Abstract
Functional collateral vessels often stem from outward remodelling of pre-existing connections between perfusion territories. Knowledge of the distribution and morphology of innate collateral connections may help in identifying myocardial areas with protection against risk for ischaemia. The coronary network of six healthy canine hearts was investigated with an imaging cryomicrotome. Innate collateral connections ranged from 286 to 1015 μm in diameter. Left ventricular collateral density (number per gram of tissue) was about five in the subendocardium vs. 2.5 in the mid-myocardium (P < 0.01) and 1.3 in the epicardium (P < 0.01). Subendocardial collateral connections were oriented parallel to the long axis of the heart. For the major coronary arteries, five times more intracoronary than intercoronary connections were found, while their median diameter and interquartile range were not significantly different, at 96.1 (16.9) vs. 94.7 (18.9) μm. Collateral vessels connecting crowns from sister branches from a stem are denoted intercrown connections and those within crowns intracrown connections. The number of intercrown connections was related to the mean tissue weight of the crowns (y = 0.73x − 0.33, r2 = 0.85, P < 0.0001). This relation was likewise found to describe intercoronary connections. The median collateral diameter and length were independent of the tissue volumes bridged. We conclude that connectivity and morphology of the innate collateral network are distributed with no preference for intra-or intercrown connections, independent of stem diameter, including epicardial arteries. This renders all sites of the myocardium equally protected in case of coronary artery disease. The orientation of subendocardial collateral vessels indicates the longitudinal direction of subendocardial collateral flow.
Introduction
When chronic coronary artery disease develops, collateral arteries may grow, providing a path for oxygen-rich blood to the perfusion region of an obstructed coronary artery (Schaper, 2009; Seiler, 2010; Meier et al. 2012). In acute coronary occlusion, the innate collateral network is not immediately able to restore adequate perfusion to the ischaemic region, as shown in dogs (Brazzamano et al. 1985; von Mutius et al. 1988). This is in agreement with observations in patients with long-standing coronary artery disease, where only in approximately one-quarter to one-third of the cases was collateral function sufficient to prevent ischaemia during brief coronary occlusion (Wustmann et al. 2003; Meier et al. 2007). However, during development of an arterial obstruction, collateral conductance increases by outward remodelling of the pre-existing collateral arteries under the influence of an increase in fluid shear stress (Ito et al. 1997; Pipp et al. 2004). The density distribution and morphology of the innate collateral vessels are important determinants for further collateralization of the heart and, consequently, the survival of collateral-dependent myocardium. An uneven density and diameter distribution would imply that some areas in the heart are more likely to benefit from collateral development than others.
Functional collateral flow in the human heart has been demonstrated by flow variations in a contralateral artery (Piek et al. 1993), by wedge pressure measurements relative to aortic pressure and by velocity measurements (Seiler et al. 1998), by angiographic blushing (Gibson et al. 1999) and by contrast echocardiography (Vogel et al. 2006). An overview of these methods was given by Traupe et al. (2010). Morphological details of collateral connections in the human heart were reported half a century ago (Baroldi et al. 1956; Fulton, 1956; Robbins et al. 1966; Kato, 1976). However, structural information on collateral segments and the arterial subtrees that were connected could not be obtained due to methodological limitations.
In the canine heart, known for its large pre-existing collateral network (Maxwell et al. 1987), some limited quantitative measurements on collateral anatomy have been obtained. Diameters of epicardial (Bloor & White, 1972; Grayson et al. 1974) and intramural collateral segments (Menick et al. 1971) could be measured accurately, but the density and position of the collateral connections with regard to myocardial depth could not be investigated.
Given the importance of pre-existing collateral arteries for perfusion during gradual and acute coronary occlusion, the goal of this study was to quantify the morphology and distribution of the innate collateral network in the healthy canine heart. The data are obtained using an imaging cryomicrotome designed to acquire high-resolution morphological data sets of three-dimensional (3D) vascular structures (Spaan et al. 2005; van den Wijngaard et al. 2010). As these hearts were devoid of coronary vascular disease, we hypothesized the absence of preferential connectivity and morphology throughout the cardiac wall. An equally distributed innate collateral network, therefore, can expand and serve to protect the myocardium against ischaemic events independent of the location of the area at risk.
Methods
Six healthy canine hearts were obtained following acute cardiovascular experiments. The protocol was approved by the local Institutional Animal Care and Use Committee of the University of Utrecht, The Netherlands. In brief, animals (mean weight 24.2 kg, range 22.5–27.9 kg) were premedicated with medetomidine (Dormitor, Orion Pharma, Espoo, Finland) (0.03 mg kg−1 i.m.), ketamine (Nimatek, Eurovet Animal Health, Bladel, The Netherlands) (0.03 mg kg−1 i.m.) and atropine (Atropinesulfaat, Pharmachemie BV, Haarlem, The Netherlands) (0.5 mg i.m.). Anaesthesia was induced by sufentanil (Sufenta Forte, Janssen-Cilag BV, Tilburg, The Netherlands) (10 μg kg−1 h−1 i.v.), the animals were intubated and mechanically ventilated with oxygen, and a surgical level of anaesthesia was maintained with a mixture of propofol (Propofol-Lipuro, B Braun, Melsungen AG, Germany) (24 mg kg−1 h−1 i.v.) and sufentanil (3 μg kg−1 h−1 i.v.). The hearts were exposed by left thoracotomy via the fourth intercostal space. No muscle relaxants were used, and anaesthetic depth was monitored by eye reflex and heart rate variation. After completion of the experiments, additional heparin (Heparin Leo Pharma, Denmark) was administered and the animals were killed by vena cava puncture. Co-ordinated heart contraction was stopped by inducing fibrillation using a 9 V battery. The heart was then excised and the coronary arteries were flushed retrogradely from the aortic root with calcium-free 3-(N-morpholino)propanesulfonic acid (MOPS) buffer containing 4.7 mmol l−1 potassium chloride, leaving the heart in a diastolic state. Mean heart weight was 247 g and ranged from 200 to 282 g, with a base-to-apex mean length of 99.2 mm, ranging from 87.7 to 129.9 mm.
Heart preparation and vessel filling
With the heart suspended from sutures around the aorta, the right and left main coronary arteries were cannulated and flushed with buffer solution until the efflux remained clear of blood. The coronary arteries were filled at physiological pressure of 80–100 mmHg with a fluorescent cast material (Batson no. 17; Polysciences, Warrington, PA, USA) consisting of a monomer base solution, a catalyst and a promoter (Spaan et al. 2005). Potomac Yellow (excitation 440 nm, emission 49 nm; Radiant Colour, Houthalen, Belgium) or UV-Blue (excitation 375 nm, emission 430 nm; VasQtec, Zuerich, Switzerland) was added as the fluorescent base. During the casting procedure, the left ventricle was not pressurized and the heart was submerged in buffer solution. The cast material was allowed to harden for 24 h at ambient temperature, and the fully prepared heart was immersed in carboxymethylcellulose sodium solvent (Brunschwig Chemie, Amsterdam, The Netherlands) mixed with 5% Indian ink (Royal Talens, Apeldoorn, The Netherlands) and frozen at −20°C.
Three-dimensional image acquisition
The cryomicrotome set-up and episcopic imaging procedure were described previously in detail (Spaan et al. 2005; van den Wijngaard et al. 2013). In brief, the frozen heart was mounted with its long axis perpendicular to the cutting plane, which was illuminated with two clusters of seven power light-emitting diodes (Luxeon V, Star, Royal Blue; Lumileds Lighting, San Jose, CA, USA), with a central wavelength at 365 nm for UV-Blue and at 455 nm through a 440 nm excitation filter for Potomac Yellow. Each specimen was cut from apex to base at 20 μm slice thickness by a fully automated cutting mechanism. After each slice, the block face of the remaining bulk material was imaged using a digital camera (Apogee Alta U-16, Roseville, CA, USA) equipped with a variable-focus lens (Nikon 70–180 mm, Japan), achieving an in-plane image resolution of 40 μm. After each cut, a black and white photograph of the outline of the heart and a fluorescence image were recorded after emission filtering at 440 or 505 nm for the respective cast material. The resulting registered stack of sequential images yielded a detailed virtual representation of the 3D morphology of the vascular network and surrounding tissue. Isotropic voxel resolution was achieved by a maximal intensity projection combining every other two adjacent slices. A typical data set for an entire heart therefore contained between 2200 and 3200 images, depending on its size.
Image correction and vascular tree segmentation
Image degradation due to scattering of emission light originating from structures beneath the imaged surface and lens blurring was corrected by deconvolution with a system-specific point spread function (Rolf et al. 2008). The correction of the entire image stack was performed in the spatial domain using a custom-made 3D iterative deconvolution program (Geenen et al. 2013) written in CUDA (NVidia, Santa Clara, CA, USA).
After deconvolution, the vessel outlines were skeletonized using a topology-preserving thinning algorithm (Palagyi & Kuba, 1998). In brief, points forming centrelines were classified according to their nearest-neighbour connections as follows: end-points, with one neighbour; mid-points, with two neighbours; and bifurcations, in the case of three neighbours. Higher-order points, such as trifurcations, were classified in a similar manner if present in the data set. Vessel segments were defined between consecutive non-mid-points and labelled to serve as a topological representation of the vascular network.
Local diameters were determined for each mid-point by examining the cross-sectional intensity profile along 64 equally spaced vectors in a plane normal to the centreline of the segment (Lagerveld et al. 2010; van den Wijngaard et al. 2011). With the vessel centre at the maximal intensity, the full width at half-maximal intensity along each vector was found and averaged for all vectors to yield the corresponding local diameter. Assuming a circular cross-section, the mean diameter of each segment was determined by averaging local diameters over its length.
Detection of collateral connections with their spatial orientation and length
Neighbouring points along the centreline were traced and labelled consecutively down the tree, starting with seed points at the origin of four major coronary arteries, i.e. left anterior descending artery (LAD), left circumflex artery (LCX), right coronary artery (RCA) and the septal branch (SB) arising from the left main coronary artery or from the LAD as described by Blair (1961). Each centre point was assigned a numerical label denoting its originating seed point. A segment that was labelled twice identified the presence of a loop in the vascular structure, as illustrated in Fig. 1. For each detected loop, the segment with the smallest diameter was designated as the collateral connection. That collateral connection was then eliminated from the tree, and the entire tree was processed iteratively, evaluating all segments until no more collateral connections were found. A reduced skeleton of vascular loops was then obtained; collateral connections were extracted and analysed further (see also Fig. 3D).
Figure 1.
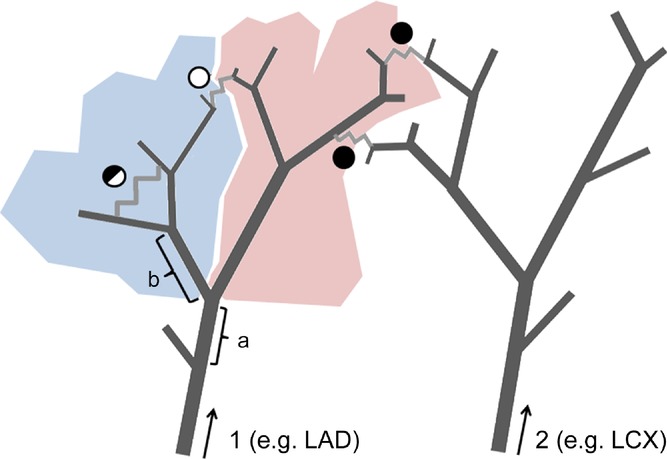
Labels indicating the originating seed points were assigned to all centre points, starting at the major coronary arteries, designated as 1 and 2. The arrows indicate the direction of the labelling. A segment that was labelled twice identified the presence of a loop. The segment with the smallest diameter in the loop was assumed to be the collateral connection (zig-zag lines). The filled circles indicate intercoronary connections between stems 1 and 2. The other two circles indicate intracoronary collaterals with respect to stem 1. Considering segment ‘a’ as a stem, two daughter crowns are depicted by two different colours. The open circle now identifies an intercrown collateral connection, while the half-open circle is now an intracrown connection. If segment ‘b’ is considered a stem, the half-open circle indicates an intercrown connection.
Figure 3.
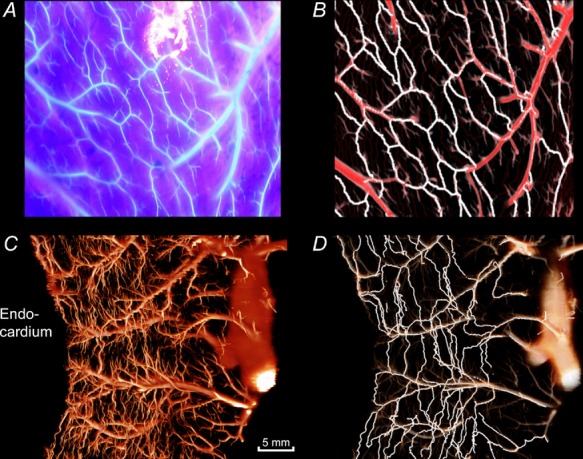
A, photograph of the epicardial fluorescence (Batson cast material with ultraviolet blue labelling) under excitation with ultraviolet light. B, reconstruction of the same epicardial area after analysis of imaging cryomicrotome data. The automatically detected collateral pathways are depicted in white and the normal vessels in red. C, transmural vessels in a 7.5-mm-thick tissue slab in the longitudinal direction of the heart. D, collateral pathways detected in the same slab are highlighted in white.
The spatial orientation of the collateral connections within the myocardial wall was evaluated by representing the collateral segment as a 3D vector between its start and end-point and defining the circumferential orientation by the angle (α) of its long axis to the normal vector of the nearest epicardial surface. An orthogonal angle (β) was calculated between the collateral segment and the longitudinal axis of the heart (Fig. 2). The length of a collateral segment was determined from the number of centre points between the start and end-point.
Figure 2.
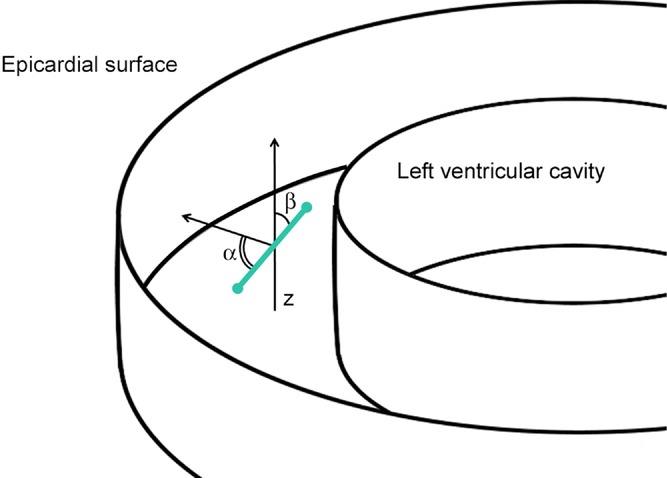
Angle α represents the angle between the long axis of the collateral vessel (collateral indicated by the green line; start and end-point of the collateral are indicated with green circles) and the normal vector to the nearest epicardial surface, and β is the angle between the collateral vessel and the longitudinal axis of the heart (z).
Classification of collateral connections
Each non-terminal segment in a branching tree can be considered a stem for daughter trees. Stems that uniquely define collateral pathways are denoted as collateral-feeding stem segments and follow from the reduced skeleton. Collateral vessels between daughter crowns are denoted as intercrown connections and those within crowns as intracrown connections. As illustrated in Fig. 1, a collateral segment can switch classification as intra-or intercrown depending on the selected stem segment along the coronary tree.
For each collateral-feeding stem in the reduced skeleton, the number of intercrown collateral vessels was counted, and that count was related to the mean tissue volume perfused by the connected crowns. Determination of crown tissue volume is described below.
The diameter distribution of the stem segments from the reduced skeleton was investigated for three different layers in the left ventricular wall, i.e. the subendocardium, mid-myocardium and subepicardium.
The LCX, LAD, SB and RCA were considered separately, and collateral connections between their territories were indicated as intercoronary connections and within their territories as intracoronary collateral connections.
Classification of regions and perfusion territories
Short-axis outline images were used to create a binary segmentation of the hearts and to distinguish three main perfusion regions of cardiac tissue, the left ventricular free wall (LVFW), right ventricular wall (RVW) and interventricular septum (SEP). The LVFW and SEP were further divided into three myocardial layers of equal thickness denoted as Endo, Mid and Epi. The RVW was divided into two layers of equal thickness denoted by Endo and Epi. Regional tissue volume was determined from the outline images by counting the corresponding bright voxels in the binary segmentation per territory. Regional collateral density was obtained by dividing the number of collateral arteries found in the region by the corresponding volume of the region. No distinction between inter-and intracrown or intra-and intercoronary collateral connections was made in this analysis.
The perfused volume for each terminal segment was derived from a Voronoi tessellation of the 3D outline data (Karch et al. 2003), with the end-points assigned as the centre of a Voronoi cell. The perfused tissue volume related to each tree segment was calculated as the sum of the Voronoi cells belonging to the crown of the segment, as shown in Fig. 1. As an estimate of the tissue volume that is influenced by a collateral segment, we arbitrarily chose the mean of the perfused tissue of the two subtrees joined by the collateral segment.
Statistical methods
D'Agostino and Pearson's omnibus normality tests were used to test for normal distribution. Normally distributed data are presented as means ± SD unless otherwise noted. Non-normally distributed data are presented as the medians ± interquartile range. The means of normally distributed data were compared using Student's paired or unpaired t test or a one-way analysis of variance followed by Tukey's post hoc multiple comparison, as appropriate. For non-normal data, a Kruskal–Wallis test followed by Dunn's multiple comparison was used. A value of P < 0.05 was considered statistically significant. All statistical analyses were performed in Graphpad Prism (GraphPad Software, Inc., La Jolla, CA, USA).
Results
In order to validate our algorithms, collateral connections at the epicardium were reconstructed after processing with the cryomicrotome and compared with their appearance on epicardial surface photographs of the fluorescent cast before processing. This is shown in Fig. 3A and B. Figure 3C shows a typical transmural reconstruction from a virtual slice of the LVFW, while Fig. 3D depicts the isolated collateral pathways found in the cast for that same area.
Collateral density and diameter distribution
Table 1 summarizes the total number of collateral segments and diameter information for all dogs. The total number of detected collateral segments varied from 286 (heart H4) to 1015 (heart H5) and was unrelated to age or sex. The diameter of the collateral connections ranged from 23 to 381 μm, with median values between 84.7 μm for H3 and 125.5 μm for H4. In H4, information on the septal branch is missing because the infusion cannula was inadvertently inserted past the ostium of that branch during casting. As illustrated in Fig. 4, overall collateral density was lowest in the RVW and about equal for LVFW and SEP. The density different between layers was only in the LVFW, i.e. the density in the subendocardium was about twice that in the mid-myocardium and triple that in the epicardial layer (P < 0.01). The subendocardial density in the LVFW was also higher than in the RVW and in the epicardial layer of the SEP (both P < 0.05). The diameter distributions of collateral vessels are summarized in Fig. 5, with the median values and interquartile range shown in the figure. Compared with the other layers, median collateral diameters in the subendocardium were about 5% smaller in both the LFVW (P < 0.001) and the RVW (P < 0.05). For the SEP, the median subendocardial diameter was about 15% smaller (P < 0.01) than for the mid-and subepicardial layers (Table 2).
Collateral vessel diameter
| Diameter (μm) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Heart no. | Age (months) | Sex | Number of collaterals | Minimum | Lower quartile | Median | Upper quartile | Maximum |
| H1 | 15 | Male | 609 | 59.8 | 85.4 | 94.9 | 103.8 | 288.8 |
| H2 | 14 | Male | 312 | 24.1 | 74.6 | 96.1 | 135.3 | 292.7 |
| H3 | 50 | Female | 364 | 23.0 | 53.8 | 84.7 | 167.3 | 381.0 |
| H4 | 51 | Female | 286 | 70.4 | 100.4 | 125.5 | 170.4 | 371.9 |
| H5 | 28 | Female | 1015 | 30.1 | 72.2 | 92.7 | 122.6 | 383.0 |
| H6 | 11 | Male | 564 | 40.8 | 71.1 | 88.8 | 106.6 | 149.2 |
| Average of all collaterals | 525 ± 274.6 | Lower quartile 305.5, median 464, upper quartile 710.5 | ||||||
Figure 4.
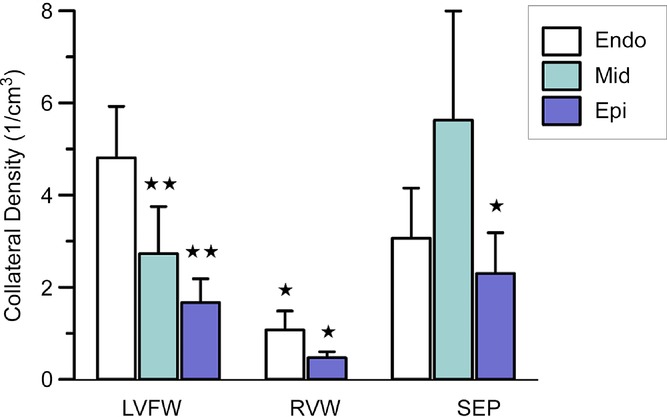
Data are shown for the total number of collaterals obtained from all study hearts. Collateral density was highest in the subendocardium of the left ventricular free wall and mid-myocardium of the septum. Abbreviations: Endo, endocardial layer; Epi, epicardial layer; LVFW, left ventricular free wall; Mid, mid-myocardial layer; RVW, right ventricular wall; and SEP, septum. *P < 0.05 and **P < 0.01, Student's t test compared with the endocardial layer of the LVFW. No significant differences exist between the other groups.
Figure 5.
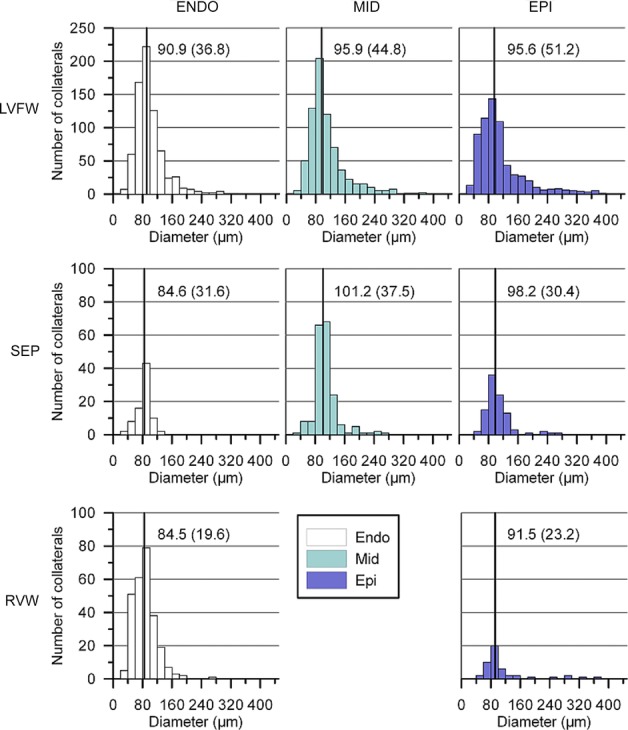
Data are shown for the total number of collaterals obtained from all study hearts. Median values are indicated by the continuous line for each distribution and are shown together with the interquartile range.
Statistical significance of differences in median diameter for the regions and their respective layers
| LVFW |
SEP |
RVW |
||||||
|---|---|---|---|---|---|---|---|---|
| Mid | Epi | Endo | Mid | Epi | Endo | Epi | ||
| LVFW | Endo | P < 0.01 | P < 0.001 | n.s. | n.s. | n.s. | P < 0.01 | n.s. |
| Mid | — | n.s. | P < 0.001 | n.s. | n.s. | P < 0.001 | n.s. | |
| Epi | — | — | P < 0.001 | n.s. | n.s. | P < 0.001 | n.s. | |
| SEP | Endo | — | — | — | P < 0.01 | P < 0.05 | n.s. | P < 0.01 |
| Mid | — | — | — | — | n.s. | P < 0.001 | n.s. | |
| Epi | — | — | — | — | — | P < 0.05 | n.s. | |
| RVW | Endo | — | — | — | — | — | — | P < 0.01 |
Abbreviations: Endo, endocardial layer; Epi, epicardial layer; LVFW, left ventricular free wall; Mid, mid-myocardial layer; n.s., not significant; RVW, right ventricular wall; and SEP, septum.
Connectivity
Approximately five times more intra-than intercoronary collateral segments were found (Fig. 6A), while the median diameter and interquartile range of the distributions was similar, at 96.1 (16.9) vs. 94.7 (18.9) μm. Most intracoronary connections were found within the LAD and LCX territories. The number of intercoronary collaterals (Fig. 6B) was highest between LAD and LCX branches (P < 0.05).
Figure 6.
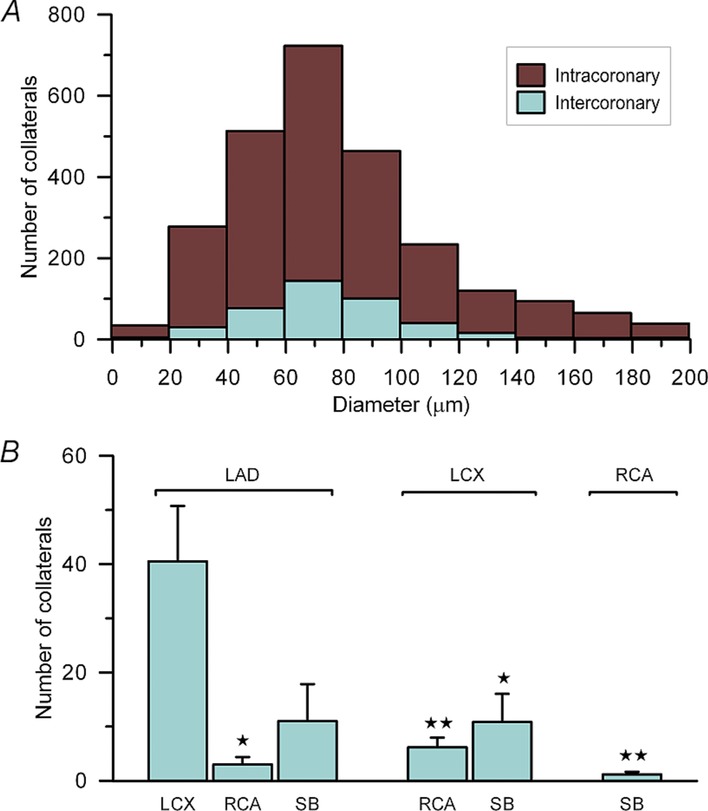
Data are shown for the total number of collaterals obtained from all study hearts. A, diameter distribution for all intra-and intercoronary collaterals. No significant difference in diameter exists between the groups. B, average number of intercoronary collateral connections separated by coronary vessels bridged. Abbreviations: LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery; and SB, septal branch. *P < 0.05 and **P < 0.01 compared with LAD–LCX connections. No other significant differences exist between the other groups. Student's unpaired t test; error bars indicate SEM.
Orientation and length
The circumferential angle of the collateral vector with the nearest tangent plane to the epicardial surface (α) is shown in Fig. 7A. A similar trend as demonstrated by Fig. 7 was observed for each of the hearts separately (not shown). The collateral orientation was predominantly parallel to the epicardial surface for all myocardial layers. In contrast, the angle β of the collateral segment with the long axis of the heart had a different distribution for different layers of the LVFW and SEP combined (Fig. 7B). At the subendocardium, the peak value of β was 20 deg and significantly (P < 0.05) smaller compared with the mid-myocardium and epicardium (peak value ∼80 deg). This implies that most endocardial collateral arteries were oriented in the direction of the cardiac long axis, while collateral vessels in the mid-myocardial and epicardial layers tended to be more horizontally oriented.
Figure 7.
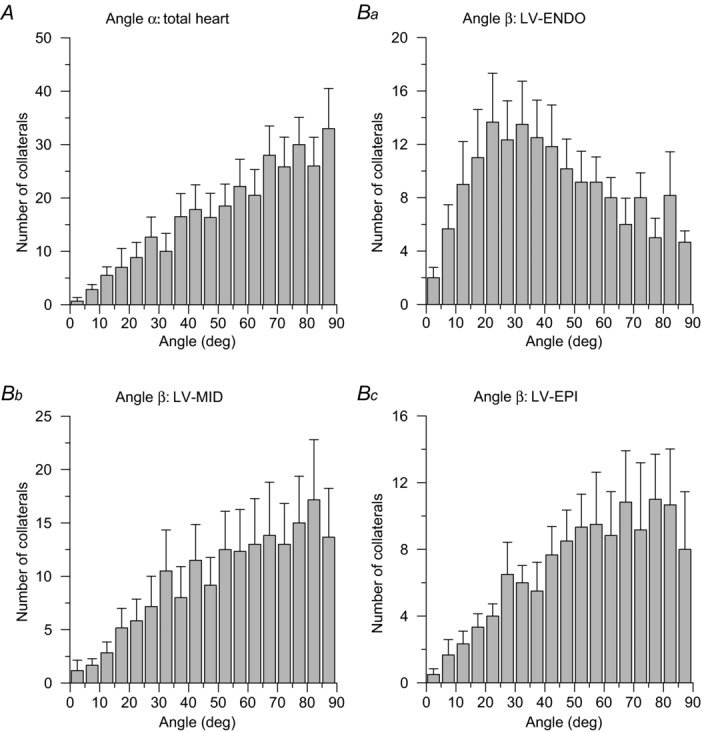
A, distribution of the circumferential angle α. Collateral vessels were predominantly oriented parallel to the epicardial surface, because the distribution of α is skewed towards 90 deg. The distribution for the total of all hearts is shown; the individual distributions are similar. Values indicate the mean number of collaterals with SEM error bars. B, distribution of the collateral longitudinal angle β for the left ventricular (LV) free wall and septum at the endocardial (Ba), mid-myocardial (Bb) and epicardial layer (Bc). Most collaterals in the LV mid-and epicardial layers were horizontally oriented, while endocardial collaterals followed a more longitudinal direction. The peak value of β was smaller for the LV-Endo layer than for LV-Mid or LV-Epi layers (P < 0.05, Student's unpaired t test).
The median length of all collateral connections was 768 μm (first to third quartile range 450–1344 μm). Intra-and intercoronary collateral connections did not statistically differ in length.
Collateral connections and perfused crown volume
The number of intercrown connections was linearly related to the average tissue volume influenced by both crowns. As demonstrated in Fig. 8A, this relationship was significant (r = 0.92, P < 0.0001), with an increase of approximately 1.4 cm3 of perfused tissue per intercrown collateral connection. Extrapolation of the regression line closely predicts the intercoronary data point shown on the far right for collateral vessels between territories of the large epicardial arteries.
Figure 8.
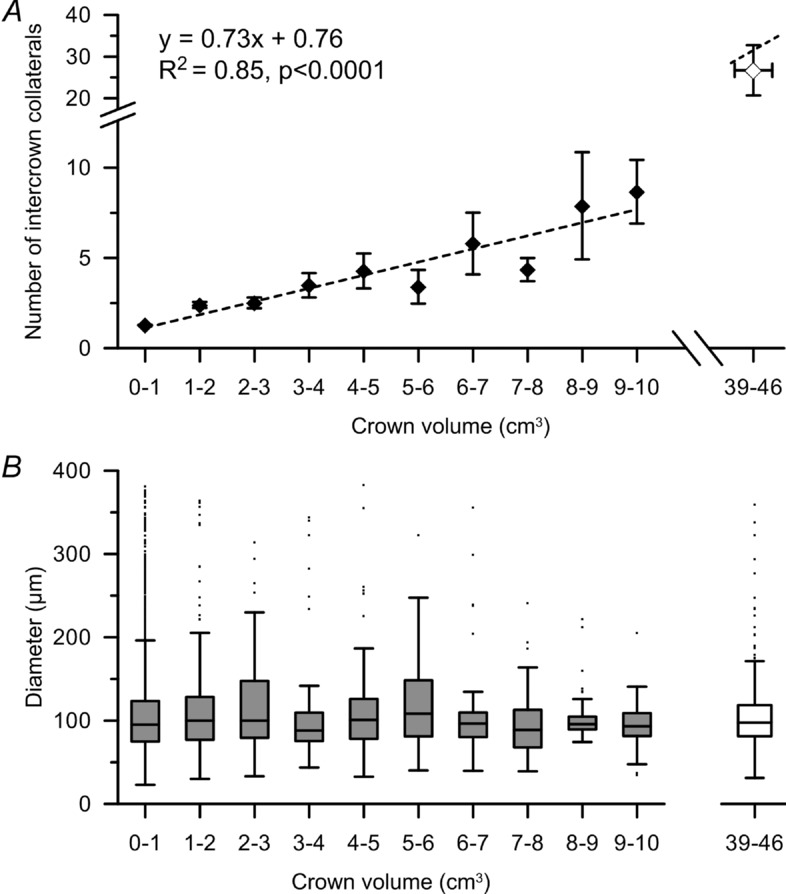
Data are shown for the total number of collaterals obtained from all study hearts. A, mean and standard error of the number of intracrown connections vs. the average of the two connected crown tissue volumes. The number of collaterals increases by one per 1.4 g of connected tissue volume. The number of intercoronary connections vs. the mean tissue weight of connected larger territories is shown at the right of the plot. Note that this data point is close to the extended regression line for smaller subtrees. B, median and interquartile range of intercrown collateral diameters for increasing average of the two tissue crown volumes connected. Larger connected crown volumes contain more collaterals, but the median and range of collateral diameter is not increased.
The median diameter of the intercrown and intercoronary connections was ∼95 μm regardless of the perfused tissue volume bridged (Fig. 8B).
Notably, twice the number of collateral-feeding stem segments were found in the subepicardium of the LVFW compared with the endocardium or mid-myocardium (P < 0.0001), which means that most collateral arteries bridged perfusion territories via penetrating arteries from the epicardium. The median diameter and first-to-third quartile range for the subepicardial layer was 521.2 (265.2–1085.0) μm, about 2.5 times higher (P < 0.0001) than in the endocardium [208.8 (145.2–324.7) μm] and mid-myocardium [265.9 (180.5–434.3) μm].
Discussion
To our knowledge, this study is the first to report on diameter, distribution, orientation and connectivity of innate collateral segments throughout the entire dog heart. Our results demonstrate the following findings. (i) Connectivity and morphological properties of the pre-existing collateral network are independent of position and with similar diameter and length distributions for the left ventricular free wall, septum and right ventricular wall. (ii) The density of collateral connections was highest at the subendocardium in the LVFW and mid-myocardium of the septum. (iii) The median diameter of collateral segments ranged from 84.7 to 125.5 μm, with smaller collateral vessels at the endocardium of all regions. (iv) Intracoronary connections were five times more frequent than intercoronary connections, with no difference in median diameter. (v) The LAD–LCX intercoronary connections by far outnumbered the other types of intercoronary connections. (vi) The number of intercrown connections relates linearly to the mean tissue volume of connected crowns independent of the collateral-feeding stem segment considered, including those of the major coronary arteries. (vii) Collateral vessels were predominantly oriented parallel to the epicardial surface and aligned with the cardiac long axis in the subendocardial layer of the left ventricle.
Comparison with previous findings
The diameter range of 23–383 μm for intramural and epicardial coronary collateral segments presented here corresponds well with that found in earlier studies in humans (20–350 μm, Baroldi et al. 1956) and in dogs (40–300 μm, Bloor & White, 1972; and 70–120 μm, (Grayson et al. 1974). Indirect information on collateral diameters has been obtained by injecting microspheres into the left coronary artery and counting them in the retrograde outflow from the right coronary artery in post-mortem isolated human hearts (Pitt, 1959). Microspheres in the ranges of 35–45 and 75–90 μm passed collateral connections in healthy and diseased hearts, indicating the presence of collateral arteries in these diameter ranges. In experiments with microsphere obstruction of microvessels in the LCX territory, intramural collateral connections were reported to have diameters between 25 and 80 μm (Scheel et al. 1990). We found larger collateral segments, with a median diameter of 90.9 μm. This difference may be explained by the outflow pressure in the right coronary artery, which was close to 0 mmHg in those earlier retrograde perfusion experiments, reducing diameters of the collateral connections.
In our study, total collateral connections per heart numbered between 286 and 1015, which is more than reported earlier for canine hearts, where counting was restricted to border zones of perfusion areas between major coronary arteries (Menick et al. 1971). An error in detecting collateral connections may have been introduced by the injection of different-coloured replica material into LCX and LAD. Colour mixing in vessels other than collaterals may result in incorrect identification of collateral connections.
Quantitative anatomical data on intracoronary collaterals are sparse due to technical limitations. Baroldi et al. (1956) made casts by injecting the coronary vessels of the human heart with a latex solution that was allowed to solidify, followed by removal of the tissue. The casts remained very elastic and, by pulling apart the territories of conduit arteries, collateral connections remained intact and became visible. Robbins et al. (1966) filled the coronary system of the human heart with a barium sulfate gel and visualized the vessels by angiographic stereoscopy. The presence of intracoronary collateral connections was determined by direct inspection of the stereo-angiograms. Although these studies demonstrated intracoronary collateral arteries in human hearts, both healthy and in the presence of coronary artery disease, quantitative data on the density or ratio between inter-and intracollateral segments were not provided.
Homogeneous distribution of collateral connections over the myocardium
Literature often focuses on the role and count of intercoronary collateral connections, which is why this category received special attention in the present study. We found that intracoronary connections were more than five times as frequent as intercoronary connections, which corresponds with earlier observations in human hearts without infarction from longitudinal and transverse sections that were obviously not covering the same tissue space (Kato, 1976). It is difficult to interpret these data in terms of intercoronary collateral density, because the direct interface between epicardial perfusion territories is two-dimensional by definition. However, intracoronary connections can be intercrown collateral connections as determined by the selection of collateral-feeding stem. In that way, the number of intercoronary connections can be related to the influenced tissue mass (equivalent to the mean tissue volume perfused by connected epicardial arteries) in the same way as the number of intercrown anastomoses. No differences were found, underlining that the role of the interface between large arteries is not different from the interface between crowns of collateral feeding stems more distal in the coronary tree.
These findings support the central hypothesis of this study that innate collateral connections are ubiquitously distributed over the myocardium and not restricted to border zones between large epicardial arteries where intercoronary collaterals are to be found. This hypothesis was further confirmed by the histograms per myocardial region, albeit with an increasing epi-to-endocardial density in the LVFW.
The relationship between the number of intercrown collateral connections and the influenced tissue volume suggests an increase of about one collateral connection per 1.4 cm3 of tissue. The same number holds for intercoronary connections, for which only the most proximal segments of the major coronary vessels are considered as stems.
There was no difference in diameter and length distribution between intra-and intercrown for all collateral feeding stem segments as well as intra-and intercoronary collateral connections, which also supports the hypothesis of functional similarity between all types of collateral connections in hearts without perfusion disturbances.
Previous studies have established that cardiac fibres are organized in sheets that spiral along the left ventricular axis (Costa et al. 1999; Lombaert et al. 2012), with a helix angle that changes with transmural depth. While we have no information on the muscle fibre direction of the hearts processed in the present study, our findings are consistent with a preferential alignment of intramural collateral segments along the transmural fibre direction, as demonstrated by Fig. 7A.
Functional and clinical implications
The major function of collateral vessels is to provide alternative pathways for blood flow to tissue territories with partly or completely impeded blood supply. In this context, the intercoronary or intercrown role of a collateral connection is important. However, the amount of tissue coupled to a collateral segment is large in comparison to its length and diameter. The role of collateral segments in compensating regional flow impediment is therefore that of precursor for enlargement of blood channels by outward remodelling, which needs time to be accomplished.
It is indeed remarkable that the median and range of innate collateral diameters are rather similar regardless of the shared crown volume. This supports our general conclusion that the distribution of innate collateral connections is rather ubiquitous over the myocardium. The reason that the collateral diameters are so similar may well be due to the fact that no ischaemic tissue was present in these healthy dog hearts that could have provided a stimulus for local outward remodelling.
For the difference between inter-and intracoronary collaterals in their potential salvaging role, one has to consider the position of a coronary stenosis in the epicardial artery considered, e.g. LCX. If the stenosis is absolutely proximal, the difference between the two types of collateral connections is clear. However, with a more distal position one has to distinguish the perfusion territories proximal and distal to the stenosis. Proximally, the intercoronary connections will not contribute to perfusion of the distal region. However, some collateral connections in this proximal region will change role from intracoronary to intercrown and contribute to perfusion of the ischaemic distal region.
There may be acute functions for innate collateral segments, for instance in preventing micro-infarction and ischaemia, and hence, protecting against local aberrations in electrical conduction (Kaneko et al. 2011). Regional ischaemia may result from local impediment of perfusion by cardiac contraction that may vary over time due to changes in cardiac function. This may explain the higher density of collateral connections in the subendocardium of the left ventricle.
Outward remodelling of intercoronary collateral connections is generally assumed to be related to wall shear stress due to the pressure difference across the collateral vessel induced by an epicardial narrowing. This was clear from early studies on the human heart (Baroldi et al. 1956; Fulton, 1956) and classical studies in dogs (Schaper et al. 1979) and was confirmed in a recent experimental pig model, where collateralization was induced by ameroid occlusion of the LCX (van den Wijngaard et al. 2011). These studies, however, investigated the morphology of the remodelled outcome after epicardial coronary occlusion.
The uniform distribution of native collateral connections appears not to be compatible with the role of shear stress in maintaining and remodelling of collateral vessels. However, pressure differences across collateral segments may also result from asymmetry in the vascular pathways proximal and distal to the collateral connections, leading to local flow heterogeneity (Austin et al. 1990, 1994; Hoffman, 1995) in tissue pieces at a scale corresponding to or smaller than the average volume per intercrown connection found in this study. This finding is also in agreement with the observation that the interface between two perfusion territories as defined by epicardial arteries may receive flow across intercoronary collateral arteries, demonstrated by injecting the LAD and LCX simultaneously with microspheres of different colours (Cicutti et al. 1992).
Early semi-quantitative studies (Baroldi et al. 1956; Laurie & Woods, 1958; Pitt, 1959) suggest that the present finding of ubiquitous distribution of native collateral connections may be extrapolated to the human heart. The dynamic nature of collateral development underlies therapeutic approaches. Fujita et al. (1994) demonstrated that differences in initial collateral presence determine further collateral development. The presence of pre-existing functional coronary arteries was demonstrated in one-fifth to one-quarter of patients with normal coronary arteries (Wustmann et al. 2003), while regression of small collaterals was demonstrated in patients with successful recanalization of a coronary occlusion (Werner et al. 2003). The extent of preformed collaterals was related to cardiovascular parameters (de Marchi et al. 2011). Patient trials in which growth factors were administered to stimulate collateral growth have thus far not shown clinical benefits (Schaper, 2009; van Royen et al. 2010). It is not clear at which moment innate collateral segments develop by angiogenesis or vasculogenesis. However, their ubiquitous presence implies that a substrate for tissue salvage by further arteriogenesis is present at all levels within the coronary circulation. The network implications for collateral stimulation should be explored further in order to understand these findings better. Lack of preferential connectivity also suggests that care should be taken with therapeutic collateral stimulation in areas that are not in need of increased blood supply.
After prolonged cardiac ischaemia and in heart failure, a vascular plexus oriented in the axial direction has been reported (Fulton, 1964; Estes et al. 1966; van den Wijngaard et al. 2010). We observed that the network of collateral vessels in the endocardial layer of the left ventricle is organized parallel to the longitudinal axis of the heart; therefore, our data suggest that the basis for such a plexus is already provided by the pre-existing collateral network.
A final implication for clinical practice may be related to the imaging of coronary perfusion, e.g. by magnetic resonance imaging or positron emission tomography. These imaging methods are far from imaging collateral segments, but perfusion through these segments is certainly feasible, especially with the application of magnetic resonance imaging, by delays in local contrast appearance in ischaemic areas. At present, imaging is mostly limited to planes perpendicular to the left ventricular axis. However, the collateral network contributes to the 3D nature of the intramural vascular network and hence its perfusion. Especially at the subendocardium, the collateral flow component, when present, will be directed parallel to the left ventricular long axis, as demonstrated by our anatomical findings. Hence, our findings advocate the development of 3D myocardial perfusion imaging in order to assess functional consequences of collateral anastomoses.
Study limitations
Naturally, the collateral network in dogs may differ from that in humans, and data on the human innate collateral network should be obtained. The canine heart contains high numbers of innate collaterals in comparison to other species, e.g. the porcine heart (Maxwell et al. 1987); therefore, the canine heart is an excellent model for studying innate collateral connectivity. Innate collateral connections were reported for healthy human hearts, including large artery inter-and intracollateral connections (Baroldi et al. 1956). In addition, the orientation of collateral segments found in the present study corresponds to observations in human hearts, at least in a qualitative way (van den Wijngaard et al. 2010).
Great care was taken to degas all fluids injected into the coronary arteries, and the filling was monitored carefully for the presence of bubbles. Lodging of a small air bubble may prevent complete filling of a major part of the coronary arterial tree distal of such an obstruction. The consequences would be visible from the 3D cast, but were not observed in these data sets. Errors resulting from an imperfect filling will reduce the absolute counts of collateral arteries in areas concerned, but will marginally affect the histograms and relationships presented. Only in heart H4, the septal branch was missing because the infusion cannula was inadvertently inserted past the origin of the septal branch during casting. This heart was excluded from the graphs related to septal branch measurements.
In previous experiments (Spaan et al. 2005; Lagerveld et al. 2010), we established that the cast material does not penetrate vessels smaller than 10 μm in diameter; hence, combined with the lower limit to optical resolution of 20 μm, this forms a limit to the size of the smallest collateral that can be detected. Therefore, the real number of collateral pathways may in fact be higher, because thinner collateral segments in particular may have been missed.
Morphometrically, the collateral segments between branching networks were uniquely defined by the collateral segment having the smallest diameter in a collateral pathway. To check the criterion of smallest diameter in a collateral path, we have inspected subsets of the automatically identified collateral segments manually and did not find any exception to the rule.
Conclusion
Pre-existing coronary collateral arteries in the healthy dog heart are abundant and lack morphological differences based on the location of the connected territories, although the density is highest at the subendocardium in the left ventricular free wall. The occurrence of both intra-and intercoronary collateral arteries and the linear relation between the number of intercrown connections and the tissue weight influenced by them showed that the pre-existing collateral segments are not preferentially located with respect to epicardial arteries prone to coronary artery disease but are evenly distributed. As a result, all areas of the myocardium are equally protected in the event of coronary artery disease.
Key points
Innate collateral arteries provide the biophysical substrate for arteriogenesis. Their distribution and morphology predestine tissue areas salvageable by collateral flow.
Fluorescent episcopic cryomicrotome imaging resulted in a three-dimensional representation of the coronary network, in which collateral segments were automatically identified.
Innate collateral segments are predominantly present in the subendocardium without preferential connectivity within the left ventricular wall of the dog heart and are preferentially oriented perpendicular to the long axis of the heart in the outer layers and parallel to this axis at the subendocardium.
These results suggest that collateral segments are maintained without local hypoxia but because of heterogeneity in pressure gradients in the arterial tree.
The high density and long-axis orientation of collateral arteries in the subendocardial region provide the substrate for arterial plexus formation and indicate the need for three-dimensional perfusion assessment in clinical perfusion imaging.
Acknowledgments
None declared.
Glossary
- Endo
endocardial layer
- Epi
epicardial layer
- LAD
left anterior descending artery
- LCX
left circumflex artery
- LVFW
left ventricular free wall
- Mid
mid-myocardial layer
- RCA
right coronary artery
- RV
right ventricle
- RVW
right ventricular wall
- SB
septal branch
- SEP
septum
- 3D
three-dimensional
Additional Information
Competing interests
None declared.
Author contributions
The experiments were performed at the University Medical Center Utrecht, and hearts were processed and analysed at the Academic Medical Center. All authors contributed to the conception and design of the experiments and data collection. Analysis and interpretation of data as well as drafting and revising the article: P.v.H., J.P.H.M.v.d.W., J.A.E.S. and M.S. All authors approved the final draft of the manuscript.
Funding
This work was supported by the European Community's Seventh Framework Program FP7-ICT grant no. 224495: euHeart (J.A.E.S.), the Netherlands Heart Foundation grant 2006B226 (J.A.E.S. and M.S.) and the Netherlands Organization for Health Research and Development (ZonMw) grant 91105008 (J.A.E.S.). J.P.H.M.v.d.W. was supported by a Veni grant from the Netherlands Organization for Scientific Research (NWO 91611171).
References
- Austin RE, Jr, Aldea GS, Coggins DL, Flynn AE, Hoffman JI. Profound spatial heterogeneity of coronary reserve. Discordance between patterns of resting and maximal myocardial blood flow. Circ Res. 1990;67:319–331. doi: 10.1161/01.res.67.2.319. [DOI] [PubMed] [Google Scholar]
- Austin RE, Jr, Smedira NG, Squiers TM, Hoffman JI. Influence of cardiac contraction and coronary vasomotor tone on regional myocardial blood flow. Am J Physiol Heart Circ Physiol. 1994;266:H2542–2553. doi: 10.1152/ajpheart.1994.266.6.H2542. [DOI] [PubMed] [Google Scholar]
- Baroldi G, Mantero O, Scomazzoni G. The collaterals of the coronary arteries in normal and pathologic hearts. Circ Res. 1956;4:223–229. doi: 10.1161/01.res.4.2.223. [DOI] [PubMed] [Google Scholar]
- Blair E. Anatomy of the ventricular coronary arteries in the dog. Circ Res. 1961;9:333–341. [Google Scholar]
- Bloor CM, White FC. Functional development of the coronary collateral circulation during coronary artery occlusion in the conscious dog. Am J Pathol. 1972;67:483–500. [PMC free article] [PubMed] [Google Scholar]
- Brazzamano S, Fedor JM, Rembert JC, Greenfield JC., Jr Collateral conductance changes during a brief coronary occlusion in awake dogs. Circulation. 1985;72:225–232. doi: 10.1161/01.cir.72.1.225. [DOI] [PubMed] [Google Scholar]
- Cicutti N, Rakusan K, Downey HF. Colored microspheres reveal interarterial microvascular anastomoses in canine myocardium. Basic Res Cardiol. 1992;87:400–409. doi: 10.1007/BF00796525. [DOI] [PubMed] [Google Scholar]
- Costa KD, Takayama Y, McCulloch AD, Covell JW. Laminar fiber architecture and three-dimensional systolic mechanics in canine ventricular myocardium. Am J Physiol Heart Circ Physiol. 1999;276:H595–H607. doi: 10.1152/ajpheart.1999.276.2.H595. [DOI] [PubMed] [Google Scholar]
- de Marchi SF, Gloekler S, Meier P, Traupe T, Steck H, Cook S, Vogel R, Seiler C. Determinants of preformed collateral vessels in the human heart without coronary artery disease. Cardiology. 2011;118:198–206. doi: 10.1159/000328648. [DOI] [PubMed] [Google Scholar]
- Estes EH, Jr, Entman ML, Dixon HB, 2nd, Hackel DB. The vascular supply of the left ventricular wall. Anatomic observations, plus a hypothesis regarding acute events in coronary artery disease. Am Heart J. 1966;71:58–67. doi: 10.1016/0002-8703(66)90657-0. [DOI] [PubMed] [Google Scholar]
- Fujita M, Yamanishi K, Araie E, Sasayama S, McKown DP, Franklin D. Determinants of collateral development in a canine model with repeated coronary occlusion. Heart Vessels. 1994;9:292–299. doi: 10.1007/BF01745094. [DOI] [PubMed] [Google Scholar]
- Fulton WF. Chronic generalized myocardial ischaemia with advanced coronary artery disease. Br Heart J. 1956;18:341–354. doi: 10.1136/hrt.18.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton WF. The dynamic factor in enlargement of coronary arterial anastomoses, and paradoxical changes in the subendocardial plexus. Br Heart J. 1964;26:39–50. doi: 10.1136/hrt.26.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geenen T, Horssen P, Spaan JE, Siebes M, Wijngaard JHM. Iterative deblurring of large 3D datasets from cryomicrotome imaging using an array of GPUs. In: Yuen DA, Wang L, Chi X, Johnsson L, Ge W, Shi Y, editors. GPU Solutions to Multi-scale Problems in Science and Engineering. Berlin, Heidelberg: Springer; 2013. pp. 573–585. [Google Scholar]
- Gibson CM, Ryan K, Sparano A, Moynihan JL, Rizzo M, Kelley M, Marble SJ, Laham R, Simons M, McClusky TR, Dodge JT., Jr Angiographic methods to assess human coronary angiogenesis. Am Heart J. 1999;137:169–179. doi: 10.1016/s0002-8703(99)70473-4. [DOI] [PubMed] [Google Scholar]
- Grayson J, Davidson JW, Fitzgerald-Finch A, Scott C. The functional morphology of the coronary microcirculation in the dog. Microvasc Res. 1974;8:20–43. doi: 10.1016/0026-2862(74)90061-2. [DOI] [PubMed] [Google Scholar]
- Hoffman JI. Heterogeneity of myocardial blood flow. Basic Res Cardiol. 1995;90:103–111. doi: 10.1007/BF00789440. [DOI] [PubMed] [Google Scholar]
- Ito WD, Arras M, Scholz D, Winkler B, Htun P, Schaper W. Angiogenesis but not collateral growth is associated with ischemia after femoral artery occlusion. Am J Physiol Heart Circ Physiol. 1997;273:H1255–H1265. doi: 10.1152/ajpheart.1997.273.3.H1255. [DOI] [PubMed] [Google Scholar]
- Kaneko N, Matsuda R, Toda M, Shimamoto K. Three-dimensional reconstruction of the human capillary network and the intramyocardial micronecrosis. Am J Physiol Heart Circ Physiol. 2011;300:H754–H761. doi: 10.1152/ajpheart.00486.2010. [DOI] [PubMed] [Google Scholar]
- Karch R, Neumann F, Neumann M, Szawlowski P, Schreiner W. Voronoi polyhedra analysis of optimized arterial tree models. Ann Biomed Eng. 2003;31:548–563. doi: 10.1114/1.1566444. [DOI] [PubMed] [Google Scholar]
- Kato T. A comparative study of the coronary arterial structure in the left ventricular free wall in infarcted and non-infarcted human hearts. Jpn Circ J. 1976;40:989–1003. doi: 10.1253/jcj.40.989. [DOI] [PubMed] [Google Scholar]
- Lagerveld BW, van Horssen P, Pes MP, van den Wijngaard JP, Streekstra GJ, de la Rosette JJ, Wijkstra H, Spaan JA. Immediate effect of kidney cryoablation on renal arterial structure in a porcine model studied by imaging cryomicrotome. J Urol. 2010;183:1221–1226. doi: 10.1016/j.juro.2009.11.064. [DOI] [PubMed] [Google Scholar]
- Laurie W, Woods JD. Anastomosis in the coronary circulation. Lancet. 1958;2:812–816. doi: 10.1016/s0140-6736(58)90374-x. [DOI] [PubMed] [Google Scholar]
- Lombaert H, Peyrat JM, Croisille P, Rapacchi S, Fanton L, Cheriet F, Clarysse P, Magnin I, Delingette H, Ayache N. Human atlas of the cardiac fiber architecture: study on a healthy population. IEEE Trans Med Imaging. 2012;31:1436–1447. doi: 10.1109/TMI.2012.2192743. [DOI] [PubMed] [Google Scholar]
- Maxwell MP, Hearse DJ, Yellon DM. Species variation in the coronary collateral circulation during regional myocardial ischaemia: a critical determinant of the rate of evolution and extent of myocardial infarction. Cardiovasc Res. 1987;21:737–746. doi: 10.1093/cvr/21.10.737. [DOI] [PubMed] [Google Scholar]
- Meier P, Gloekler S, Zbinden R, Beckh S, de Marchi SF, Zbinden S, Wustmann K, Billinger M, Vogel R, Cook S, Wenaweser P, Togni M, Windecker S, Meier B, Seiler C. Beneficial effect of recruitable collaterals: a 10-year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation. 2007;116:975–983. doi: 10.1161/CIRCULATIONAHA.107.703959. [DOI] [PubMed] [Google Scholar]
- Meier P, Hemingway H, Lansky AJ, Knapp G, Pitt B, Seiler C. The impact of the coronary collateral circulation on mortality: a meta-analysis. Eur Heart J. 2012;33:614–621. doi: 10.1093/eurheartj/ehr308. [DOI] [PubMed] [Google Scholar]
- Menick FJ, White FC, Bloor CM. Coronary collateral circulation: determination of an anatomical anastomotic index of functional collateral flow capacity. Am Heart J. 1971;82:503–510. doi: 10.1016/0002-8703(71)90235-3. [DOI] [PubMed] [Google Scholar]
- Palagyi K, Kuba A. A 3D 6-subiteration thinning algorithm for extracting medial lines. Pattern Recognit Lett. 1998;19:613–627. [Google Scholar]
- Piek JJ, Koolen JJ, Metting van Rijn AC, Bot H, Hoedemaker G, David GK, Dunning AJ, Spaan JA, Visser CA. Spectral analysis of flow velocity in the contralateral artery during coronary angioplasty: a new method for assessing collateral flow. J Am Coll Cardiol. 1993;21:1574–1582. doi: 10.1016/0735-1097(93)90371-7. [DOI] [PubMed] [Google Scholar]
- Pipp F, Boehm S, Cai WJ, Adili F, Ziegler B, Karanovic G, Ritter R, Balzer J, Scheler C, Schaper W, Schmitz-Rixen T. Elevated fluid shear stress enhances postocclusive collateral artery growth and gene expression in the pig hind limb. Arterioscler Thromb Vasc Biol. 2004;24:1664–1668. doi: 10.1161/01.ATV.0000138028.14390.e4. [DOI] [PubMed] [Google Scholar]
- Pitt B. Interarterial coronary anastomoses. Occurrence in normal hearts and in certain pathologic conditions. Circulation. 1959;20:816–822. doi: 10.1161/01.cir.20.5.816. [DOI] [PubMed] [Google Scholar]
- Robbins SL, Solomon M, Bennett A. Demonstration of intercoronary anastomoses in human hearts with a low viscosity perfusion mass. Circulation. 1966;33:733–743. doi: 10.1161/01.cir.33.5.733. [DOI] [PubMed] [Google Scholar]
- Rolf MP, ter Wee R, van Leeuwen TG, Spaan JA, Streekstra GJ. Diameter measurement from images of fluorescent cylinders embedded in tissue. Med Biol Eng Comput. 2008;46:589–596. doi: 10.1007/s11517-008-0328-9. [DOI] [PubMed] [Google Scholar]
- Schaper W. Collateral circulation: past and present. Basic Res Cardiol. 2009;104:5–21. doi: 10.1007/s00395-008-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaper W, Frenzel H, Hort W, Winkler B. Experimental coronary artery occlusion. II. Spatial and temporal evolution of infarcts in the dog heart. Basic Res Cardiol. 1979;74:233–239. doi: 10.1007/BF01907740. [DOI] [PubMed] [Google Scholar]
- Scheel KW, Daulat G, Williams SE. Functional anatomical site of intramural collaterals in dogs. Am J Physiol Heart Circ Physiol. 1990;259:H706–H711. doi: 10.1152/ajpheart.1990.259.3.H706. [DOI] [PubMed] [Google Scholar]
- Seiler C. The human coronary collateral circulation. Eur J Clin Invest. 2010;40:465–476. doi: 10.1111/j.1365-2362.2010.02282.x. [DOI] [PubMed] [Google Scholar]
- Seiler C, Fleisch M, Garachemani A, Meier B. Coronary collateral quantitation in patients with coronary artery disease using intravascular flow velocity or pressure measurements. J Am Coll Cardiol. 1998;32:1272–1279. doi: 10.1016/s0735-1097(98)00384-2. [DOI] [PubMed] [Google Scholar]
- Spaan JA, ter Wee R, van Teeffelen JW, Streekstra G, Siebes M, Kolyva C, Vink H, Fokkema DS, VanBavel E. Visualisation of intramural coronary vasculature by an imaging cryomicrotome suggests compartmentalisation of myocardial perfusion areas. Med Biol Eng Comput. 2005;43:431–435. doi: 10.1007/BF02344722. [DOI] [PubMed] [Google Scholar]
- Traupe T, Gloekler S, de Marchi SF, Werner GS, Seiler C. Assessment of the human coronary collateral circulation. Circulation. 2010;122:1210–1220. doi: 10.1161/CIRCULATIONAHA.109.930651. [DOI] [PubMed] [Google Scholar]
- van den Wijngaard JP, Schulten H, van Horssen P, Ter Wee RD, Siebes M, Post MJ, Spaan JA. Porcine coronary collateral formation in the absence of a pressure gradient remote of the ischemic border zone. Am J Physiol Heart Circ Physiol. 2011;300:H1930–H1937. doi: 10.1152/ajpheart.00403.2010. [DOI] [PubMed] [Google Scholar]
- van den Wijngaard JP, Schwarz JC, van Horssen P, van Lier MG, Dobbe JG, Spaan JA, Siebes M. 3D Imaging of vascular networks for biophysical modeling of perfusion distribution within the heart. J Biomech. 2013;46:229–239. doi: 10.1016/j.jbiomech.2012.11.027. [DOI] [PubMed] [Google Scholar]
- van den Wijngaard JP, van Horssen P, ter Wee R, Coronel R, de Bakker JM, de Jonge N, Siebes M, Spaan JA. Organization and collateralization of a subendocardial plexus in end-stage human heart failure. Am J Physiol Heart Circ Physiol. 2010;298:H158–H162. doi: 10.1152/ajpheart.00654.2009. [DOI] [PubMed] [Google Scholar]
- van Royen N, Piek JJ, Schaper W, Fulton WF. A critical review of clinical arteriogenesis research. J Am Coll Cardiol. 2010;55:17–25. doi: 10.1016/j.jacc.2009.06.058. [DOI] [PubMed] [Google Scholar]
- Vogel R, Zbinden R, Indermühle A, Windecker S, Meier B, Seiler C. Collateral-flow measurements in humans by myocardial contrast echocardiography: validation of coronary pressure-derived collateral-flow assessment. Eur Heart J. 2006;27:157–165. doi: 10.1093/eurheartj/ehi585. [DOI] [PubMed] [Google Scholar]
- von Mutius S, Neumann M, Meesmann W. Early changes in collateral blood flow to ischemic myocardium and their influence on bimodal vulnerability during the first 30 min of acute coronary artery occlusion in dogs. Basic Res Cardiol. 1988;83:94–106. doi: 10.1007/BF01907109. [DOI] [PubMed] [Google Scholar]
- Werner GS, Emig U, Mutschke O, Schwarz G, Bahrmann P, Figulla HR. Regression of collateral function after recanalization of chronic total coronary occlusions: a serial assessment by intracoronary pressure and Doppler recordings. Circulation. 2003;108:2877–2882. doi: 10.1161/01.CIR.0000100724.44398.01. [DOI] [PubMed] [Google Scholar]
- Wustmann K, Zbinden S, Windecker S, Meier B, Seiler C. Is there functional collateral flow during vascular occlusion in angiographically normal coronary arteries? Circulation. 2003;107:2213–2220. doi: 10.1161/01.CIR.0000066321.03474.DA. [DOI] [PubMed] [Google Scholar]


