensis is well-known for containing a yellow isoquinoline alkaloid, berberine (Fig. 12) [112, 113]. Berberine itself is isolated from many other plants and was previously suggested to be effective in the treatment of cancer, diabetes, and obesity [114–119]. Its use as an agent for treatment of diabetes and obesity might have suggested the possible use of berberine as an anti-LDA agent. Early in vitro and in vivo studies on the effect of berberine indicated that it inhibits ACC, one of the key lipogenesis enzymes which mediate activation of AMPK. AMPK inhibits the ACC activity via phosphorylation of ACC, as demonstrated in myoblasts and adipocyte in vitro and also in db/db mice in vivo [120]. Similar results were also reciprocated by another study, which reported a similar result showing that berberine also causes activation of AMPK in HepG2, the human hepatocellular carcinoma cell line [121]. In addition to the inhibition of lipogenesis, the anti-adipogenesis effect of berberine was also reported. In summary, berberine has been shown to inhibit the expression of PPARγ, and C/EBPα while up-regulating GATA binding protein-2 (GATA-2) and -3 that functions as an adipocyte differentiation suppressor [7, 122, 123].
Fig. 12.
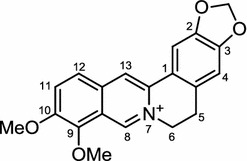
Structure of berberine
Curcuma longa (L.)—Zingiberaceae
Curcuma longa, more commonly known as turmeric, is cultivated in many parts of the tropical countries, from the root of which a member of compounds have been isolated, including curcumin, demethoxycurcumin and bisdemethoxycurcumin (Fig. 13). Studies on the mixtures of the above mentioned compounds have shown that in rats fed with HFD, administration of such mixtures caused reduction in the body weight gain and LDL-c [10]. In another study, an ethyl acetate fraction from a C. longa root methanol extract was shown to cause partial inhibition of lipid synthesis in 3T3–L1 cells, which was suggested to be via the suppression of GLUT4 expression and stimulation of lipolysis via induction of hormone sensitive lipase (HSL) and ATGL, where both lipases played a role as a rate-limiting enzyme in a lipolysis process [124]. Further investigations of those above mentioned, suggested lipolysis activity may be mediated by curcumin. An in vivo study showed that when added as a supplement to HFD at 500 mg/kg, curcumin itself caused the body weight reduction of C57BL/6J mice. Further studies indicated that when curcumin is administrated to C57BL/6J mice fed with HFD at 500 mg/kg, it increased AMPK activity and CPT1 expression while reducing glycerol-3-phosphate acyl transferase-1. Thus, it causes increases in oxidation and decreases in fatty acid esterification. Subsequent confirmation with RT-PCR, showed that curcumin also lowered the expression of PPARγ, and C/EBPα [125]. Investigation by another researcher indicated that curcumin also reduced the expression of PPARγ, and C/EBPα in 3T3–L1 cells, further supporting the hypothesis of curcumin influence on PPARγ, and C/EBPα expression [126]. Participation of the Wnt signaling pathway in the curcumin-induced suppression of adipogenesis in 3T3–L1 cells was reported. Curcumin was shown to dose dependently restored nuclear translocation of β-catenin, a major component of Wnt signaling, though the definite relationship between Wnt signaling pathway and reduction in PPARγ, and C/EBPα expression remains unclear (Table 2) [127].
Fig. 13.
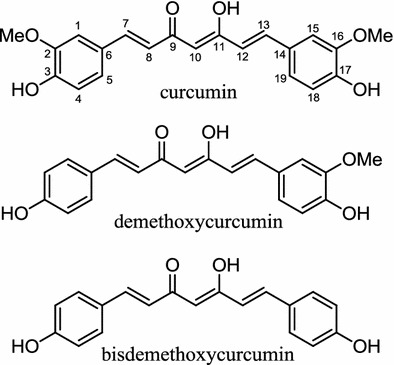
Structures of curcumin, demethoxycurcumin, and bisdemethoxycurcumin
Table 2.
List of plants possibly working as inhibitors of lipid droplets synthesis or as promoter of lypolysis
| Species name (family name) |
Plant parts used | Bioactive anti-LDA fraction(s) or compound(s) | Mechanism of action | Ref. no. |
|---|---|---|---|---|
| Bergenia crassifolia (L.) Fritsch. (Saxifragaceae) | Root | 3,11-di-O-galloylbergenin, 4,11-di-O-galloylbergenin | Not reported | [128] |
| Citrus sunki Hort. ex. Tanaka. (Rutaceae) | Fruit Peel | Ethanol extract | Activation of AMPK and ACC inhibitor | [129] |
| Elsholtzia ciliata (Thunb.) Hyl. (Lamiaceae) | Whole | Ethanol extract | Inhibition of expression of PPARγ, and subsequently FAS and aP2 | [130] |
| Galega officinalis (L.) (Fabaceae) | Whole | Galegine | ACC inhibitor | [131] |
| Nelumbo nucifera Gaertn. (Nelumbonaceae) | Leaf and Seed | Ethanol extract | Inhibition of expression of FAS and SREBP-1c and acting as ACC inhibitor, and down-regulater of PPARγ | [132–136] |
| Nepeta japonica Maximowicz (Lamiaceae) | Whole | Ethanol extract | Inhibition of pancreatic lipase activity | [137] |
| Peucedanum japonicum Thunb. (Apiaceae) | Leaf and Stem | Ethanol extract | Increase of expression of ATGL | [138–141] |
| Sasa quelpaertensis Nakai. (Poaceae) | Leaf | p-coumaric acid | Activation of AMPK leading to increase in fatty acid oxidation activity | [142–145] |
| Zingiber mioga Rosc. (Zingiberaceae) | Shoot | Ethanol extract | Reduction TG and G3PDH whose exact mechanism is not yet elucidated | [146] |
Conclusions
Concerns over various understandable health implications possibly caused by excessive accumulation of lipid droplets have stimulated studies in the search for anti-LDA agents. However the body energy homeostasis system is all too complex to allow easy solution to the problem. This fact, in a way allows researchers to approach this problem from various wider aspects. This paper is a summary of such attempts from the plant chemistry view point. Recent identification of several plants and their contents showing significant potential activity in inhibiting LDA both in vitro and in vivo has been reported. Many studies are still conducted by using crude extracts, but cases supporting their bioactive compounds that are responsible for the expected activity are isolated and, nevertheless, their mechanisms of action involved have also been reported. As to date, the studies on plant-derived anti-LDA agents are considered to be in their early stages and more in-depth studies are necessary. Nonetheless, from the plant chemistry viewpoint, plant derived anti-LDA agents hold great potential to be developed as a remedy for diseases caused by excessive accumulation of lipid droplets in the future.
References
- 1.Trayhum P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc. 2001;60:329–339. doi: 10.1079/pns200194. [DOI] [PubMed] [Google Scholar]
- 2.Vidal-Puig AJ, Sethi JK. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007;48:1253–1262. doi: 10.1194/jlr.R700005-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. Int J Clin Endocrinol Metab. 2004;89(6):2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 4.Willett WC, Manson JE, Stampfer MJ, Colditz GA, Rosner B, Speizer FE, Hennekens CH. Weight, weight change, and coronary heart disease in women: risk within the “normal” weight range. J Am Med Assoc. 1995;273:461–465. doi: 10.1001/jama.1995.03520300035033. [DOI] [PubMed] [Google Scholar]
- 5.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 6.Hu Y, Davies GE. Berberine inhibits adipogenesis in high-fat diet-induced obesity mice. Fitoterapia. 2010;81:358–366. doi: 10.1016/j.fitote.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Beekum O, Fleskens V, Kalkhoven E (2009) Posttranslational modifications of PPAR-γ: fine-tuning the metabolic master regulator. Obesity 17:213–219 [DOI] [PubMed]
- 8.Kim HK, Nelson-Dooley C, Della-Fera MA, Yang JY, Zhang W, Duan J, Hartzell DL, Hamrick MW, Baile CA. Genistein decreases food intake, body weight, and fat pad weight and causes adipose tissue apoptosis in ovariectomized female mice. J Nutr. 2006;136(2):409–414. doi: 10.1093/jn/136.2.409. [DOI] [PubMed] [Google Scholar]
- 9.Sarma DN, Barrett ML, Chavez ML, Gardiner P, Ko R, Mahady GB, Marles RJ, Pellicore LS, Giancaspro GI, Low DT. Safety of green tea extracts: a systematic review by the US Pharmacopeia. Drug Saf. 2008;31:469–484. doi: 10.2165/00002018-200831060-00003. [DOI] [PubMed] [Google Scholar]
- 10.Asai A, Miyazawa T. Dietary curcuminoids prevent high-fat diet-induced lipid accumulation in rat liver and epididymal adipose tissue. J Nutr. 2001;131(11):2932–2935. doi: 10.1093/jn/131.11.2932. [DOI] [PubMed] [Google Scholar]
- 11.Lowell BB, Flier JS. Brown adipose tissue, beta 3-adrenergic receptors and obesity. Annu Rev Med. 1997;48:307–316. doi: 10.1146/annurev.med.48.1.307. [DOI] [PubMed] [Google Scholar]
- 12.Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 13.Huang H, Song TJ, Li X, Hu L, He Q, Liu M, Lane MD, Tang QQ. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci USA. 2009;106:12670–12675. doi: 10.1073/pnas.0906266106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zamani N, Brown C. Emerging roles for the transforming growth factor-beta superfamily in regulating adiposity and energy expenditure. Endrocr Rev. 2010;32:387–403. doi: 10.1210/er.2010-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawai M, Rosen CJ. The IGF-I regulatory system and its impact on skeletal and energy homeostasis. J Cell Biochem. 2010;111(1):14–19. doi: 10.1002/jcb.22678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zúñiga LA, Shen WJ, Joyce-Shaikh B, Pyatnova EA, Richards AG, Thom C, Andrade SM, Cua DJ, Kraemer FB, Butcher EC. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol. 2010;185:6947–6959. doi: 10.4049/jimmunol.1001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Widberg CH, Newell FS, Bachmann AW, Ramnoruth SN, Spelta MC, Whitehead JP, Hutley LJ, Prins JB. Fibroblast growth factor receptor 1 is a key regulator of early adipogenic events in human preadipocytes. Am J Physiol Endocinol Metab. 2009;296:121–131. doi: 10.1152/ajpendo.90602.2008. [DOI] [PubMed] [Google Scholar]
- 18.Xiao L, Sobue T, Esliger A, Kronenberg MS, Coffin JD, Doetschman T, Hurley MM. Disruption of the Fgf2 gene activates the adipogenic and suppresses the osteogenic program in mesenchymal marrow stromal stem cells. Bone. 2010;47:360–370. doi: 10.1016/j.bone.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaragosi LE, Wdziekonski B, Villageois P, Keophiphath M, Maumus M, Tchkonia T, Bourlier V, Mohsen-Kanson T, Ladoux A, Elabd C, Scheideler M, Trajanoski Z, Takashima Y, Amri E, Lacasa D, Sengenes C, Ailhaud G, Clément K, Bouloumie A, Kirkland J, Dani C. Activin A plays a critical role in proliferation and differentiation of human adipose progenitors. Diabetes. 2010;59:2513–2521. doi: 10.2337/db10-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawai M, Namba N, Mushiake S, Etani Y, Nishimura R, Makishima M, Ozono K. Growth hormone stimulates adipogenesis of 3T3–L1 cells through activation of the Stat5A/5B-PPARgamma pathway. J Mol Endocrinol. 2007;38:19–34. doi: 10.1677/jme.1.02154. [DOI] [PubMed] [Google Scholar]
- 21.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 22.White UA, Stephens JM. Transcriptional factors that promote formation of white adipose tissue. Mol Cell Endocrinol. 2010;318:10–14. doi: 10.1016/j.mce.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.James DE, Brown R, Navarro J, Pilch PF. Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature. 1988;333(6169):183–185. doi: 10.1038/333183a0. [DOI] [PubMed] [Google Scholar]
- 24.Mead JR, Irvine SA, Ramji DP. Lipoprotein lipase: structure, function, regulation, and role in disease. J Mol Med. 2002;80(12):753–769. doi: 10.1007/s00109-002-0384-9. [DOI] [PubMed] [Google Scholar]
- 25.Seckl JR, Walker BR. Minireview: 11β-hydroxysteroid dehydrogenase type 1- a tissue-specific amplifier of glucocorticoid action. Endocrinology. 2001;142(4):1371–1376. doi: 10.1210/endo.142.4.8114. [DOI] [PubMed] [Google Scholar]
- 26.Seckl JR. 11beta-Hydroxysteroid dehydrogenase in the brain: a novel regulator of glucocorticoid action? Front Neuroendocrinol. 1997;18(1):49–99. doi: 10.1006/frne.1996.0143. [DOI] [PubMed] [Google Scholar]
- 27.Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. The pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab. 2009;94(8):2692–2701. doi: 10.1210/jc.2009-0370. [DOI] [PubMed] [Google Scholar]
- 28.Baxa CA, Sha RS, Buelt MK, Smith AJ, Matarese V, Chinander LL, Boundy KL, Bernlohr DA. Human adipocyte lipid-binding protein: purification of the protein and cloning of its complementary DNA. Biochemistry. 1989;28(22):8683–8690. doi: 10.1021/bi00448a003. [DOI] [PubMed] [Google Scholar]
- 29.Christiansen K, Jensen PK. Membrane-bound lipid particles from beef heart. Chemical composition and structure. Biochim Biophys Acta. 1972;260:449–459. doi: 10.1016/0005-2760(72)90060-4. [DOI] [PubMed] [Google Scholar]
- 30.Lass A, Zimmermann R, Oberer M, Zechner R. Lipolysis—A highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog Lipid Res. 2011;50:14–27. doi: 10.1016/j.plipres.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zweytick D, Athenstaedt K, Daum G. Intracellular lipid particles of eukaryotic cells. Biochim Biophys Acta. 2000;1469:101–120. doi: 10.1016/s0005-2736(00)00294-7. [DOI] [PubMed] [Google Scholar]
- 32.Ohsaki Y, Cheng J, Suzuki M, Shinohara Y, Fujita A, Fujimoto T. Biogenesis of cytoplasmic lipid droplets: from the lipid ester globule in the membrane to the visible structure. Biochim Biophys Acta. 2009;1791:399–407. doi: 10.1016/j.bbalip.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Reue K. A thematic review series: lipid droplet storage and metabolism: from yeast to man. J Lipid Res. 2011;52:1865–1868. doi: 10.1194/jlr.E020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS. Regulation of lipolysis in adipocytes. Annu Rev Nutr. 2007;27:79–101. doi: 10.1146/annurev.nutr.27.061406.093734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 37.Fredrickson G, Strålfors P, Nilsson NÖ, Belfrage P. Hormone-sensitive lipase of rat adipose tissue. Purification and some properties. J Biol Chem. 1981;256:6311–6320. [PubMed] [Google Scholar]
- 38.Kwak DH, Lee JH, Kim T, Ahn HS, Cho WK, Ha H, Hwang YH, Ma JY (2012) Aristolochia manshuriensis Kom inhibits adipocyte differentiation by regulation of ERK1/2 and Akt pathway. PLOS one 7(11):e49530 [DOI] [PMC free article] [PubMed]
- 39.Shibata S. A drug over the millennia: pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi. 2000;120:849–862. doi: 10.1248/yakushi1947.120.10_849. [DOI] [PubMed] [Google Scholar]
- 40.Quan HY, Baek NI, Chung SH. Licochalcone A prevents adipocyte differentiation and lipogenesis via suppression of peroxisome proliferator-activated receptor γ and sterol regulatory element-binding protein pathways. J Agri Chem. 2012;60:5112–5120. doi: 10.1021/jf2050763. [DOI] [PubMed] [Google Scholar]
- 41.Lin J, Della-Fera MA, Baile CA. Green tea polyphenol epigallocatechin gallate inhibits adipogenesis and induces apoptosis in 3T3–L1 adipocytes. Obes Res. 2005;13:982–990. doi: 10.1038/oby.2005.115. [DOI] [PubMed] [Google Scholar]
- 42.Söhle J, Knott A, Holtzmann U, Siegner R, Grönniger E, Schepky A, Gallinat S, Wenck H, Stäb F, Winnefeld M (2009) White tea extract induces lipolytic activity and inhibits adipogenesis in human subcutaneous (pre)-adipocytes. Nutr Metab (Lond). 20(6) [DOI] [PMC free article] [PubMed]
- 43.Kim H, Sakamoto K. (-)-Epigallocatechin gallate suppresses adipocyte differentiation through the MEK/ERK and PI3K/Akt pathways. Cell Biol Int. 2012;36(2):147–153. doi: 10.1042/CBI20110047. [DOI] [PubMed] [Google Scholar]
- 44.Han LK, Takaku T, Li J, Kimura Y, Okuda H. Anti-obesity action of oolong tea. Int J Obes. 1999;23:98–105. doi: 10.1038/sj.ijo.0800766. [DOI] [PubMed] [Google Scholar]
- 45.Sayama K, Lin S, Zheng G, Oguni I. Effects of green tea on growth, food utilization and lipid metabolism in mice. In Vivo. 2000;14:481–484. [PubMed] [Google Scholar]
- 46.Han LK, Kimura Y, Kawashima M, Takaku T, Taniyama T, Hayashi T, Zheng YN, Okuda H. Anti-obesity effects in rodents of dietary tea saponin, a lipase inhibitor. Int J Obes. 2001;25:1459–1464. doi: 10.1038/sj.ijo.0801747. [DOI] [PubMed] [Google Scholar]
- 47.Mohamad K, Hirasawa Y, Lim CS, Awang K, Hadi AHA, Takeya K, Morita H. Ceramicine A and walsogyne A, novel limonoids from two species of Meliaceae. Tetrahedron Lett. 2008;49:4276–4278. [Google Scholar]
- 48.Mohamad K, Hirasawa Y, Litaudon M, Awang K, Hadi AHA, Takeya K, Ekasari W, Widyawaruyanti A, Zaini NC, Morita H. Ceramicines B–D, new antiplasmodial limonoids from Chisocheton ceramicus. Bioorg Med Chem. 2009;17:727–730. doi: 10.1016/j.bmc.2008.11.048. [DOI] [PubMed] [Google Scholar]
- 49.Wong CP, Shimada M, Nagakura Y, Nugroho AE, Hirasawa Y, Kaneda T, Awang K, Hadi AHA, Mohamad K, Shiro M, Morita H. Ceramicines E–I, New Limonoids from Chisocheton ceramicus. Chem Pharm Bull. 2011;59:407–411. doi: 10.1248/cpb.59.407. [DOI] [PubMed] [Google Scholar]
- 50.Wong CP, Shimada M, Nugroho AE, Hirasawa Y, Kaneda T, Awang K, Hadi AHA, Mohamad K, Shiro M, Morita H. Ceramicines J–L, New Limonoids from Chisocheton ceramicus. J Nat Med. 2012;66:566–570. doi: 10.1007/s11418-011-0616-9. [DOI] [PubMed] [Google Scholar]
- 51.Najmuldeen IA, Hadi AH, Awang K, Mohamad K, Chong SL, Chan G, Nafiah MA, Weng NS, Shirota O, Hosoya T, Nugroho AE, Morita H. Chisomicines A–C, Limonoids from Chisocheton ceramicus. J Nat Prod. 2011;74(5):1313–1317. doi: 10.1021/np200013g. [DOI] [PubMed] [Google Scholar]
- 52.Najmuldeen IA, Hadi AH, Mohamad K, Awang K, Ketuly KA, Mukhtar MR, Taha H, Nordin N, Litaudon M, Guéritte F, Nugroho AE, Morita H. Chisomicines A–C, Two New Limonoids from Chisocheton ceramicus. Heterocycles. 2012;84(2):1265–1270. [Google Scholar]
- 53.Najmuldeen IA, Hadi AH, Awang K, Mohamad K, Ng SW. 14-Deoxyxyloccensin K from Chisocheton ceramicus (Meliaceae) Acta Crystallographica Section E. 2010;66:1927. doi: 10.1107/S160053681002564X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong CP, Kaneda T, Hadi AHA, Morita H (2013) Ceramicine B, a limonoid with anti-lipid droplets accumulation activity from Chisocheton ceramicus. J Nat Med 68(1):22–30 [DOI] [PubMed]
- 55.Wong CP, Deguchi J, Nugroho AE, Kaneda T, Hadi AHA, Morita H. Ceramcines from Chisocheton ceramicus as lipid-droplets accumulation inhibitors. Bioorg Med Chem Lett. 2013;23:1786–1788. doi: 10.1016/j.bmcl.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 56.Munekata K, Sakamoto K. Forkhead transcription factor Foxo1 is essential for adipocyte differentiation. In Vitro Cell Dev Biol. 2009;45:642–651. doi: 10.1007/s11626-009-9230-5. [DOI] [PubMed] [Google Scholar]
- 57.Armoni M, Harel C, Karni S, Chen H, Bar-Yoseph F, Ver MR, Quon MJ, Karnieli E. FOXO1 represses PPARγ1 and PPARγ2 gene promoters in primary adipocytes: a novel paradigm to increase insulin sensitivity. J Bio Chem. 2006;281(29):19881–19891. doi: 10.1074/jbc.M600320200. [DOI] [PubMed] [Google Scholar]
- 58.Seo JB, Choe SS, Jeong HW, Park SW, Shin HJ, Choi SM, Park JY, Choi EW, Kim JB, Seen DS, Jeong JY, Lee TG. Anti-obesity effect of Lysimachia foenum-graecum characterized by decreased adipogenesis and regulated lipid metabolism. Exp Mol Med. 2011;43(4):205–215. doi: 10.3858/emm.2011.43.4.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen YH, Weng ZY, Zhao QS, Zeng YQ, Rios JL, Xiao WL, Xu G, Sun HD. Five new triterpene glycosides from Lysimachia foenum-graecum and evaluation of their effect on arachidonic acid metabolizing enzyme. Planta Med. 2005;71:770–775. doi: 10.1055/s-2005-871289. [DOI] [PubMed] [Google Scholar]
- 60.Li XR, Li ZM, Du SS, Wang GL, Lin RC. Two triterpenes from Lysimachia foenum-graecum. J Asian Nat Prod Res. 2009;11:128–131. doi: 10.1080/10286020802573859. [DOI] [PubMed] [Google Scholar]
- 61.Li XR, Li ZM, Lin RC. Two triterpenes from Lysimachia foenum-graecum. J Asian Nat Prod Res. 2009;11:529–533. doi: 10.1080/10286020902930884. [DOI] [PubMed] [Google Scholar]
- 62.Seo JB, Park SW, Choe SS, Jeong HW, Park JY, Choi EW, Seen DS, Jeong JY, Lee TG. Foenumoside B from Lysimachia foenum-graecum inhibits adipocyte differentiation and obesity induced by high-fat diet. Biochem Biophys Res Commun. 2012;417(2):800–806. doi: 10.1016/j.bbrc.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 63.Kong C, Lee JI, Kim J, Seo Y. In vitro evaluation on the antiobesity effect of lignin from the flower buds of Magnolia denudate. J Agric Food Chem. 2011;59:5665–5670. doi: 10.1021/jf200230s. [DOI] [PubMed] [Google Scholar]
- 64.Martineau LC, Muhammad A, Saleem A, Hervé J, Harris CS, Arnason JT, Haddad PS. Anti-adipogenic activities of Alnus incana and Populus balsamifera bark extracts, part II: bioassay-guided identification of actives salicortin and oregonin. Planta Med. 2010;76(14):1519–1524. doi: 10.1055/s-0029-1240991. [DOI] [PubMed] [Google Scholar]
- 65.Harbilas D, Vallerand D, Brault A, Saleem A, Arnason JT, Musallam L, Haddad PS (2013) Populus balsamifera extract and its active component salicortin reduce obesity and attenuate insulin resistance in a diet-induced obese mouse model. Evid Based Complement Alternat Med 2013:172537 [DOI] [PMC free article] [PubMed]
- 66.Lee M, Lee SH, Kang J, Yang H, Jeong EJ, Kim HP, Kim YC, Sung SH. Salicortin-derivatives from Salix pseudo-lasiogyne Twigs inhibit adipogenesis in 3T3–L1 cells via modulation of C/EBPα and SREBP-1c dependent pathway. Molecules. 2013;18:10484–10496. doi: 10.3390/molecules180910484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jung CH, Jang SJ, Ahn J, Gwon SY, Jeon TI, Kim TW, Ha TY. Alpinia officinarum inhibits adipocyte differentiation and high-fat diet-induced obesity in mice through regulation of adipogenesis and lipogenesis. J Med Food. 2012;15(11):959–967. doi: 10.1089/jmf.2012.2286. [DOI] [PubMed] [Google Scholar]
- 68.Choi HK, Won EK, Jang YP, Choung SY (2013) Antiobesity effect of Codonopsis lanceolata in high-calorie/high-fat-diet-induced obese rats. Evid Based Complement Alternat Med 2013:210297 [DOI] [PMC free article] [PubMed]
- 69.Lee YJ, Kim DB, Lee JS, Cho JH, Kim BK, Choi HS, Lee BY, Lee OH. Antioxidant activity and anti-adipogenic effects of wild herbs mainly cultivated in Korea. Molecules. 2013;18(10):12937–12950. doi: 10.3390/molecules181012937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cho K, Kim SJ, Park SH, Kim S, Park T. Protective effect of Codonopsis lanceolata root extract against alcoholic fatty liver in the rat. J Med Food. 2009;12(6):1293–1301. doi: 10.1089/jmf.2009.0085. [DOI] [PubMed] [Google Scholar]
- 71.Jadeja RN, Thounaojam MC, Ramani UV, Devkar RV, Ramachandran AV. Anti-obesity potential of Clerodendron glandulosum Coleb leaf aqueous extract. J Ethnopharm. 2011;135:338–343. doi: 10.1016/j.jep.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 72.Jadeja RN, Thounaojam MC, Singh TB, Devkar RV, Ramachandran AV. Traditional uses, phytochemistry and pharmacology of Clerodendron glandulosum Coleb-A review. Asian Pac J Trop Med. 2012;5(1):1–6. doi: 10.1016/S1995-7645(11)60236-8. [DOI] [PubMed] [Google Scholar]
- 73.Lee J, Kim D, Choi J, Choi H, Ryu JH, Jeong J, Park EJ, Kim SH, Kim S. Dehydrodiconiferyl alcohol isolated from Cucurbita moschata shows anti-adipogenic and anti-lipogenic effects in 3T3–L1 cells and primary mouse embryonic fibroblasts. J Biol Chem. 2012;287(12):8839–8851. doi: 10.1074/jbc.M111.263434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xiao J, Wang NL, Sun B, Cai GP. Estrogen receptor mediates the effects of pseudoprotodiocsin on adipogenesis in 3T3–L1 cells. Am J Physiol Cell Physiol. 2010;299(1):128–138. doi: 10.1152/ajpcell.00538.2009. [DOI] [PubMed] [Google Scholar]
- 75.Kwon CS, Sohn HY, Kim SH, Kim JH, Son KH, Lee JS, Lim JK, Kim JS. Anti-obesity effect of Dioscorea nipponica Makino with lipase-inhibitory activity in rodents. Biosci Biotechnol Biochem. 2003;67(7):1451–1456. doi: 10.1271/bbb.67.1451. [DOI] [PubMed] [Google Scholar]
- 76.Jung HA, Jung HJ, Jeong HY, Kwon HJ, Kim M-S, Choi JS (2013) Anti-adipogenic activity of the edible brown alga Ecklonia stolonifera and its constituent fucosterol in 3T3–L1 adipocytes. Arch Pharm Res. doi:10.1007/s12272-013-0237-9 [DOI] [PubMed]
- 77.Kobayashi Y, Nakano Y, Kizaki M, Hoshikuma K, Yokoo Y, Kamiya T. Capsaicin-like anti-obese activities of evodiamine from fruits of Evodia rutaecarpa, a vanilloid receptor agonist. Planta Med. 2001;67:628–633. doi: 10.1055/s-2001-17353. [DOI] [PubMed] [Google Scholar]
- 78.Wang T, Wang Y, Yamashita H. Evodiamine inhibits adipogenesis via the EGFR-PKCalpha-ERK signaling pathway. FEBS Lett. 2009;583:3655–3659. doi: 10.1016/j.febslet.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 79.Kim MS, Kim JK, Kim HJ, Moon SR, Shin BC, Park KW, Yang HO, Kim SM, Kim KY, Park R. Hibiscus extract inhibits the lipid droplet accumulation and adipogenic transcription factors expression of 3T3–L1 preadipocytes. J Altern Complement Med. 2003;9:499–504. doi: 10.1089/107555303322284785. [DOI] [PubMed] [Google Scholar]
- 80.Kim JY, So H, Youn M, Kim HJ, Kim Y, Park C, Kim SJ, Ha YA, Chai KY, Kim SM, Kim KY, Park R. Hibiscus sabdariffa L. water extract inhibits the adipocyte differentiation through the PI3-K and MAPK pathway. J Ethnopharm. 2007;114:260–267. doi: 10.1016/j.jep.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 81.Oben JE, Ngondi JL, Blum K (2008) Inhibition of Irvingia gabonensis seed extract (OB131) on adipogenesis as mediated via down regulation of the PPARgamma and leptin genes and up-regulation of the adiponectin gene. Lipids Health Dis 7:44 [DOI] [PMC free article] [PubMed]
- 82.Klein G, Kim J, Himmeldirk K, Cao Y, Chen X. Anti-diabetes and anti-obesity activity of Lagerstroemia speciosa. Evid Based Complement Altern Med. 2007;4:401–407. doi: 10.1093/ecam/nem013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu F, Kim J, Li Y, Liu X, Li J, Chen X. An extract of Lagerstroemia speciosa L. has insulin-like glucose uptake-stimulatory and adipocyte differentiation-inhibitory activities in 3T3–L1 cells. J Nutr. 2001;131:2242–2247. doi: 10.1093/jn/131.9.2242. [DOI] [PubMed] [Google Scholar]
- 84.Liu X, Kim J, Li Y, Li J, Liu F, Chen X. Tannic acid stimulates glucose transport and inhibits adipocyte differentiation in 3T3–L1 cells. J Nutri. 2005;135:165–171. doi: 10.1093/jn/135.2.165. [DOI] [PubMed] [Google Scholar]
- 85.Hsieh YH, Wang SY. Lucidone from Lindera erythrocarpa Makino fruits suppresses adipogenesis in 3T3–L1 cells and attenuates obesity and consequent metabolic disorders in high-fat diet C57BL/6 mice. Phytomedicine. 2013;20(5):394–400. doi: 10.1016/j.phymed.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 86.Nerurkar PV, Lee YK, Nerurkar VR (2010) Momordica charantia (bitter melon) inhibits primary human adipocyte differentiation by modulating adipogenic genes. BMC Complement Altern Med 10:34 [DOI] [PMC free article] [PubMed]
- 87.Hwang JT, Lee MS, Kim HJ, Sung MJ, Kim HY, Kim MS, Kwon DY. Antiobesity effect of ginsenoside Rg3 involves the AMPK and PPAR-gamma signal pathways. Phytother Res. 2009;23:262–266. doi: 10.1002/ptr.2606. [DOI] [PubMed] [Google Scholar]
- 88.Hwang JT, Kim SH, Lee MS, Kim SH, Yang HJ, Kim MJ, Kim HS, Ha J, Kim MS, Kwon DY. Anti-obesity effects of ginsenoside Rh2 are associated with the activation of AMPK signaling pathway in 3T3–L1 adipocyte. Biochem Biophys Res Commun. 2007;364(4):1002–1008. doi: 10.1016/j.bbrc.2007.10.125. [DOI] [PubMed] [Google Scholar]
- 89.Gu W, Kim K, Kim DH. Ginsenoside Rh1 ameliorates high fat diet-induced obesity in mice by inhibiting adipocyte differentiation. Biol Pharm Bull. 2013;36(1):102–107. doi: 10.1248/bpb.b12-00558. [DOI] [PubMed] [Google Scholar]
- 90.Watanabe T, Hata K, Hiwatashi K, Hori K, Suzuki N, Itoh H. Suppression of murine preadipocyte differentiation and reduction of visceral fat accumulation by a Petasites japonicus ethanol extract in mice fed a high-fat diet. Biosci Biotechnol Biochem. 2010;74(3):499–503. doi: 10.1271/bbb.90684. [DOI] [PubMed] [Google Scholar]
- 91.Shimada T, Nagai E, Harasawa Y, Watanabe M, Negishi K, Akase T, Sai Y, Miyamoto K, Aburada M. Salacia reticulata inhibits differentiation of 3T3–L1 adipocytes. J Ethnopharm. 2011;136:67–74. doi: 10.1016/j.jep.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 92.Shimada T, Nagai E, Harasawa Y, Akase T, Aburada T, Iizuka S, Miyamoto K, Aburada M. Metabolic disease prevention and suppression of fat accumulation by Salacia reticulata. J Nat Med. 2010;64(3):266–274. doi: 10.1007/s11418-010-0401-1. [DOI] [PubMed] [Google Scholar]
- 93.Son Y, Nam JS, Jang MK, Jung IA, Cho SI, Jung MH. Antiobesity activity of Vigna nakashimae extract in high-fat diet-induced obesity. Biosci Biotechnol Biochem. 2013;77(2):332–338. doi: 10.1271/bbb.120755. [DOI] [PubMed] [Google Scholar]
- 94.Kim Sh, Park HS, Lee MS, Cho YJ, Kim YS, Hwang JT, Sung MJ, Kim MS, Kwon DY. Vitisin A inhibits adipocyte differentiation through cell cycle arrest in 3T3–L1 cells. Biochem Biophys Res Commun. 2008;372:108–113. doi: 10.1016/j.bbrc.2008.04.188. [DOI] [PubMed] [Google Scholar]
- 95.Ogawa T, Tabata H, Katsube T, Ohta Y, Yamasaki Y, Yamasaki M, Shiwaku K. Suppressive effect of hot water extract of wasabi (Wasabia japonica Matsum.) leaves on the differentiation of 3T3–L1 preadipocytes. Food Chem. 2010;118(2):239–244. [Google Scholar]
- 96.Yamasaki M, Ogawa T, Wang L, Katsube T, Yamasaki Y, Sun X, Shiwaku K. Anti-obesity effects of hot water extract from Wasabi (Wasabia japonica Matsum.) leaves in mice fed high-fat diets. Nutr Res Pract. 2013;7(4):267–272. doi: 10.4162/nrp.2013.7.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gwon SY, Ahn JY, Kim TW, Ha TY. Zanthoxylum piperitum DC ethanol extract suppresses fat accumulation in adipocytes and high fat diet-induced obese mice by regulating adipogenesis. J Nutr Sci Vitaminol. 2012;58:393–401. doi: 10.3177/jnsv.58.393. [DOI] [PubMed] [Google Scholar]
- 98.Yahagi T, Daikonya A, Kitanaka S. Flavonol acylglycosides from flower of Albizia julibrissin and their inhibitory effects on lipid accumulation in 3T3–L1 cells. Chem Pharm Bull. 2012;60(1):129–136. doi: 10.1248/cpb.60.129. [DOI] [PubMed] [Google Scholar]
- 99.Dragull K, Breksa AP, 3rd, Cain B. Synephrine content of juice from Satsuma mandarins (Citrus unshiu Marcovitch) J Agric Food Chem. 2008;56(19):8874–8878. doi: 10.1021/jf801225n. [DOI] [PubMed] [Google Scholar]
- 100.Tsujita T, Takaku T. Lipolysis induced by segment wall extract from Satsuma mandarin orange (Citrus unshu Mark) J Nutri Sci Vitaminol. 2007;53:547–551. doi: 10.3177/jnsv.53.547. [DOI] [PubMed] [Google Scholar]
- 101.Jung HK, Jeong YS, Park CD, Park CH, Hong JH. Inhibitory effect of citrus peel extract on lipid accumulation of 3T3–L1 adipocytes. J Korean Soc Appl Biol Chem. 2011;54(2):169–176. [Google Scholar]
- 102.Takayanagi K, Morimoto S, Shirakura Y, Mukai K, Sugiyama T, Tokuji Y, Ohnishi M. Mechanism of visceral fat reduction in Tsumura Suzuki obese, diabetes (TSOD) mice orally administered β-cryptoxanthin from Satsuma mandarin oranges (Citrus unshiu Marc) J Agric Food Chem. 2011;59(23):12342–12351. doi: 10.1021/jf202821u. [DOI] [PubMed] [Google Scholar]
- 103.Baek J, Lee J, Kim K, Kim T, Kim D, Kim C, Tsutomu K, Ochir S, Lee K, Park HC, Lee YJ, Choe M. Inhibitory effects of Capsicum annuum L. water extracts on lipoprotein lipase activity in 3T3–L1 cells. Nutr Res Pract. 2013;7(2):96–102. doi: 10.4162/nrp.2013.7.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Diepvens K, Westerterp KR, Westerterp-Plantenga MS. Obesity and thermogenesis related to the consumption of caffeine, ephedrine, capsaicin, and green tea. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):77–85. doi: 10.1152/ajpregu.00832.2005. [DOI] [PubMed] [Google Scholar]
- 105.Hwang JT, Park IJ, Shin JI, Lee YK, Lee SK, Baik HW, Ha J, Park OJ. Genistein, EGCG, and capsaicin inhibit adipocyte differentiation process via activating AMP-activated protein kinase. Biochem Biophys Res Commun. 2005;338(2):694–699. doi: 10.1016/j.bbrc.2005.09.195. [DOI] [PubMed] [Google Scholar]
- 106.Kawabata F, Inoue N, Yazawa S, Kawada T, Inoue K, Fushiki T. Effects of CH-19 sweet, a non-pungent cultivar of red pepper, in decreasing the body weight and suppressing body fat accumulation by sympathetic nerve activation in humans. Biosci Biotechnol Biochem. 2006;70(12):2824–2835. doi: 10.1271/bbb.60206. [DOI] [PubMed] [Google Scholar]
- 107.Ludy MJ, Moore GE, Mattes RD. The effects of capsaicin and capsiate on energy balance: critical review and meta-analyses of studies in humans. Chem Senses. 2012;37(2):103–121. doi: 10.1093/chemse/bjr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Masuda Y, Haramizu S, Oki K, Ohnuki K, Watanabe T, Yazawa S, Kawada T, Hashizume S, Fushiki T. Upregulation of uncoupling proteins by oral administration of capsiate, a nonpungent capsaicin analog. J Appl Physiol. 2003;95(6):2408–2415. doi: 10.1152/japplphysiol.00828.2002. [DOI] [PubMed] [Google Scholar]
- 109.Watanabe J, Kawabata J, Kasai T. 9-Oxooctadeca-10,12-dienoic Acids as Acetyl CoA Carboxylase Inhibitors from Red Pepper (Capsicum annuum L.) Biosci Biotechnol Biochem. 1999;63(3):489–493. doi: 10.1271/bbb.63.489. [DOI] [PubMed] [Google Scholar]
- 110.Jeon G, Choi Y, Lee SM, Kim Y, Jeong HS, Lee J. Anti-obesity activity of methanol extract from hot pepper (Capsicum annuum L.) seeds in 3T3–L1 adipocyte. Food Sci Biotechnol. 2010;19(4):1123–1127. [Google Scholar]
- 111.Arumugam M, Vijayan P, Raghu C, Ashok G, Dhanaraj SA, Kumarappan CT (2008) Anti-adipogenic activity of Capsicum annum (Solanaceae) in 3T3 L1. J Complement Integr Med 5(1):1–9
- 112.Affuso F, Mercurio V, Fazio V, Fazio S. Cardiovascular and metabolic effects of berberine. World J Cardiol. 2010;2(4):71–77. doi: 10.4330/wjc.v2.i4.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kwak DH, Lee JH, Kim DG, Kim T, Lee KJ, Ma JY (2013) Inhibitory effects of Hwangryunhaedok-Tang in 3T3-L1 adipogenesis by regulation of Raf/MEK1/ERK1/2 pathway and PDK1/Akt phosphorylation. Evid Based Complement Alternat Med 2013:413906 [DOI] [PMC free article] [PubMed]
- 114.Schor J (2012) Clinical applications for Berberine: Potential therapeutic applications in metabolic syndrome, type 2 diabetes, and dyslipidemia. Nat Med J. http://www.naturalmedicinejournal.com/article_content.asp?article=384
- 115.Cernáková M, Kostálová D. Antimicrobial activity of berberine–a constituent of Mahonia aquifolium. Folia Microbiol (Praha) 2002;47(4):375–378. doi: 10.1007/BF02818693. [DOI] [PubMed] [Google Scholar]
- 116.Yu HH, Kim KJ, Cha JD, Kim HK, Lee YE, Choi NY, You YO. Antimicrobial activity of berberine alone and in combination with ampicillin or oxacillin against methicillin-resistant Staphylococcus aureus. J Med Food. 2005;8(4):454–461. doi: 10.1089/jmf.2005.8.454. [DOI] [PubMed] [Google Scholar]
- 117.Tan W, Li Y, Chen M, Wang Y. Berberine hydrochloride: anti-cancer activity and nanoparticulate delivery system. Int J Nanomed. 2011;6:1773–1777. doi: 10.2147/IJN.S22683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Choi MS, Yuk DY, Oh JH, Jung HY, Han SB, Moon DC, Hong JT. Berberine inhibits human neuroblastoma cell growth through induction of p53-dependent apoptosis. Anticancer Res. 2008;28(6A):3777–3784. [PubMed] [Google Scholar]
- 119.Choi MS, Oh JH, Kim SM, Jung HY, Yoo HS, Lee YM, Moon DC, Han SB, Hong JT. Berberine inhibits p53-dependent cell growth through induction of apoptosis of prostate cancer cells. Int J Oncol. 2009;34(5):1221–1230. [PubMed] [Google Scholar]
- 120.Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT, Hohnen-Behrens C, Gosby A, Kraegen EW, James DE, Kim JB. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55(8):2256–2264. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- 121.Brusq JM, Ancellin N, Grondin P, Guillard R, Martin S, Saintillan Y, Issandou M. Inhibition of lipid synthesis through activation of AMP kinase: an additional mechanism for the hypolipidemic effects of berberine. J Lipid Res. 2006;47(6):1281–1288. doi: 10.1194/jlr.M600020-JLR200. [DOI] [PubMed] [Google Scholar]
- 122.Choi BH, Ahn IS, Kim YH, Park JW, Lee SY, Hyun CK, Do MS. Berberine reduces the expression of adipogenic enzymes and inflammatory molecules of 3T3–L1 adipocyte. Exp Mol Med. 2006;38(6):599–605. doi: 10.1038/emm.2006.71. [DOI] [PubMed] [Google Scholar]
- 123.Hu Y, Kutscher E, Davies GE. Berberine inhibits SREBP-1-related clozapine and risperidone induced adipogenesis in 3T3–L1 cells. Phytother Res. 2010;24(12):1831–1838. doi: 10.1002/ptr.3204. [DOI] [PubMed] [Google Scholar]
- 124.Lee J, Yoon HG, Lee YH, Park J, You Y, Kim K, Jang JY, Yang JW, Jun W. The potential effects of ethyl acetate fraction from Curcuma longa L. on lipolysis in differentiated 3T3–L1 adipocytes. J Med Food. 2010;13(2):364–370. doi: 10.1089/jmf.2009.1276. [DOI] [PubMed] [Google Scholar]
- 125.Ejaz A, Wu D, Kwan P, Meydani M. Curcumin inhibits adipogenesis in 3T3–L1 adipocytes and angiogenesis and obesity in C57/BL mice. J Nutr. 2009;139(5):919–925. doi: 10.3945/jn.108.100966. [DOI] [PubMed] [Google Scholar]
- 126.Kim CY, Le TT, Chen C, Cheng JX, Kim KH. Curcumin inhibits adipocyte differentiation through modulation of mitotic clonal expansion. J Nutr Biochem. 2011;10:910–920. doi: 10.1016/j.jnutbio.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 127.Ahn J, Lee H, Kim S, Ha T. Curcumin-induced suppression of adipogenic differentiation is accompanied by activation of Wnt/beta-catenin signaling. Am J Physiol Cell Physiol. 2010;298(6):C1510–C1516. doi: 10.1152/ajpcell.00369.2009. [DOI] [PubMed] [Google Scholar]
- 128.Janar J, Fang L, Wong CP, Kaneda T, Hirasawa Y, Shahmanova BG, Abduahitovich AZ, Morita H. A new galloylbergenin from Bergenia crassifolia with anti-lipid droplet accumulation activity. Heterocycles. 2012;86(2):1591–1595. [Google Scholar]
- 129.Kang SI, Shin HS, Kim HM, Hong YS, Yoon SA, Kang SW, Kim JH, Kim MH, Ko HC, Kim SJ. Immature Citrus sunki peel extract exhibits antiobesity effects by β-oxidation and lipolysis in high-fat diet-induced obese mice. Biol Pharm Bull. 2012;35(2):223–230. doi: 10.1248/bpb.35.223. [DOI] [PubMed] [Google Scholar]
- 130.Sung YY, Yoon T, Yang WK, Kim SJ, Kim HK. Inhibitory effects of Elsholtzia ciliata extract on fat accumulation in high-fat diet-induced obese mice. J Korean Soc Appl Biol Chem. 2011;54(3):388–394. [Google Scholar]
- 131.Mooney MH, Fogarty S, Stevenson C, Gallagher AM, Palit P, Hawley SA, Hardie DG, Coxon GD, Waigh RD, Tate RJ, Harvey AL, Furman BL. Mechanisms underlying the metabolic actions of galegine that contribute to weight loss in mice. Br J Pharmacol. 2008;153(8):1669–1677. doi: 10.1038/bjp.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ono Y, Hattori E, Fukuya Y, Imai S, Ohzumi Y. Anti-obesity effect of Nelumbo nucifera leaves extract in mice and rats. J Ethnopharm. 2006;106:238–244. doi: 10.1016/j.jep.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 133.Du H, You JS, Zhao X, Park JY, Kim SH, Chang KJ (2010) Antiobesity and hypolipidemic effects of lotus leaf hot water extract with taurine supplementation in rats fed a high fat diet. J Biomed Sci 17(Suppl 1):S42 [DOI] [PMC free article] [PubMed]
- 134.Seigner R, Heuser S, Holtzmann U, Söhle J, Schepky T, Raschke F, Stäb H, Wenck H, Winnefeld M. Lotus leaf extract and L-carnitine influence different processes during the adipocyte life cycle. Nutr Metabol. 2010;7:66. doi: 10.1186/1743-7075-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ahn JH, Kim ES, Lee C, Kim S, Cho SH, Hwang BY, Lee MK. Chemical constituents from Nelumbo nucifera leaves and their anti-obesity effects. Bioorg Med Chem Lett. 2013;23(12):3604–3608. doi: 10.1016/j.bmcl.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 136.You JS, Lee YJ, Kim KS, Kim SH, Chang KJ (2014) Anti-obesity and hypolipidaemic effects of Nelumbo nucifera seed ethanol extract in human pre-adipocytes and rats fed a high-fat diet. J Sci Food Agri 94(3):568–575 [DOI] [PubMed]
- 137.Roh C, Jung U. Nepeta japonica Maximowicz extract from natural products inhibits lipid accumulation. J Sci Food Agri. 2012;92(10):2195–2199. doi: 10.1002/jsfa.5608. [DOI] [PubMed] [Google Scholar]
- 138.Nukitrangsan N, Okabe T, Toda T, Inafuku M, Iwasaki H, Yanagita T, Oku H. Effect of Peucedanum japonicum Thunb on the expression of obesity-related genes in mice on a high-fat diet. J Oleo Sci. 2011;60(10):527–536. doi: 10.5650/jos.60.527. [DOI] [PubMed] [Google Scholar]
- 139.Okabe T, Toda T, Nukitrangsan N, Inafuku M, Iwasaki H, Oku H. Peucedanum japonicum Thunb inhibits high-fat diet induced obesity in mice. Phytother Res. 2011;6:870–877. doi: 10.1002/ptr.3355. [DOI] [PubMed] [Google Scholar]
- 140.Nukitrangsan N, Okabe T, Toda T, Inafuku M, Iwasaki H, Oku H. Effect of Peucedanum japonicum Thunb extract on high-fat diet-induced obesity and gene expression in mice. J Oleo Sci. 2012;61(2):89–101. doi: 10.5650/jos.61.89. [DOI] [PubMed] [Google Scholar]
- 141.Nugara RN, Inafuku M, Iwasaki H, Oku H (2013) Partially purified Peucedanum japonicum Thunb extracts exert anti-obesity effects in vitro. Nutrition. doi:10.1016/j.nut.2013.09.017 [DOI] [PubMed]
- 142.Kang SI, Shin HS, Kim HM, Hong YS, Yoon SA, Kang SW, Kim JH, Ko HC, Kim SJ. Anti-obesity properties of a Sasa quelpaertensis extract in high-fat diet-induced obese mice. Biosci Biotechnol Biochem. 2012;76(4):755–761. doi: 10.1271/bbb.110868. [DOI] [PubMed] [Google Scholar]
- 143.Kang SW, Kang SI, Shin HS, Yoon SA, Kim JH, Ko HC, Kim SJ. Sasa quelpaertensis Nakai extract and its constituent p-coumaric acid inhibit adipogenesis in 3T3–L1 cells through activation of the AMPK pathway. Food Chem Toxicol. 2013;59:380–385. doi: 10.1016/j.fct.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 144.Kim JH, Kang SI, Shin HS, Yoon SA, Kang SW, Ko HC, Kim SJ. Sasa quelpaertensis and p-coumaric acid attenuate oleic acid-induced lipid accumulation in HepG2 cells. Biosci Biotechnol Biochem. 2013;77(7):1595–1598. doi: 10.1271/bbb.130167. [DOI] [PubMed] [Google Scholar]
- 145.Yoon SA, Kang SI, Shin HS, Kang SW, Kim JH, Ko HC, Kim SJ. p-Coumaric acid modulates glucose and lipid metabolism via AMP-activated protein kinase in L6 skeletal muscle cells. Biochem Biophys Res Commun. 2013;432(4):553–557. doi: 10.1016/j.bbrc.2013.02.067. [DOI] [PubMed] [Google Scholar]
- 146.Iwashita K, Yamaki K, Tsushida T. Mioga (Zingiber mioga Rosc.) extract prevents 3T3–L1 differentiation into adipocytes and obesity in mice. Food Sci Technol Res. 2001;7(2):164–170. [Google Scholar]


