Abstract
Neurotransmitter transporters are ion-coupled symporters that drive the uptake of neurotransmitters from neural synapses. In the past decade, the structure of a bacterial amino acid transporter, leucine transporter (LeuT), has given valuable insights into the understanding of architecture and mechanism of mammalian neurotransmitter transporters. Different conformations of LeuT, including a substrate-free state, inward-open state, and competitive and non-competitive inhibitor-bound states, have revealed a mechanistic framework for the transport and transport inhibition of neurotransmitters. The current review integrates our understanding of the mechanistic and pharmacological properties of eukaryotic neurotransmitter transporters obtained through structural snapshots of LeuT.
Introduction
Chemical neurotransmission in neural synapses is initiated by the calcium-dependent release of neurotransmitters from a presynaptic neuron and activation of neurotransmitter-dependent ion channels and receptors primarily localized on the postsynaptic neuron (Jessell & Kandel, 2006). A wide range of neurotransmitters, including glutamate, acetylcholine, noradrenaline, dopamine, serotonin, GABA and glycine, are involved in the modulation of neuronal activity through excitatory or inhibitory mechanisms (Amara & Kuhar, 2002). The synaptic levels of released neurotransmitters are controlled by their uptake into presynaptic cells or surrounding glial cells through neurotransmitter transporters (Amara & Kuhar, 2002). These secondary active transporters harness the electrochemical gradients of ions, particularly Na+, to drive the uphill transport of neurotransmitters, which is why they are referred to as neurotransmitter sodium symporters (NSSs) (Rudnick, 2009). Solute carrier 1 (SLC1) members, referred to as excitatory amino acid transporters, transport glutamate with the aid of Na+/K+ and H+ (Amara & Fontana, 2002). A majority of neurotransmitters, including the biogenic amines – dopamine, noradrenaline and serotonin – as well as glycine and GABA, are transported by the solute carrier 6 (SLC6) family of transporters that are dependent upon sodium and chloride for neurotransmitter transport (Krishnamurthy et al. 2008). Transport in secondary active transporters can be understood in terms of the mechanism of alternating access (Mitchell, 2011; Jardetzky, 2002), which involves the movement of substrate across a membrane barrier by conformational rearrangements of a transporter which is accessible to only one side of the membrane at any given time.
Because of the critical roles played by SLC6 family members in the spatiotemporal control of neurotransmitter levels, perturbation of transporter activity by mutations or by pharmacophores can disrupt normal neurological activity. Dysfunction of transport activity in SLC6 members has been associated with Parkinson's disease, depression, schizophrenia, Tourette's syndrome, attention deficit hyperactivity disorder (ADHD) and orthostatic intolerance (Masson et al. 2009; Shannon et al. 1998; Kristensen et al. 2012). Dopamine, noradrenaline and serotonin transporters are the primary targets for a wide array of antidepressant medications, including the selective serotonin reuptake inhibitors, drugs to target ADHD or neuropathic pain, and drugs of abuse, such as cocaine and amphetamines (Torres et al. 1983).
Prior to the structural determination of the leucine transporter (LeuT), studies to understand NSS topology were focused on information derived from mutagenic/chimeric studies, partial proteolysis, cysteine accessibility analysis, antibody localization studies and hydrophobicity analyses (Amara & Kuhar, 2002). Information garnered from such experiments suggested that these transporters contain 11–13 transmembrane helices (Amara & Kuhar, 2002). Moreover, NSS proteins have a high level of sequence conservation, thus allowing findings from one member to be extrapolated to members with different substrate specificity. Interestingly, bacterial genome sequences revealed proteins with 20–30% amino acid sequence identity to eukaryotic NSS members (Androutsellis-Theotokis et al. 1993). Thus bacterial transporters presented themselves as tractable models for the understanding of mammalian neurotransmitter transporter structure and function. Shortly thereafter, the crystal structure of LeuT, determined at a resolution of 1.65 Å, gave unprecedented insights into the structure and organization of the SLC6 family of sodium-coupled transporters (Yamashita et al. 2010).
Structure of LeuT, a neurotransmitter transporter homologue
LeuT is composed of 12 transmembrane helices, the first 10 of which are related by an inverted topological repeat. Helices 1–5 and 6–10 are related by a pseudo two-fold axis of symmetry, with the two units mutually superimposable upon rotation by 173 deg about an axis oriented approximately parallel to the membrane plane (Yamashita et al. 2010) (Fig. 1). This organization immediately suggested a structural relationship between the molecular architecture of SLC6 transporters and the mechanism of alternating access. Helices 1 and 6 have a discontinuous architecture with the loops connecting regions ‘a’ and ‘b’ of the two helices harbouring the substrate and ion binding sites (Fig. 1). The structure of LeuT revealed one leucine molecule bound in the substrate binding pocket, which is occluded from solvent access from the extracellular vestibule by the presence of a gating residue F253 (TM6a), whose side chain forms a barrier between the extracellular vestibule and the binding pocket (Fig. 2C, G). This conformational state, deemed the occluded conformation, represents an intermediate state in the transport cycle (Yamashita et al. 2010).
Figure 1.
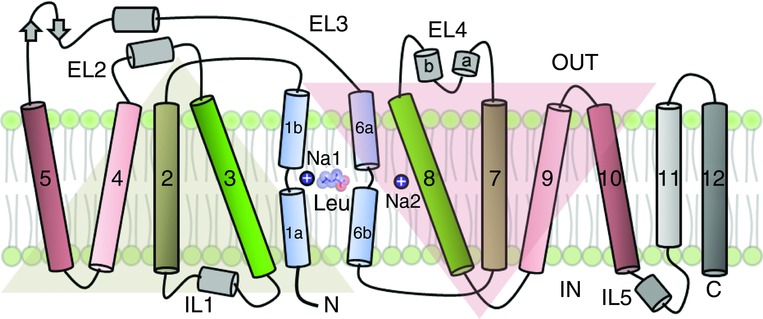
Structural organization in LeuT, depicted as a pseudosymmetric arrangement of helices. Helices 1–5 and 6–10 are mutually superimposable upon a 173 deg rotation. Helices 1 and 6 are discontinuous and connected by short loops. The two helices harbour the substrate and ion binding sites.
Figure 2.

A, competitive inhibitor-bound state of LeuT (cyan; pdb id. 3F3a). B, substrate-free LeuT (salmon colour; pdb id. 3TT1) is in an outward-open state which could bind inhibitor or bind substrate and C, form an intermediate state by occlusion of the substrate binding pocket (magenta; pdb id. 2A65). D, a noncompetitive inhibitor [tricyclic antidepressant (TCA) clomipramine (CMI)] could bind to the substrate-bound occluded state and stabilize it (green; pdb id. 2Q6H). E, the substrate-bound occluded state isomerizes to release the substrate and ions inside the cytoplasm (inward-open state; orange; pdb id. 3TT3) and regains the outward-open state of the transporter. F–H, close-up images of the substrate binding pocket and extracellular vestibule (EV) in tryptophan-bound, leucine-bound and leucine + clomipramine-bound states, respectively, with gating residues F253 and Y108 labelled.
The two sodium ion binding sites are in close proximity to the substrate, with sodium at site 1 in direct coordination with the carboxylate group of the substrate. In mammalian biogenic amine transporters, the lack of a carboxylate group in substrates is compensated by a conserved aspartic acid residue in TM1 which takes the place of Gly24 in LeuT (Yamashita et al. 2010; Beuming et al. 2003). Accordingly, a glycine residue in this position is conserved in NSS members specific for the substrates glycine and GABA, both of which harbour a carboxylate group (Beuming et al. 2003). The two sodium sites in LeuT differ in terms of coordination geometry. Specific coordination geometries are inherent for most ions. Coordination numbers of 6 (octahedral geometry) and 5 (trigonal bipyramidal geometry) support a mean ion coordinating radius of 2.4 Å between Na+ and surrounding oxygen atoms (Harding, 2007). The ion at the Na 1 site is coordinated predominantly by side chain residues with an octahedral geometry and the ion at the Na 2 site is coordinated primarily by main chain carbonyls and side chain atoms derived from residues on TM1 and TM8 with trigonal bipyramidal geometry. Interestingly, some transporters that have a LeuT-like fold, such as the Vibrio parahaemolyticus Na+/galactose cotransporter (vSGLT), lack sodium site 1 and instead have only one sodium site similar in location to site 2 of LeuT (Faham et al. 1997). Furthermore, a Na+-independent amino acid, polyamine and organocation transporter (ApcT), which ferries nutrients with the aid of proton gradients, exploits a lysine side chain (Lys158) whose ɛ-amino group is located in a position equivalent to that of the sodium site 2 of LeuT (Shaffer et al. 2009). This lysine residue plays an essential role in H+-dependent amino acid transport of ApcT (Shaffer et al. 2009).
Despite the similarities in sodium stoichiometries with mammalian NSS members, amino acid transport in LeuT is independent of chloride, which is in stark contrast to eukaryotic NSSs, which are known to require both sodium and chloride for effective transport activity (Yamashita et al. 2010). LeuT has proven to be useful in the identification of a putative chloride site through homology modelling and site-directed mutagenesis on mammalian serotonin transporter (SERT; SLC6A4) by Forrest et al. (2010). These authors suggested that chloride binds in a pocket close to the sodium site 1 with residues in TM7 involved in anion coordination. They also demonstrated that the S372E (E290 in LeuT) and N368D (N286 in LeuT) mutations in SERT reduce the chloride dependence of transport (Forrest et al. 2010). In a separate study, Kanner and colleagues predicted that Ser331 in the GABA transporter (GAT1; SLC6A1) coordinates the Cl− ion with contributions from Asn327 (Zomot et al. 2005), corroborating this conclusion by way of mutations S331E in GAT1 and E290S in LeuT. Strikingly, these mutants confer Cl−-independent uptake in GAT1 (S331E) and Cl−-dependent uptake in LeuT (E290S) (Zomot et al. 2005). A recently reported crystal structure of a LeuT-E290S mutant has validated this predicted site with the presence of Cl− and Br− ions coordinated by residues Thr254 (TM6a), Gln250 (TM6a), Tyr47 (TM2) and Ser290 (TM7) (Kantcheva et al. 1966). Taken together, these studies have pinpointed the location of the Cl− binding site and furthered our understanding of its role in neurotransmitter transport.
Pharmacological insights
Inhibitors of NSSs are widespread therapeutic agents employed to treat depression, ADHD and fibromyalgia, as a few of many examples. By contrast, inhibitors, such as cocaine, and substrate mimics, such as methamphetamine and 3,4-methylene dioxy-N-methylamphetamine (ecstasy), are widespread substances of abuse (Kristensen et al. 2012). In this context, LeuT was employed to obtain complexes with antidepressant drugs. Tricyclic antidepressants, such as clomipramine, bind to LeuT with relatively weak affinity at sites located in the extracellular vestibule about 11 Å above the substrate binding site (Singh et al. 2010), consistent with the observation that clomipramine is a noncompetitive inhibitor of transport in LeuT (Fig. 2D, H). Thus, although the complexes of tricyclic antidepressants with LeuT provide insight into the mechanism of LeuT-catalysed transport and noncompetitive inhibition, they do not define how antidepressants act on eukaryotic NSSs (Singh et al. 2010, 2000). Indeed, multiple studies on NSS members indicate that antidepressants inhibit biogenic amine transport through competitive binding at the substrate binding pocket (Talvenheimo et al. 2007; Henry et al. 2009; Andersen et al. 2002).
To illuminate the mechanism of competitive inhibition, amino acids with different side chains were tested to find candidates that could inhibit transport by way of a competitive mechanism (Singh et al. 2000). Tryptophan was observed to be a competitive inhibitor of transport and a crystal structure of the LeuT–Trp complex yielded a substrate binding pocket that was not occluded (Fig. 2A, F). The indole ring of the tryptophan sterically prevents closure of helices TM1b, TM6a and TM2 to keep the substrate binding pocket open to the extracellular vestibule and to thereby render the substrate site accessible to solvent (Singh et al. 2000). This structure provided the first hint that competitive inhibitors of neurotransmitter transporters act through the stabilization of the open-to-out state of the transporter (Fig. 2A, F). Studies based on LeuT-derived homology models of NSS members, such as the dopamine transporter (SLC6A3), suggested that drugs such as cocaine and benztropine, the latter of which is a nonaddictive cocaine analogue, have binding sites that overlap with the dopamine binding site (Beuming et al. 2011; Bisgaard et al. 2008).
Kristensen and colleagues have successfully transferred, using only five mutations, the drug specificity of a highly specific SERT inhibitor citalopram into the noradrenaline transporter (NET; SLC6A2) (Andersen et al. 2002). They determined that residues that control the drug specificity also reside in the substrate binding pocket of the two neurotransmitter transporters. Using LeuT as a molecular map, residues lining the substrate binding pocket in TMs 1, 3, 6, 8 and 9 in NET were modified to equivalent residues in SERT. This resulted in a complete shift in the specificity of binding affinity of the modified NET from talopram to citalopram, which is the most selective SERT antagonist (Andersen et al. 2002). More recently, Kristensen and colleagues have studied the selectivities of antidepressants on SERT and NET by altering residues in the substrate binding site and swapping residues between the two paralogues (Sorensen et al. 2008). All of these detailed pharmacological studies were made possible by the availability of LeuT as a template to model the structures of mammalian neurotransmitter transporters.
Transport cycle
To provide a molecular basis for the transport cycle of LeuT and NSSs, structural snapshots of LeuT were required in the substrate-free outward-and inward-open states. Prior to obtaining alternative conformations of LeuT, crystal structures of other transporters belonging to the LeuT fold were determined in a variety of conformational states. These included an outward-open state of a hydantoin transporter (MHP1) (Weyand et al. 2012) and arginine:agmatine antiporter (AdiC) (Gao et al. 2008), occluded states of a betaine transporter (BetP) (Ressl et al. 2012) and ApcT (Shaffer et al. 2009), and inward-open states of vSGLT (Faham et al. 1997), MHP1 (Shimamura et al. 2009) and a carnitine–betaine antiporter (CaiT) (Tang et al. 2012).
The x-ray structure of a substrate-free outward-open transporter was made possible by weakening of the substrate binding using the Y108F mutant (Krishnamurthy & Gouaux, 2013) by ablating a hydrogen bond between the hydroxyl group of tyrosine and the carboxylate of the substrate leucine. Tyrosine at this position is extensively conserved in mammalian NSSs. A Y to F mutant was first characterized in GAT1, where it was observed to reduce substrate binding (Bismuth et al. 2006). Nevertheless, mutations in critical regions, such as in the core of the transporter, have an inherent risk of disturbing the native structure. To more completely understand the effects of such perturbations, we attempted to measure the functional activity by way of binding and transport assays, to investigate the binding of conformation-specific antibodies and to determine additional crystal structures. In the end, however, additional studies are needed that include complementary biophysical, spectroscopic and functional experiments. The crystal structure of the corresponding LeuT–Fab complex in a leucine-free/Na+-bound state revealed an outward-open conformation (Fig. 2B) with helical positions similar to the tryptophan-bound state of the transporter (Krishnamurthy & Gouaux, 2013). Shifts in TM1b, TM6a and TM2 away from their equivalent positions in the substrate-bound state (Fig. 3A), together with movements of the gating residue F253 away from the substrate binding pocket, open the substrate site to extracellular solution (Krishnamurthy & Gouaux, 2013) (Fig. 2B). This supports the conclusion that the transporter occupies an open-to-out conformation in the substrate-free Na+-bound state and, upon binding substrate, transitions to the occluded state. This structural observation is also bolstered through solution studies of LeuT using spin labels, which show that accessibility to the extracellular vestibule is enhanced upon binding of sodium ions in the absence of substrate (Claxton et al. 2011).
Figure 3.
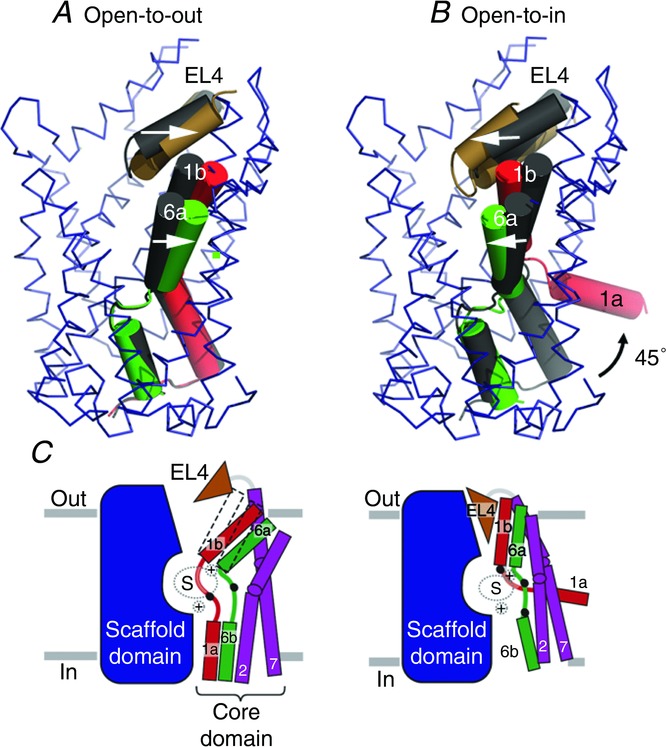
A, movement of helices TM1 and TM6 and EL4 region compared between substrate-free (coloured helices) and substrate-bound (grey) states. B, conformational changes involving 45 deg outward movement of helix 1a towards the intracellular side. TM11 and TM12 are clipped to give a clearer view. Arrows point to the changes in helical positions upon moving from a substrate-free to a substrate-bound state (A) followed by a substrate bound to an inward-open state (B). C, outward-open and inward-open states of LeuT with core and scaffold helices represented as cartoons (Krishnamurthy & Gouaux, 2013).
To obtain an inward-facing conformation of LeuT, alterations had to be made at multiple regions in the molecule. It has been demonstrated previously in the case of MHP1 and vSGLT, through molecular dynamic simulations, that weakening of the Na 2 site is crucial for the transporter to release its cargo towards the cytoplasmic face (Shimamura et al. 2009; Watanabe et al. 2003).
In addition, it has been shown in eukaryotic NSS members that disruption of intracellular gating networks at the cytoplasmic face of the molecule shifts the conformational equilibrium of the transporters towards an inward-open conformation (Kniazeff et al. 1993). On the basis of the two findings, a potentially inward-facing LeuT was designed by mutating residues T354 and S355 to weaken the Na 2 site and mutating Y268 to alanine to disrupt intracellular π-stacking and hydrogen-bond networks in LeuT. The structure of LeuT, in an inward-facing conformation, revealed conformational changes at both the extracellular and intracellular portions of the transporter (Krishnamurthy & Gouaux, 2013). The molecule has a group of invariant helices which act as a scaffold (3, 4, 8 and 9) and a core group of helices (1, 2, 6 and 7) which undergo substantial changes in positions. Helices 1b and 6a have maximal shifts and move about 20 deg inward to close the extracellular gate and partially occlude the extracellular vestibule (Fig. 3B). EL4 plays a significant role in this context by an inward movement resulting in plugging of the extracellular vestibule. On the cytoplasmic side, TM1a undergoes a profound conformational shift of 45 deg which creates access to the substrate binding pocket from the intracellular side (Krishnamurthy & Gouaux, 2013) (Fig. 2E). This large reorientation leads to conformational changes in the positions of TM5 and TM7 that result in the molecule attaining an inward-open state.
Alternating access in LeuT
Although structural and biophysical studies of LeuT support an alternating access mechanism in the form of an alternate closure and opening of solvent access between the intracellular and extracellular compartments, the movements associated with transport are substantially different from those predicted by rigid helical movements modelled by swapping the pseudosymmetric domains of 1–5 and 6–10. Rigid helical movements that create open-to-out and open-to-in states in LeuT fold proteins have been found in the case of MHP1, where a four helix bundle (hash motif comprising of helices 3, 4, 8 and 9) moves against a rigid core to provide substrate access to either side of the transporter (Shimamura et al. 2009). BetP, which transports the osmolyte betaine, has helical elements that work as rigid bodies coupled with subtle conformational changes (Perez et al. 1999). TM1a is displaced by as much as 45 deg in LeuT (Fig. 3B, C), but has a minimal displacement of 18 deg in BetP (Perez et al. 1999). As a validation to the large-scale changes in the position of helix 1a in LeuT, single molecule FRET studies have reported that movements in TM1a are coupled to the release of substrate in LeuT in solution (Zhao et al. 2008).
Conclusions
The different snapshots of LeuT have allowed a near-complete understanding of the transport cycle. Models of mammalian NSS members based on LeuT have provided substantial pharmacological and mechanistic insights into the workings of these molecular machines. Despite controversies concerning substrate stoichiometry (Quick et al. 2010, 2012; Piscitelli et al. 1957; Wang et al. 2010), LeuT has served as an invaluable spyglass to further our understanding of mammalian neurotransmitter transporters, whose structure determination remains a challenging task.
Acknowledgments
We thank Kevin Wang, Hui Wang and Derek Claxton for helpful comments and suggestions.
Glossary
- ADHD
attention deficit hyperactivity disorder
- ApcT
amino acid, polyamine and organocation transporter
- BetP
betaine transporter
- GAT
GABA transporter
- LeuT
leucine transporter
- Mhp1
Microbacterium liquefaciens sodium-hydantoin transporter
- NET
noradrenaline transporter
- NSS
neurotransmitter sodium symporter
- SERT
serotonin transporter
- SLC1/6
solute carrier 1/6
- vSGLT
Vibrio parahaemolyticus Na+/galactose cotransporter
Biographies
Aravind Penmatsa is a postdoctoral fellow in the Gouaux laboratory. After obtaining a PhD from the Centre for Cellular andMolecular Biology (CCMB), India, studying structural and biophysical properties of calcium-binding proteins, Aravind is currently involved in the study of sodium-coupled neurotransmitter transporters.
Eric Gouaux is an investigator with the Howard Hughes Medical Institute (HHMI) and a senior scientist at Oregon Health & Science University (OHSU). His PhD research involved structural studies of aspartate transcarbamoylase with William N. Lipscomb and, during his postdoctoral fellowship, he initiated crystallographic studies of integral membrane channels and receptors with H. Gobind Khorana. At present, the Gouaux laboratory studies the molecular mechanisms of synaptic transmission through structural studies of ligand-gated ion channels and neurotransmitter transporters.
Additional information
Competing interests
None.
Author contributions
A.P. and E.G. wrote the manuscript.
Funding
A.P. is supported by a postdoctoral fellowship from the American Heart Association (11POST7370096). E.G. is an investigator with the Howard Hughes Medical Institute.
References
- Amara SG, Fontana AC. Excitatory amino acid transporters: keeping up with glutamate. Neurochem Int. 41:313–318. doi: 10.1016/s0197-0186(02)00018-9. [DOI] [PubMed] [Google Scholar]
- Amara SG, Kuhar MJ. Neurotransmitter transporters: recent progress. Annu Rev Neurosci. 16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- Andersen J, Stuhr-Hansen N, Zachariassen L, Toubro S, Hansen SM, Eildal JN, Bond AD, Bogeso KP, Bang-Andersen B, Kristensen AS, Stromgaard K. Molecular determinants for selective recognition of antidepressants in the human serotonin and norepinephrine transporters. Proc Natl Acad Sci U S A. 2002;108:12137–12142. doi: 10.1073/pnas.1103060108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androutsellis-Theotokis A, Goldberg NR, Ueda K, Beppu T, Beckman ML, Das S, Javitch JA, Rudnick G. Characterization of a functional bacterial homologue of sodium-dependent neurotransmitter transporters. J Biol Chem. 1993;278:12703–12709. doi: 10.1074/jbc.M206563200. [DOI] [PubMed] [Google Scholar]
- Beuming T, Kniazeff J, Bergmann ML, Shi L, Gracia L, Raniszewska K, Newman AH, Javitch JA, Weinstein H, Gether U, Loland CJ. The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat Neurosci. 2011;11:780–789. doi: 10.1038/nn.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuming T, Shi L, Javitch JA, Weinstein H. A comprehensive structure-based alignment of prokaryotic and eukaryotic neurotransmitter/Na+ symporters (NSS) aids in the use of the LeuT structure to probe NSS structure and function. Mol Pharmacol. 2003;70:1630–1642. doi: 10.1124/mol.106.026120. [DOI] [PubMed] [Google Scholar]
- Bisgaard H, Larsen MA, Mazier S, Beuming T, Newman AH, Weinstein H, Shi L, Loland CJ, Gether U. The binding sites for benztropines and dopamine in the dopamine transporter overlap. Neuropharmacology. 2008;60:182–190. doi: 10.1016/j.neuropharm.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bismuth Y, Kavanaugh MP, Kanner BI. Tyrosine 140 of the gamma-aminobutyric acid transporter GAT-1 plays a critical role in neurotransmitter recognition. J Biol Chem. 2006;272:16096–16102. doi: 10.1074/jbc.272.26.16096. [DOI] [PubMed] [Google Scholar]
- Claxton DP, Quick M, Shi L, de Carvalho FD, Weinstein H, Javitch JA, McHaourab HS. Ion/substrate-dependent conformational dynamics of a bacterial homolog of neurotransmitter:sodium symporters. Nat Struct Mol Biol. 2011;17:822–829. doi: 10.1038/nsmb.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faham S, Watanabe A, Besserer GM, Cascio D, Specht A, Hirayama BA, Wright EM, Abramson J. The crystal structure of a sodium galactose transporter reveals mechanistic insights into Na+/sugar symport. Science. 1997;321:810–814. doi: 10.1126/science.1160406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest LR, Tavoulari S, Zhang YW, Rudnick G, Honig B. Identification of a chloride ion binding site in Na-/Cl-dependent transporters. Proc Natl Acad Sci U S A. 2010;104:12761–12766. doi: 10.1073/pnas.0705600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Lu F, Zhou L, Dang S, Sun L, Li X, Wang J, Shi Y. Structure and mechanism of an amino acid antiporter. Science. 2008;324:1565–1568. doi: 10.1126/science.1173654. [DOI] [PubMed] [Google Scholar]
- Harding MM. Metal-ligand geometry relevant to proteins and in proteins: sodium and potassium. Acta Crystallogr D Biol Crystallogr. 2007;58:872–874. doi: 10.1107/s0907444902003712. [DOI] [PubMed] [Google Scholar]
- Henry LK, Field JR, Adkins EM, Parnas ML, Vaughan RA, Zou MF, Newman AH, Blakely RD. Tyr-95 and Ile-172 in transmembrane segments 1 and 3 of human serotonin transporters interact to establish high affinity recognition of antidepressants. J Biol Chem. 2009;281:2012–2023. doi: 10.1074/jbc.M505055200. [DOI] [PubMed] [Google Scholar]
- Jardetzky O. Simple allosteric model for membrane pumps. Nature. 2002;211:969–970. doi: 10.1038/211969a0. [DOI] [PubMed] [Google Scholar]
- Jessell TM, Kandel ER. Synaptic transmission: a bidirectional and self-modifiable form of cell–cell communication. Cell. 2006;72(Suppl):1–30. doi: 10.1016/s0092-8674(05)80025-x. [DOI] [PubMed] [Google Scholar]
- Kantcheva AK, Quick M, Shi L, Winther AM, Stolzenberg S, Weinstein H, Javitch JA, Nissen P. Chloride binding site of neurotransmitter sodium symporters. Proc Natl Acad Sci U S A. 1966;110:8489–8494. doi: 10.1073/pnas.1221279110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniazeff J, Shi L, Loland CJ, Javitch JA, Weinstein H, Gether U. An intracellular interaction network regulates conformational transitions in the dopamine transporter. J Biol Chem. 1993;283:17691–17701. doi: 10.1074/jbc.M800475200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy H, Gouaux E. X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature. 2013;481:469–474. doi: 10.1038/nature10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy H, Piscitelli CL, Gouaux E. Unlocking the molecular secrets of sodium-coupled transporters. Nature. 2008;459:347–355. doi: 10.1038/nature08143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen AS, Andersen J, Jorgensen TN, Sorensen L, Eriksen J, Loland CJ, Stromgaard K, Gether U. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev. 2012;63:585–640. doi: 10.1124/pr.108.000869. [DOI] [PubMed] [Google Scholar]
- Masson J, Sagne C, Hamon M, El Mestikawy S. Neurotransmitter transporters in the central nervous system. Pharmacol Rev. 2009;51:439–464. [PubMed] [Google Scholar]
- Mitchell P. A general theory of membrane transport from studies of bacteria. Nature. 2011;180:134–136. doi: 10.1038/180134a0. [DOI] [PubMed] [Google Scholar]
- Perez C, Koshy C, Yildiz O, Ziegler C. Alternating-access mechanism in conformationally asymmetric trimers of the betaine transporter BetP. Nature. 1999;490:126–130. doi: 10.1038/nature11403. [DOI] [PubMed] [Google Scholar]
- Piscitelli CL, Krishnamurthy H, Gouaux E. Neurotransmitter/sodium symporter orthologue LeuT has a single high-affinity substrate site. Nature. 1957;468:1129–1132. doi: 10.1038/nature09581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick M, Shi L, Zehnpfennig B, Weinstein H, Javitch JA. Experimental conditions can obscure the second high-affinity site in LeuT. Nat Struct Mol Biol. 2012;19:207–211. doi: 10.1038/nsmb.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick M, Winther AM, Shi L, Nissen P, Weinstein H, Javitch JA. Binding of an octylglucoside detergent molecule in the second substrate (S2) site of LeuT establishes an inhibitor-bound conformation. Proc Natl Acad Sci U S A. 2010;106:5563–5568. doi: 10.1073/pnas.0811322106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressl S, Terwisscha van Scheltinga AC, Vonrhein C, Ott V, Ziegler C. Molecular basis of transport and regulation in the Na(+)/betaine symporter BetP. Nature. 2012;458:47–52. doi: 10.1038/nature07819. [DOI] [PubMed] [Google Scholar]
- Rudnick G. Ion-coupled neurotransmitter transport: thermodynamic vs kinetic determinations of stoichiometry. Methods Enzymol. 2009;296:233–247. doi: 10.1016/s0076-6879(98)96018-9. [DOI] [PubMed] [Google Scholar]
- Shaffer PL, Goehring A, Shankaranarayanan A, Gouaux E. Structure and mechanism of a Na+-independent amino acid transporter. Science. 2009;325:1010–1014. doi: 10.1126/science.1176088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon JR, Flattem NL, Jordan J, Jacob G, Black BK, Biaggioni I, Blakely RD, Robertson D. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N Engl J Med. 1998;342:541–549. doi: 10.1056/NEJM200002243420803. [DOI] [PubMed] [Google Scholar]
- Shimamura T, Weyand S, Beckstein O, Rutherford NG, Hadden JM, Sharples D, Sansom MS, Iwata S, Henderson PJ, Cameron AD. Molecular basis of alternating access membrane transport by the sodium-hydantoin transporter Mhp1. Science. 2009;328:470–473. doi: 10.1126/science.1186303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Piscitelli CL, Yamashita A, Gouaux E. A competitive inhibitor traps LeuT in an open-to-out conformation. Science. 2000;322:1655–1661. doi: 10.1126/science.1166777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Yamashita A, Gouaux E. Antidepressant binding site in a bacterial homologue of neurotransmitter transporters. Nature. 2010;448:952–956. doi: 10.1038/nature06038. [DOI] [PubMed] [Google Scholar]
- Sorensen L, Andersen J, Thomsen M, Hansen SM, Zhao X, Sandelin A, Stromgaard K, Kristensen AS. Interaction of antidepressants with the serotonin and norepinephrine transporters: mutational studies of the S1 substrate binding pocket. J Biol Chem. 2008;287:43694–43707. doi: 10.1074/jbc.M112.342212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talvenheimo J, Fishkes H, Nelson PJ, Rudnick G. The serotonin transporter-imipramine “receptor. J Biol Chem. 2007;258:6115–6119. [PubMed] [Google Scholar]
- Tang L, Bai L, Wang WH, Jiang T. Crystal structure of the carnitine transporter and insights into the antiport mechanism. Nat Struct Mol Biol. 2012;17:492–496. doi: 10.1038/nsmb.1788. [DOI] [PubMed] [Google Scholar]
- Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci. 1983;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- Wang H, Elferich J, Gouaux E. Structures of LeuT in bicelles define conformation and substrate binding in a membrane-like context. Nat Struct Mol Biol. 2010;19:212–219. doi: 10.1038/nsmb.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, Choe S, Chaptal V, Rosenberg JM, Wright EM, Grabe M, Abramson J. The mechanism of sodium and substrate release from the binding pocket of vSGLT. Nature. 2003;468:988–991. doi: 10.1038/nature09580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand S, Shimamura T, Yajima S, Suzuki S, Mirza O, Krusong K, Carpenter EP, Rutherford NG, Hadden JM, O'Reilly J, Ma P, Saidijam M, Patching SG, Hope RJ, Norbertczak HT, Roach PC, Iwata S, Henderson PJ, Cameron AD. Structure and molecular mechanism of a nucleobasecation-symport-1 family transporter. Science. 2012;322:709–713. doi: 10.1126/science.1164440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl-dependent neurotransmitter transporters. Nature. 2010;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Terry DS, Shi L, Quick M, Weinstein H, Blanchard SC, Javitch JA. Substrate-modulated gating dynamics in a Na+-coupled neurotransmitter transporter homologue. Nature. 2008;474:109–113. doi: 10.1038/nature09971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomot E, Bendahan A, Quick M, Zhao Y, Javitch JA, Kanner BI. Mechanism of chloride interaction with neurotransmitter:sodium symporters. Nature. 2007;449:726–730. doi: 10.1038/nature06133. [DOI] [PubMed] [Google Scholar]


