Abstract
Introduction: The presence of positive nodal disease (LND) and the number of lymph nodes involved (LNB) are known to be significant prognostic markers for resected adenocarcinoma of the pancreas. In addition, the ratio of the number of involved nodes to the number of nodes resected known as the lymph node ratio (LNR) is emerging as an important prognostic marker. The role of the resection margin (RM) as presently defined (R1 ≤ 1 mm) is unclear as results differ based on the dataset. The aim of this study was to assess the impact of nodal disease and a redefined RM on outcome.
Material and methods: Retrospective analysis of pancreatic head resections for adenocarcinomas from 2003–2009. The RM was re-analysed based on tumour clearance and categorized into: histopathological evidence of a tumour; ≤0.5 mm, ≤1 mm, ≤1.5 mm, or ≤2.0 mm of the actual surgical resection margin. The impact of histopathological variables on cancer-specific survival (CSS) and disease-free survival (DFS) was analysed.
Results: LND, LNB and LNR were independent prognostic markers for CSS (P = 0.048, 0.003, 0.016) but, did not influence DFS. A LNR < 0.143 was associated with a higher CSS [38.16 ± 4.69 versus 20.59 ± 2.20 months, P = 0.0042, hazard ratio (HR) 3.74 (95% confidence interval (CI) 1.52–9.23)]. An R1 RM was not associated with CSS or DFS on multivariate analysis, irrespective of the distance. LNB and LNR maintained independent significance irrespective of the size of the RM.
Conclusion: LNB and LNR are the only prognostic factors for CSS in patients with pancreatic head adenocarcinoma, but do not predict recurrence. Microscopic RMs does not seem to influence the outcome even when redefined. Further prospective studies are indicated to substantiate these findings.
Introduction
The long-term prognosis for patients with pancreatic cancer remains dismal with 5-year survival ranging from 15–25%.1–4 A surgical resection offers the best chance for a cure and remains the mainstay of therapy. However, only 10–20% of patients present with resectable disease.5,6 Adjuvant chemotherapy has been shown to almost double the 5-year survival after a successful resection.7,8 The failure to significantly influence outcome in pancreatic cancer in contrast to other site cancers reflects a lack of understanding of pancreatic tumour biology.
Numerous studies have evaluated histological parameters that predict outcome after surgery.3,4,9,10 While lymph node positivity, tumour stage and grade, microscopic resection margins, lymphovascular and perineural invasion and tumour size have all been associated with a poor outcome, results vary based on the dataset.3,4,9–11 Lymph nodal metastases is probably the most consistent factor associated with poor outcome although the exact nature of its association is as yet unclear. Three patterns of this association have been identified: (i) the presence of ≥1 involved nodes (LND, lymph node disease); (ii) an increasing number of nodes infiltrated by the tumour (LNB, lymph node burden); and (iii) an increasing ratio of the positive to examined nodes (lymph node ratio, LNR).1,3,12 The association between lymph nodal metastases and local disease recurrence/disease-free survival (DFS) has not been previously reported.
Although there is little doubt that an incomplete resection portents poor outcome, the significance of the negative resection margin is unclear. Currently, a resection margin >1 mm is considered negative.13 Unlike the data supporting the importance of a 1-mm circumferential resection margin (CRM) in rectal cancers,14,15 robust scientific evidence is lacking in pancreatic cancers. Further, the significance of a R0 resection in predicting outcome has been variable depending on the dataset analysed.3,16–24 It is likely that these outcomes may be influenced by the definition of R0, which may in itself be intrinsically flawed or, may be variably influenced by specific margins in relation to the tumour or, by the size of the study.
The aim of this study was to determine the histopathological variables that influenced the outcome with particular emphasis on the lymph node status and the resection margins (RM). The current definition of an R0 resection was redefined and the influence of specific margin status on DFS and overall survival (OS) was assessed.
Material and methods
Retrospective analysis of prospectively collected data on all patients undergoing a potentially curative pancreaticoduodenectomy for pancreatic ductal adenocarcinoma at the Royal Free hospital, London, UK, from 2003–2009. Data collected included demographic variables, details of surgery, time to recurrence or date of latest clinical review, time to death or latest clinical review and the use of adjuvant therapy.
The pancreaticoduodenectomy specimens were all dissected according to The Royal College of Pathologists (RCP) guidelines.13 Margins were inked prior to sectioning with different colours for each margin and the specimen sliced in the axial plane. Six different resection margins were measured microscopically and recorded, namely proximal duodenal (or gastric), distal duodenal, pancreatic, common bile duct, posterior and superior mesenteric (SMV)/ portal vein (PV) groove. A margin of >1 mm was reported as negative (R0). Distance from the tumour to the anterior surface was also noted. Other data included the type, grade and size of the cancer, the total number of nodes within the resected specimen and the total number of nodes involved with cancer. Lymph nodal disease (LND) was defined as the presence of ≥1 metastatic nodes, lymph nodal burden (LNB) was the number of nodes infiltrated by a tumour and the lymph nodal ratio (LNR) was the ratio of metastatic nodes to total nodes examined.
All the available histopathological specimens were re-examined by independent pathologists (J.W. and A.I.) to accurately define the R0 status based on tumour clearance and to re-categorize the R1 status based on histopathological evidence of a tumour ≤0.5 mm, ≤1 mm, ≤1.5 mm or ≤2.0 mm of the actual surgical resection margin (re-defined status).
All clinicopathological variables were correlated with cancer-specific survival (CSS) and DFS. DFS was based on the diagnosis of local or systemic recurrence identified through a combination of radiological imaging, tumour marker levels and biopsy. Local disease recurrence was confirmed either by biopsy and or the progression of lesions on subsequent follow-up scans. All patients underwent 3-monthly CT scans (chest, abdomen and pelvis) and serum tumour marker (CEA and CA19-9) analysis for at least 2 years after a surgical resection. Hence, all patients that were operated locally but followed up at other centres were excluded to ensure accuracy.
Specific exclusions included patients with adenocarcinoma arising in the background of an intraductal papillary mucinous neoplasm (IPMN), non-cancerous pathology, patients who underwent a total pancreatectomy, patients who died in the peri-operative period or within 30 days of discharge and deaths unrelated to tumour recurrence.
Statistical analysis
All the analysis was done using STATVIEW (SAS Institute Inc., Cary, NC, USA). Descriptive statistics was used to present demographic and treatment-related data. OS and DFS were univarietly analysed using the Kaplan–Meier method. The log-rank test was used to compare subgroups. Factors found to be associated with P ≤ 0.1 on univariate analysis were included in the multivariate analysis. Cox's proportional hazard was used for multivariate analysis. Statistical significance was set at P ≤ 0.05. Lymph nodal data were analysed as both nominal and continuous variables.
Results
A total of 86 patients identified from the pathological specimen database had undergone a laparotomy for resection of a pancreatic head adenocarcinoma from 2003–2009. Of these, 70 fulfilled all the criteria to be included in the study. Sixteen (19%) patients were excluded: six distal cholangiocarcinomas (incorrect coding), three total pancreatectomies, four periampullary tumours, one peri-operative death and two adenocarcinoma with background IPMN. The clinicopathological characteristics of the cohort and the operation performed are listed in Table 1. The median follow-up period was 19.5 months (range 2.5–101.3). 24/70 (34.3%) were alive at the time of inclusion.
Table 1.
Demographic, operative and histopathological variables
| Variable | Value | |
|---|---|---|
| Median age in years (interquartile range) | 65.79 (16.77) | |
| Gender | Male 35; female 35 | |
| Operation | PPPD | 60 (85.70%) |
| Whipples | 10 (14.30%) | |
| Vascular resection | 11 (15.70%) | |
| Max. tumour size in mm – median (interquartile range) | 30.00 (16) | |
| Differentiation | Well | 7 (10%) |
| Moderate | 37 (52.90%) | |
| Poor | 26 (37.14%) | |
| Surgical margins | R0/R1 | R0–18 (25.7%); |
| R1–52 (74.3%) | ||
| Anterior +ve | 21 (30%) | |
| Posterior +ve | 32 (45.70%) | |
| SMV/PV groove +ve | 33 (47.10%) | |
| CBD +ve | 2 (2.9%) | |
| Pancreatic edge +ve | 2 (2.9%) | |
| Perineural invasion +ve | 65 (92.90%) | |
| Lymphovascular invasion +ve | 38 (54.30%) | |
| Nodal status | Nodal status +ve | 55 (78.60%) |
| Median nodes examined | 14 (IQR 8) | |
| Median +ve nodes examined | 2.0 (IQR 3) | |
PPPD, pylorus-preserving pancreaticoduodenectomy; SMV/PV, superior mesenteric/portal vein; CBD, common bile duct.
Survival
CSS data were available in all 70 patients. The mean time to death was 22.90 ± 17.40 months (range: 3–101.13). On univariate analysis, the degree of differentiation (P = 0.031), lymphovascular invasion (P = 0.013), LND (presence of ≥1 positive nodes, P = 0.005) significantly affected CSS. (Table 2, Fig. 1) LNB (P = 0.0005), total nodes examined (P = 0.034) and the LNR (P = 0.018) were significantly associated with CSS when analysed as continuous variables. On multivariate analysis only the LNB, LNR and LND maintained significance (P = 0.0029, 0.016 and 0.048, respectively) (Table 2).
Table 2.
Impact of demographic, surgical and histopathological variables on survival
| Variables | Overall survival |
Disease-free survival |
|||||
|---|---|---|---|---|---|---|---|
| Median OS (IQR) – mths | Univariate P-value | Multivariate P-value | Median OS (IQR) – months | Univariate P-value | Multivariate P-value | ||
| Gender | 0.618 | 0.819 | |||||
| Vascular resection | 0.674 | 0.678 | |||||
| Type of reconstruction | 0.754 | 0.844 | |||||
| Tumour size | 0.13 | 0.955 | |||||
| Differentiation (Binomial Variable) | Well/Mod | 18.57 (16.65) | 0.031 | 0.161 | 7.42 (6.37) | 0.06 | 0.30 |
| Poorly | 13.53 (19.68) | 6.08 (6.08) | |||||
| Neural invasion | 0.973 | 0.631 | |||||
| Lymphovascular invasion | −ve | 24.26 (26.56) | 0.013 | 0.631 | 8.3 (13.1) | 0.022 | 0.155 |
| +ve | 16.37 (15.65) | 7.42 (7.0) | |||||
| SMV/PV groove invasion | −ve | 19.57 (27.98) | 0.065 | 0.167 | 0.363 | ||
| +ve | 14.47 (18.84) | ||||||
| Resection status (R0/R1) | −ve | 22.43 (28.53) | 0.015 | 0.840 | 0.301 | ||
| +ve | 16.30 (18.48) | ||||||
| Ant. margin status | 0.275 | 0.838 | |||||
| Post. margin status | −ve | 18.57 (22.58) | 0.062 | 0.652 | 0.012 | 0.292 | |
| +ve | 16.30 (17.78) | ||||||
| CBD margin | 0.762 | 0.507 | |||||
| +ve lymph node status | −ve | 26.13 (32.26) | 0.005 | 0.048 | 10.34 (17.52) | 0.016 | 0.075 |
| +ve | 16.30 (16.27) | 7.10 (6.80) | |||||
| Lymph node ratio (LNR)* | 0.018 | 0.016 | 0.287 | ||||
| Lymph node burden (LNB)* | 0.0005 | 0.0029 | 0.207 | ||||
| Total nodes* | 0.034 | 0.106 | 0.444 | ||||
CBD, common bile duct; LNR, lymph node ratio; LNB, lymph node burden.
Figure 1.
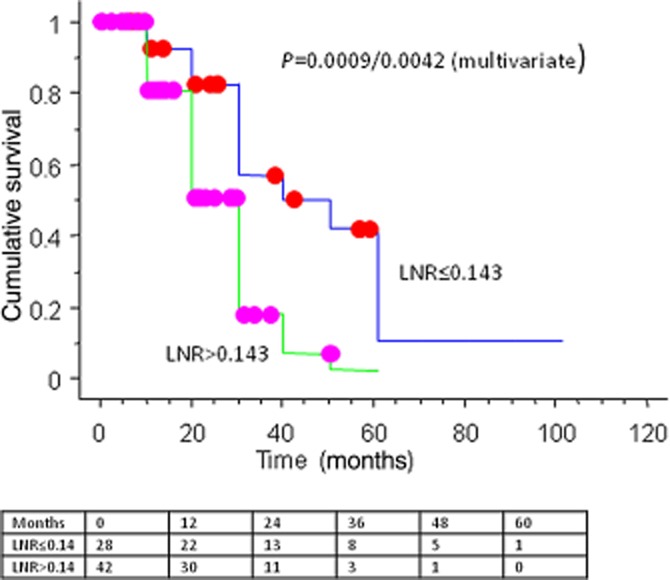
Influence of the lymph node ratio (LNR) stratification on overall survival (OS) – Kaplan–Meier survival curves and the numbers at the risk table
DFS was determined in 34 patients in whom all data were available. The mean time to recurrence was 9.89 ± 7.54 months (range: 1.27–58.35). On univariate analysis, lymphovascular invasion (P = 0.022) and positive lymph node status (P = 0.016) significantly affected DFS (Table 2).
Receiver-operator curve (ROC) analysis was used to determine the association between the LNR and CSS based on mortality during the study period. The best cut-off for LNR with regards to survival was determined at 0.143 (area under curve = 0.730). Patients with a LNR < 0.143 had a significantly higher survival than those with a higher LNR both on univariate and multivariate analyses [38.16 ± 4.69 versus 20.59 ± 2.20 months, P = 0.0042, hazard ratio (HR) 3.74 (95% CI 1.52–9.23)] (Fig. 1). There was no significant difference in the total number of examined nodes between these two groups (P = 0.81).
RM status and survival
The overall R0 resection rate based on the RCP guidelines was 25.7%, and was significantly associated with OS (P = 0.015) on univariate but not on multivariate analysis (P = 0.84). No association was found with DFS. The SMV/PV groove margin was the most common resection margin involvement (Table 1). A significant correlation was found between lymph node and margin positivity [P = 0.01, OR4.50 (95% CI 1.3–15.70)]. However, sub-grouping patients into node negative and node positive groups did not reveal any significant correlation between resection margin status and CSS (Table 5).
Table 5.
Impact of the resection margin (RM) status on cancer-specific survival (CSS) among node-ve and node +ve patients analysed individually
| Nodal status | Number of patients (%) | R0/R1 status [Number of patients (%)] |
Impact of R0/R1 status on CSS (P-value) | |
|---|---|---|---|---|
| R0 | R1 | |||
| Negative | 15 (21.5) | 7 (53) | 8 (47) | 0.20 |
| Positive | 55 (78.5) | 11 (20) | 44 (80) | 0.80 |
Redefining the RM status demonstrated a significant association with OS for completeness of resection (R0), and for the specific margins involving the PV/SMV groove and posterior resection margin for all groups (0.5, 1, 1.5 and 2 mm) (Table 3). However, these were not maintained on multivariate analysis.
Table 3.
Impact of varying the resection margin (RM) status on cancer-specific survival (CSS) and disease-free survival (DFS) (re-reported specimens)
| Margin value (mm) | Margin site | OS |
DFS |
||
|---|---|---|---|---|---|
| Univariate P-value | Multivariate P-value | Univariate P-value | Multivariate P-value | ||
| ≤0.5 | Ant. margin | 0.708 | 0.837 | ||
| Post. margin | 0.196 | 0.097 | 0.683 | ||
| SMV groove | 0.051 | 0.130 | 0.156 | ||
| R0/R1 | 0.064 | 0.846 | 0.670 | ||
| ≤1.0 | Ant. margin | 0.275 | 0.838 | ||
| Post. margin | 0.062 | 0.652 | 0.012 | 0.292 | |
| SMV groove | 0.065 | 0.167 | 0.363 | ||
| R0/R1 | 0.015 | 0.840 | 0.301 | ||
| ≤1.5 | Ant. margin | 0.163 | 0.461 | ||
| Post. margin | 0.087 | 0.486 | 0.066 | 0.963 | |
| SMV groove | 0.062 | 0.115 | 0.536 | ||
| R0/R1 | 0.016 | 0.810 | 0.267 | ||
| ≤2.0 | Ant. margin | 0.179 | 0.575 | ||
| Post. margin | 0.11 | 0.106 | |||
| SMV groove | 0.031 | 0.093 | 0.544 | ||
| R0/R1 | 0.007 | 0.54 | 0.262 | ||
OS, overall survival.
Multivariate regression analysis confirmed that the LNR and LNB maintained their independent influence on CSS irrespective of the chosen cut-off for what was considered to be completeness of resection in all groups, whereas lymph nodal status maintained significance only for a RM status ≤1 and 1.5 mm (Table 4).
Table 4.
Influence of the redefined resection margin status on the association between lymph nodal positivity (LNP), lymph node burden (LNB), lymph node ratio (LNR) and cancer-specific Survival (CSS)
| Resection margin (R0 defined as under) | LNP Multivariate P-value | LNB Multivariate P-value | LNR Multivariate P-value |
|---|---|---|---|
| ≤0.5 | 0.105 | 0.007 | 0.032 |
| ≤1.0 | 0.048 | 0.0029 | 0.016 |
| ≤1.5 | 0.041 | 0.003 | 0.010 |
| ≤2.0 | 0.156 | 0.004 | 0.018 |
Discussion
This study reaffirms the adverse prognostic effect of lymph nodal metastases on survival after a pancreatic head resection for ductal adenocarcinoma. This study has shown that LND, LNB and LNR independently influence outcome. However, these findings are not entirely consistent with previous studies and opinion is still guarded as to the exact nature of the relationship between lymph nodal metastases and outcome.1,3,12
A number of retrospective studies have analysed the relationship between survival and extent of lymph node assessment after resection of pancreatic tumours.1,12,25 An inadequate surgical lymphadenectomy or pathological assessment would understage patients owing to the potential of missing metastatic nodes. A large review of 1666 patients within the Surveillance, Epidemiology and End results (SEER) database for pancreatic cancer suggested that an attempt to harvest and resect at least 15 lymph nodes was essential for accurate staging.26 Higher nodal yields were not associated with significant survival differences at any stage.26 Similarly, a study by the Memorial Sloan–Kettering Cancer Centre group showed that a lymph node harvest of 12 nodes provided accurate survival estimates in patients with node negative disease.1 The median nodes examined in this study was 14 (IQR 8) and, unlike the SEER database study, there was no significant difference in the total number of nodes examined between the nodes positive and nodes negative group [median 15.0 (range: 3–49) versus 10.0 (range: 4–25); P = 0.140].
It has been suggested that the use of LND alone or LNB may carry the bias of an inadequate lymphadenectomy or histopathological examination. Previous studies in both colorectal and gastric cancer have shown that a ratio-based classification of lymph nodes was far superior to LNB and LND in predicting survival.27–29 The same seems to be true for pancreatic tumours where the LNR is emerging as the candidate marker for prognosis as opposed to the LND or LNB.1,12,25 These data showed that a LNR ≥ 0.143 was associated with reduced survival [HR 3.74 (95% CI 1.52–9.23)]. Unlike this study, an association between LNB and LND has not been a consistent finding in previous studies, and this disparity can be explained as a result of a number of clinical and pathological differences in the datasets.1,3,4,9,10,12 As it stands, LNR appears to be the most consistent marker for stratification of prognosis.
The majority of reported series have included tumours not confined to the head of the pancreas, tumours other than ductal adenocarcinomas, and above all there appears to be a huge difference in the reporting of a resection margin status suggesting inconsistent reporting of histopathological specimens.1,12,16,25,30–32 The large population-based study by Slidell et al. included histological variants such as adenosquamous and signet ring cell carcinomas; the biology of these are likely to differ influencing the outcome.32 Numerous other studies reporting on lymph nodes included resections for tumours in the head, body and tail, requiring anatomically varied resections. Their patterns of spread are different, as are their outcomes.1,12,25,29 Such confounding variables have been eliminated in this dataset. Unlike previous studies, this dataset included a homogenous population of patients with primary adenocarcinoma of the head of the pancreas who have undergone a pancreaticoduodenectomy alone.
A recent study by Murikami et al. factored in the role of adjuvant chemotherapy and showed that LNB and lymph node positivity status predicted outcome, but not LNR.33 A key contributor to their observations may have been the fact that an extended lymphadenectomy was performed in the vast majority of their patient cohort (mean number of nodes examined was 29), perhaps allowing a better nodal staging of the disease. As the chemotherapy practice was variable, we could not include data in our analysis. Randomized controlled trials have confirmed the positive effect of chemotherapy on survival and is likely to influence the results.8,19
This study also shows that lymph nodal metastasis is not a marker for early recurrence as evidenced by the lack of an association between LND, LNB and LNR, and DFS. This finding assumes significance as most previous studies reporting on the prognostic significance of lymph nodes take into account the CSS but not DFS. As routine surveillance does not improve outcome even if metastases are detected early, estimation of DFS is often inaccurate. Owing to our surveillance policy of 3 monthly CT scanning even in patients not receiving adjuvant therapy, we could determine DFS. However, the numbers were significantly smaller than those analysed for CSS owing to our referral practice. This lack of an association between lymph nodal status and DFS suggests that patients with lymph node metastases do not recur earlier than those without, but once recurrence occurs they succumb faster. This could result from differences in tumour biology, differential sites of metastases or possibly the tendency of these tumours to de-differentiate as recurrence manifests. It would be interesting to compare the sites of recurrence in these groups of patients. We acknowledge the limitations of our study in that the dataset for the DFS group is significantly smaller than the CSS group and hence, the possibility of a type2 error.
Previous studies have shown a variable effect of the RM on survival. Differences in pathological reporting, surgical technique, definition of positivity and group stratification affects outcome analyses.34 Additionally, margin positivity has not been shown to be significant on multivariate analysis in a number of studies.34 Campbell et al. reviewed the histology of 128 patients undergoing a pancreatic resection for adenocarcinoma reported as having an R1 resection.35 Of these, 57 (45%) were based on equivocal margins (tumour involvement within 1 mm of, but not directly reaching, one or more resection margins). There was no difference in survival between the equivocal and unequivocal groups. All R1 resections had a poorer survival as compared with R0 resections on univariate but not on multivariate analysis. Menon et al. demonstrated that R1 resections adversely affected survival on univariate analysis alone.36 R0 resection rates of 15–30% is noted in centres that use the RCP guidelines for pathological reporting; the rate in this study is 25.7%.36,37 In contrast, the reported R0 resection rates from the US are much higher (55–85%) suggesting a difference in pathological staging standards.1,12,25,31 In spite of these differences in pathological reporting, RM status was not associated with survival on multivariate analyses in any of these studies. This would suggest that the RM is not a significant prognostic factor for survival. Interestingly in our dataset, there is a significant correlation between lymph node and margin positivity (P = 0.001). Further 78% of this dataset had positive lymph node disease. This could suggest that lymph node positivity has a stronger influence on outcome as compared with RM status. When the RM in node negative patients alone was examined no significance was found in the outcome between R0 and R1 resections on univariate analysis (Table 5 ). However, the numbers were too small for any meaningful interpretation of and the chance of a type2 error is highly likely.
Redefining the resection margin did not impact the prognostic significance on multivariate analysis. Incorporating the margin status into a multivariate analysis showed that the LNR and LNB maintained their significance throughout all margin sizes, whereas the lymph nodal positivity status was variable depending upon the margin cutoff. This suggests that the LNR and LNB are better prognostic indicators than the RM.4
Conclusion
This study is the first report to demonstrate a clear and independent association of tumour lymph node burden and the LNR with OS irrespective of the resection margin status as it stands currently or, if redefined. The presence of LND did not seem to affect DFS. However, the small size of the study and the lack of chemotherapy data might significantly influence the results. Hence, large controlled prospective studies to define the exact relationship between lymph nodes and RM, and outcome are indicated.
Conflicts of interest
None declared.
References
- 1.House MG, Gonen M, Jamagin WR, DÁngelica M, DeMatteo RP, Fong Y, et al. Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. J Gastrointest Surg. 2007;11:1549–1555. doi: 10.1007/s11605-007-0243-7. [DOI] [PubMed] [Google Scholar]
- 2.Tseng JF, Raut CP, Lee JE, Pisters PW, Vauthey JN, Abdalla EK, et al. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg. 2004;8:935–949. doi: 10.1016/j.gassur.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 3.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 pancreaticoduodenctomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Richter A, Niedergethmann M, Sturm JW, Lorenz D, Post S, Trede M. Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World J Surg. 2003;27:324–329. doi: 10.1007/s00268-002-6659-z. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer Statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 6.Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100, 313 patients diagnosed from 1985–1995, using the National Cancer database. J Am Coll Surg. 1999;189:1–7. doi: 10.1016/s1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 7.Neoptolemos JP, Stocken DD, Dunn JA, Almond J, Beger HG, Pederzoli P, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg. 2001;234:758–768. doi: 10.1097/00000658-200112000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 9.Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74–85. doi: 10.1097/00000658-200301000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breslin TM, Hess KR, Harbison DB, Jean ME, Cleary KR, Dackiw AP, et al. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: treatment variables and survival duration. Ann Surg Oncol. 2001;8:123–132. doi: 10.1007/s10434-001-0123-4. [DOI] [PubMed] [Google Scholar]
- 11.Chen JWC, Bhandari M, Astill DS, Wilson TG, Kow L, Brooke-Smith M, et al. Predicting patient survival after pancreaticoduodenctomy for malignancy: histological criteria based on perinueral infiltration and lymphovascular invasion. HPB. 2010;12:101–108. doi: 10.1111/j.1477-2574.2009.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pawlik TM, Gleisner AL. Cameron JL, Winter JM, Assumpcao L, Lillemoe KD, et al. Prognostic relevance of lymph node ratio following pacreatiduodenctomy for pancreatic cancer. Surgery. 2007;141:610–618. doi: 10.1016/j.surg.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 13.The Royal College of Pathologists. Standards and Minimum Datasets for Reporting Cancers. Minimum Dataset for the Histopathological Reporting of Pancreatic, Ampulla of Vater and Bile Duct Carcinoma. London: The Royal College of Pathologists; 2002. [Google Scholar]
- 14.Adam IJ, Mohamdee MO, Martin IG, Scott N, Finan PJ, Johnston D, et al. Role of circumferential resection margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344:707–711. doi: 10.1016/s0140-6736(94)92206-3. [DOI] [PubMed] [Google Scholar]
- 15.Birbeck KF, Macklin CP, Tiffin NJ, Parsons W, Dixon MF, Mapstone NP, et al. Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery. Ann Surg. 2002;235:449–457. doi: 10.1097/00000658-200204000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jamieson NB, Foulis AK, Oien KA, Going JJ, Glen P, Dickson EJ, et al. Positive mobilization margins alone do not influence survival following pancreatico-duodenctomy for pancreatic ductal adenocarcinoma. Ann Surg. 2010;251:1003–1010. doi: 10.1097/SLA.0b013e3181d77369. [DOI] [PubMed] [Google Scholar]
- 17.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RS, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 18.Yeo CJ, Cameron JL, Lillemoe KD, Sitzmann JV, Hruban RH, Goodman SN, et al. Pancreaticoduodenctomy for cancer of the head of the pancreas. 201 patients. Ann Surg. 1995;221:721–731. doi: 10.1097/00000658-199506000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, et al. Adjuvant Chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomized controlled trial. Lancet. 2001;358:1576–1585. doi: 10.1016/s0140-6736(01)06651-x. [DOI] [PubMed] [Google Scholar]
- 20.Bouvet M, Gamagami RA, Gilpin EA, Romeo O, Sasson A, Easter DW, et al. Factors influencing survival after resection for periampullary neoplasm. Am J Surg. 2000;180:13–17. doi: 10.1016/s0002-9610(00)00405-0. [DOI] [PubMed] [Google Scholar]
- 21.Fusai G, Warnaar N, Sabin CA, Archibong S, Davidson BR. Outcome of R1 resection in patients undergoing pancreatico-duodenctomy for pancreatic cancer. Eur J Surg Oncol. 2008;34:1309–1315. doi: 10.1016/j.ejso.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Jarufe NP, Coldham C, Mayer AD, Mirza DF, Buckels JA, Bramhall SR. Favourable prognostic factors in a large UK experience adenocarcinoma of the head of the pancreas and periampullary region. Dig Surg. 2004;21:202–209. doi: 10.1159/000079346. 718-725. discussion 725–727. [DOI] [PubMed] [Google Scholar]
- 23.Raut CP, Tseng JF, Sun CC, Wang H, Wolff RA, Crane CH, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52–60. doi: 10.1097/01.sla.0000259391.84304.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt CM, Powell ES, Yiannoustsos CT, Howard TJ, Wiebke EA, Wiesenauer CA, et al. Pancreaticoduodenectomy: a 20-year experience in 516 patients. Arch Surg. 2004;139:725–727. doi: 10.1001/archsurg.139.7.718. [DOI] [PubMed] [Google Scholar]
- 25.Riediger H, Keck T, Wellner U, zur Hausen A, Adam U, Hopt UT, et al. The Lymph Node ratio is the strongest prognostic factor after resection of pancreatic cancer. J Gastrointest Surg. 2009;13:1337–1344. doi: 10.1007/s11605-009-0919-2. [DOI] [PubMed] [Google Scholar]
- 26.Schwarz RE, Smith DD. Extent of lymph node retrieval and pancreatic cancer survival: information from a large US population database. Ann Surg Oncol. 2006;13:1189–1200. doi: 10.1245/s10434-006-9016-x. [DOI] [PubMed] [Google Scholar]
- 27.Bando E, Yonemura Y, Taniguchi K, Fushida S, Fujimura T, Miwa K. Outcome of ratio of lymph node metastasis in gastric carcinoma. Ann Surg Oncol. 2002;9:775–784. doi: 10.1007/BF02574500. [DOI] [PubMed] [Google Scholar]
- 28.Berger AC, Sigurdson ER, LeVoyer T, Hanlon A, Mayer RJ, Macdonald JS, et al. Colon Cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol. 2005;23:8706–8712. doi: 10.1200/JCO.2005.02.8852. [DOI] [PubMed] [Google Scholar]
- 29.Le Voyer TE, Sigurdson ER, Hanlon AL, Mayer RJ, Macdonald JS, Catalano PJ, et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol. 2003;21:2912–2919. doi: 10.1200/JCO.2003.05.062. [DOI] [PubMed] [Google Scholar]
- 30.Bhatti I, Peacock O, Awan AK, Semeraro D, Larvin M, Hall RI. Lymph node ratio versus number of affected lymph nodes as predictors of survival for resected pancreatic adenocarcinoma. World J Surg. 2010;34:768–775. doi: 10.1007/s00268-009-0336-4. [DOI] [PubMed] [Google Scholar]
- 31.Katz MHG, Want H, Fleming J, Sun CC, Hwang RF, Wolff RA, et al. Long-Term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16:836–847. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slidell MB, Chang DC, Cameron JL, Wolfgang C, Herman JM, Schulick RD, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol. 2008;15:165–174. doi: 10.1245/s10434-007-9587-1. [DOI] [PubMed] [Google Scholar]
- 33.Murakami Y, Uemura K, Sudo T, Hayashidani Y, Hashimoto Y, Nakashima A, et al. Number of metastatic lymph nodes, but not lymph node ratio, is an independent prognostic factor after resection of pancreatic carcinoma. J Am Coll Surg. 2010;211:196–204. doi: 10.1016/j.jamcollsurg.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 34.Evans DB, Farnell MB, Lillemoe KD, Vollmer C, Jr, Strasberg SM, Schulick RD. Surgical Treatment of Resectable and Borderline Resectable Pancreas Cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1736–1744. doi: 10.1245/s10434-009-0416-6. [DOI] [PubMed] [Google Scholar]
- 35.Campbell F, Smith RA, Whelan P, Sutton R, Raraty M, Neoptolemus JP, et al. Classification of R1 resections for pancreatic cancer: the prognostic relevance of tumour involvement within 1mm of a resection margin. Histopathology. 2009;55:277–283. doi: 10.1111/j.1365-2559.2009.03376.x. [DOI] [PubMed] [Google Scholar]
- 36.Menon KV, Gomez D, Smith AM, Anthony A, Verbeke CS. Impact of margin status on survival following pancreatoduodenectomy for cancer: the Leeds Pathology Protocol (LEEPP) HPB. 2009;11:18–24. doi: 10.1111/j.1477-2574.2008.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esposito I, Kleeff J, Bergmann F, Reiser C, Herpel E, Friess H, et al. Most pancreatic cancer resections are R1 resections. Ann Surg Oncol. 2008;15:1651–1660. doi: 10.1245/s10434-008-9839-8. [DOI] [PubMed] [Google Scholar]


