Abstract
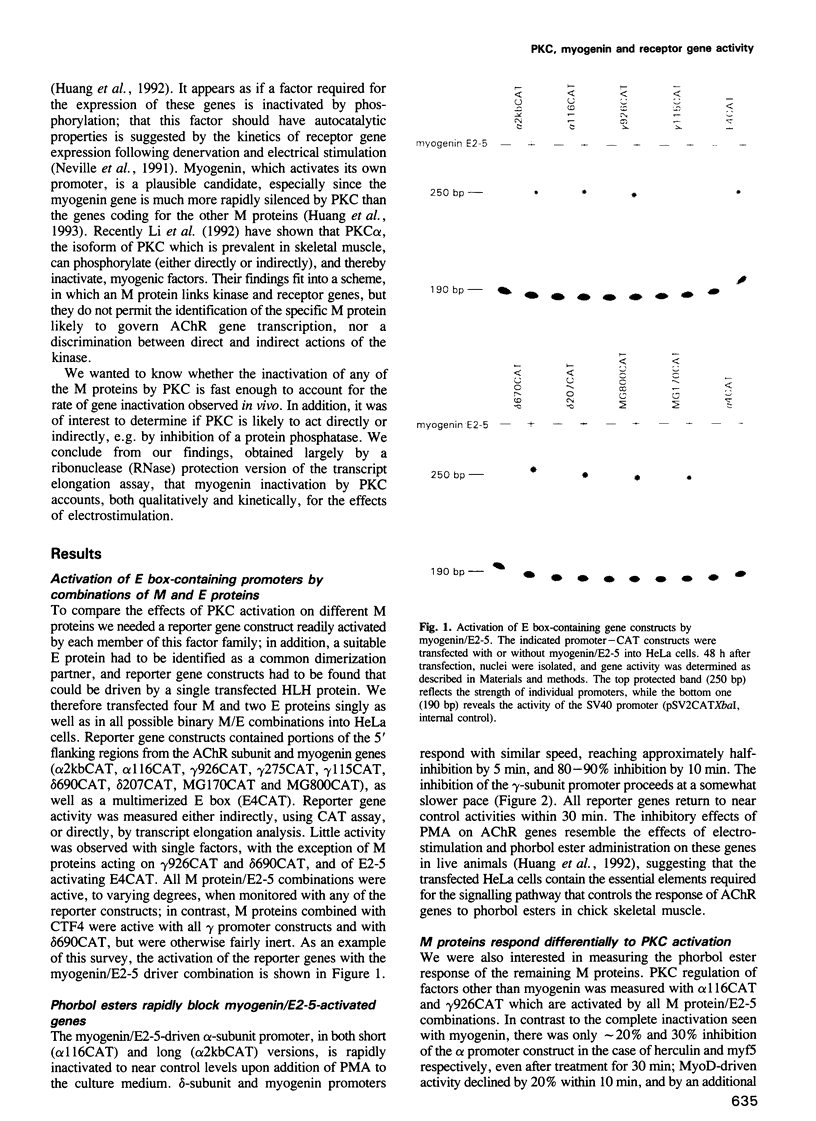
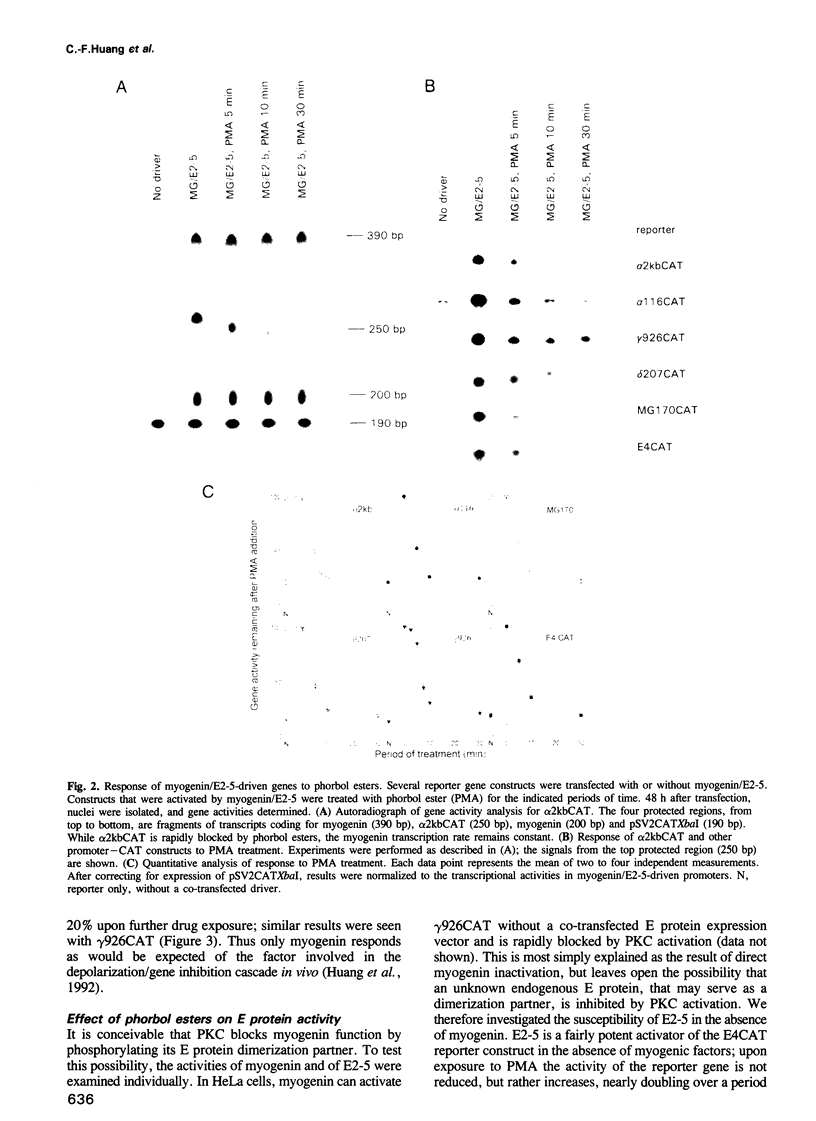
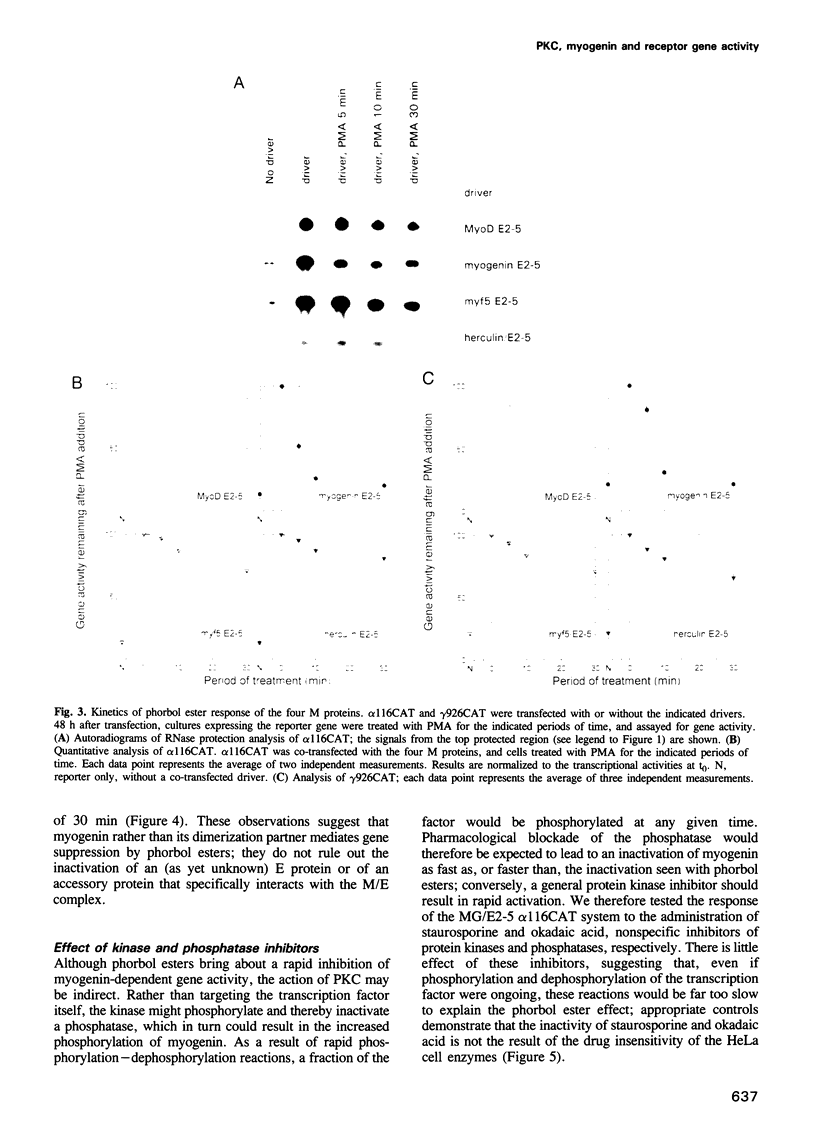
In investigating the coupling of depolarization and transcription in skeletal muscle we have focused on how protein kinase C suppresses acetylcholine receptor subunit genes. The activity of acetylcholine receptor subunit promoters in non-muscle cells co-transfected with myogenic factors and E proteins was measured, and their response to protein kinase C activation analyzed. To simplify interpretation of results, gene activities rather than levels of reporter enzymes were assayed, transcriptional effects of phorbol esters were determined, with drug exposures brief enough to preclude kinase depletion, and analysis was carried out with HeLa cells, which are not liable to myogenic conversion. Myogenin, which had been postulated previously to play a role in denervation supersensitivity (Neville et al., Mol. Cell. Neurobiol., 12, 511-527, 1992), was found to be the only myogenic factor whose inactivation kinetics can account for the plasma membrane-protein kinase C-receptor gene cascade observed in intact muscle (Huang et al., Neuron, 9, 671-678, 1992).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdul-Ghani M., Kravitz E. A., Meiri H., Rahamimoff R. Protein phosphatase inhibitor okadaic acid enhances transmitter release at neuromuscular junctions. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1803–1807. doi: 10.1073/pnas.88.5.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin T. J., Burden S. J. Isolation and characterization of the mouse acetylcholine receptor delta subunit gene: identification of a 148-bp cis-acting region that confers myotube-specific expression. J Cell Biol. 1988 Dec;107(6 Pt 1):2271–2279. doi: 10.1083/jcb.107.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A., Kozikowski A. P., Lazo J. S. Structural requirements of lyngbyatoxin A for activation and downregulation of protein kinase C. Biochemistry. 1992 Apr 21;31(15):3824–3830. doi: 10.1021/bi00130a013. [DOI] [PubMed] [Google Scholar]
- Birnbaum M., Reis M. A., Shainberg A. Role of calcium in the regulation of acetylcholine receptor synthese in cultured muscle cells*. Pflugers Arch. 1980 May;385(1):37–43. doi: 10.1007/BF00583913. [DOI] [PubMed] [Google Scholar]
- Braun T., Bober E., Buschhausen-Denker G., Kohtz S., Grzeschik K. H., Arnold H. H., Kotz S. Differential expression of myogenic determination genes in muscle cells: possible autoactivation by the Myf gene products. EMBO J. 1989 Dec 1;8(12):3617–3625. doi: 10.1002/j.1460-2075.1989.tb08535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T., Bober E., Winter B., Rosenthal N., Arnold H. H. Myf-6, a new member of the human gene family of myogenic determination factors: evidence for a gene cluster on chromosome 12. EMBO J. 1990 Mar;9(3):821–831. doi: 10.1002/j.1460-2075.1990.tb08179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T., Buschhausen-Denker G., Bober E., Tannich E., Arnold H. H. A novel human muscle factor related to but distinct from MyoD1 induces myogenic conversion in 10T1/2 fibroblasts. EMBO J. 1989 Mar;8(3):701–709. doi: 10.1002/j.1460-2075.1989.tb03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursztajn S., Schneider L. W., Jong Y. J., Berman S. A. Phorbol esters inhibit the synthesis of acetylcholine receptors in cultured muscle cells. Biol Cell. 1988;63(1):57–65. [PubMed] [Google Scholar]
- Chahine K. G., Walke W., Goldman D. A 102 base pair sequence of the nicotinic acetylcholine receptor delta-subunit gene confers regulation by muscle electrical activity. Development. 1992 May;115(1):213–219. doi: 10.1242/dev.115.1.213. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Danchin A. Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976 Dec 23;264(5588):705–712. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- Church G. M., Ephrussi A., Gilbert W., Tonegawa S. Cell-type-specific contacts to immunoglobulin enhancers in nuclei. 1985 Feb 28-Mar 6Nature. 313(6005):798–801. doi: 10.1038/313798a0. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Duclert A., Piette J., Changeux J. P. Influence of innervation of myogenic factors and acetylcholine receptor alpha-subunit mRNAs. Neuroreport. 1991 Jan;2(1):25–28. doi: 10.1097/00001756-199101000-00006. [DOI] [PubMed] [Google Scholar]
- Dutton E. K., Simon A. M., Burden S. J. Electrical activity-dependent regulation of the acetylcholine receptor delta-subunit gene, MyoD, and myogenin in primary myotubes. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2040–2044. doi: 10.1073/pnas.90.5.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D. G., Brennan T. J., Olson E. N. Mitogenic repression of myogenin autoregulation. J Biol Chem. 1991 Nov 15;266(32):21343–21346. [PubMed] [Google Scholar]
- Edmondson D. G., Olson E. N. Helix-loop-helix proteins as regulators of muscle-specific transcription. J Biol Chem. 1993 Jan 15;268(2):755–758. [PubMed] [Google Scholar]
- Eftimie R., Brenner H. R., Buonanno A. Myogenin and MyoD join a family of skeletal muscle genes regulated by electrical activity. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1349–1353. doi: 10.1073/pnas.88.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi A., Church G. M., Tonegawa S., Gilbert W. B lineage--specific interactions of an immunoglobulin enhancer with cellular factors in vivo. Science. 1985 Jan 11;227(4683):134–140. doi: 10.1126/science.3917574. [DOI] [PubMed] [Google Scholar]
- Gilmour B. P., Fanger G. R., Newton C., Evans S. M., Gardner P. D. Multiple binding sites for myogenic regulatory factors are required for expression of the acetylcholine receptor gamma-subunit gene. J Biol Chem. 1991 Oct 25;266(30):19871–19874. [PubMed] [Google Scholar]
- Gu W., Schneider J. W., Condorelli G., Kaushal S., Mahdavi V., Nadal-Ginard B. Interaction of myogenic factors and the retinoblastoma protein mediates muscle cell commitment and differentiation. Cell. 1993 Feb 12;72(3):309–324. doi: 10.1016/0092-8674(93)90110-c. [DOI] [PubMed] [Google Scholar]
- Henthorn P., Kiledjian M., Kadesch T. Two distinct transcription factors that bind the immunoglobulin enhancer microE5/kappa 2 motif. Science. 1990 Jan 26;247(4941):467–470. doi: 10.1126/science.2105528. [DOI] [PubMed] [Google Scholar]
- Hu J. S., Olson E. N., Kingston R. E. HEB, a helix-loop-helix protein related to E2A and ITF2 that can modulate the DNA-binding ability of myogenic regulatory factors. Mol Cell Biol. 1992 Mar;12(3):1031–1042. doi: 10.1128/mcb.12.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. F., Neville C. M., Schmidt J. Control of myogenic factor genes by the membrane depolarization/protein kinase C cascade in chick skeletal muscle. FEBS Lett. 1993 Mar 15;319(1-2):21–25. doi: 10.1016/0014-5793(93)80029-t. [DOI] [PubMed] [Google Scholar]
- Huang C. F., Tong J., Schmidt J. Protein kinase C couples membrane excitation to acetylcholine receptor gene inactivation in chick skeletal muscle. Neuron. 1992 Oct;9(4):671–678. doi: 10.1016/0896-6273(92)90030-h. [DOI] [PubMed] [Google Scholar]
- Jia H. T., Tsay H. J., Schmidt J. Analysis of binding and activating functions of the chick muscle acetylcholine receptor gamma-subunit upstream sequence. Cell Mol Neurobiol. 1992 Jun;12(3):241–258. doi: 10.1007/BF00712929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarsfeld A., Daubas P., Bourachot B., Changeux J. P. A 5'-flanking region of the chicken acetylcholine receptor alpha-subunit gene confers tissue specificity and developmental control of expression in transfected cells. Mol Cell Biol. 1987 Feb;7(2):951–955. doi: 10.1128/mcb.7.2.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarsfeld A., Laufer R., Fontaine B., Devillers-Thiéry A., Dubreuil C., Changeux J. P. Regulation of muscle AChR alpha subunit gene expression by electrical activity: involvement of protein kinase C and Ca2+. Neuron. 1989 Mar;2(3):1229–1236. doi: 10.1016/0896-6273(89)90307-3. [DOI] [PubMed] [Google Scholar]
- Lassar A. B., Davis R. L., Wright W. E., Kadesch T., Murre C., Voronova A., Baltimore D., Weintraub H. Functional activity of myogenic HLH proteins requires hetero-oligomerization with E12/E47-like proteins in vivo. Cell. 1991 Jul 26;66(2):305–315. doi: 10.1016/0092-8674(91)90620-e. [DOI] [PubMed] [Google Scholar]
- Laufer R., Klarsfeld A., Changeux J. P. Phorbol esters inhibit the activity of the chicken acetylcholine receptor alpha-subunit gene promoter. Role of myogenic regulators. Eur J Biochem. 1991 Dec 18;202(3):813–818. doi: 10.1111/j.1432-1033.1991.tb16437.x. [DOI] [PubMed] [Google Scholar]
- Levy D. N., Fernandes L. S., Williams W. V., Weiner D. B. Induction of cell differentiation by human immunodeficiency virus 1 vpr. Cell. 1993 Feb 26;72(4):541–550. doi: 10.1016/0092-8674(93)90073-y. [DOI] [PubMed] [Google Scholar]
- Li L., Zhou J., James G., Heller-Harrison R., Czech M. P., Olson E. N. FGF inactivates myogenic helix-loop-helix proteins through phosphorylation of a conserved protein kinase C site in their DNA-binding domains. Cell. 1992 Dec 24;71(7):1181–1194. doi: 10.1016/s0092-8674(05)80066-2. [DOI] [PubMed] [Google Scholar]
- Lomo T., Westgaard R. H. Further studies on the control of ACh sensitivity by muscle activity in the rat. J Physiol. 1975 Nov;252(3):603–626. doi: 10.1113/jphysiol.1975.sp011161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H., Sasaki Y. Staurosporine, a protein kinase C inhibitor interferes with proliferation of arterial smooth muscle cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):105–109. doi: 10.1016/s0006-291x(89)80183-4. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlie J. P., Kornhauser J. M. Neural regulation of gene expression by an acetylcholine receptor promoter in muscle of transgenic mice. Neuron. 1989 Apr;2(4):1295–1300. doi: 10.1016/0896-6273(89)90067-6. [DOI] [PubMed] [Google Scholar]
- Murre C., Voronova A., Baltimore D. B-cell- and myocyte-specific E2-box-binding factors contain E12/E47-like subunits. Mol Cell Biol. 1991 Feb;11(2):1156–1160. doi: 10.1128/mcb.11.2.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C., Shen L. P., Meister A., Fodor E., Rutter W. J. Pan: a transcriptional regulator that binds chymotrypsin, insulin, and AP-4 enhancer motifs. Genes Dev. 1990 Jun;4(6):1035–1043. doi: 10.1101/gad.4.6.1035. [DOI] [PubMed] [Google Scholar]
- Neville C. M., Schmidt M., Schmidt J. Response of myogenic determination factors to cessation and resumption of electrical activity in skeletal muscle: a possible role for myogenin in denervation supersensitivity. Cell Mol Neurobiol. 1992 Dec;12(6):511–527. doi: 10.1007/BF00711232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville C., Schmidt M., Schmidt J. Kinetics of expression of ACh receptor alpha-subunit mRNA in denervated and stimulated muscle. Neuroreport. 1991 Nov;2(11):655–657. doi: 10.1097/00001756-199111000-00005. [DOI] [PubMed] [Google Scholar]
- Numberger M., Dürr I., Kues W., Koenen M., Witzemann V. Different mechanisms regulate muscle-specific AChR gamma- and epsilon-subunit gene expression. EMBO J. 1991 Oct;10(10):2957–2964. doi: 10.1002/j.1460-2075.1991.tb07846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. N. MyoD family: a paradigm for development? Genes Dev. 1990 Sep;4(9):1454–1461. doi: 10.1101/gad.4.9.1454. [DOI] [PubMed] [Google Scholar]
- Osada S., Mizuno K., Saido T. C., Suzuki K., Kuroki T., Ohno S. A new member of the protein kinase C family, nPKC theta, predominantly expressed in skeletal muscle. Mol Cell Biol. 1992 Sep;12(9):3930–3938. doi: 10.1128/mcb.12.9.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzementi L., Schmidt J. Ryanodine alters the rate of acetylcholine receptor synthesis in chick skeletal muscle cell cultures. J Biol Chem. 1981 Dec 25;256(24):12651–12654. [PubMed] [Google Scholar]
- Piette J., Bessereau J. L., Huchet M., Changeux J. P. Two adjacent MyoD1-binding sites regulate expression of the acetylcholine receptor alpha-subunit gene. Nature. 1990 May 24;345(6273):353–355. doi: 10.1038/345353a0. [DOI] [PubMed] [Google Scholar]
- Prody C. A., Merlie J. P. The 5'-flanking region of the mouse muscle nicotinic acetylcholine receptor beta subunit gene promotes expression in cultured muscle cells and is activated by MRF4, myogenin and myoD. Nucleic Acids Res. 1992 May 11;20(9):2367–2372. doi: 10.1093/nar/20.9.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich N. C., Pfeffer L. M. Evidence for involvement of protein kinase C in the cellular response to interferon alpha. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8761–8765. doi: 10.1073/pnas.87.22.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh B. H., Pezzementi L., Schmidt J. Extracellular potassium and the regulation of acetylcholine receptor synthesis in embryonic chick muscle cells. Brain Res. 1983 Mar 21;263(2):259–265. doi: 10.1016/0006-8993(83)90318-9. [DOI] [PubMed] [Google Scholar]
- Sun X. H., Baltimore D. An inhibitory domain of E12 transcription factor prevents DNA binding in E12 homodimers but not in E12 heterodimers. Cell. 1991 Jan 25;64(2):459–470. doi: 10.1016/0092-8674(91)90653-g. [DOI] [PubMed] [Google Scholar]
- Tapscott S. J., Thayer M. J., Weintraub H. Deficiency in rhabdomyosarcomas of a factor required for MyoD activity and myogenesis. Science. 1993 Mar 5;259(5100):1450–1453. doi: 10.1126/science.8383879. [DOI] [PubMed] [Google Scholar]
- Tsay H. J., Choe Y. H., Neville C. M., Schmidt J. CTF4, a chicken transcription factor of the helix-loop-helix class A family. Nucleic Acids Res. 1992 May 25;20(10):2624–2624. doi: 10.1093/nar/20.10.2624-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. M., Tsay H. J., Schmidt J. Expression of the acetylcholine receptor delta-subunit gene in differentiating chick muscle cells is activated by an element that contains two 16 bp copies of a segment of the alpha-subunit enhancer. EMBO J. 1990 Mar;9(3):783–790. doi: 10.1002/j.1460-2075.1990.tb08174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Xu H. P., Wang X. M., Ballivet M., Schmidt J. A cell type-specific enhancer drives expression of the chick muscle acetylcholine receptor alpha-subunit gene. Neuron. 1988 Aug;1(6):527–534. doi: 10.1016/0896-6273(88)90183-3. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Davis R., Tapscott S., Thayer M., Krause M., Benezra R., Blackwell T. K., Turner D., Rupp R., Hollenberg S. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991 Feb 15;251(4995):761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Tapscott S. J., Davis R. L., Thayer M. J., Adam M. A., Lassar A. B., Miller A. D. Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5434–5438. doi: 10.1073/pnas.86.14.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B., Standaert M., Arnold T., Hernandez H., Watson J., Ways K., Cooper D. R., Farese R. V. Effects of insulin on diacylglycerol/protein kinase-C signalling and glucose transport in rat skeletal muscles in vivo and in vitro. Endocrinology. 1992 Jun;130(6):3345–3355. doi: 10.1210/endo.130.6.1597146. [DOI] [PubMed] [Google Scholar]
- Zawel L., Reinberg D. Initiation of transcription by RNA polymerase II: a multi-step process. Prog Nucleic Acid Res Mol Biol. 1993;44:67–108. doi: 10.1016/s0079-6603(08)60217-2. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Babin J., Feldhaus A. L., Singh H., Sharp P. A., Bina M. HTF4: a new human helix-loop-helix protein. Nucleic Acids Res. 1991 Aug 25;19(16):4555–4555. doi: 10.1093/nar/19.16.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. Y., Schwartz R. J., Crow M. T. Phorbol esters selectively downregulate contractile protein gene expression in terminally differentiated myotubes through transcriptional repression and message destabilization. J Cell Biol. 1991 Nov;115(3):745–754. doi: 10.1083/jcb.115.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]








