Abstract
Dietary proteins are cleaved within the intestinal lumen to oligopeptides which are further processed to small peptides (di-and tripeptides) and free amino acids. Although the transport of amino acids is mediated by several specific amino acid transporters, the proton-coupled uptake of the more than 8000 different di-and tripeptides is performed by the high-capacity/low-affinity peptide transporter isoform PEPT1 (SLC15A1). Its wide substrate tolerance also allows the transport of a repertoire of structurally closely related compounds and drugs, which explains their high oral bioavailability and brings PEPT1 into focus for medical and pharmaceutical approaches. Although the first evidence for the interplay of nutrient supply and PEPT1 expression and function was described over 20 years ago, many aspects of the molecular processes controlling its transcription and translation and modifying its transporter properties are still awaiting discovery. The present review summarizes the recent knowledge on the factors modulating PEPT1 expression and function in Caenorhabditis elegans, Danio rerio, Mus musculus and Homo sapiens, with focus on dietary ingredients, transcription factors and functional modulators, such as the sodium–proton exchanger NHE3 and selected scaffold proteins.
Introduction
The digestion of dietary proteins in the intestinal lumen results in the release of free amino acids and small peptides. Although the transport of amino acids via the enterocyte plasma membrane is mediated by several classes of amino acid transporter (for a review, see Broer, 2008), the transport of di-and tripeptides is driven solely by the intestinal peptide transporter isoform PEPT1. PEPT1 (SLC15A1; SLC, solute carrier) is a proton-dependent peptide transporter and belongs to the SLC15 family of membrane transporters, which also includes the renal peptide transporter isoform PEPT2 (SLC15A2) and two PHT proteins that transport histidine and selected peptides (Daniel & Kottra, 2008). The electrochemical proton gradient across the membrane allows the uptake of di-and tripeptides against a concentration gradient, enabling higher intracellular than extracellular peptide concentrations, and is strictly dependent on the function of the sodium–proton exchanger NHE3 in the apical membrane (Kennedy et al. 2010; Watanabe et al. 2005). With their proton gradient-dependent uptake mechanism, peptide transporters belong to the group of ‘archaic’ transporters. They are present in all living organisms and developed early in evolution. Various isoforms are present in the cell membranes of prokaryotes and simple eukaryotic cells (Daniel et al. 1994). In multicellular eukaryotes with a defined tissue distribution, the separation into the intestinal PEPT1-like form and the renal PEPT2-like form can be found from worms (Caenorhabditis elegans) (Fei et al. 2006; Meissner et al. 2012) to fish (Danio rerio, Gadus morhua) (Verri et al. 1999; Ronnestad et al. 1999) and birds (Gallus gallus domesticus) (Chen et al. 2008) up to mammals (Oryctolagus cuniculus, Mus musculus, Homo sapiens) (Fei et al. 2009, 1994; Liang et al. 2002). Both PEPT isoforms have a broad substrate pattern that includes mostly all di-and tripeptides formed from l-alpha amino acids, as well as a large variety of derivatives, including drugs such as beta-lactam antibiotics, selected angiotensin-converting enzyme inhibitors, protease inhibitors and antivirals (for a review, see Rubio-Aliaga & Daniel, 2010). As a result of their role in mediating the absorption and bioavailability of drugs, they are of considerable importance for pharmacology. Therefore, detailed knowledge about the mechanisms modulating PEPT1 expression and function will enhance its potential in clinical aspects of nutritional and drug therapy. The present review focuses on the known transcriptional, translational and post-translational regulatory mechanisms for the intestinal peptide transporter PEPT1 and summarizes data from studies in various organisms.
PEPT1 regulation by nutrient supply
Historical background
The daily absorption of amino acids, carbohydrates and fatty acids from the diet provides the nutritional requirements for the survival of an organism. During evolution, often parallel uptake routes for a macronutrient were developed to enable compensation for the loss of one transporter by another. Fatty acid uptake via the intestinal plasma membrane, for example, is mediated by various fatty acid transporters and, in the case of a high dietary fatty acid concentration, is performed by a proton gradient-dependent ‘flip–flop’ mechanism (Hamilton, 2000). For a long time, it was believed that proteins were completely hydrolysed to free amino acids in the intestinal lumen and that their absorption was mediated by various amino acid transporters. However, in the 1970s, studies revealed that small peptides of two and three amino acids in length (di-and tripeptides) were the main product of intestinal protein digestion (Adibi & Mercer, 1973), which initiated the exploration of the corresponding transport system. About 20 years later, the successful cloning of the intestinal di-and tripeptide transporter of rabbit (Boll et al. 2011; Fei et al. 2009), human (Liang et al. 2002), rat (Saito et al. 2007), worm (Fei et al. 2006) and mouse (Fei et al. 1994b) opened up a new field in macronutrient physiology.
It is well known that intestinal anatomy and the overall expression and function of nutrient transporters in enterocytes are triggered by the dietary composition and nutrient supply. As amino acids are building blocks for cellular peptides and proteins, the efficient uptake of amino acids in the form of di-and tripeptides via PEPT1 is tightly linked to the luminal peptide/amino acid concentration. In 1998, Walker and colleagues reported a peptide-mediated increased Pept1 mRNA transcription in human colon carcinoma Caco-2 cells (Walker et al. 2003). In this context, protein-rich diets (>20% casein) and supplementation with the dipeptide glycyl-phenylalanine or the amino acid phenylalanine more than doubled the mRNA/protein expression and transport function of PEPT1 in rats (Shiraga et al. 2006a). In addition, a low supply of PEPT1 substrates in a short-term fasting state also enhances the expression and membrane abundance of their intestinal transporter, as observed in rats (Ogihara et al. 2003; Thamotharan et al. 2009; Ihara et al. 1998), mice (Ma et al. 2013), chicken (Madsen & Wong, 1995) and human volunteers (Vazquez et al. 2008). These controversial regulations can be explained by the fact that, during short-term fasting, the intestine is prepared for efficient di-and tripeptide transport in the refeeding phase. The increased intestinal PEPT1 expression in a fasting state is dependent on the peroxisome proliferator-activated receptor alpha (PPARalpha). In fasted PPARalpha-deficient mice, the PEPT1 protein expression was unchanged when compared with ad libitum-fed animals (Shimakura et al. 2001a). These studies have identified the products of protein digestion as metabolic signals for modulation of Pept1 mRNA transcription/stability and of the intracellular trafficking of the PEPT1 protein.
Current results in C. elegans and human colon cells
Based on these findings and on the knowledge that the intracellular amino acid pool is also dependent on the peptidase-driven breakdown rate of di-and tripeptides, further research was performed. In 2001, Wenzel and colleagues demonstrated in Caco-2 cells that PEPT1-driven peptide uptake loads the cells with small peptides which are hydrolysed to amino acids, which, in turn, trans-stimulate the uptake of essential amino acids (l-arginine, l-lysine) via the transport system b0,+ (Wenzel et al. 1998). These mechanisms were dependent on intracellular peptide hydrolysis, as no trans-stimulation was detectable in the presence of the aminopeptidase inhibitor amastatin. However, the impact of amastatin on PEPT1 function was not analysed. For in vivo studies, we chose the nematode C. elegans, which expresses three peptide transporter isoforms: the intestinal high-capacity/low-affinity PEPT-1 (K04E7.2, formerly OPT-2, PEP-2), the broader expressed (excretory cells, muscles) PEPT-2 (C06G8.2, formerly OPT-1, PEP-1) (Meissner et al. 2012) and the neuronal PEPT-3 (F56F4.5, formerly OPT-3), which mainly acts as a proton channel (Fei et al. 1998a). In a recent study, we demonstrated that RNA interference (RNAi) gene silencing (Fraser et al. 2000a) of two predicted cytosolic peptidases, ZC416.6 and R11H6.1/PES-9, was sufficient to reduce intestinal C. elegans PEPT-1 expression and function (Benner et al. 1973). ZC416.9 is an orthologue of the mammalian bifunctional leukotriene A4 hydrolase/aminopeptidase LTA4H, but, as C. elegans lacks leukotrienes (Morgan et al. 2011), ZC416.6 may act exclusively as an aminopeptidase in the nematode. R11H6.1/PES-9 is structurally related to the mammalian cytosolic dipeptidase CNDP2. As both mammalian peptidases LTA4H and CNDP2 are sensitive to the aminopeptidase inhibitors bestatin (Davies et al. 2004) and amastatin (Daniel & Adibi, 2010), the impact of both compounds on C. elegans PEPT-1 function was analysed. By taking into account that bestatin itself is a PEPT1 substrate and to exclude competition at the binding site of the transporter, bestatin concentrations lower than 0.1 mm were used. Both inhibitors reduced C. elegans PEPT-1 activity in a concentration-dependent manner without changing its mRNA or protein abundance, indicating that both peptidases modulate the intracellular amino acid pool, which, in turn, affects the transport capacity of C. elegans PEPT-1. To test whether the mechanism is also conserved in mammals, LTA4H and CNDP2 gene silencing by siRNA was performed in human colon carcinoma Caco-2 cells and resulted in a significantly reduced PEPT1 protein expression (Benner et al. 1973). These results support the finding that the intracellular amino acid pool, modulated either by changes in the extracellular peptide supply or by a reduced intracellular peptide hydrolysis, is an evolutionarily conserved key regulator for the intestinal peptide transporter PEPT1.
Transcription factors regulating pept1 gene expression
Although studies on the regulation of Pept1 gene expression in mammals by dietary factors have been known for several years, studies on the underlying molecular mechanisms are limited. Shiraga et al. (2006a) found that selected amino acids (including leucine and phenylalanine) can control the promoter activity of rat-Pept1 via the amino acid responsive element (AARE). The AARE-like motif in the rat-PEPT1 promoter is located between −277 and −271 bp (5′-CATGGTG-3′) upstream of the start codon and shows high homology to the AARE motif from asparagine synthetase. Furthermore, the authors described a predicted Cdx2 binding site around 640 bp upstream of the start codon in the antisense direction. The systematic analysis of the mouse-Pept1 promoter revealed the relevant cis-and trans-elements within 1140 bp upstream of the transcription start site, including three GC boxes for the binding of the transcription factor Sp1 (Fei et al. 1994b). This regulatory mechanism is also conserved in humans, where the basal human-Pept1 gene expression is regulated by Sp1 (Shimakura et al. 1998) (summarized in Table 1). The direct interaction of Sp1 with two GC boxes in the first 170 bp upstream of the start codon highlights its role in mammalian Pept1 expression. One year later, the same group reported that the intestine-specific expression of Pept1 is controlled by Cdx2. However, the human-Pept1 promoter lacks typical binding motifs for Cdx2. Although the binding site of Cdx2 is not yet known, further studies have uncovered that Cdx2 binding is dependent on Sp1 (Shimakura et al. 2005b) and on butyrate (Dalmasso et al. 2002). In 2008, Inui and colleagues found a circadian regulation of rat-Pept1 transcriptional activation by the clock gene-controlled protein albumin D site-binding protein, which binds to its corresponding binding motif in the distal promoter (Saito et al. 2008). These binding motifs were established late in evolution, as the C. elegans pept-1 core promoter (600 bp upstream of the transcriptional start site) lacks Sp1-and Cdx2-related binding sites, and, in a recent study, we found that knockdown of the homologous genes had no impact on C. elegans PEPT-1 protein expression. However, in C. elegans, the FOXO transcription factor DAF-16, the key transcription factor of the insulin/insulin growth factor-like signalling pathway, was predicted to be a repressor of pept-1 gene expression (Meissner et al. 2012).
Table 1.
Overview of selected factors modulating the gene and/or protein expression and function of the intestinal peptide transporter PEPT1
| Modulator | Effect on PEPT1 | Kind of modulation | References |
|---|---|---|---|
| Amino acids leucine and phenylalanine | Control of pept1 promoter activity via the AARE in rats | Transcriptional | Shiraga et al. (2006a) |
| Transcription factor SP1 | Binds to the PEPT1 promoter and modulates the basal pept1 transcription | Transcriptional | Shimakura et al. (1998) |
| Transcription factor Cdx2 | Modulates pept1 transcription in concert with SP1 and with butyrate in mammals | Transcriptional | Shimakura et al. (2005b) Dalmasso et al. (2002) |
| Transcription factor DAF-16 | Represses pept1 transcription in Caenorhabditis elegans | Transcriptional | Meissner et al. (2012) |
| Peptidase inhibitors amastatin and bestatin | Decrease intracellular peptide hydrolysis and reduce pept1 function in C. elegans | Functional | Benner et al. (1973) |
| RNA interference of intracellular peptidases | Reduced protein expression and function of PEPT1 in Caco-2 cells and C. elegans | Translational, functional | Benner et al. (1973) |
| Sodium–proton exchanger NHE3/NHX-2 | Proton export is necessary for correct function of PEPT1 in mammalian cells and in C. elegans | Functional interaction via proton gradient (protein–protein interaction not yet proven) | Kennedy et al. (2010) Watanabe et al. (2005) Pieri et al. (2006) Benner et al. (1973) |
| Scaffold protein PDZK1 | Interacts with mouse PEPT1, trafficking to and anchoring in the plasma membrane | Direct protein–protein interaction | Sugiura et al. (1999) |
AARE, amino acid responsive element; PDZ, PSD95-disc large-ZO1.
PEPT1 regulation by modification of the transmembrane proton flux and the intracellular pH
The function of PEPT1 is strictly dependent on the transmembrane proton gradient in combination with an inside-negative membrane potential. Both factors provide a powerful driving force for the proton-coupled transport of di-and tripeptides into enterocytes. In the presence of the dipeptide glycyl-sarcosine, a decrease in the intracellular pH was detectable in enterocytes of wild-type mice, which was absent in PEPT1-deficient mice, indicating the acid loader function of PEPT1 (Chen et al. 2009). To avoid acidification of the cells, Na+/H+ exchangers mediate the electroneutral efflux of protons into the gut lumen in exchange with extracellular Na+, which leaves the cell via the Na+/K+-ATPase at the basolateral side. It has been demonstrated in mammalian cell models that NHE3 activity is required for the correct function of PEPT1 in the intestinal epithelium (Kennedy et al. 2010; Watanabe et al. 2005; Pieri et al. 2006), whereas the function of the renal form PEPT2 is dependent on NHE1 and/or NHE2 (Wada et al. 1985). These processes are evolutionarily conserved, and the essential action of NHE3 in the mammalian enterocytes has already been established in the C. elegans intestine by its orthologue NHX-2 (Nehrke, 2011a). We showed in vivo that RNAi gene silencing of NHX-2 in C. elegans, which results in a moderate but significant decrease in intracellular pH (Nehrke, 2011a), leads to reduced protein expression and function of C. elegans PEPT-1 (Benner et al. 1973) (Fig. 1). Furthermore, both were found to alter other central proton-dependent nutrient transport processes in nematodes. As PEPT1-deficient C. elegans accumulates large quantities of body fat (Brooks et al. 1994; Spanier et al. 2006b) and nhx-2(RNAi) C. elegans is extremely lean (Nehrke, 2011a), a general impact of PEPT1 and NHX-2 function on fatty acid uptake was predicted. Indeed, part of the cellular fatty acid uptake is mediated via a pH-dependent mechanism called the ‘fatty acid flip–flop’ (Hamilton, 2000). Uncharged fatty acids enter the phospholipid bilayer of the plasma membrane and move to the cytosol by releasing a proton. An increase in intracellular pH, as in nematodes with reduced PEPT1 expression and function, supports the flip–flop mechanism and induces fat accumulation. In contrast, a reduced NHX-2 function slows down the ‘fatty acid flip–flop’ as a result of a decrease in the intracellular pH, finally leading to lean nematodes (Spanier et al. 2006b) (Fig. 2).
Figure 1.
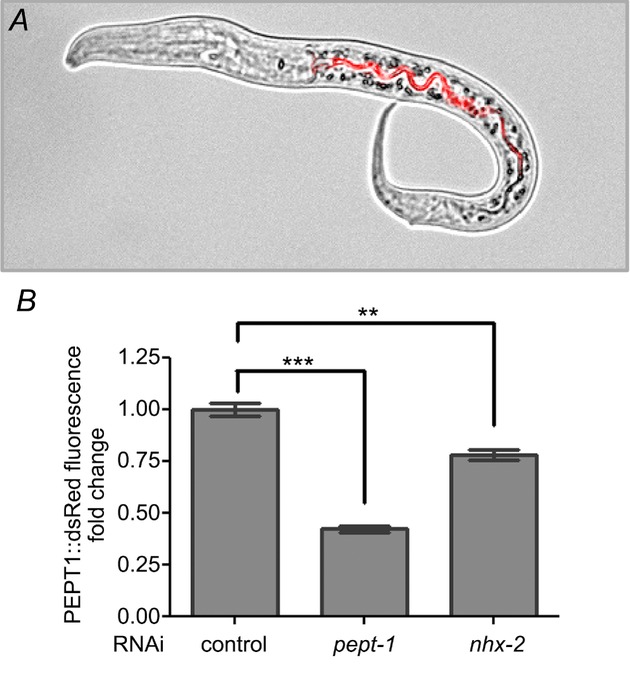
A, representative Caenorhabditis elegans MZE91R (unc-119(ed3) III; cbgIs91[pPept-1:pept-1::DsRed;unc-119(+)]; rrf-3(pk1426) II) expressing the PEPT1::DsRed fusion protein at the intestinal brush border membrane (red signal). B, PEPT1::DsRed expression in MZE91R C. elegans after RNA interference (RNAi) gene silencing of pept-1 and nhx-2. The RNAi feeding protocol described in Fraser et al. (2000a) was used. Controls were fed on bacteria carrying the empty pPD129.36 vector. Values are based on four independent experiments showing mean ± SEM. For statistical analysis, the Kruskal–Wallis test was performed with **P ≤ 0.01 and ***P ≤ 0.001.
Figure 2.
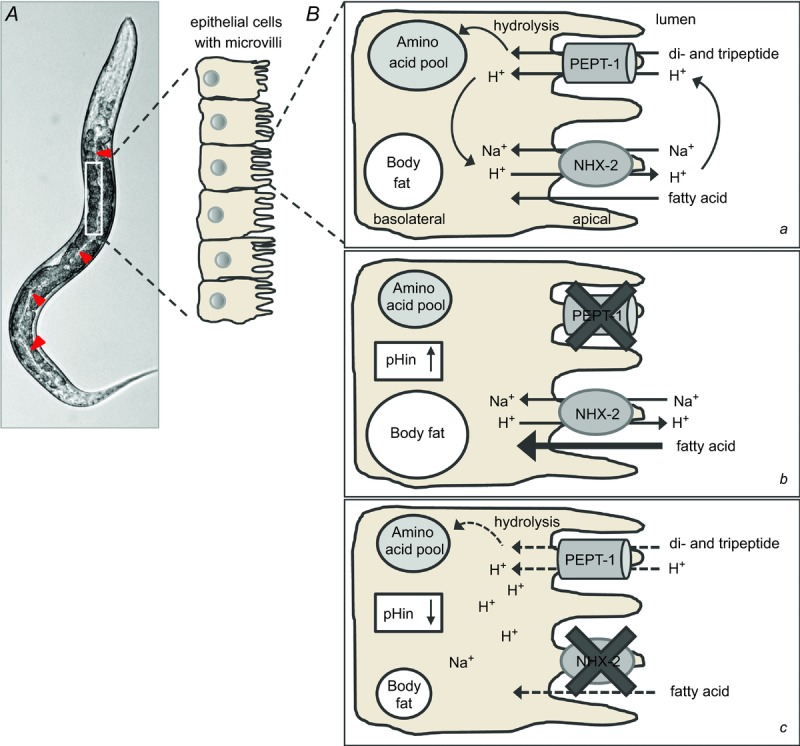
A, image of Caenorhabditis elegans and schematic magnification of the intestinal epithelium. The nematode's head faces to the top and arrowheads mark the intestinal lumen. The scheme focuses on epithelial cells with the basolateral side to the left and the apical side to the right side. B, interplay of peptide transporter PEPT-1 and sodium–proton exchanger NHX-2 in enterocytes. Ba, in the wild-type situation, both proteins are active in the apical membrane and stabilize amino acid homeostasis and intracellular pH (pHin). Bb, loss of PEPT-1 protein stops the peptide and proton influx, leading to lower intracellular amino acid concentrations and an increase in pHin. Uptake of fatty acids via the fatty acid flip–flop mechanism is supported and induces obesity. Bc, when NHX-2 is missing, proton export stops and pHin decreases. Uptake of fatty acids via the flip–flop mechanism is decreased, leading to nematodes with empty body fat depots.
Diet-dependent phenotypic alterations in PEPT1-deficient mice
Although the loss of the intestinal peptide transporter induces severe phenotypic alterations in C. elegans, the phenotype of PEPT1-deficient mice is no different from that of wild-type mice when they are fed on a carbohydrate-rich standard diet (Hu et al. 2012). However, when fed a diet containing 45% of its energy as protein, PEPT1 knockout mice were found to show major changes in the plasma amino acid pattern, whereas the pattern was unchanged when the diet contained less than 21% protein (Nassl et al. 2004a). Therefore, PEPT1 function is essential under nutritional conditions with high protein content, when the amino acid transporters are saturated and the uptake of small peptides offers additional nutrient supply. As a high-protein diet increases satiety and promotes weight loss, it was tested whether the intestinal peptide transporter is involved in these processes. Interestingly, loss of PEPT1 reduced the food intake in the first days on a high-protein diet more severely than in wild-type mice, an effect that might be driven by increased arginine and corresponding low leptin concentrations in plasma (Nassl et al. 2005b).
To induce obesity in PEPT1-deficient mice, the knockout animals were challenged with a diet containing 48% of its energy as fat. Interestingly, these mice showed a significantly lower weight gain than their wild-type littermates. This can be explained by a reduced energy intake as a result of nutrient maldigestion/malabsorption in the small intestine and a higher energy loss in faeces. Investigating the origin of the impaired nutrient digestion/absorption, we observed that PEPT1-deficient mice lack the diet-induced ability to modify the architecture of the intestinal mucosa. In contrast with wild-type mice, in which a high-fat diet increases villus length and surface area of the upper small intestine, both factors remain unchanged in PEPT1-deficient animals (Kolodziejczak et al. 2002). The gut architecture is modified by various factors in mammals and, recently, interleukin-6 has been reported to be an essential growth promotion chemokine that supports villus elongation (Jin et al. 2008). However, PEPT1-deficient animals have a markedly lower systemic interleukin-6 concentration than wild-type mice, which finally might result in reduced intestinal surface area. Furthermore, the loss of PEPT1 as cellular acid loader modifies the proton export of NHE3. As NHE3 also contributes to water absorption, PEPT1-deficient mice show reduced water absorption from the small intestine (Chen et al. 2009). Although they do not develop diarrhoea, this impairment could also contribute to the diet-insensitive villus architecture, for example via changes in intestinal trans-mural pressure, which has also been shown to affect interleukin-6 secretion (Kishikawa et al. 2004). Most interestingly, mice lacking NHE3 have reduced body weight, increased gut length and increased mass of caecum and colon tissue and content (Schultheis et al. 1995), and they display, in part, phenotypic features of PEPT1-deficient mice. Based on these observations, we hypothesize that the different phenotypic outcomes with respect to the body fat content in mice and C. elegans might be caused by the ability to modify the surface area of their intestinal epithelium and therefore compensate for food energy absorption. Although the surface of the murine intestinal epithelium is highly flexible depending on the diet, the surface of the 20 enterocytes in C. elegans is predicted to be stable. Yet, no studies have been published on the modifications of the intestinal microvilli structure and enterocyte surface area of C. elegans based on food source and diet composition.
PEPT regulation by PDZ (PSD95-disc large-ZO1) domain proteins
In polarized cells, including all kinds of epithelial cells, the defined localization of membrane proteins is essential for their correct function. Protein–protein interactions with scaffold proteins, which target them to appropriate regions of the plasma membrane and regulate their activity, have been shown for various xenobiotic transporters (Kato et al. 2000). However, information on direct protein–protein interactions with peptide transporters is scarce. Both mammalian peptide transporter isoforms PEPT1 and PEPT2 contain the class I PDZ binding motif S/T-X-Ø (S/T, serine/threonine; X, any residue; Ø, hydrophobic residue) at their C-terminus and are recognized by PDZ domain proteins. The PDZ domain is typical for scaffold proteins and essential for protein–protein contact (Sheng & Sala, 2008). A direct interaction of mouse PEPT1 with the scaffold protein PDZK1 has been reported (Sugiura et al. 1999), whereas binding of human-PEPT1 to sodium–proton exchanger regulation factor 1 (NHERF1) and NHERF2 was undetectable (Boehmer et al. 1973). However, interaction of human-PEPT2 with PDZK1 (Noshiro et al. 2011b) and NHERF2 (Boehmer et al. 1973) was described. Just recently the homologous gene for the mammalian NHERF family of PDZ domain proteins was found in C. elegans. The nematode contains only one NHERF family protein, named NRFL-1 (C01F6.6, formerly TAG-60), which is essential for the anchoring of the amino acid transporter AAT-6 (homologue of the light subunit of heteromeric amino acid transporters) to the apical plasma membrane (Hagiwara et al. 2000b). Whether C. elegans PEPT-1, which contains a predicted class I PDZ binding motif (TFD) at its C-terminus, is also an interaction partner of NRFL-1 awaits further analysis.
Concluding remarks
During the last two decades, many factors that directly or indirectly influence the expression and function of the intestinal peptide transporter PEPT1 have been evaluated. Without any doubt, the close cooperation of PEPT1 with the sodium–proton exchanger NHE3 in enterocytes is of high relevance and influences other central nutrient transport processes, such as fatty acid transport via the brush border membrane. However, evidence for direct protein–protein interactions with PEPT1 is scarce. Therefore, future research should focus on the identification of interaction partner proteins to PEPT1 and NHE3 in the apical membrane of enterocytes. Although technically challenging, as the proteins of interest are membrane proteins, this will expand the repertoire of modulators of this nutrient transport system.
Acknowledgments
The Caenorhabditis elegans strain MZE91R was kindly provided by Marino Zerial, MPI of Molecular Cell Biology and Genomics, Dresden, Germany. Many thanks to all past and present members of my working group for their contribution to the results presented in the report, to Tamara Zietek and Manuela Rist for comments on the manuscript, and to Hannelore Daniel for support of the worm projects. The author apologizes for all studies that were not cited because of space limitations.
Glossary
- AARE
amino acid responsive element
- NHE3
sodium–proton exchanger 3
- NHERF
sodium–proton exchanger regulation factor
- PDZ
PSD95-disc large-ZO1
- PEPT
di-and tripeptide transporter
- PPAR
peroxisome proliferator-activated receptor
- RNAi
RNA interference
- SLC
solute carrier
Biography
Britta Spanier obtained her Diploma in Biology and started to work with C. elegans as amodel for oxidative stress resistance and longevity during her doctoral studies with Kimberly Henkle-Dührsen, both at the Heinrich-Heine-Universität in Düsseldorf, Germany. Shemoved to the laboratory of Ralf Baumeister, Ludwig-Maximilians-Universität,München for a 2-year postdoctoral fellowship on cell surface protease genetics in C. elegans, before she started to investigate the function of peptide transporters in Hannelore Daniels' laboratory at the Technische Universität München. The current projects in her working group focus on the regulation of peptide transporter function and its interplay with other pH-dependent processes in the small and large intestine.
Additional information
Competing interests
No conflicts of interest exist for the author.
Author contributions
BS planned, wrote and revised the manuscript.
Funding
The research is funded by the Deutsche Forschungsgemeinschaft (DFG).
References
- Adibi SA, Mercer DW. Protein digestion in human intestine as reflected in luminal, mucosal, and plasma amino acid concentrations after meals. J Clin Invest. 52:1586–1594. doi: 10.1172/JCI107335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner J, Daniel H, Spanier B. A glutathione peroxidase, intracellular peptidases and the TOR complexes regulate peptide transporter PEPT-1 in C. elegans. PLoS One. 6:e25624. doi: 10.1371/journal.pone.0025624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmer C, Palmada M, Klaus F, Jeyaraj S, Lindner R, Laufer J, Daniel H, Lang F. The peptide transporter PEPT2 is targeted by the protein kinase SGK1 and the scaffold protein NHERF2. Cell Physiol Biochem. 1973;22:705–714. doi: 10.1159/000185554. [DOI] [PubMed] [Google Scholar]
- Boll M, Markovich D, Weber WM, Korte H, Daniel H, Murer H. Expression cloning of a cDNA from rabbit small intestine related to proton-coupled transport of peptides, beta-lactam antibiotics and ACE-inhibitors. Pflugers Arch. 2011;429:146–149. doi: 10.1007/BF02584043. [DOI] [PubMed] [Google Scholar]
- Broer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev. 2008;88:249–286. doi: 10.1152/physrev.00018.2006. [DOI] [PubMed] [Google Scholar]
- Brooks KK, Liang B, Watts JL. The influence of bacterial diet on fat storage in C. elegans. PLoS One. 1994;4:e7545. doi: 10.1371/journal.pone.0007545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Pan Y, Wong EA, Bloomquist JR, Webb KE., Jr Molecular cloning and functional expression of a chicken intestinal peptide transporter (cPepT1) in Xenopus oocytes and Chinese hamster ovary cells. J Nutr. 2008;132:387–393. doi: 10.1093/jn/132.3.387. [DOI] [PubMed] [Google Scholar]
- Chen M, Singh A, Xiao F, Dringenberg U, Wang J, Engelhardt R, Yeruva S, Rubio-Aliaga I, Nassl AM, Kottra G, Daniel H, Seidler U. Gene ablation for PEPT1 in mice abolishes the effects of dipeptides on small intestinal fluid absorption, short-circuit current, and intracellular pH. Am J Physiol Gastrointest Liver Physiol. 2009;299:G265–G274. doi: 10.1152/ajpgi.00055.2010. [DOI] [PubMed] [Google Scholar]
- Dalmasso G, Nguyen HT, Yan Y, Charrier-Hisamuddin L, Sitaraman SV, Merlin D. Butyrate transcriptionally enhances peptide transporter PepT1 expression and activity. PLoS One. 2002;3:e2476. doi: 10.1371/journal.pone.0002476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel H, Adibi SA. Functional separation of dipeptide transport and hydrolysis in kidney brush border membrane vesicles. FASEB J. 2010;8:753–759. doi: 10.1096/fasebj.8.10.8050675. [DOI] [PubMed] [Google Scholar]
- Daniel H, Kottra G. The proton oligopeptide cotransporter family SLC15 in physiology and pharmacology. Pflugers Arch. 2008;447:610–618. doi: 10.1007/s00424-003-1101-4. [DOI] [PubMed] [Google Scholar]
- Daniel H, Spanier B, Kottra G, Weitz D. From bacteria to man: archaic proton-dependent peptide transporters at work. Physiology (Bethesda) 1994;21:93–102. doi: 10.1152/physiol.00054.2005. [DOI] [PubMed] [Google Scholar]
- Davies DR, Mamat B, Magnusson OT, Christensen J, Haraldsson MH, Mishra R, Pease B, Hansen E, Singh J, Zembower D, Kim H, Kiselyov AS, Burgin AB, Gurney ME, Stewart LJ. Discovery of leukotriene A4 hydrolase inhibitors using metabolomics biased fragment crystallography. J Med Chem. 2004;52:4694–4715. doi: 10.1021/jm900259h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei YJ, Fujita T, Lapp DF, Ganapathy V, Leibach FH. Two oligopeptide transporters from Caenorhabditis elegans: molecular cloning and functional expression. Biochem J. 2006;332:565–572. doi: 10.1042/bj3320565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei YJ, Kanai Y, Nussberger S, Ganapathy V, Leibach FH, Romero MF, Singh SK, Boron WF, Hediger MA. Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature. 2009;368:563–566. doi: 10.1038/368563a0. [DOI] [PubMed] [Google Scholar]
- Fei YJ, Romero MF, Krause M, Liu JC, Huang W, Ganapathy V, Leibach FH. A novel H+-coupled oligopeptide transporter (OPT3) from Caenorhabditis elegans with a predominant function as a H+ channel and an exclusive expression in neurons. J Biol Chem. 1998;275:9563–9571. doi: 10.1074/jbc.275.13.9563. [DOI] [PubMed] [Google Scholar]
- Fei YJ, Sugawara M, Liu JC, Li HW, Ganapathy V, Ganapathy ME, Leibach FH. cDNA structure, genomic organization, and promoter analysis of the mouse intestinal peptide transporter PEPT1. Biochim Biophys Acta. 1994;1492:145–154. doi: 10.1016/s0167-4781(00)00101-9. [DOI] [PubMed] [Google Scholar]
- Fraser AG, Kamath RS, Zipperlen P, Martinez-Campos M, Sohrmann M, Ahringer J. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature. 2000a;408:325–330. doi: 10.1038/35042517. [DOI] [PubMed] [Google Scholar]
- Hagiwara K, Nagamori S, Umemura YM, Ohgaki R, Tanaka H, Murata D, Nakagomi S, Nomura KH, Kage-Nakadai E, Mitani S, Nomura K, Kanai Y. NRFL-1, the C. elegans NHERF orthologue, interacts with amino acid transporter 6 (AAT-6) for age-dependent maintenance of AAT-6 on the membrane. PLoS One. 2000b;7:e43050. doi: 10.1371/journal.pone.0043050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JA. Fatty acid transport: difficult or easy. J Lipid Res. 2000;39:467–481. [PubMed] [Google Scholar]
- Hu Y, Smith DE, Ma K, Jappar D, Thomas W, Hillgren KM. Targeted disruption of peptide transporter Pept1 gene in mice significantly reduces dipeptide absorption in intestine. Mol Pharm. 2012;5:1122–1130. doi: 10.1021/mp8001655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara T, Tsujikawa T, Fujiyama Y, Bamba T. Regulation of PepT1 peptide transporter expression in the rat small intestine under malnourished conditions. Digestion. 1998;61:59–67. doi: 10.1159/000007736. [DOI] [PubMed] [Google Scholar]
- Jin X, Zimmers TA, Zhang Z, Pierce RH, Koniaris LG. Interleukin-6 is an important in vivo inhibitor of intestinal epithelial cell death in mice. Gut. 2008;59:186–196. doi: 10.1136/gut.2008.151175. [DOI] [PubMed] [Google Scholar]
- Kato Y, Yoshida K, Watanabe C, Sai Y, Tsuji A. Screening of the interaction between xenobiotic transporters and PDZ proteins. Pharm Res. 2000;21:1886–1894. doi: 10.1023/b:pham.0000045244.83999.43. [DOI] [PubMed] [Google Scholar]
- Kennedy DJ, Leibach FH, Ganapathy V, Thwaites DT. Optimal absorptive transport of the dipeptide glycylsarcosine is dependent on functional Na+/H+ exchange activity. Pflugers Arch. 2010;445:139–146. doi: 10.1007/s00424-002-0910-1. [DOI] [PubMed] [Google Scholar]
- Kishikawa H, Miura S, Yoshida H, Hirokawa M, Nakamizo H, Higuchi H, Adachi M, Nakatsumi RC, Suzuki H, Saito H, Ishii H. Transmural pressure induces IL-6 secretion by intestinal epithelial cells. Clin Exp Immunol. 2004;129:86–91. doi: 10.1046/j.1365-2249.2002.01895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziejczak D, Spanier B, Pais R, Kraiczy J, Stelzl T, Gedrich K, Scherling C, Zietek T, Daniel H. Mice lacking the intestinal peptide transporter display reduced energy intake and a subtle maldigestion/malabsorption that protects them from diet-induced obesity. Am J Physiol Gastrointest Liver Physiol. 2002;304:G897–G907. doi: 10.1152/ajpgi.00160.2012. [DOI] [PubMed] [Google Scholar]
- Liang R, Fei YJ, Prasad PD, Ramamoorthy S, Han H, Yang-Feng TL, Hediger MA, Ganapathy V, Leibach FH. Human intestinal H+/peptide cotransporter. Cloning, functional expression, and chromosomal localization. J Biol Chem. 2002;270:6456–6463. doi: 10.1074/jbc.270.12.6456. [DOI] [PubMed] [Google Scholar]
- Ma K, Hu Y, Smith DE. Influence of fed-fasted state on intestinal PEPT1 expression and in vivo pharmacokinetics of glycylsarcosine in wild-type and pept1 knockout mice. Pharm Res. 2013;29:535–545. doi: 10.1007/s11095-011-0580-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen SL, Wong EA. Expression of the chicken peptide transporter 1 and the peroxisome proliferator-activated receptor alpha following feed restriction and subsequent refeeding. Poult Sci. 1995;90:2295–2300. doi: 10.3382/ps.2010-01173. [DOI] [PubMed] [Google Scholar]
- Meissner B, Boll M, Daniel H, Baumeister R. Deletion of the intestinal peptide transporter affects insulin and TOR signalling in Caenorhabditis elegans. J Biol Chem. 2012;279:36739–36745. doi: 10.1074/jbc.M403415200. [DOI] [PubMed] [Google Scholar]
- Morgan EL, Maskrey BH, Rowley AF. At what stage in metazoan evolution did leukotriene generation first appear?–key insights from cartilaginous fish. Dev Comp Immunol. 2011;29:53–59. doi: 10.1016/j.dci.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Nassl AM, Rubio-Aliaga I, Fenselau H, Marth MK, Kottra G, Daniel H. Amino acid absorption and homeostasis in mice lacking the intestinal peptide transporter PEPT1. Am J Physiol Gastrointest Liver Physiol. 2004;301:G128–G137. doi: 10.1152/ajpgi.00017.2011. [DOI] [PubMed] [Google Scholar]
- Nassl AM, Rubio-Aliaga I, Sailer M, Daniel H. The intestinal peptide transporter PEPT1 is involved in food intake regulation in mice fed a high-protein diet. PLoS One. 2005;6:e26407. doi: 10.1371/journal.pone.0026407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehrke K. A reduction in intestinal cell pHi due to loss of the Caenorhabditis elegans Na+/H+ exchanger NHX-2 increases life span. J Biol Chem. 2011a;278:44657–44666. doi: 10.1074/jbc.M307351200. [DOI] [PubMed] [Google Scholar]
- Noshiro R, Anzai N, Sakata T, Miyazaki H, Terada T, Shin HJ, He X, Miura D, Inui K, Kanai Y, Endou H. The PDZ domain protein PDZK1 interacts with human peptide transporter PEPT2 and enhances its transport activity. Kidney Int. 2011b;70:275–282. doi: 10.1038/sj.ki.5001522. [DOI] [PubMed] [Google Scholar]
- Ogihara H, Suzuki T, Nagamachi Y, Inui K, Takata K. Peptide transporter in the rat small intestine: ultrastructural localization and the effect of starvation and administration of amino acids. Histochem J. 2003;31:169–174. doi: 10.1023/a:1003515413550. [DOI] [PubMed] [Google Scholar]
- Pieri M, Christian HC, Wilkins RJ, Boyd CA, Meredith D. The apical (hPepT1) and basolateral peptide transport systems of Caco-2 cells are regulated by AMP-activated protein kinase. Am J Physiol Gastrointest Liver Physiol. 2006;299:G136–G143. doi: 10.1152/ajpgi.00014.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnestad I, Gavaia PJ, Viegas CS, Verri T, Romano A, Nilsen TO, Jordal AE, Kamisaka Y, Cancela ML. Oligopeptide transporter PepT1 in Atlantic cod (Gadus morhua L.): cloning, tissue expression and comparative aspects. J Exp Biol. 1999;210:3883–3896. doi: 10.1242/jeb.007898. [DOI] [PubMed] [Google Scholar]
- Rubio-Aliaga I, Daniel H. Peptide transporters and their roles in physiological processes and drug disposition. Xenobiotica. 2010;38:1022–1042. doi: 10.1080/00498250701875254. [DOI] [PubMed] [Google Scholar]
- Saito H, Okuda M, Terada T, Sasaki S, Inui K. Cloning and characterization of a rat H+/peptide cotransporter mediating absorption of beta-lactam antibiotics in the intestine and kidney. J Pharmacol Exp Ther. 2007;275:1631–1637. [PubMed] [Google Scholar]
- Saito H, Terada T, Shimakura J, Katsura T, Inui K. Regulatory mechanism governing the diurnal rhythm of intestinal H+/peptide cotransporter 1 (PEPT1) Am J Physiol Gastrointest Liver Physiol. 2008;295:G395–G402. doi: 10.1152/ajpgi.90317.2008. [DOI] [PubMed] [Google Scholar]
- Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet. 1995;19:282–285. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- Sheng M, Sala C. PDZ domains and the organization of supramolecular complexes. Annu Rev Neurosci. 2008;24:1–29. doi: 10.1146/annurev.neuro.24.1.1. [DOI] [PubMed] [Google Scholar]
- Shimakura J, Terada T, Katsura T, Inui K. Characterization of the human peptide transporter PEPT1 promoter: Sp1 functions as a basal transcriptional regulator of human PEPT1. Am J Physiol Gastrointest Liver Physiol. 1998;289:G471–G477. doi: 10.1152/ajpgi.00025.2005. [DOI] [PubMed] [Google Scholar]
- Shimakura J, Terada T, Saito H, Katsura T, Inui K. Induction of intestinal peptide transporter 1 expression during fasting is mediated via peroxisome proliferator-activated receptor alpha. Am J Physiol Gastrointest Liver Physiol. 2001;291:G851–G856. doi: 10.1152/ajpgi.00171.2006. [DOI] [PubMed] [Google Scholar]
- Shimakura J, Terada T, Shimada Y, Katsura T, Inui K. The transcription factor Cdx2 regulates the intestine-specific expression of human peptide transporter 1 through functional interaction with Sp1. Biochem Pharmacol. 2005;71:1581–1588. doi: 10.1016/j.bcp.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Shiraga T, Miyamoto K, Tanaka H, Yamamoto H, Taketani Y, Morita K, Tamai I, Tsuji A, Takeda E. Cellular and molecular mechanisms of dietary regulation on rat intestinal H+/peptide transporter PepT1. Gastroenterology. 2006a;116:354–362. doi: 10.1016/s0016-5085(99)70132-0. [DOI] [PubMed] [Google Scholar]
- Spanier B, Lasch K, Marsch S, Benner J, Liao W, Hu H, Kienberger H, Eisenreich W, Daniel H. How the intestinal peptide transporter PEPT-1 contributes to an obesity phenotype in Caenorhabditits elegans. PLoS One. 2006b;4:e6279. doi: 10.1371/journal.pone.0006279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Kato Y, Wakayama T, Silver DL, Kubo Y, Iseki S, Tsuji A. PDZK1 regulates two intestinal solute carriers (Slc15a1 and Slc22a5) in mice. Drug Metab Dispos. 1999;36:1181–1188. doi: 10.1124/dmd.107.020321. [DOI] [PubMed] [Google Scholar]
- Thamotharan M, Bawani SZ, Zhou X, Adibi SA. Functional and molecular expression of intestinal oligopeptide transporter (Pept-1) after a brief fast. Metabolism. 2009;48:681–684. doi: 10.1016/s0026-0495(99)90164-6. [DOI] [PubMed] [Google Scholar]
- Vazquez JA, Morse EL, Adibi SA. Effect of starvation on amino acid and peptide transport and peptide hydrolysis in humans. Am J Physiol Gastrointest Liver Physiol. 2008;249:G563–G566. doi: 10.1152/ajpgi.1985.249.5.G563. [DOI] [PubMed] [Google Scholar]
- Verri T, Kottra G, Romano A, Tiso N, Peric M, Maffia M, Boll M, Argenton F, Daniel H, Storelli C. Molecular and functional characterisation of the zebrafish (Danio rerio) PEPT1-type peptide transporter. FEBS Lett. 1999;549:115–122. doi: 10.1016/s0014-5793(03)00759-2. [DOI] [PubMed] [Google Scholar]
- Wada M, Miyakawa S, Shimada A, Okada N, Yamamoto A, Fujita T. Functional linkage of H+/peptide transporter PEPT2 and Na+/H+ exchanger in primary cultures of astrocytes from mouse cerebral cortex. Brain Res. 1985;1044:33–41. doi: 10.1016/j.brainres.2005.02.064. [DOI] [PubMed] [Google Scholar]
- Walker D, Thwaites DT, Simmons NL, Gilbert HJ, Hirst BH. Substrate upregulation of the human small intestinal peptide transporter, hPepT1. J Physiol. 2003;507(Pt 3):697–706. doi: 10.1111/j.1469-7793.1998.697bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe C, Kato Y, Ito S, Kubo Y, Sai Y, Tsuji A. Na+/H+ exchanger 3 affects transport property of H+/oligopeptide transporter 1. Drug Metab Pharmacokinet. 2005;20:443–451. doi: 10.2133/dmpk.20.443. [DOI] [PubMed] [Google Scholar]
- Wenzel U, Meissner B, Doring F, Daniel H. PEPT1-mediated uptake of dipeptides enhances the intestinal absorption of amino acids via transport system b(0,+) J Cell Physiol. 2001;186:251–259. doi: 10.1002/1097-4652(200102)186:2<251::AID-JCP1027>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]


