Abstract
Background: Neo-adjuvant chemo-radiotherapy has been proposed to improve resectability of locally-advanced pancreatic cancer (LAPC). However, the ability of neo-adjuvant therapy to induce radiological tumour regression has not been reported.
Methods: Pre-and post-treatment computed tomography (CT) scans of patients undergoing neo-adjuvant chemo-radiotherapy for LAPC were reviewed. LAPC was sub-classified into borderline resectable disease [≤180° involvement of the superior mesenteric artery (SMA); short-segment encasement/abutment of the common hepatic artery; or tumour-associated deformity, abutment or short-segment occlusion of the superior mesenteric vein (SMV)/ portal vein (PV) that was amenable to vascular resection and reconstruction] and locally advanced un-resectable pancreatic cancer (vascular involvement more than that described for borderline resectable pancreatic cancer). The radiological response and surgical resection rates were assessed.
Results: Sixteen patients received neo-adjuvant therapy for LAPC during 2005–2008. Regression of major vascular involvement, i.e. un-encasement or regression of abutment of any involved vessels was not observed in any patient. Pre-and post-treatment tumour densities were not statistically different. Fifty per cent of patients with borderline resectable disease and none of the patients with locally advanced un-resectable pancreatic cancer eventually underwent surgical resection.
Conclusion: Neo-adjuvant treatment does not induce radiological tumour regression of LAPC with major vascular involvement. Patient selection for neo-adjuvant trial enrolment should remain focused on borderline disease which may have a potential for surgical resection.
Introduction
Pancreatic cancer is the fourth leading cause of cancer-related mortality in the United States.1 The all-stage 5-year survival rate for this cancer is a dismal 5% which has largely unchanged over three decades.1 Surgical resection offers the only opportunity of a cure. However, only 10–20% of patients present early enough to be considered a candidate for curative surgery.2 Forty per cent of patients have metastatic disease at presentation and are mainly considered for palliative therapy. The remaining 40–50% have locally advanced disease which is not amenable to immediate surgical resection,2 especially without any additional therapy.
Given that surgical extirpation is the only chance of a cure, neo-adjuvant therapy has been used to induce tumour regression and convert locally advanced, non-metastatic disease to one that is surgically resectable, thus potentially improving outcomes. After neo-adjuvant therapy for locally advanced pancreatic cancer, surgical resection rates of 1–81%3–6 have been reported in literature. This wide variation in the reported rates of resection after neo-adjuvant therapy could be explained by the use of a non-standard definition of locally advanced pancreatic cancer and a varying extent of surgical resection. Increasing the understanding of this problem has led to the emergence of consistent definitions of locally advanced pancreatic cancer.7–9 Furthermore, the best way to monitor a response to neo-adjuvant therapy is also not established. Typically, patients undergoing neo-adjuvant therapy for locally advanced pancreatic cancer undergo serial imaging [e.g. computed tomography (CT) scan] to evaluate a response and resectability. However, the rate of radiological responses to neo-adjuvant therapy has not been reported before. The aim of the present study was to evaluate the radiological response of patients with locally advanced pancreatic cancer to neo-adjuvant therapy.
Methods
Patient selection and treatment algorithm
Patients included in this study were treated with a combination of cisplatin, interferon-α, fluorouracil (5-FU) and radiation based on the phase II treatment protocol for locally advanced pancreatic cancer from 2005–2008 but included patients treated off protocol as well. Sixteen patients diagnosed with locally advanced pancreatic adenocarcinoma from 2005–2008 were included. Locally advanced pancreatic cancer was defined as pancreatic cancer with vascular involvement precluding immediate surgical resection and was further subdivided into those with ‘Borderline Resectable Pancreatic Cancer’8 and those with ‘Locally Advanced Un-resectable Pancreatic Cancer’. Borderline resectable pancreatic cancer was defined as the absence of distant metastasis and one or more of the following: (i) tumor-associated deformity of the superior mesenteric vein (SMV) and portal vein (PV); (ii) abutment of the SMV or PV ≥ 180°; (iii) short-segment occlusion of the SMV or PV amenable to resection and venous reconstruction; (iv) short-segment involvement of the hepatic artery or its branches amenable to resection and reconstruction; and (v) abutment of the SMA <180°.10 ‘Locally advanced un-resectable’ disease was defined as patients who were un-resectable owing to vascular involvement but the disease was more extensive than as defined for borderline resectable disease.
All patients had histological confirmation of pancreatic adenocarcinoma before initiation of interferon-based chemo-radiation (interferon-α, cisplatin, 5-FU and radiation). Five out of 16 patients were explored before neo-adjuvant therapy with the intent of surgical resection but were found to be un-resectable and were subsequently included in the neo-adjvuant therapy protocol. A pancreas protocol CT was obtained before initiation of neo-adjuvant therapy. After therapy patients were restaged by a pancreas protocol CT scan and a resection was re-considered in a multi-disciplinary conference. All except two patients had carbohydrate antigen (CA) 19-9 measured before initiation of chemotherapy and after completion of chemotherapy. All pathological specimens underwent standard pathological examination. The bile duct margin, pancreatic margin and SMA margin were systematically analysed.
Data collection and statistical analysis
The study was approved by Institutional Review Board at the University of Minnesota. Patient demographics and CA 19-9 levels (before initiation of chemo-radiation and at completion of chemo-radiation) were collected. Detailed evaluation of pre-and post-adjuvant chemo-radiation cross-sectional imaging for the presence and degree of SMV/PV, SMA, celiac axis and hepatic artery involvement was performed. The size and location of the pancreatic tumour before and after chemo-radiation was also noted. The development of metastatic disease was also evaluated. The end point of analysis included the proportion of patients with a radiological response and rates of tumour resection. A single, body imaging radiologist (S.W.) reviewed all films to ensure uniform interpretation. A paired two sample t-test was used to compare pre-and post-chemo-radiation tumour size, CA 19-9 levels and tumour density in Hounsfield units.
Results
From 2005 to 2008, 16 patients with locally advanced pancreatic adenocarcinoma were enrolled in a neo-adjuvant chemo-radiation protocol based on cisplatin, α-interferon, 5-FU and radiotherapy. The patient and tumour characteristics of this group are shown in Table 1. The median age of the cohort was 64 years (range 45–78) and 69% of the patients were male. Five patients out of 16 had an exploratory laparotomy with the intention of surgical resection and were found to be un-resectable and were thus included in the study. One patient (Patient 1, Table 1) did not have clear vascular involvement on the CT scan but was found to have portal vein involvement on endoscopic ultrasound and thus was included in the study. The tumour size (P = 0.14), CA 19-9 levels (P = 0.12) and tumour density (P = 0.36) expressed in Hounsfield units did not significantly change with treatment (Table 1).
Table 1.
Patient and tumour characteristics
| Age in years | |
| Median (range) | 64 (45–78) |
| Gender: % (n) | |
| Male | 69% (11) |
| Female | 31% (5) |
| Explored before neoadjuvant chemoradiation: % (n) | |
| Yes | 31% (5) |
| No | 69% (11) |
| Location of the tumour: % (n) | |
| Head | 69% (11) |
| Body | 18% (3) |
| Tail | 13% (2) |
| Tumour size (mean ± SD) | |
| Pre-treatment | 3.85 ± 1.92NS (P = 0.1423) |
| Post-treatment | 3.39 ± 1.81 |
| Tumour extension at presentation: % (n) | |
| Borderline resectable | 62.5% (10) |
| Locally advanced | 37.5% (6) |
| CA 19-9 levels: | |
| Pre-treatment | 1436 ± 772NS (P = 0.12) |
| Post-treatment | 772 ± 220 |
| Tumour density in Hounsfield units | |
| Pre-treatment | 60.4 ± 6.5NS (P = 0.3698) |
| Post-treatment | 58.2 ± 6.9 |
| Radiological response: % (n) | |
| Regression | 0% (0) |
| Stable | 69% (11) |
| Progression | 31% (5) |
| Surgical resection of cancer after neo-adjuvant chemoradiation: % (n) | |
| Yes | 31% (5) |
| No | 69% (11) |
| Patients undergoing surgical resection classified by tumour extension at presentation: % (n) | |
| Borderline resectable | 50% (5) |
| Locally advanced | 0% (0) |
| Pathological response in those undergoing a resection (n = 5) | |
| Macroscopic tumour | 4 |
| Microscopic tumour only | 1 |
NS, non-significant.
The imaging characteristics and vascular involvement before and after treatment of the individual patients is presented in Table 2. Patients were further classified as having a borderline resectable tumour or locally advanced un-resectable tumour. Locally advanced un-resectable tumours were those which were un-resectable based on their vascular involvement characteristics and did not have the characteristics of a borderline resectable tumour as defined in the methods section, typically as a result of arterial encasement. Sixty-two percent of patients at presentation had borderline resectable tumour characteristics and the remaining were locally advanced un-resectable. Representative imaging of a resectable pancreatic cancer (not from the current study), borderline resectable with intra-operative correlation and two patients with locally advanced un-resectable disease is shown in figure (Fig. 1). As seen in Tables 2, none of the 16 patients had radiological regression and either had stable disease or disease progression with additional vascular involvement or development of distant disease. Furthermore, none of the patients with locally advanced un-resectable cancer had regression of disease to render them resectable (Tables 2). Specifically, no encased vessels became ‘un-encased’. Out of 10 patients with borderline resectable disease only 5 underwent a resection after neo-adjuvant therapy (Tables 2). All these patients had a margin negative resection. The remaining five patients with borderline resectable disease were unable to undergo a resection owing to poor performance status (3 patients), progression of vascular involvement (1 patient) or because of the development of distant disease (1 patient) (Table 2). CA 19-9 levels before and after treatments are shown in Table 2. In the subgroup of patients who underwent a resection (n = 5), one patient had no macroscopic tumour and had only microscopic disease. The four remaining patients had persistent macroscopic disease on pathological examination.
Table 2.
Imaging characteristics, biochemical response, imaging response and post-treatment resection
| Patient no. | Tumour extension at presentation (On imaging) | SMA |
SMV/PV |
Celiac artery |
Hepatic artery |
CT response to treatment | Surgery | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |||||
| 1 | BR | U | U | A | A | U | U | U | U | Stable | Yes | Involvement based on EUS and intra-operative findings |
| 2 | LA | U | U | A | A | E | E | E | E | Stable | No | |
| 3 | BR | U | U | A | E | U | A | U | E | Progression | No | Pre chemo-rad exploration |
| 4 | LA | E | E | E | E | A | E | E | E | Progression | No | |
| 5 | LA | U | U | E | E | A | E | E | E | Progression | No | |
| 6 | BR | A | A | A | A | U | U | U | U | Stable | Yes | Pre chemo-rad exploration |
| 7 | BR | U | U | E | E | U | U | U | U | Stable | No | Development of Malignant ascites |
| 8 | LA | U | U | E | E | U | U | E | E | Stable | No | |
| 9 | BR | U | U | E | E | U | U | U | U | Stable | No | Poor performance status |
| 10 | BR | U | U | A | A | U | U | U | U | Stable | Yes | Excision and replacement of vein. |
| 11 | LA | U | A | E | E | E | E | E | E | Progression | No | |
| 12 | BR | U | U | U | E | U | U | U | U | Progression | No | Pre chemo-rad exploration. Involvement of base of mesentery precluded resection on pre-treatment exploration, Poor performance status precluded resection after treatment |
| 13 | BR | A | A | U | U | U | U | U | U | Stable | No | Pre chemo-rad exploration. Poor performance status precluded resection. |
| 14 | LA | A | A | E | E | E | E | E | E | Stable | No | |
| 15 | BR | U | U | A | A | U | U | U | U | Stable | Yes | Pre chemo-rad exploration |
| 16 | BR | U | U | A | A | U | U | U | U | Stable | Yes | Vein resection |
E, encasement; A, abutment; U, uninvolved; BR, borderline resectable; LA, locally advanced; SMA, superior mesenteric artery; NA, not available.
Figure 1.
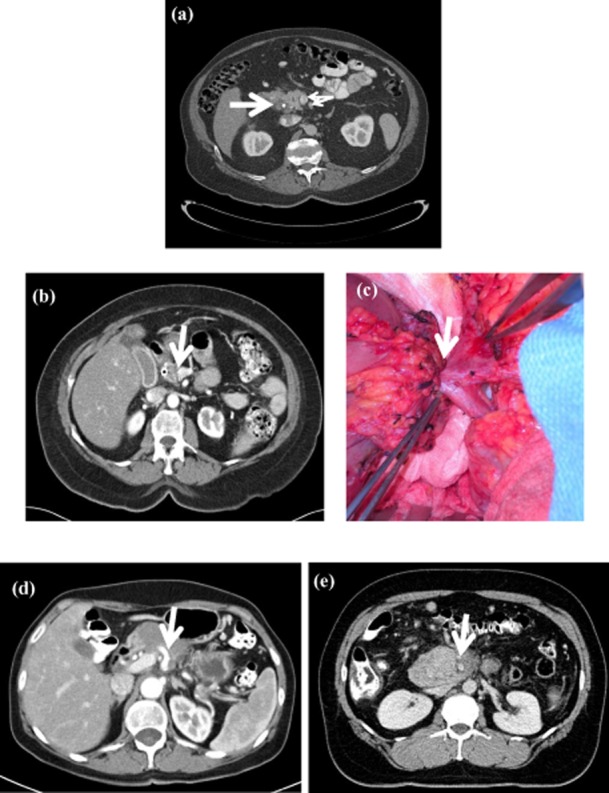
Representative imaging characteristics of patients with resectable, borderline resectable and locally advanced un-resectable pancreatic cancer. (a) Patient with resectable pancreatic cancer. The bold arrow indicates a hypodense mass. Small arrows indicate the superior mesenteric vein (SMV)/ superior mesenteric artery (SMA), both of which have a clear fat plane around them. (b) A patient with borderline resectable pancreatic cancer. The bold arrow indicates the site where the tumour involves the left side of the portal vein (PV). (c) Intra-operative picture of a borderline resectable tumour involving the PV. This tumour required the resection of the PV. (d) A locally advanced un-resectable tumour encasing the celiac axis depicted by a bold arrow. (e) Locally advanced un-resectable pancreatic cancer encasing the SMA depicted by a bold arrow
All of the five patients who had an exploratory laparotomy with the intent of resection before enrolment in this neo-adjuvant chemo-radiotherapy trial had borderline resectable disease (Table 2, comment section). Out of these five patients, two underwent a surgical resection after neo-adjuvant chemo-radiation therapy, two could not be resected owing to poor performance status and one had progression of the disease.
Discussion
In the current series of patients with borderline resectable and locally advanced un-resectable pancreatic cancer, an aggressive neo-adjuvant treatment regimen11,12 did not lead to radiological tumour regression. Furthermore, none of the patients with locally advanced un-resectable pancreatic cancer and only half of the patients with a borderline resectable tumour eventually underwent surgical resection. These data suggests that with currently available chemo-radiation, radiological regression of an un-resectable locally advanced tumour to one that is surgically resectable is an unlikely event. Furthermore, CT is not a reliable indicator of tumour response. Although imaging may not change in response to neo-adjuvant treatment, patients with borderline resectable tumours should undergo tumour resection after completion of neo-adjuvant therapy, in a patient with good performance status and in the absence of tumour progression. As has been shown, in spite of the lack of a radiological response, a margin negative resection can frequently be performed.
The issue of neo-adjuvant therapy for locally advanced pancreatic cancer has been addressed in previous studies with mixed results. White et al.6 retrospectively analysed the data of 25 patients with locally advanced pancreatic cancer who received neo-adjuvant chemo-radiation. In this study, locally advanced pancreatic cancer was loosely defined as disease that abutted the SMV or SMA or involved lymph nodes. In this study, about 10% of patients had disease regression on imaging and overall only 20% patients underwent a tumour resection. In another previous study by Wanebo et al.,5 14 patients with locally advanced pancreatic cancer were treated with 5-FU, cisplatin and radiation, 11 patients underwent surgical exploration and 9 received a definitive resection thus reporting a success rate of ∼80%. However, on a closer examination, out of 11 patients who eventually underwent surgery, 2 patients had T2 disease without any vascular involvement before neo-adjuvant treatment and 4 patients required an arterial resection which would not be recommended routinely. Remarkably, 7 out of 9 patients either had only microscopic disease or no residual cancer in the resected specimen. Whether the neo-adjuvant therapy provided radiological regression in this study is unclear. In another retrospective review of 83 patients3 with locally advanced pancreatic cancer who received neo-adjuvant chemoradiation from 1993–1999 at Memorial Sloan-Kettering Cancer Center, only 3.4% had a sufficient radiological response to consider exploration. In this study, only patients with extensive retropancreatic venous involvement or encasement of the celiac axis, hepatic artery or SMA were included. In a recent single institution retrospective review of 215 patients with locally advanced un-resectable pancreatic cancer treated with gemcitabine-based neo-adjuvant chemo-radiation, Habermehl et al.13 demonstrated that 90% of the patients had stable or progressive disease and only about 10% of patients had a partial response. Whether there was regression of vascular involvement, the key determinant of resectability in pancreatic cancer, is unclear. Furthermore, it is intriguing that even although all patients had un-resectable disease at presentation and that only 10% of patients had a partial response on imaging, up to one-fourth of these patients underwent a surgical resection. Whether there was concomitant vascular resection-reconstruction is also unclear. In another study by Arvold et al.,14 the effect of neo-adjuvant therapy on the radiological regression and resection rates of locally advanced pancreatic cancer was studied. In this study, neo-adjuvant therapy followed by chemo-radiotherapy led to radiological regression and surgical resection in 30% and 20% of the patients, respectively. However, whether there was regression of vascular involvement is unclear and only 10% of patients in the locally advanced unresectable group could undergo a resection.
In the present study, none of the 16 patients had radiological disease regression. If one was to use the RECIST criteria15 the radiological responses in the present study would be classified either as progression or stable disease without any partial or complete responses. The fact that five patients with borderline resectable disease with continued vascular involvement were taken to the operating room but only one patient required vascular resection again highlights that a CT scan may not correctly predict regression of vascular involvement. Taken together the data from the present study and from the published literature3,5,6 suggests that with currently available neo-adjuvant therapy protocol a radiological response with regression of an un-resectable locally advanced tumour to one that is surgically resectable, is an unlikely event.
Recently, a new subgroup of pancreatic cancer has been recognized, namely borderline resectable pancreatic cancer which is considered unique from resectable as well as locally advanced un-resectable pancreatic cancer. Borderline resectable pancreatic cancer is defined as a tumour which has one or more of the following criteria: (i) tumour-associated deformity of the SMV and PV; (ii) abutment of the SMV or PV ≥ 180°; (iii) short-segment occlusion of the SMV or PV amenable to resection and venous reconstruction; (iv) short-segment involvement of the hepatic artery or its branches amenable to resection and reconstruction; and (v) abutment of the SMA <180°.10 Recognition of this group of patients is important as the pancreatic resection in this unique subgroup of patients, although technically possible, is associated with a high likelihood of R1 and R2 resections. Current guidelines recommend neo-adjuvant therapy followed by surgery to improve the likelihood of a margin negative resection.8,16 As discussed before, and seen in this study, radiological tumour regression in response to neo-adjuvant therapy is an unlikely outcome. However, a pathological response to neo-adjuvant therapy has been described and may facilitate a margin negative resection. In the study from MD Anderson Cancer Center,8 56% of the patients who underwent a pancreatectomy after neo-adjuvant therapy demonstrated a partial pathological response (<50% viable tumour). In our series, out of five patients who underwent a resection, one patient had no macroscopic tumour and had only microscopic disease on pathological examination. Since the radiological and pathological response may not correlate, all patients with borderline resectable pancreatic cancer who do not show disease progression and can tolerate the surgical procedure should undergo exploration with the intent of a R0 resection.
Given the discrepancy between the radiological and pathological response, functional imaging techniques such as positron emission tomography (PET) may have a role in monitoring the response to neo-adjuvant therapy. In a recent study by Patel et al.17 evaluating the effect of gemcitabine, docetaxal and capecitabine-based neo-adjuvant chemo-radiation on resectability and imaging characteristics of patients with borderline resectable pancreatic cancer, there was marked reduction in the standard uptake value (SUV) on PET. The SUV max on PET scan did not correlate significantly with tumour size as measured by the CT scan. Furthermore, patients who underwent a resection had minimal residual disease on pathological analysis. In another study, out of nine patients undergoing18 FDG-PET imaging before and after neoadjuvant chemoradiation, four had evidence of tumour regression by PET. These four patients went on to undergo resection of their tumour and histological analysis demonstrated 20–80% tumour necrosis in the surgical specimen. Of note, the CT was unable to detect any response to neo-adjuvant therapy in this group. Based on these limited data, a PET scan may have a role in following the tumour response to therapy and should be included in future studies and trials of neo-adjuvant therapy in pancreatic cancer.
Another common theme, which emerges on close review of the literature on neo-adjuvant therapy in locally advanced pancreatic cancer, is the absence of a standard definition of locally advanced pancreatic cancer. If the results from various studies and trials are to be comparable it is imperative that a standard definition of locally advanced pancreatic cancer needs to be employed. It is logical to separate patients with locally advanced pancreatic cancer into borderline resectable pancreatic cancer8,16 and locally advanced unresectable disease. Given that borderline resectable pancreatic cancer patients are technically resectable and neoadjuvant therapy improves the likelihood of a margin negative resection, it is reasonable to include these patients in trials of neo-adjuvant therapy. However, given that disease regression with neo-adjuvant therapy is unlikely, the rational of including locally advanced unresectable pancreatic cancer in a trial of neo-adjuvant therapy is unclear. Until more effective therapies are available these patients should probably be considered for palliative chemotherapy only.
This study is limited by the small sample size and the number of patients eventually undergoing surgery thus limiting the subgroup of patients in whom the radiological response could be correlated with the intra-operative and histological findings. However, we are able to provide detailed analysis of vascular involvement by a single, fellowship-trained, body imaging radiologist who specializes in the evaluation of pancreatic disease. In this way, meticulous assessment of vascular involvement could be performed according to currently recognized, standardized definitions of ‘borderline resectable’ and ‘locally advanced un-resectable’ disease. Even although the neo-adjuvant chemo-radiation regimen used in the present study has been shown to be equally if not more effective as compared with contemporary regimens,18,19 the possibility that the low rates of regression of vascular involvement observed in the present study is as a result of the use of a non-standard neo-adjvuant protocol cannot be ruled out.
In summary, these data suggest that with currently available adjuvant therapy, regression of an un-resectable locally advanced tumour to one that is surgically resectable is an unlikely event. Consideration may be given to functional imaging modalities such as PET to follow the tumour response to neo-adjuvant therapy. Future clinical trials of neo-adjuvant therapy in pancreatic cancer should continue to focus on borderline resectable pancreatic cancer, which is the group most likely to benefit from a neo-adjuvant approach. Patients with borderline resectable pancreatic cancer, in the absence of disease progression, should be routinely explored after neo-adjuvant therapy as imaging may not accurately reflect a biological/pathological response of the tumour.
Conflicts of interest
None declared.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Warshaw AL, Gu ZY, Wittenberg J, Waltman AC. Preoperative staging and assessment of resectability of pancreatic cancer. Arch Surg. 1990;125:230–233. doi: 10.1001/archsurg.1990.01410140108018. [DOI] [PubMed] [Google Scholar]
- 3.Kim HJ, Czischke K, Brennan MF, Conlon KC. Does neoadjuvant chemoradiation downstage locally advanced pancreatic cancer? J Gastrointest Surg. 2002;6:763–769. doi: 10.1016/s1091-255x(02)00017-3. [DOI] [PubMed] [Google Scholar]
- 4.Mehta VK, Fisher G, Ford JA, Poen JC, Vierra MA, Oberhelman H, et al. Preoperative chemoradiation for marginally resectable adenocarcinoma of the pancreas. J Gastrointest Surg. 2001;5:27–35. doi: 10.1016/s1091-255x(01)80010-x. [DOI] [PubMed] [Google Scholar]
- 5.Wanebo HJ, Glicksman AS, Vezeridis MP, Clark J, Tibbetts L, Koness RJ, et al. Preoperative chemotherapy, radiotherapy, and surgical resection of locally advanced pancreatic cancer. Arch Surg. 2000;135:81–87. doi: 10.1001/archsurg.135.1.81. discussion 8. [DOI] [PubMed] [Google Scholar]
- 6.White R, Lee C, Anscher M, Gottfried M, Wolff R, Keogan M, et al. Preoperative chemoradiation for patients with locally advanced adenocarcinoma of the pancreas. Ann Surg Oncol. 1999;6:38–45. doi: 10.1007/s10434-999-0038-z. [DOI] [PubMed] [Google Scholar]
- 7.Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1727–1733. doi: 10.1245/s10434-009-0408-6. [DOI] [PubMed] [Google Scholar]
- 8.Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833–846. doi: 10.1016/j.jamcollsurg.2007.12.020. discussion 46–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varadhachary GR, Wolff RA, Crane CH, Sun CC, Lee JE, Pisters PW, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3487–3495. doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]
- 10.Abrams RA, Lowy AM, O'Reilly EM, Wolff RA, Picozzi VJ, Pisters PW. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1751–1756. doi: 10.1245/s10434-009-0413-9. [DOI] [PubMed] [Google Scholar]
- 11.Katz MH, Wolff R, Crane CH, Varadhachary G, Javle M, Lin E, et al. Survival and quality of life of patients with resected pancreatic adenocarcinoma treated with adjuvant interferon-based chemoradiation: a phase II trial. Ann Surg Oncol. 2011;18:3615–3622. doi: 10.1245/s10434-011-1847-4. [DOI] [PubMed] [Google Scholar]
- 12.Picozzi VJ, Kozarek RA, Traverso LW. Interferon-based adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg. 2003;185:476–480. doi: 10.1016/s0002-9610(03)00051-5. [DOI] [PubMed] [Google Scholar]
- 13.Habermehl D, Kessel K, Welzel T, Hof H, Abdollahi A, Bergmann F, et al. Neoadjuvant chemoradiation with Gemcitabine for locally advanced pancreatic cancer. Radiat Oncol. 2012;7:28. doi: 10.1186/1748-717X-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arvold ND, Ryan DP, Niemierko A, Blaszkowsky LS, Kwak EL, Wo JY, et al. Long-term outcomes of neoadjuvant chemotherapy before chemoradiation for locally advanced pancreatic cancer. Cancer. 2012;118:3026–3035. doi: 10.1002/cncr.26633. [DOI] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Evans DB, Erickson BA, Ritch P. Borderline resectable pancreatic cancer: definitions and the importance of multimodality therapy. Ann Surg Oncol. 2010;17:2803–2805. doi: 10.1245/s10434-010-1285-8. [DOI] [PubMed] [Google Scholar]
- 17.Patel M, Hoffe S, Malafa M, Hodul P, Klapman J, Centeno B, et al. Neoadjuvant GTX chemotherapy and IMRT-based chemoradiation for borderline resectable pancreatic cancer. J Surg Oncol. 2011;104:155–161. doi: 10.1002/jso.21954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nukui Y, Picozzi VJ, Traverso LW. Interferon-based adjuvant chemoradiation therapy improves survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg. 2000;179:367–371. doi: 10.1016/s0002-9610(00)00369-x. [DOI] [PubMed] [Google Scholar]
- 19.Picozzi VJ, Abrams RA, Decker PA, Traverso W, O'Reilly EM, Greeno E, et al. Multicenter phase II trial of adjuvant therapy for resected pancreatic cancer using cisplatin, 5-fluorouracil, and interferon-alfa-2b-based chemoradiation: ACOSOG Trial Z05031. Ann Oncol. 2011;22:348–354. doi: 10.1093/annonc/mdq384. [DOI] [PMC free article] [PubMed] [Google Scholar]


