Abstract
Introduction: The aim of this study was to analyse the influence of factors reported in the minimum histopathology dataset for colorectal liver metastases (CRLM) and other pre-operative factors compared with additional data relating to the presence of tumour pseudocapsules and necrosis on recurrence 1 year after a resection.
Methods: For a period of 14 months, extended histological reporting of CRLM specimens was performed, including the presence of pseudocapsules and necrosis in each tumour. The details of recurrence were obtained from surveillance imaging.
Results: In 66 patients there were 27 recurrences within 1 year. The rates were lower for patients with tumour pseudocapsules (8/27) than for patients without (19/36) (P = 0.030). Pseudocapsules were associated with a younger age (P = 0.005), nodal stage of the primary colorectal tumour (P = 0.025) and metachronous tumours (P = 0.004). In patients with synchronous disease and pseudocapsules, the recurrence rate was 2/12 compared with 13/23 patients without pseudocapsules (P = 0.026).
Discussion: These findings demonstrate that histological examination of resection specimens can provide significant additional prognostic information for patients after resection of CRLM, compared with clinical and radiological data. The present finding that the absence of a pseudocapsule in patients with synchronous CRLM is associated with a dramatically worse outcome may help direct patient-specific adjuvant treatment and care.
Introduction
Although resection of colorectal liver metastases (CRLM) offers overall 5-year survival rates ranging from 32–65%,1,2 there is a spectrum of outcomes after surgery with some individuals remaining disease free and potentially being cured, whereas others will recur early with a poor outcome.3,4 A number of risk scoring systems exist to stratify patients according to likely 5-year survival. These systems predominantly use factors measurable pre-operatively which have been shown to be markers of prognosis, such as carcinoembryonic antigen (CEA) estimation,5 tumour number,5–15 tumour size,5,12–15 resection margin clearance,5,8–10,12,16–20 the presence of satellite lesions18 and the ratio of neutrophils to lymphocytes amongst white cells in a full blood count measured pre-operatively.21 Liver specimens are routinely sent for pathological analysis after resection and the UK Royal College of Pathologists (RCPath) minimum dataset for liver specimens with colorectal metastases includes details of tumour number, size, location, resection margin clearance, capsular invasion, degree of differentiation, the presence of tumour necrosis, vascular and lymphatic invasion, the presence of satellite lesions, invasion of adherent tissue and lymph node status if sampled.22 With regards to prognostic factors, the most important additional information the pathology report reveals which is not available pre-operatively is the resection margin status. However, of the 15 risk scoring systems available, only 3 have shown the presence of an involved resection margin to be a significant prognostic factor.23 Therefore histological examination of CRLM specimens may add relatively little additional prognostic information compared with clinical, radiological and laboratory data in the currently used scoring systems.
Extended examination of resection specimens may reveal other features whose prognostic significance has not been rigorously assessed, including details of a fibrous pseudocapsule around the tumour and the degree of tumour necrosis. The presence of a pseudocapsule has been associated with a better overall survival after resection of CRLM.24–26 Tumour necrosis can result from chemotherapy use27 and is also seen in tumours with high rates of cellular turnover in rapidly expanding tumours.28 Therefore tumour necrosis may be associated with more aggressive tumours and a worse prognosis.
The aim of this study was to analyse the relative significance of factors reported in the minimum histopathology dataset and other pre-operative factors compared with additional data relating to the presence of tumour pseudocapsules and necrosis on tumour recurrence 1 year after resection of CRLM.
Methods
Between March 2010 and May 2011, the Histopathology Department at Derriford Hospital performed extended reporting of CRLM specimens as an experimental protocol. Histology reports documented the presence or absence of a pseudocapsule, as well as how much of each tumour diameter was encompassed (zero, <50% or >50%). The presence and degree of necrosis observed in each tumour (nil, <33%, 33–66% and complete necrosis) was also recorded. Up to a maximum of the three largest tumours in each patient were assessed and relevant features recorded. The pseudocapsule was identified as a paucicellular collagenous band present between the tumour cells and the adjacent hepatocytes, which measured at least 0.1 mm in thickness (Fig. 1). Tumour necrosis was characterized as discrete foci of cellular debris indicative of coagulative cell death (Fig. 2). A proforma was designed and agreed within the Histopathology department to standardize reporting of resection specimens. In cases of heterogeneity between tumours, the amount of pseudocapsule in up to the three largest tumours was measured and an average figure calculated according to a simple formula (>50% = 2, <50% = 1, no pseudocapsule = 0) and used in analyses. The amount of necrosis was determined for the largest lesion only.
Figure 1.
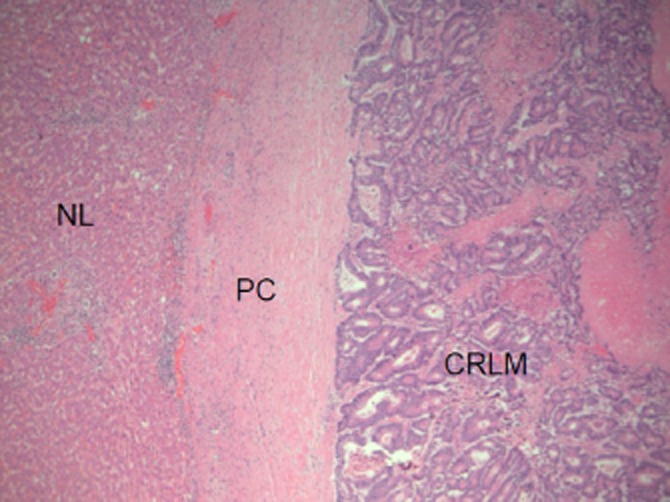
Pathological examination showing a pseudocapsule (PC), non-neoplastic liver (NL) and a colorectal liver metastasis (CRLM). Original magnification x50 using haematoxylin and eosin stain
Figure 2.
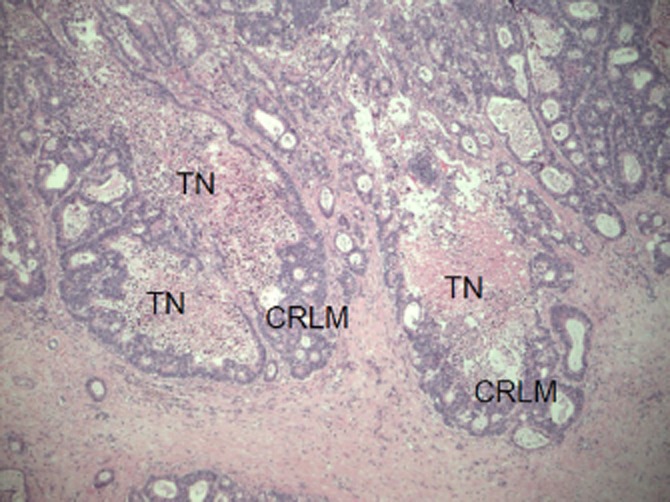
Pathological examination showing tumour necrosis (TN) in colorectal liver metastases (CRLM). Original magnification x50 using haematoxylin and eosin stain
All patients underwent tumour staging with a computed tomography scan prior to surgery. In addition, 46 patients had a pre-operative magnetic resonance imaging (MRI) scan and 50 patients a pre-operative positron emission tomography (PET) scan, at the discretion of the referring clinician.
A prospective database is maintained of all patients undergoing resection for CRLM and a review of these patients was performed when all had been followed up for a minimum of 1 year. The database holds information on primary histology, timing of detection of metastatic disease (synchronous tumours were defined as those discovered pre-operatively or within 2 months of primary surgery), the neutrophil to lymphocyte ratio, the use of chemotherapy as well as the histological features of the resected CRLM. Details of tumour recurrence were identified from surveillance imaging which is performed according to published guidelines.22 CEA estimation was not used routinely in post-operative surveillance. One patient did not have surveillance imaging in the first post-operative year and was excluded from recurrence analysis. One-year recurrence was chosen as the primary end point because a high proportion of CRLM recur within this timeframe and early recurrence is associated with a worse overall survival.3,4
Potential associations between 1-year tumour recurrence and clinical and histological characteristics were tested initially using univariate logistic regression or the chi-square test at the level of P < 0.25,29 as appropriate. The association between clinical and histological characteristics and the presence of a tumour pseudocapsule in individual tumours was tested in a similar fashion. Significant variables in the univariate analysis were included in the multivariate logistic regression model and were considered to be significant if P < 0.05. All analyses were carried out using the statistical package R 2.1.14.30
Results
Sixty-six patients were identified who underwent surgery for CRLM of whom 65 were available for recurrence analysis. Additional staging MRI scans were performed in 28 of 38 patients with synchronous tumours and 18 of 28 with metachronous tumours (P = 0.431). Additional staging PET scans were performed in 28 of 38 patients with synchronous tumours and 22 of 28 with metachronous tumours (P = 0.774). The median number of surveillance scans performed was one (1–4) in patients who recurred and two (1–5) in patients who had not recurred at 1 year.
In addition to surgery, four patients had intra-operative radiofrequency ablation (RFA). Six patients died of recurrent cancer in the first year of follow-up. Twenty-eight patients (43.1%) developed recurrent cancer within the first year of follow-up. Eight of these recurred in the liver only, 12 had extrahepatic recurrence only and 8 had both hepatic and extrahepatic recurrence. Patient characteristics are displayed in Table 1.
Table 1.
Pre-operative details of 66 patients undergoing extended histological reporting of resection of hepatic colorectal metastases
| n = 66 | Median (range) | Count (%) | |
|---|---|---|---|
| Age | 65 (33–84) | ||
| Gender | Male | 40 (60.6) | |
| Female | 26 (39.4) | ||
| Primary T stage | 0 | 2 (3.0) | |
| 1 | 3 (4.5) | ||
| 2 | 7 (10.6) | ||
| 3 | 29 (43.9) | ||
| 4 | 23 (34.8) | ||
| Unavailable | 2 (3.0) | ||
| Primary N stage | 0 | 34 (51.5) | |
| 1 | 18 (27.2) | ||
| 2 | 11 (16.7) | ||
| Unavailable | 3 (4.5) | ||
| Timing | Synchronous | 38 (57.6) | |
| Metachronous | 28 (42.4) | ||
| Liver-directed chemotherapy | Synchronous | 35 (92.1) | |
| Metachronous | 11 (39.2) | ||
| Neutrophil lymphocyte ratio | Less than 5 | 57 (86.4) | |
| More than 5 | 9 (13.6) | ||
From the total patient group, 132 lesions were examined histologically in the extended dataset. In two patients, three tumours had responded completely to chemotherapy and were only identifiable microscopically as areas of complete necrosis. In these tumours, the presence of a pseudocapsule could not be assessed. Histological details of the resected specimens including the RCPath dataset and the presence of a pseudocapsule and degree of tumour necrosis for the 65 patients included in the recurrence analysis are shown in Table 2. Heterogeneity in the presence of tumour pseudocapsules in multiple metastases was observed in 6 of 27 patients, where pseudocapsules were absent in some tumours, and in 5 of 27 patients where a differing amount of pseudocapsule was noted between tumours.
Table 2.
Histopathological features and 1-year recurrence of 65 patients undergoing extended histological reporting of resection of hepatic colorectal metastases with 1-year follow-up
| n = 65 | 1-year recurrence |
||||
|---|---|---|---|---|---|
| No (n = 37) | Yes (n = 28) | ||||
| Median (range) | Count | Median (range) | Count | ||
| Number of lesions identified | 2 | 3 | |||
| (1–10) | (1–10) | ||||
| Max diameter at histology (mm) | 27 | 43 | |||
| (3–119) | (7–120) | ||||
| Satellite lesions | Yes (0) | 0 | 0 | ||
| No (65) | 37 | 28 | |||
| Margin less than 10 mm | Yes (45) | 24 | 21 | ||
| No (20) | 13 | 7 | |||
| Margin less than 1 mm | Yes (22) | 9 | 13 | ||
| No (43) | 28 | 15 | |||
| Liver capsule smooth and intact | Yes (55) | 33 | 22 | ||
| No (10) | 4 | 6 | |||
| Invasion of adherent tissue | Yes (1) | 0 | 1 | ||
| No (64) | 37 | 27 | |||
| Differentiation | No tumour (3) | 2 | 1 | ||
| Well/moderate (62) | 35 | 27 | |||
| Vascular invasion | Yes (9) | 3 | 6 | ||
| No (56) | 34 | 22 | |||
| Histological evidence of response to chemotherapy | No response (4) | 2 | 2 | ||
| Response (19) | 13 | 6 | |||
| Uncertain (7) | 4 | 3 | |||
| Not recorded (35) | 18 | 17 | |||
| Average amount of pseudocapsule | Nil (36) | 17 | 19 | ||
| <50% (17) | 12 | 5 | |||
| >50% (10) | 7 | 3 | |||
| N/A (2) | 1 | 1 | |||
| Amount of necrosis of the largest tumour | Nil (4) | 3 | 1 | ||
| <33% (29) | 16 | 13 | |||
| 33–66% (21) | 13 | 8 | |||
| >66% (11) | 5 | 6 | |||
In two patients (three tumours) a complete response to chemotherapy was noted and therefore a pseudocapsule could not be identified (NA).
Analysis of factors associated with 1-year recurrence in 65 patients
Univariate analysis of pre-operative and histological factors and 1-year recurrence revealed potential associations with age, number of metastases, a resection margin of less than 1 mm and the presence or absence of a pseudocapsule (P < 0.250) (Table 3). Multivariate analysis revealed that only the absence of a pseudocapsule and a resection margin of less than 1 mm were significantly associated with early tumour recurrence (Table 3). One-year recurrence rates were lower for patients with tumour pseudocapsules (8/27) than for patients with no pseudocapsule (19/36) (P = 0.030). There was no significant difference in tumour recurrence rates according to the amount (< or >50%) of pseudocapsule present (P = 0.750) (Fig. 3). The recurrence rate in patients with a resection margin of <1 mm was 13/22 compared with 15/43 in those with a margin of >1 mm (P = 0.045).
Table 3.
Univariate and multivariate analysis of pre-operative and histological factors affecting 1-year recurrence after resection of hepatic colorectal metastases in 65 patients
| Factor (n = 65) | Univariate P-value | Multivariate P-value | Incidence ratio (95% CI) |
|---|---|---|---|
| Age | 0.025a | 0.876 | |
| Gender | 0.506 | ||
| Max diameter of tumour at histology | 0.323 | ||
| Number of lesions | 0.240a | 0.831 | |
| Capsule smooth and intact | 0.408 | ||
| Margin less than 1 mm | 0.109a | 0.045b | 2.89 (1.61– 5.18) |
| Margin less than 10 mm | 0.545 | ||
| Histological response to chemotherapy | 0.674 | ||
| T stage of primary tumour | 0.571 | ||
| N stage of primary tumour | 0.381 | ||
| Synchronous versus metachronous | 0.824 | ||
| Liver directed chemotherapy (yes/no) | 0.710 | ||
| Neutrophil lymphocyte ratio (>5) | 0.320 | ||
| Pseudocapsule present | 0.114a | 0.030b | 0.30 (0.174–0.524) |
| Necrosis of largest lesion | 0.886 |
Significant at the level of 0.25 for univariate analysis and included in multivariate analysis.
Significant at the level of 0.05 for multivariate analysis.
Figure 3.
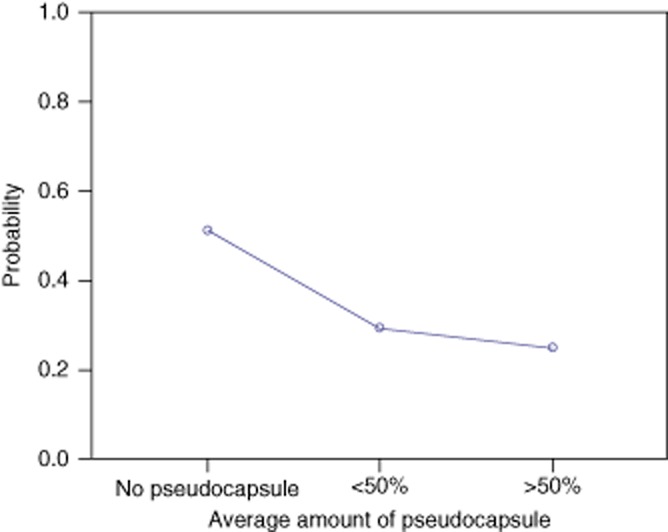
Probability of 1 year recurrence according to the amount of pseudocapsule present for 65 patients undergoing extended histological reporting of resection of hepatic colorectal metastases. No significant difference between <50% and >50% (P = 0.75)
Analysis of factors associated with the presence of a pseudocapsule in 132 tumours
Uni-and multivariate analysis was undertaken and revealed that increasing age, nodal status of the primary colorectal cancer and metachronous liver metastases were associated with the presence of a tumour pseudocapsule (Table 4). The size of individual tumours was not associated with the presence of a pseudocapsule. For each year of age the incidence of a pseudocapsule falls by 0.073 (Fig. 4). Similarly as the N stage increases by 1, the incidence of a pseudocapsule falls by 0.566 (Fig. 5). Pseudocapsules occurred more commonly in tumours with a metachronous presentation (25/51) compared with a synchronous presentation (20/81) (P = 0.004). Resection margin positivity was noted in 11 of 52 tumours with a pseudocapsule and 8 of 77 tumours without a pseudocapsule (P = 0.105).
Table 4.
Univariate and multivariate analysis of factors associated with the presence of a tumour pseudocapsule (n = 132) in 66 patients undergoing resection of hepatic colorectal metastases
| Factor (n = 132) | Univariate P-value | Multivariate P-value | Incidence ratio (95% CI) |
|---|---|---|---|
| Age (per year) | 0.011a | 0.005b | 0.937 (0.90–0.98) |
| Gender | 0.090a | 0.142 | |
| T stage of primary tumour | 0.290 | ||
| N stage of primary tumour | <0.001a | 0.025b | 0.434 (0.24–0.77) |
| Metachronous versus synchronous | 0.004a | 0.004b | 2.622 (1.13–6.09) |
| Neutrophil lymphocyte ratio | 0.348 | ||
| Tumour size | 0.167a | 0.405 | |
| Resection margin < 1 mm | 0.150a | 0.105 |
Significant at the level of 0.25 for univariate analysis and included in multivariate analysis.
Significant at the level of 0.05 for multivariate analysis.
Figure 4.
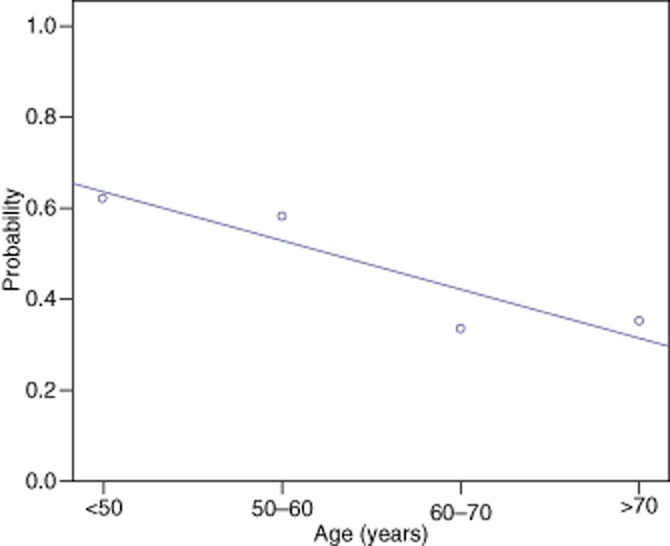
Probability of pseudocapsule presence according to age for 132 tumours in patients undergoing resection of hepatic colorectal metastases
Figure 5.
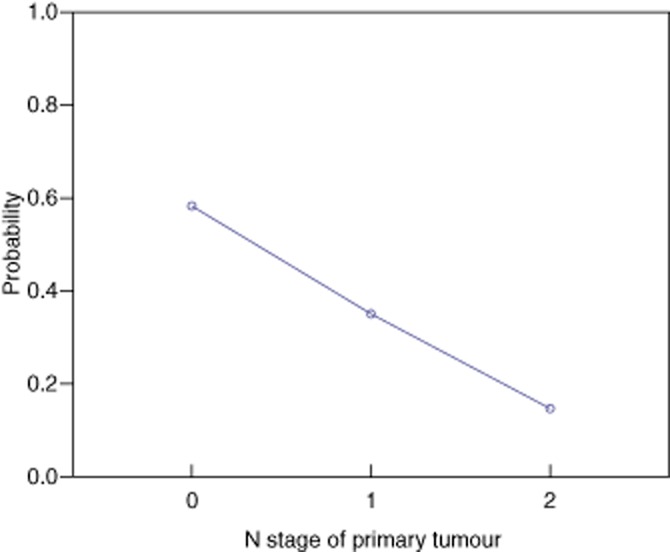
Probability of a pseudocapsule according to N stage of primary colorectal tumour for 132 tumours in patients undergoing resection of hepatic colorectal metastases
The presence of a pseudocapsule had no significant association with 1-year recurrence in patients with metachronous CRLM. However, in individuals with synchronous lesions the presence of a pseudocapsule was associated with a lower 1-year recurrence rate (2/12 versus 13/23) (P = 0.026) (Table 5).
Table 5.
Relationship between pseudocapsule and 1-year recurrence in synchronous and metachronous lesions in patients undergoing resection of hepatic colorectal metastases
| n = 65 | 1-year recurrence |
|||||
|---|---|---|---|---|---|---|
| No | Yes | P-value | ||||
| Timing | Synchronous (37) | Pseudocapsule | Absent (23) | 10 | 13 | 0.026a |
| Present (12) | 10 | 2 | ||||
| N/A (2) | 1 | 1 | ||||
| Metachronous (28) | Pseudocapsule | Absent (13) | 7 | 6 | 0.521 | |
| Present (15) | 9 | 6 | ||||
Significant at the level of 0.05 on Fisher's exact test.
Discussion
The principal finding of this study is that a fibrous pseudocapsule is a common histological feature in patients undergoing resection of CRLM and is associated with a lower 1-year tumour recurrence rate. This study extends earlier reports by showing that the benefit of a pseudocapsule occurs predominantly in patients with synchronous hepatic metastases. In these patients, only one-third develop a pseudocapsule but have a dramatically reduced incidence of 1-year tumour recurrence (2/12) compared with patients without a pseudocapsule (13/23). The study also confirms that the presence of an involved resection margin is an independent predictor of early tumour recurrence. These two findings demonstrate that histological examination of resection specimens can provide significant additional prognostic information for patients after resection of CRLM, compared with clinical and radiological data available pre-operatively.
The strength of the study lies in its prospective and unselected design, including all patients over a defined period with standardization of reporting within predetermined guidelines. Specimens were reported by pathologists with a subspecialty interest in gastrointestinal disease who collectively approved the experimental protocol. The value of these findings to clinical practice is significant as the identification of a tumour pseudocapsule is readily performed on standard histology specimens without the need for special stains. A potential weakness of the study is that estimation of the extent of the pseudocapsule is subjective and semi-quantitative; however, the present data show that the extent of the pseudocapsule is less important than its simple presence.
Although three previously published studies have shown that the presence of a pseudocapsule is associated with improved long-term survival after resection of CRLM24–26 it is not commonly reported in this setting. Our series is the first to report lower recurrence rates in the presence of a tumour pseudocapsule in a Western population and adds further evidence of the benefit of adding this finding to the core data set in histology reporting of CRLM. Further follow-up will determine if lower early recurrence rates in this group are associated with an improved survival.
It is not known what stimulates the formation of a fibrous pseudocapsule and what role it plays in preventing early recurrence. The capsule develops at the interface between tumour tissue and normal liver tissue and the proliferating stromal cells in the capsule have been shown to be myofibroblasts.26 It has been suggested that CRLM activate hepatic stellate cells to form myofibroblasts and that this is a host defence response, similar to an inflammatory response, creating a mechanical and chemical barrier around the tumour preventing further vascular and intrabiliary invasion.26 The present finding that the absence of a tumour pseudocapsule is associated with a more aggressive primary tumour with nodal metastases supports this hypothesis, although we did not find any association with the neutrophil to lymphocyte ratio among circulating leucocytes, which has also been shown to be a marker of an inflammatory response to the tumour.21 It is also possible that older patients are less able to generate an inflammatory response to the tumour, accounting for the finding of a smaller proportion with tumour pseudocapsules in this age group.
The present finding that the absence of a pseudocapsule in patients with synchronous CRLM is associated with a higher tumour recurrence may help direct patient-specific adjuvant treatment and care. For example, these patients may benefit from an increased frequency of post-operative imaging surveillance. Although post-operative chemotherapy after resection of CRLM has been shown to be of a limited value,31 future trials of this modality may be developed to target treatment to high-risk groups, such as patients with synchronous tumours with no pseudocapsules.
Further research needs to be undertaken to confirm the potential association of a lower tumour recurrence rate in patients with tumour pseudocapsule in larger series, in addition to correlating this finding with improved survival. Further data may allow the development of a risk scoring system incorporating this finding.
Acknowledgments
The authors would like to thank to Dr N. Robertson and Dr J. Denson (Consultant Histopatholgists) for their work in performing extended reporting during this study.
Conflicts of interest
None declared.
References
- 1.Ahmed I, Lobo D. Malignant tumours of the liver. Surgery. 2009;27:30–37. [Google Scholar]
- 2.Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006;94:982–999. doi: 10.1038/sj.bjc.6603033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malik HZ, Gomez D, Wong V, Al-Mukthar A, Toogood GJ, Lodge JPA, et al. Predictors of early disease recurrence following hepatic resection for colorectal cancer metastasis. Eur J Surg Oncol. 2007;33:1003–1009. doi: 10.1016/j.ejso.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi S, Konishi M, Nakagohri T, Gotohda N, Saito N, Kinoshita T. Short time to recurrence after hepatic resection correlates with poor prognosis in colorectal hepatic metastasis. Jpn J Clin Oncol. 2006;36:368–375. doi: 10.1093/jjco/hyl027. [DOI] [PubMed] [Google Scholar]
- 5.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 6.Minagawa M, Yamamoto J, Kosuge T, Matsuyama Y, Miyagawa SMM. Simplified staging system for predicting the prognosis of patients with resectable liver metastasis. Arch Surg. 2007;142:269–276. doi: 10.1001/archsurg.142.3.269. [DOI] [PubMed] [Google Scholar]
- 7.Yamashita Y, Adachi E, Toh Y, Ohgaki K, Ikeda O, Oki E, et al. Risk factors for early recurrence after curative hepatectomy for colorectal liver metastases. Surg Today. 2011;41:526–532. doi: 10.1007/s00595-010-4471-1. [DOI] [PubMed] [Google Scholar]
- 8.Cady B, Jenkins RL, Steele GD, Lewis WD, Stone MD, McDermott WV, et al. Surgical margin in hepatic resection for colorectal metastasis: a critical and improvable determinant of outcome. Ann Surg. 1998;227:566–571. doi: 10.1097/00000658-199804000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbas S, Lam V, Hollands M. Ten-year survival after liver resection for colorectal metastases: systematic review and meta-analysis. Int Sch Res Netw Oncol. 2011;2011:1–11. doi: 10.5402/2011/763245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambiru S, Miyazaki M, Isono T, Ito H, Nakagawa K, Shimizu H, et al. Hepatic resection for colorectal metastases: analysis of prognostic factors. Dis Colon Rectum. 1999;42:632–639. doi: 10.1007/BF02234142. [DOI] [PubMed] [Google Scholar]
- 11.Maithel SK, Gönen M, Ito H, Dematteo RP, Allen PJ, Fong Y, et al. Improving the clinical risk score: an analysis of molecular biomarkers in the era of modern chemotherapy for resectable hepatic colorectal cancer metastases. Surgery. 2012;151:162–170. doi: 10.1016/j.surg.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–321. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwatsuki S, Dvorchik I, Madariaga JR, Marsh JW, Dodson F, Bonham AC, et al. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 1999;189:291–299. doi: 10.1016/s1072-7515(99)00089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagashima I, Takada T, Adachi M, Nagawa H, Muto T, Okinaga K. Proposal of criteria to select candidates with colorectal liver metastases for hepatic resection: comparison of our scoring system to the positive number of risk factors. World J Gastroenterol. 2006;12:6305–6309. doi: 10.3748/wjg.v12.i39.6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kavlakoglu B, Ustun I, Oksuz O, Pekcici R, Ergocen S, Oral S. Surgical treatment of liver metastases from colorectal cancer: experience of a single institution. Arch Iran Med. 2011;14:120–125. [PubMed] [Google Scholar]
- 16.Hayashi M, Inoue Y, Komeda K, Shimizu T, Asakuma M, Hirokawa F, et al. Clinicopathological analysis of recurrence patterns and prognostic factors for survival after hepatectomy for colorectal liver metastasis. BMC Surg. 2010;10:27. doi: 10.1186/1471-2482-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandeweyer D, Neo EL, Chen JWC, Maddern GJ, Wilson TG, Padbury RTA. Influence of resection margin on survival in hepatic resections for colorectal liver metastases. HPB. 2009;11:499–504. doi: 10.1111/j.1477-2574.2009.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altendorf-Hofmann A, Scheele J. A critical review of the major indicators of prognosis after resection of hepatic metastases from colorectal carcinoma. Surg Oncol Clin N Am. 2003;12:165–192. doi: 10.1016/s1055-3207(02)00091-1. xi. [DOI] [PubMed] [Google Scholar]
- 19.Gomez D, Morris-Stiff G, Toogood GJ, Lodge JPA, Prasad KR. Interaction of tumour biology and tumour burden in determining outcome after hepatic resection for colorectal metastases. HPB. 2010;12:84–93. doi: 10.1111/j.1477-2574.2009.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, et al. Effect of Surgical Margin Status on Survival and Site of Recurrence After Hepatic Resection for Colorectal Metastases. Ann Surg. 2005;241:715–724. doi: 10.1097/01.sla.0000160703.75808.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halazun KJ, Aldoori A, Malik HZ, Al-Mukhtar A, Prasad KR, Toogood GJ, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol. 2008;34:55–60. doi: 10.1016/j.ejso.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Garden OJ, Rees M, Poston GJ, Mirza D, Saunders M, Ledermann J, et al. Guidelines for resection of colorectal cancer liver metastases. Gut. 2006;55:iii1–iii8. doi: 10.1136/gut.2006.098053. (Suppl 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spelt L, Andersson B, Nilsson J, Andersson R. Prognostic models for outcome following liver resection for colorectal cancer metastases: a systematic review. Eur J Surg Oncol. 2012;38:16–24. doi: 10.1016/j.ejso.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Okano K, Yamamoto J, Kosuge T, Yamamoto S, Sakamoto M, Nakanishi Y, et al. Fibrous pseudocapsule of metastatic liver tumors from colorectal carcinoma. Clinicopathologic study of 152 first resection cases. Cancer. 2000;89:267–275. [PubMed] [Google Scholar]
- 25.Nanashima A, Araki M, Tobinaga S, Kunizaki M, Hidaka S, Shibata K, et al. Relationship between period of survival and clinicopathological characteristics in patients with colorectal liver metastasis. Eur J Surg Oncol. 2009;35:504–509. doi: 10.1016/j.ejso.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Lunevicius R, Nakanishi H, Ito S, Kozaki K, Kato T, Tatematsu M, et al. Clinicopathological significance of fibrotic capsule formation around liver metastasis from colorectal cancer. J Cancer Res Clin Oncol. 2001;127:193–199. doi: 10.1007/s004320000199. [DOI] [PubMed] [Google Scholar]
- 27.Chang HHL, Leeper WR, Chan G, Quan D, Driman DK. Infarct-like necrosis: a distinct form of necrosis seen in colorectal carcinoma liver metastases treated with perioperative chemotherapy. Am J Surg Pathol. 2012;36:570–576. doi: 10.1097/PAS.0b013e31824057e7. [DOI] [PubMed] [Google Scholar]
- 28.Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, Andres A, Audard V, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18:299–304. doi: 10.1093/annonc/mdl386. [DOI] [PubMed] [Google Scholar]
- 29.Agresti A. An Introduction to Categorical Data Analysis. 2nd edn. Hoboken, NJ: John Wiley; 2002. [Google Scholar]
- 30.2011. R”–project for statistical computing; 170. Available at http://www.r-project.org/ (accessed 28/11/2012)
- 31.Nordlinger B, Vauthey J-N, Poston G, Benoist S, Rougier P, Van Cutsem E. The timing of chemotherapy and surgery for the treatment of colorectal liver metastases. Clin Colorectal Cancer. 2010;9:212–218. doi: 10.3816/CCC.2010.n.031. [DOI] [PubMed] [Google Scholar]


