Abstract
Background: Hepatitis C infection (HCV) and hepatocellular carcinoma (HCC), the two main causes of liver transplantation (LT), have reduced survival post-LT. The impact of HCV, HCC and their coexistence on post-LT survival were assessed.
Methodology: All 601 LT patients from 1992 to 2011 were reviewed. Those deceased within 30 days (n = 69) and re-transplants (n = 49) were excluded. Recipients were divided into four groups: (a) HCC-/HCV-(n = 252) (b) HCC+/HCV-(n = 58), (c) HCC-/HCV+ (n = 106) and (d) HCC+/HCV+ (n = 67). Demographics, the donor risk index (DRI), Model for End-Stage Liver Disease (MELD) score, survival, complications and tumour characteristics were collected. Statistical analysis included anova, chi-square, Fisher's exact tests and Cox and Kaplan–Meier for overall survival.
Results: Groups were comparable with regards to baseline characteristics, but HCC patients were older. After adjusting for age, MELD, gender and the donor risk index (DRI), survival was lower in the HCC+/HCV+ group (59.5% at 5 yrs) and the hazard ratio (HR) was 1.90 [95% confidence interval (CI),1.24–2.95, P = 0.003] and 1.45 (95% CI, 0.99–2.12, P = 0.054) for HCC-/HCV+. HCC survival was similar to controls (HR 1.18, 95% CI, 0.71–1.93, P = 0.508). HCC+/HCV-patients exceeded the Milan criteria (50% versus 31%, P < 0.04) and had more micro-vascular invasion (37.5% versus 20.6%, P = 0.042). HCC+/HCV+ versus HCC+/HCV-survival remained lower (HR 1.94, 95% CI, 1.06–3.81, P = 0.041) after correcting for tumour characteristics and treatment.
Conclusion: HCV patients had lower survival post-LT. HCC alone had no impact on survival. Patient survival decreased in the HCC+/HCV+ group and this appears to be as a consequence of HCV recurrence.
Introduction
Hepatitis C virus (HCV) is one of the leading causes of end-stage liver disease (ESLD), as 3% of the world population is chronically infected.1–4 Liver transplantation (LT) remains the only curative treatment.5 While long-term survival after orthotopic LT (OLT) has greatly improved over the past decades, the 10-year survival post-LT for HCV is reported to be 60%,6,7 remaining inferior to many other causes of ESLD. The 5-year cumulative risk of developing hepatocellular carcinoma (HCC) for patients with ESLD is up to 30%.8 HCV infection increases the risk of HCC by 14-to 22-fold when compared with HCV-negative patients.9
According to the International Agency for Research on Cancer, HCC is currently the fifth most common cancer worldwide.4 The pivotal work by Mazaferro et al. successfully identified a group of patients with a post-LT survival similar to that of non-cancer patients.10 However 15 years later, the literature still fails to reach a consensus on the impact of HCV on survival post-LT in patients with HCC. Some previous studies have demonstrated the independent negative impact of HCV on survival in patients transplanted with HCC11–15 whereas others have not.16–18 These studies are however limited at 5-year survival.
We believe that the effect of HCV on survival in patients transplanted for HCC becomes significantly detrimental beyond 5 years post-LT. The goal of this study was to assess the long-term impact of the coexistence of HCV and HCC on recipient and graft survival post-LT. The secondary outcomes were the cause of death (COD) for all our patients, the 90-day complications and the length of intensive care unit (ICU) stay and admission.
Methodology
Patient selection
A retrospective study of this prospectively collected transplant database was conducted. All LTs performed at the McGill University Health Center from January 1st 1992 to December 1st 2010 were reviewed (n = 601). Given that the study's primary outcome was long-term survival, patients were excluded if they required re-transplantation or died within 30 days (n = 69). Only the primary transplant was analysed in this study, thus second or third LTs for the same patient were excluded (n = 49). The remaining 483 recipients were divided into four groups (Fig. 1): patients transplanted for causes other than HCV and HCC (HCC-/HCV-) (n = 252), those transplanted for HCC alone and other causes of liver cirrhosis (HCC+/HCV-) (n = 58), patients with HCV cirrhosis alone (HCC+/HCV-) (n = 106) and those with both HCV cirrhosis and HCC (HCC+/HCV+) (n = 67). This grouping enables us to assess the independent impact of HCC and HCV on outcome, as well as that of their conjoint presentation.
Figure 1.
Patient selection flowchart. OLT, orthotopic liver transplantation; MUHC, McGill University Health Center, HCC, hepatocellular carcinoma; HCV, hepatitis C infection
Baseline characteristics
Recipient demographics, the natural Model for End-Stage Liver Disease (MELD) score and the donor risk index (DRI) were retrieved. The DRI score is based on donor age, race, height, COD, donation after cardiac death, use of a split liver, type of allocation and cold-ischemia time.19 Adherence to Milan criteria, as determined at the time of listing using pre-operative radiological imaging [computed tomography (CT) scan, ultrasound (US) and magnetic resonance imaging], was also collected. Tumour characteristics were established using explant histopathology: the grade of the tumour based on (2 and less versus more than 2), the number of tumours (3 and less versus more than 3), the total tumour size (less than 5 cm versus 5 cm and larger) and the presence of micro-vascular invasion. In this institution, HCC bridging treatments are reserved for patients with lesions with demonstrated growth as demonstrated by radiological modalities or lesions greater than 3 cm, while awaiting transplantation. Bridging therapy consisted mainly of trans-arterial chemo-embolization (TACE). In addition to TACE, some patients received percutaneous ethanol injections, systemic chemotherapy and intra-arterial chemotherapy. No patients underwent resection for downstaging. HCV diagnosis was performed through serology and confirmed by serum HCV rNA as of 1996. Patients with advanced cirrhosis requiring LT were not treated prior to transplantation.
In this cohort, eligibility for LT was determined by the transplant selection committee based on underlying cause of ESLD, HCC size and characteristics. Absolute contraindications were radiological evidence of HCC macrovascular invasion or extra-hepatic disease. LT listings were performed on the base of patient status (whether the patient was at home, hospitalized, in the ICU or on life-support) as per Quebec transplant guidelines at the time of listing until 2009 and based on the MELD score with adjustment for HCC patients thereafter. Selection based on Milan criteria was only formally introduced in this cohort in 2009. The immunosuppression in most patients consisted of induction with Thymoglobulin® (Pfizer, Saint-Laurent, Quebec. Canada), a calcineurin inhibitior Tacrolimus® (Astellas, Markham, Ontario, Canada) or Neoral® (Novartis, Dorval Montreal, Quebec, Canada), azathyropine (GlaxoSmithKline, Mississauga, Ontario, Canada) until 1997 and mycophenolate mofetil (Roche, Mississauga, Ontario, Canada) after 2007. More than 80% of the patients had prednisone discontinued after the first year post-OLT. Post-LT HCV treatment was initiated in patients with evidence of HCV recurrence on a liver biopsy performed based on clinical grounds of elevated or rising liver enzymes. The treatment consisted of alpha interferon from 1995 to 2000 and as of 2000 this institution started using pegalated alpha interferon and ribavirin.
Follow-up
The primary outcome was survival at last follow-up contact (ranging from 18 months to 20 years). Patients that were not deceased at the end of the study were censored at that time. Graft survival was also considered in the same fashion. Secondary outcomes were complications within 90 days of transplantation classified as operative, cardiac, pulmonary, renal, gastrointestinal, infectious and wound complications. The length of ICU stay and admission were also examined. HCV recurrence was ascertained through serology and histologically confirmed after liver biopsy for cause of elevation of liver transaminases.
HCC patients were followed by US and alpha-feto protein levels twice a year post-OLT. If an abnormality was detected, HCC recurrence was assessed by CT scan, PET scan, biopsy or autopsy. HCV genotyping was not routinely available at this centre until 1999 and was thus not included in this study. A 98.2% complete follow-up was achieved with losses to follow-up owing to LT recipients leaving the province. A great majority of deaths occurred in this hospital centre and where the primary cause of death was recorded in the database as indicated on the death certificate and the physician notes or from autopsy reports. In the minority of cases where the patients died in another hospital or at home, communication with the family of the recipient or primary physician confirmed the cause of death and was prospectively collected in this database.
Statistical analysis
Statistical analysis of donor and recipient baseline characteristics, length of admission and ICU stay was performed using anova. For tumour characteristics, the chi-square or Fisher's exact tests were used.
Survival at 5 and 10 years for each group was estimated using the Kaplan–Meier curve and a log rank test was used to assess whether the observed differences were statistically significant. After verifying compliance with the proportionality assumption, a Cox multivariate regression was performed to assess independent predictors of outcome. The selection of variables included in the model was predetermined based on our literature review. It was also assessed whether there was a potential interaction effect between HCC and HCV. A Cox multivariate sub-analysis was performed to assess an impendent predictor of survival in the HCC+ patients.
When comparing different causes of death across the four groups, the chi-square or Fisher's exact tests were used when appropriate.
Results
Baseline characteristics
Recipients were comparable for natural MELD and DRI between the four groups (Table 1). Recipients in the two HCC+ groups were older (61.2 in HCV-and 58.4 in HCV+) when compared with the cancer-free groups (53.9 and 55.3, P < 0.000) and were predominantly male (P = 0.032).
Table 1.
Basic characteristics presented in terms of means and standard deviation (SD), the P-value results from the anova test performed to compare the means
| HCC- | HCC+ | HCC- | HCC+ | P-value | |
|---|---|---|---|---|---|
| HCV- | HCV- | HCV+ | HCV+ | ||
| n = 253 | n = 57 | n = 106 | n = 67 | ||
| Age | 53.9 ± 11.8 | 61.2 ± 6.2 | 55.3 ± 9.2 | 58.4 ± 8.2 | <0.000* |
| Male | 63.4% | 86.2% | 65.1% | 76.1% | 0.032* |
| MELD score | 22.7 ± 9.6 | 20.7 ± 2.2 | 21.5 ± 8.4 | 20.6 ± 3.4 | 0.128 |
| DRI | 1.55 ± 0.34 | 1.57 ± 0.35 | 1.55 ± 0.35 | 1.62 ± 0.38 | 0.469 |
| ICU stay (days) | 8.1 ± 9.4 | 5.6 ± 6.6 | 7.2 ± 8.7 | 6.5 ± 7.3 | 0.183 |
| Length of admission (days) | 52.2 ± 50.4 | 49.7 ± 67.2 | 50.9 ± 47.1 | 44.5 ± 46.9 | 0.758 |
| Other causes of cirrhosis | Total | ||||
| HBV | 11.9% | 42.1% | 4.72% | 2.9% | 12.63% |
| ALD | 31.6% | 29.8% | 22.6% | 11.9% | 26.7% |
| NASH | 11.3% | 10.5% | 0.0% | 0.0% | 7.9% |
| PBC | 10.7% | 1.8% | 0.0% | 0.0% | 5.8% |
| PSC | 9.5% | 0.0% | 0.0% | 1.5% | 5.2% |
| Cryptogenic | 7.9% | 7.0% | 0.0% | 0.0% | 5.0% |
| Toxic | 2.8% | 0.0% | 0.0% | 0.0% | 1.5% |
HCC, hepatocellular carcinoma; HCV, hepatitis C infection; MELD, model for end-stage liver disease; DRI, donor risk index; ICU, intensive care unit; HBV, heptatitis B virus; ALD, alcohol-related liver disease; NASH, non-alcoholic steatohepatitis; PBC, primary biliary cirrhosis; PSC, primary sclerosis cholangitis.
Tumour characteristics
The non-HCV group (HCC+/HCV-) had a significantly greater rate of microvascular invasion than the HCV+ group (HCC+/HCV+) (37.5% versus 20.6%, P = 0.042). The HCC+/HCV-group tended to exceed Milan criteria when compared with the infected group (50% versus 26%, P = 0.027). The two HCC+ groups were otherwise comparable for the number of lesions, the total tumour size and pre-LT bridging treatments (Table 2).
Table 2.
Tumour characteristics by HCC/HCV group and in all the HCC populations
| Total HCC+ | HCC+ HCV- | HCC+ HCV+ | P-value | |
|---|---|---|---|---|
| n = 119* | n = 56 | n = 63 | ||
| Number of lesions | ||||
| 1 | 49 | 37.5% | 50.8% | 0.265 |
| 1 to 3 | 36 | 37.5% | 25.4% | |
| More than 3 | 28 | 25.0% | 23.8% | |
| Tumour grade | ||||
| 1 and 2 | 93 | 71.4% | 84.1% | 0.128 |
| 3 and 4 | 26 | 28.6% | 15.9% | |
| Microvascular invasion | 34 | 37.5% | 20.6% | 0.042* |
| Total tumour size | ||||
| Smaller than 5 cm | 86 | 67.9% | 76.2% | 0.311 |
| 5 cm or more | 33 | 32.1% | 23.8% | |
| Outside the Milan criteria | 34 | 50.0% | 20.6% | 0.027* |
| Pre-operative treatment | 39 | 39.6% | 20.6% | 0.283 |
HCC, hepatocellular carcinoma; HCV, hepatitis C infection.
In six patients, not all tumour characteristics were available.
Chi-square or Fisher's exact tests were performed to compare the two groups.
Survival analysis
The Kaplan–Meier survival curves are presented in Fig. 2. The survival estimates (Table 3) were similar among the first three groups at 1 year: 89.4% for the HCC-/HCV-group, 87.2% for the HCC+/HCV-and 89.5% for the HCC-/HCV+. There was a trend in lower survival in patients with HCC+/HCV+ one cancer HCV group (78.7%). At 5 years, the survival curves continue to diverge (78.6%, 70.4%, 68.2% and 59.5%, respectively). The difference in survival becomes striking at 10 years when the HCC+/HCV+ group has half the estimated survival (35.3%) when compared with the control group (67.2%). The HCC control group and HCV control group had survival rates of 57.1% and 57.5%, respectively, similar to those of the control group. These results translate into a median survival of the HCC+/HCV+ group approaching one-third (6.7 years) of that of the HCC-/HCV-group (>15.8 years). The HCC+/HCV-and HCC-/HCV+ have median survivals of 12.3 and 11.8 years, respectively.
Figure 2.
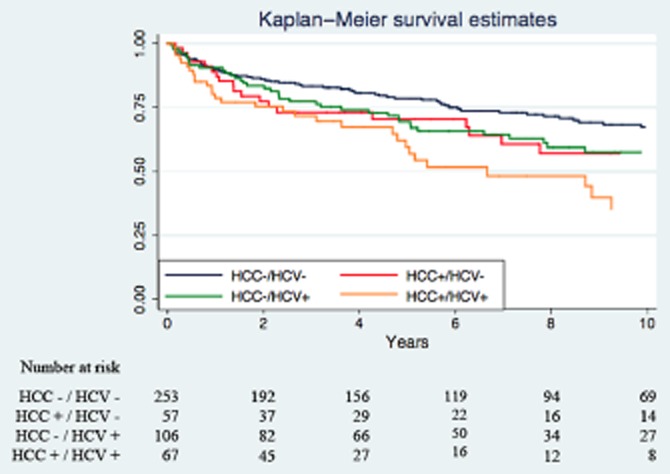
Kaplan–Meier survival curves and numbers at risk
Table 3.
Kaplan–Meier estimates by HCC/HCV group
| HCC- | HCC+ | HCC- | HCC+ | |
|---|---|---|---|---|
| HCV- | HCV- | HCV+ | HCV+ | |
| n = 252 | n = 58 | n = 106 | n = 67 | |
| Survival at 1 year | 89.4% | 87.2% | 89.5% | 78.7% |
| Survival at 5 years | 78.6% | 70.4% | 68.2% | 59.5% |
| Survival at 10 years | 67.2% | 57.1% | 57.5% | 35.3% |
| Survival at 15years | 61.1% | 46.1% | 40.8% | 18.8% |
| Median survival (years) | >15.8 | 12.3 | 11.8 | 6.7 |
HCC, hepatocellular carcinoma; HCV, hepatitis C infection.
In the multivariate COX regression analysis for patient survival (Table 4), after adjusting for age, MELD, gender and DRI, patients with HCC+/HCV+ was the strongest predictor of death with a hazard ratio (HR) of 1.91 (95% CI 1.24–2.95, P = 0.003). Having HCV cirrhosis alone was also a negative predictor of survival with a HR of 1.45 (95% CI, 0.99–2.12, P = 0.054) when compared with the HCC-/HCV-group. The hazard of death in the HCC alone group was comparable to that of controls (HR 1.18, 95% CI, 0.71–1.93, P = 0.508). Other than the cause of ESLD, DRI (HR 1.68, 95% CI, 1.11–2.56, P = 0.015) and recipient age (HR 1.04, 95% CI, 1.02–1.06, P < 0.000) were also independent predictors of mortality. The MELD score was not found to be a significant predictor of mortality.
Table 4.
Results of the multivariate Cox regression analysis for patient survival
| Hazard ratio | 95% CI | P-value | |
|---|---|---|---|
| HCC+ HCV- | 1.18 | 0.71–1.93 | 0.508 |
| HCC-HCV+ | 1.45 | 0.99–2.12 | 0.054 |
| HCC+ HCV+ | 1.91 | 1.24–2.95 | 0.003* |
| Recipient age | 1.04 | 1.02–1.06 | <0.000* |
| Male | 1.06 | 0.75–1.48 | 0.245 |
| MELD score | 1.01 | 0.99–1.03 | 0.716 |
| DRI | 1.68 | 1.11–2.56 | 0.015* |
CI, confidence interval; HCC, hepatocellular carcinoma; HCV, hepatitis C infection; MELD, model for end-stage liver disease; DRI, donor risk index.
After correcting for DRI, recipient age and MELD score, results for graft survival (Table 5) were similar to those of patient survival. In summary, the HCC+/HCV+ had twice the hazard of mortality when compared with the HCC+/HCV-. The HCV alone group had the second highest risk of mortality with a HR of 1.61 (95% CI, 1.05–2.48, P = 0.028) whereas the HCC alone group had a risk comparable to the controls. In this multivariate analysis, DRI was a significant predictor of survival whereas recipient age was not.
Table 5.
Results of the multivariate Cox regression analysis for graft survival
| Hazard ratio | 95% CI | P-value | |
|---|---|---|---|
| HCC+ HCV- | 1.13 | 0.61–2.08 | 0.744 |
| HCC-HCV+ | 1.61 | 1.05–2.48 | 0.028* |
| HCC+ HCV+ | 1.86 | 1.14–3.08 | 0.014* |
| Recipient age | 1.02 | 1.00–1.03 | 0.093 |
| Male | 1.15 | 0.75–1.49 | 0.512 |
| MELD score | 1.00 | 0.98–1.03 | 0.716 |
| DRI | 1.73 | 1.06–2.82 | 0.028* |
CI, confidence interval; HCC, hepatocellular carcinoma; HCV, hepatitis C infection; MELD, model for end-stage liver disease; DRI, donor risk index.
HCC survival
In spite of a clear advantage in tumour characteristics for the HCC+/HCV+ group (Table 2), survival was twice as low in this group when compared with the HCC+/HCV-group as can be seen on the Kaplan–Meier curve (Fig. 2) and in the survival estimates (Table 3). In this Cox sub-analysis, after correcting for tumour characteristics and treatment, this trend persisted (HR 1.94, 95% CI, 1.06–3.81, P = 0.041) (Table 6). Total tumour size, being outside Milan criteria, the presence of microvascular invasion, recipient age and DRI did not have an impact on survival.
Table 6.
Results of the multivariate Cox regression, a sub-analysis of all patients with hepatocellular carcinoma (HCC)
| Hazard ratio | 95% CI | P-value | |
|---|---|---|---|
| HCC+ HCV- | Baseline risk | ||
| HCC+ HCV+ | 1.94 | 1.06–3.81 | 0.041* |
| Recipient age | 1.03 | 0.99–1.03 | 0.171 |
| Male | 0.89 | 0.46–2.63 | 0.793 |
| MELD score | 1.04 | 0.92–1.15 | 0.613 |
| DRI | 1.35 | 0.66–2.93 | 0.446 |
| Micro-vascular invasion | 1.52 | 0.88–3.93 | 0.103 |
| Treatment | 1.35 | 0.72–2.53 | 0.353 |
| Outside Milan criteria | 1.22 | 0.60–2.45 | 0.200 |
HCV, hepatitis C infection; MELD, model for end-stage liver disease; DRI, donor risk index.
Cause of death (COD)
The COD as they occurred in the four groups are presented in Table 7. There was a higher incidence of death owing to cardiovascular disease in the HCC+/HCV-group (P = 0.064) and a higher incidence of COD owing to other causes (including accidents) in the HCC+/HCV+ group (P = 0.038). The most common COD by groups were malignancy in the HCC-/HCV-group (8.3%), cardiovascular and respiratory in the HCC alone group (15.8%) and HCV recurrence in the HCV alone group (23.6%). The most common cause of COD in the HCC+/HCV+ study group was also cirrhosis related to HCV recurrence, occurring in 17.9% of patients. HCC recurrence was low in this cohort of patients as well as it was not the COD in the majority of HCC patients in both HCV-and HCV+ patients.
Table 7.
Causes of death by hepatocellular carcinoma/hepatitis C infection (HCC/HCV) group (A chi-squared or Fisher's analysis was performed to compare the prevalence)
| Cause of death | HCC- | HCC+ | HCC- | HCC+ | P-value |
|---|---|---|---|---|---|
| HCV- | HCV- | HCV+ | HCV+ | ||
| n = 252 | n = 58 | n = 106 | n = 67 | ||
| Cardiovascular and respiratory | 7.5% | 15.8% | 3.8% | 6.0% | 0.064 |
| Sepsis | 4.7% | 5.3% | 3.8% | 1.5% | 0.665 |
| Biliary sepsis | 1.2% | 0% | 2.9% | 1.5% | 0.739 |
| Renal | 1.2% | 0% | 0% | 0% | 0.819 |
| Malignancy | 8.3% | 7.0% | 5.7% | 1.5% | 0.223 |
| Transplant related complications | 2.0% | 1.8% | 2.8% | 1.5% | 0.958 |
| Other causes | 3.2% | 0% | 0% | 6.0% | 0.038* |
| HCV recurrence | 0% | 0% | 23.6% | 17.9% | 0.245 |
| HCC recurrence | 0% | 7.0% | 0% | 10.5% | 0.365 |
| Total | 27.8% | 37.9% | 40.6% | 46.3% |
Discussion
This study is the first in the literature to assess the long-term impact of HCV on post-LT survival in patients with HCC. It was shown that the coexistence of HCC and HCV has the most deleterious impact on long-term patient and graft survival, doubling the risk of mortality. These results persist even after correcting for all other covariates.
Two previous studies have found results that differ from the present.16,17 A study of the United Network for Organ Sharing (UNOS) database comparing changes in survival in patients with HCC and HCV found no independent impact on survival in HCV and HCC patients.17 In spite of being the largest described cohort, the survival assessment in that study was limited at 5 years. At the 5-year point, this study describes similar results, suggesting that the impact of HCV might not be that important in the short term. However, it was demonstrated that HCV bears a significant toll on long-term survival (10 years). Furthermore, although the Thuluvath et al. study accounted for numerous peri-operative variables, the authors did not correct for tumour characteristics, did not report COD and had limited ascertainment of mortality.
A single-centre study by Doyle et al. concluded that in the MELD era, there was no significant difference in survival between patients with hepatitis C and HCC and patients with HCC alone on Kaplan–Meier analysis.16 However, this study also did not adjust for tumour characteristics and other variables that are known to be independent predictors of survival.20 In this study, it was found that in spite of having more advanced tumour characteristics, the HCC+/HCV-group had a better survival on Kaplan–Meier analysis. In this multivariate sub-analysis of survival in all HCC+ patients, HCV remained an independent predictor of survival, increasing the mortality risk by almost two-fold.
Four studies have reported results supporting our conclusions.11–13,15 However, these studies only have a 5-year survival analysis. Moreover, only one study performed a multivariate analysis for overall survival. The Moya et al. study found that viral-related aetiology of cirrhosis, tumour recurrence and older donor age remained the only independent predictors of survival after correcting for other variables.14,15
In the present multivariate Cox regression, the DRI emerged as an independent predictor of survival. The DRI, established in 2006 by Feng et al.,19 is a proven and validated negative predictor of survival.21,22 This cohort is a homogenous Caucasian population, living in proximity to the transplanting centre, in which the use of deceased-after-cardiac-death donors is very limited and few split-liver transplants are performed. Therefore, the main variations in the DRI are attributed to donor age. This is in agreement with previous studies that have shown that donor age is a significant independent predictor of poor survival14,15,17 and even more so in HCV+ patients.23,24 Moreover, a study of the UNOS database found a synergistic interaction between DRI and recipient HCV status;25 patients with a high DRI were reported to have a worse survival in HCV+ recipients than in HCV-recipients.
In the same analysis, recipient age was also an independent predictor of survival. This finding is in accordance with older and more recent UNOS studies,17,26 as well as with the United Kingdom (UK) transplant database results.27 We found that patients with HCC tend to be, on average, 5 years older than their non-HCC counterparts, independent of HCV status. This is probably secondary to the fact that the lead-time for HCC recurrence after liver cirrhosis is longer than the development of cirrhosis itself.
Another important result is that the major COD in all HCV+ patients, whether HCC-or HCC+ (23.6% and 17.9% respectively), was HCV recurrence. Doyle et al. found that HCC+/HCV+ recipients in their cohort did not die from recurrent HCV (3.9%).16 However, Moya et al. found similar results with their COD in all HCC patients being HCV-related graft cirrhosis (8%).15 Shimoda et al. also found HCV recurrence to be the second COD in HCC+/HCV+ recipients, whereas HCC recurrence was the third most common COD,18 supporting the present results. A study on the pattern of late mortality post-LT in the UK found that graft failure (including HCV recurrence) was the main COD at 10 years (20%) in all LT patients.27 Their second most prevalent cause of mortality was cardiac (12%), similar to our HCC+/HCV-recipients (15.8%).
To summarize, in this cohort, a high DRI (mainly older donor age) and an older recipient age, together with HCV, interacted to yield a lower overall survival for HCC+/HCV+ patients. It is recognized that almost all HCV+ patients receiving a liver allograft tend to recur.28,29 Donor age is a significant predictor of graft quality and may increase the impact of HCV recurrence on graft survival.27 This explains why the main COD in all HCV+ patients in our cohort is HCV recurrence. It is possible that HCV recurrence may have less of a detrimental effect in younger donor livers. Given that our donor pool is significantly older than other centres, this risk factor might not be modifiable but a better optimization in donor selection should be the aim. The main remediable risk factor remains HCV recurrence. Although there has been an improvement in treatment strategies and immunosuppression, the benefits of these new strategies remain contentious.30–33
In the present cohort, tumour characteristics were not found to be significant predictors of long-term survival. This is probably as a result of the fact that the HCC recurrence rate was low (18.4%), possibly creating a small number of effects. Moreover, HCC alone had no increased risk of death when compared with the HCC-/HCV-patients. It is interesting to note that many of the HCC+/HCV-patients were HBV positive (42.1%, Table 1). The comparable survival is probably as a result of the fact that transplantation is curative in HBV-positive recipients and graft re-infection is now a rarity since the introduction of hepatitis B immune globulin and nucleoside analogue prophylaxis.25,34
It is noteworthy that the underlying cause of ESLD in HCC differs between regions.4,35 While HCV is the leading cause of cirrhosis in Europe, North America and Japan, HBV is the main cause of ESLD in Africa and Asia.36 In our cohort, HCV is the first cause of ESLD at 36.3%, three times more than HBV (12.6%). The HCV+ population transplanted at this centre is different from many other centres, as it is constituted of immigrants infected in Southern and Eastern Europe and the Middle East. This renders the present population somewhat unique and may limit the external validity of our results.
The limitations of this study are those inherent to a retrospective study although data collection was prospective and performed in a systematic and standardized fashion. The advantage of using our institutional database when compared with national databases is that this cohort has near complete follow-up (98.8%). This enables a long follow-up (up to 19 years in some cases) and a very accurate outcome ascertainment, shedding light on the long-term causes of mortality.
Results are less prone to misclassification and recall bias error; however, a random misclassification error can still be present. There is little selection bias introduced in this study as all LT performed at our centre were considered and only patients that died or underwent a redo within the first 30 days were excluded. While no sensitivity analysis was performed, the 90-day complication causes and rates were equally distributed among the groups. The rationale behind this exclusion was to focus on long-term survival and not be influenced by what are probably technical errors. Only first grafts in the graft survival analysis were included; however, survival of subsequent grafts was indirectly included in the overall patient survival. Given that the patient and graft survival analysis yield very similar results, a selection bias introduced by this exclusion criterion is highly unlikely.
Conclusion
This study is the first to assess the long-term impact of HCV on post-LT survival in HCC patients. Patients with HCV have a significantly lower survival after LT when compared with HCV negative patients. HCC alone has almost no impact on patient survival in our cohort. Patient survival is dramatically decreased when HCC and HVC are both present. Nevertheless, the attributable impact of HCV is greater than that of HCC. Controlling HCV recurrence and other modifiable variables such as donor age and DRI, especially in HCV positive patients, could greatly improve the long-term survival.
Acknowledgments
The MUHC database is supported via an unrestricted grant by Astellas fund and the Avrith Liver Transplant fund of McGill University.
Conflicts of interest
None declared.
References
- 1.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 2.European Association of the Study of the Liver. 2011 European Association of the Study of the Liver hepatitis C virus clinical practice guidelines. Liver Int. 2012;32(Suppl. 1):2–8. doi: 10.1111/j.1478-3231.2011.02703.x. [DOI] [PubMed] [Google Scholar]
- 3.Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29(Suppl. 1):74–81. doi: 10.1111/j.1478-3231.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1731. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown RS. Hepatitis C and liver transplantation. Nature. 2005;436:973–978. doi: 10.1038/nature04083. [DOI] [PubMed] [Google Scholar]
- 6.Belli LS, Burroughs AK, Burra P, Alberti AB, Samonakis D, Camma C, et al. Liver transplantation for HCV cirrhosis: improved survival in recent years and increased severity of recurrent disease in female recipients: results of a long term retrospective study. Liver Transpl. 2007;13:733–740. doi: 10.1002/lt.21093. [DOI] [PubMed] [Google Scholar]
- 7.Russo MW, Galanko JA, Zacks SL, Beavers KL, Fried MW, Shrestha R. Impact of donor age and year of transplant on graft survival in liver transplant recipients with chronic hepatitis C. Am J Transpl. 2004;4:1133–1138. doi: 10.1111/j.1600-6143.2004.00470.x. [DOI] [PubMed] [Google Scholar]
- 8.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5 Suppl. 1):S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Donato F, Boffetta P, Puoti M. A meta-analysis of epidemiological studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma. Int J Cancer. 1998;75:347–354. doi: 10.1002/(sici)1097-0215(19980130)75:3<347::aid-ijc4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 10.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 11.Zendejas-Ruiz I, Hemming AW, Chen C, Schwartz JJ, Sorensen JB, Kim RD. Recurrent hepatocellular carcinoma in liver transplant recipients with hepatitis C. Int J Gastrointest Cancer. 2012;43:229–235. doi: 10.1007/s12029-010-9230-4. [DOI] [PubMed] [Google Scholar]
- 12.Bozorgzadeh A, Orloff M, Abt P, Tsoulfas G, Younan D, Kashyap R, et al. Survival outcomes in liver transplantation for hepatocellular carcinoma, comparing impact of hepatitis C versus other etiology of cirrhosis. Liver Transpl. 2007;13:807–813. doi: 10.1002/lt.21054. [DOI] [PubMed] [Google Scholar]
- 13.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Difference in tumor invasiveness in cirrhotic patients with hepatocellular carcinoma fulfilling the Milan criteria treated by resection and transplantation: impact on long-term survival. Ann Surg. 2007;245:51–58. doi: 10.1097/01.sla.0000225255.01668.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melum E, Friman S, Bjoro K, Rasmussen A, Isoniemi H, Gjertsen H, et al. Hepatitis C impairs survival following liver transplantation irrespective of concomitant hepatocellular carcinoma. J Hepatol. 2007;47:777–783. doi: 10.1016/j.jhep.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Moya A, Berenguer M, Aguilera V, Juan FS, Nicolas D, Pastor M, et al. Hepatocellular carcinoma: can it be considered a controversial indication for liver transplantation in centers with high rates of hepatitis C? Liver Transpl. 2002;8:1020–1027. doi: 10.1053/jlts.2002.35664. [DOI] [PubMed] [Google Scholar]
- 16.Doyle MB, Vachharajani N, Maynard E, Shenoy S, Anderson C, Wellen JR, et al. Liver transplantation for hepatocellular carcinoma: long-term results suggest excellent outcomes. J Am Coll Surg. 2012;215:19–28. doi: 10.1016/j.jamcollsurg.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 17.Thuluvath PJ, Maheshwari A, Thuluvath NP, Nguyen GC, Segev DL. Survival after liver transplantation for hepatocellular carcinoma in the model for end-stage liver disease and pre-model for end-stage liver disease eras and the independent impact of hepatitis C virus. Liver Transpl. 2009;15:754–762. doi: 10.1002/lt.21744. [DOI] [PubMed] [Google Scholar]
- 18.Shimoda M, Ghobrial RM, Carmody IC, Anselmo DM, Farmer DG, Yersiz H, et al. Predictors of survival after liver transplantation for hepatocellular carcinoma associated with Hepatitis C. Liver Transpl. 2004;10:1478–1486. doi: 10.1002/lt.20303. [DOI] [PubMed] [Google Scholar]
- 19.Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transpl. 2006;6:783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 20.Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17(Suppl. 2):S44–S57. doi: 10.1002/lt.22365. Epub 2011/06/23. [DOI] [PubMed] [Google Scholar]
- 21.Blok JJ, Braat AE, Adam R, Burroughs AK, Putter H, Kooreman NG, et al. Validation of the donor risk index in orthotopic liver transplantation within the Eurotransplant region. Liver Transpl. 2012;18:112–119. doi: 10.1002/lt.22447. [DOI] [PubMed] [Google Scholar]
- 22.Schaubel DE, Sima CS, Goodrich NP, Feng S, Merion RM. The survival benefit of deceased donor liver transplantation as a function of candidate disease severity and donor quality. Am J Transpl. 2008;8:419–425. doi: 10.1111/j.1600-6143.2007.02086.x. [DOI] [PubMed] [Google Scholar]
- 23.Mutimer DJ, Gunson B, Chen J, Berenguer J, Neuhaus P, Castaing D, et al. Impact of donor age and year of transplantation on graft and patient survival following liver transplantation for hepatitis C virus. Transplantation. 2006;81:7–14. doi: 10.1097/01.tp.0000188619.30677.84. [DOI] [PubMed] [Google Scholar]
- 24.Berenguer M, Prieto M, San Juan F, Rayon JM, Martinez F, Carrasco D, et al. Contribution of donor age to the recent decrease in patient survival among HCV-infected liver transplant recipients. Hepatology. 2002;36:202–210. doi: 10.1053/jhep.2002.33993. [DOI] [PubMed] [Google Scholar]
- 25.Maluf DG, Edwards EB, Stravitz RT, Kauffman HM. Impact of the donor risk index on the outcome of hepatitis C virus-positive liver transplant recipients. Liver Transpl. 2009;15:592–599. doi: 10.1002/lt.21699. [DOI] [PubMed] [Google Scholar]
- 26.Condron SL, Heneghan MA, Patel K, Dev A, McHutchison JG, Muir AJ. Effect of donor age on survival of liver transplant recipients with hepatitis C virus infection. Transplantation. 2005;80:145–148. doi: 10.1097/01.tp.0000164291.35925.7a. [DOI] [PubMed] [Google Scholar]
- 27.Gelson W, Hoare M, Dawwas MF, Vowler S, Gibbs P, Alexander G. The pattern of late mortality in liver transplant recipients in the United Kingdom. Transplantation. 2011;91:1240–1244. doi: 10.1097/TP.0b013e31821841ba. [DOI] [PubMed] [Google Scholar]
- 28.Wiesner RH, Sorrell M, Villamil F. Report of the first International Liver Transplantation Society expert panel consensus conference on liver transplantation and hepatitis C. Liver Transpl. 2003;9:S1–S9. doi: 10.1053/jlts.2003.50268. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Retortillo M, Forns X, Feliu A, Moitinho E, Costa J, Navasa M, et al. Hepatitis C virus kinetics during and immediately after liver transplantation. Hepatology. 2002;35:680–687. doi: 10.1053/jhep.2002.31773. [DOI] [PubMed] [Google Scholar]
- 30.Everson GT, Terrault NA, Lok AS, Rodrigo DR, Brown RS, Jr, Saab S, et al. A randomized controlled trial of pretransplant antiviral therapy to prevent recurrence of hepatitis c after liver transplantation. Hepatology. 2012 doi: 10.1002/hep.25976. Epub 2012/07/24. doi: 10.1002/hep.25976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gurusamy KS, Tsochatzis E, Xirouchakis E, Burroughs AK, Davidson BR. Antiviral therapy for recurrent liver graft infection with hepatitis C virus. Cochrane Database Syst Rev. 2010;(1) doi: 10.1002/14651858.CD006803.pub3. CD006803. [DOI] [PubMed] [Google Scholar]
- 32.Manousou P, Samonakis D, Cholongitas E, Patch D, O'Beirne J, Dhillon AP, et al. Outcome of recurrent hepatitis C virus after liver transplantation in a randomized trial of tacrolimus monotherapy versus triple therapy. Liver Transpl. 2009;15:1783–1791. doi: 10.1002/lt.21907. [DOI] [PubMed] [Google Scholar]
- 33.Rabie R, Mumtaz K, Renner EL. Efficacy of antiviral therapy for hepatitis C after liver transplantion with cyclosporine and tacrolimus: a systematic review and meta-analysis. Liver Transpl. 2013;19:36–48. doi: 10.1002/lt.23516. [DOI] [PubMed] [Google Scholar]
- 34.Poordad FF. Liver transplant and recurrent disease. Clin Liver Dis. 2004;8:461–473. doi: 10.1016/j.cld.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 35.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 36.Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. 5 (Suppl. 1) [DOI] [PubMed] [Google Scholar]


