Abstract
Irisin was identified as a myokine secreted by contracting skeletal muscle, possibly mediating some exercise health benefits via ‘browning’ of white adipose tissue. However, a controversy exists concerning irisin origin, regulation and function in humans. Thus, we have explored Fndc5 gene and irisin protein in two clinical studies: (i) a cross-sectional study (effects of type 2 diabetes (T2D) in drug-naive men) and (ii) an intervention study (exercise effects in sedentary, overweight/obese individuals). Glucose tolerance and insulin sensitivity were assessed. Maximal aerobic capacity and muscle strength were measured before and after training. Body composition (magnetic resonance imaging), muscle and liver fat content (1H-magnetic resonance spectroscopy (MRS)) and in vivo muscle metabolism (32P-MRS) were determined. Skeletal muscle and subcutaneous abdominal adipose tissue samples were taken in the fasted state and during euglycaemic hyperinsulinaemia (adipose tissue) and before/after exercise training (muscle). We found that muscle Fndc5 mRNA was increased in prediabetes but not T2D. Fndc5 in adipose tissue and irisin in plasma were reduced in T2D by 40% and 50%, respectively. In contrast, T2D-derived myotubes expressed/secreted the highest levels of Fndc5/irisin. Neither hyperinsulinaemia (adipose tissue/plasma) nor exercise (muscle/plasma) affected Fndc5/irisin in vivo. Circulating irisin was positively associated with muscle mass, strength and metabolism and negatively with fasting glycaemia. Glucose and palmitate decreased Fndc5 mRNA in myotubes in vitro. We conclude that distinct patterns of Fndc5/irisin in muscle, adipose tissue and circulation, and concordant in vivo down-regulation in T2D, indicate that irisin might distinguish metabolic health and disease. Moreover, Fndc5/irisin was discordantly regulated in diabetic muscle and myotubes in vitro, suggesting that whole body factors, such as glucose and fatty acids, might be important for irisin regulation. Exercise did not affect Fndc5/irisin. However, irisin was positively linked to muscle mass, strength and metabolism, pointing to common regulatory factors and/or the potential for irisin to modify muscle phenotype.
Introduction
Regular exercise is known to have substantial positive effects on the whole body metabolic health. This beneficial impact is at least to some extent mediated by skeletal muscle which is, with respect to its size and ability to utilize large amounts of energy, an important player in the whole body energy homeostasis. Over the last decade, another level of complexity has been revealed. Contracting skeletal muscle secretes the spectrum of bioactive molecules, ‘myokines’, coordinating the energy flow needed to sustain the muscle activity as well as stimulating adaptive plasticity of muscle and multiple tissues and organs in response to repeated exercise stimulus (Pedersen & Febbraio, 2008). One of the key molecules orchestrating many exercise-induced changes, including mitochondrial biogenesis and/or adipose tissue browning, is a transcription cofactor, peroxisome proliferator-activated receptor-γ coactivator 1α (PGC1α; Boström et al. 2012; Spiegelman, 2013). Interestingly, a link between myokines and PGC1α was recently discovered in the form of the new attractive PGC1α-driven myokine, irisin. Irisin was identified as a proteolytic cleavage product of the fibronectin type III domain-containing protein 5 (FNDC5), a transmembrane protein present mainly in skeletal muscle (Boström et al. 2012). In the original report by Boström et al., FNDC5/irisin was induced by both Pgc1α overexpression and physical activity in mice, and circulating irisin was shown to increase in humans in response to exercise (Boström et al. 2012). The evidence from in vitro and animal studies suggested the induction of the brown fat-like phenotype by FNDC5/irisin (Boström et al. 2012). Furthermore, an overexpression of Fndc5 gene in animal models of obesity resulted in marked up-regulation of uncoupling protein 1 (UCP1) and several mitochondrial genes, increase in oxygen consumption, amelioration of glucose tolerance and reduction of insulinaemia, demonstrating a greatly improved metabolic profile most likely via elevated energy expenditure (Boström et al. 2012). However, conflicting data on the role of exercise in the regulation of irisin in humans have recently emerged. The original study by Boström et al. showed an up-regulation of irisin by exercise in humans (Boström et al. 2012). However, other studies observed an acute exercise-induced increase in muscle Fndc5 mRNA only in sedentary seniors (Timmons et al. 2012) and in a small cohort of young healthy untrained individuals (Huh et al. 2012) completely failing to demonstrate the induction of Fndc5/irisin in muscle or circulation by prolonged exercise training (Huh et al. 2012; Timmons et al. 2012). Controversy exists regarding a putative role of irisin in the protection against obesity-related metabolic disease. Two independent studies showed a reduction of circulating irisin in both lean (Choi et al. 2013) and obese (Moreno-Navarrete et al. 2013) patients with type 2 diabetes. However, a few recent studies observed positive associations of muscle Fndc5 with insulin resistance (Huh et al. 2012; Roca-Rivada et al. 2013; Stengel et al. 2013), speculating on the negative, desensitizing effects of irisin on insulin action. The sources and mechanisms regulating the circulating irisin in humans also remain obscure. Although skeletal muscle, liver, heart and brain are tissues with the highest levels of Fndc5 expression in humans and animal models, other tissues might still contribute to the circulating irisin pool (Teufel et al. 2002; Huh et al. 2012). It has been shown in both animals and humans that irisin is expressed and secreted by adipose tissue (Moreno-Navarrete et al. 2013; Roca-Rivada et al. 2013). In humans, adipose tissue Fndc5 expression seems to represent only a fraction of that expressed in muscle, but it is adipose tissue not skeletal muscle expression that correlates with levels of circulating irisin (Moreno-Navarrete et al. 2013). The regulation of Fndc5/irisin production and the function of irisin in modulating metabolic disease progression in humans is far from being elucidated. The principal aim of our work was to study the Fndc5 gene expression and irisin secretion in human skeletal muscle, human primary muscle cells and in subcutaneous adipose tissue from lean, obese, prediabetic and type 2 diabetic individuals. Irisin content was assessed in plasma and in muscle cell conditioned media. We have also explored the regulation of Fndc5 expression and irisin secretion by insulin (euglycaemic–hyperinsulinaemic clamp) and by acute and regular exercise. In an in vitro system we evaluated the possible regulatory role of saturated fatty acids and glucose (diabetes-mimicking treatment). With this complex approach we aim to gain a better understanding of the role of irisin in the human pathophysiology of metabolic disease.
Methods
Ethical approval
All studies were approved by the ethics committee of the University Hospital Bratislava, Comenius University Bratislava and/or the Ethics Committee of the Bratislava Region Office and conform to the ethical guidelines of the Declaration of Helsinki 2000. All participants provided witnessed written informed consent prior to entering the study.
Study design
Study in sedentary men with obesity and type 2 diabetes (Study 1)
Ninety-nine middle-aged sedentary men were recruited and classified into groups of (i) lean healthy (control) individuals (n = 29), (ii) healthy overweight/obese patients (n = 29), (iii) patients with impaired glucose tolerance (prediabetes; n = 25), and (iv) those with newly diagnosed yet untreated type 2 diabetes (n = 16). The obesity level in the last three groups was comparable. Individuals with any chronic disease or regular use of pharmacotherapy were not eligible to enter the study. The characteristics of the study population are summarized in Table 1.
Table 1.
Characteristics of Study 1 population
| Lean (n = 29) | Obese (n = 29) | Prediabetic (n = 25) | T2D (n = 16) | |
|---|---|---|---|---|
| Age (years) | 37.3 ± 1.8 | 37.6 ± 1.4 | 44.0 ± 2.0* | 49.6 ± 2.1*‡ |
| Weight (kg) | 75.2 ± 1.8 | 99.2 ± 2.6* | 101.4 ± 3.1* | 99.3 ± 3.5* |
| BMI (kg m–2) | 23.3 ± 0.4 | 30.4 ± 0.5* | 31.0 ± 0.7* | 31.2 ± 1.0* |
| Waist (cm) | 87.9 ± 1.6 | 105.6 ± 1.5* | 110.0 ± 2.1* | 108.2 ± 3.1* |
| Body fat (%) | 18.6 ± 0.9 | 28.6 ± 0.8* | 29.7 ± 1.0* | 29.9 ± 1.4* |
| Lean body mass (kg) | 61.1 ± 1.3 | 70.5 ± 1.4* | 70.5 ± 1.6* | 70.2 ± 1.6* |
| Adipocyte diameter (μm) | 94.9 ± 2.8 | 118.0 ± 2.1* | 117.8 ± 2.9* | 118.8 ± 4.0* |
| Subcutaneous AT (cm2) | 137.1 ± 10.5 | 277.8 ± 14.8* | 310.2 ± 21.4* | 337.5 ± 33.9* |
| Visceral AT (cm2) | 62.1 ± 2.8 | 78.3 ± 3.9 | 94.0 ± 7.4* | 126.6 ± 7.4*‡† |
| Hepatic lipids (% of water resonance) | 1.29 ± 0.23 | 5.45 ± 1.36 | 17.65 ± 3.92*‡ | 22.24 ± 5.84*‡ |
| IMCL (relative to creatine) | 1.13 ± 0.14 | 1.24 ± 0.15 | 1.68 ± 0.36 | 1.21 ± 0.25 |
| EMCL (relative to creatine) | 4.08 ± 0.66 | 5.52 ± 0.59 | 6.65 ± 1.13 | 7.91 ± 1.73* |
| Fasting glucose (mmol l–1) | 4.7 ± 0.1 | 4.9 ± 0.1 | 5.7 ± 0.1* | 8.9 ± 0.7*‡† |
| 2 h glucose (mmol l–1) | 5.4 ± 0.2 | 5.4 ± 0.3 | 8.1 ± 0.3*‡ | 13.6 ± 1.1*‡† |
| M-value (mg kg−1 min−1) | 8.18 ± 0.46 | 4.90 ± 0.32* | 3.89 ± 0.42* | 3.06 ± 0.56 ‡* |
| M-value/insulin (mg kg−1 min−1 per μU ml−1 of insulin) | 0.15 ± 0.01 | 0.08 ± 0.01* | 0.08 ± 0.01* | 0.06 ± 0.01* |
| Triglycerides (mmol l–1) | 1.08 ± 0.09 | 1.43 ± 0.13 | 1.91 ± 0.20* | 2.15 ± 0.33*‡ |
| Cholesterol (mmol l–1) | 4.23 ± 0.15 | 4.58 ± 0.15 | 5.02 ± 0.24* | 5.22 ± 0.20* |
| HDL (mmol l–1) | 1.37 ± 0.05 | 1.25 ± 0.05 | 1.28 ± 0.05 | 1.27 ± 0.06 |
| FFAs (mmol l–1) | 0.58 ± 0.04 | 0.61 ± 0.03 | 0.70 ± 0.04 | 0.65 ± 0.07 |
| FFAs SS-clamp (mmol l–1) | 0.12 ± 0.01 | 0.15 ± 0.01 | 0.17 ± 0.02 | 0.17 ± 0.03 |
| Fasting insulin (μU ml−1) | 4.99 ± 0.71 | 10.92 ± 1.72* | 12.24 ± 1.58* | 13.34 ± 2.08* |
Data are expressed as mean ± SEM.
Significant compared to lean.
Significant compared to obese.
Significant compared to prediabetic group. Comparisons between groups were assessed by Tukey's HSD. P < 0.05. AT, adipose tissue; EMCL, extramyocellular lipids; FFAs, free fatty acids; FFAs SS-clamp, FFAs measured in a steady state of the euglycaemic–hyperinsulinaemic clamp; IMCL, intramyocellular lipids; 2 h glucose, serum glucose in oral glucose tolerance test; M-value, insulin sensitivity index measured by clamp.
Exercise intervention study (Study 2)
Sixteen sedentary overweight/obese individuals (10/6 male/female; age: 36.5 ± 1.1 years; body mass index (BMI): 31.8 ± 0.6 kg m−2; homeostasis model of assessment, insulin resistance (HOMA-IR): 2.99 ± 0.81; maximal aerobic capacity (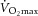 ): 31.79 ± 2.75 ml O2 min−1 (kg lean body mass (LBM))−1) completed a 12 week strength/endurance training programme, designed and supervised by exercise professionals. Exercise intervention resulted in a 25% increase in
): 31.79 ± 2.75 ml O2 min−1 (kg lean body mass (LBM))−1) completed a 12 week strength/endurance training programme, designed and supervised by exercise professionals. Exercise intervention resulted in a 25% increase in 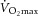 (P < 0.01) and, in accordance with the original study design, it produced no change in BMI. Fasting and 2 h plasma glucose or insulin resistance index (HOMA-IR) remained within the normal range. Supervised training sessions lasting 1 h, preceded by 10 min warm-up and followed by cool-down and stretching exercises, were performed 3 times per week, with strength and endurance components equally introduced during the training. The backbone of the training programme was the circuit training, combining strength and aerobic activities, which was specifically designed for each participant according to maximum heart rate, one repetition maximum (1RM) or maximum voluntary contraction force, and aimed at improving the performance of the major muscle groups. It consisted of upper-body exercises (squat-ups, bench dips, back extensions, bench lift, inclined press-up), core and trunk exercises (sit ups – lower abdominals, stomach crunch – upper abdominals, back extension, chest raise), lower-body exercises (squat jumps, astride jumps, step-ups, bench squats) and total-body exercises (burpees, squat thrusts, squat press, skipping), and was supplemented by sessions of aerobic dance, running and spinning. Aerobic performance and cardiovascular health status were assessed by a cardiologist in the recruitment process, and after completing the 3 month exercise intervention. Maximal aerobic capacity (
(P < 0.01) and, in accordance with the original study design, it produced no change in BMI. Fasting and 2 h plasma glucose or insulin resistance index (HOMA-IR) remained within the normal range. Supervised training sessions lasting 1 h, preceded by 10 min warm-up and followed by cool-down and stretching exercises, were performed 3 times per week, with strength and endurance components equally introduced during the training. The backbone of the training programme was the circuit training, combining strength and aerobic activities, which was specifically designed for each participant according to maximum heart rate, one repetition maximum (1RM) or maximum voluntary contraction force, and aimed at improving the performance of the major muscle groups. It consisted of upper-body exercises (squat-ups, bench dips, back extensions, bench lift, inclined press-up), core and trunk exercises (sit ups – lower abdominals, stomach crunch – upper abdominals, back extension, chest raise), lower-body exercises (squat jumps, astride jumps, step-ups, bench squats) and total-body exercises (burpees, squat thrusts, squat press, skipping), and was supplemented by sessions of aerobic dance, running and spinning. Aerobic performance and cardiovascular health status were assessed by a cardiologist in the recruitment process, and after completing the 3 month exercise intervention. Maximal aerobic capacity (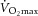 ) was calculated from the continuous measurement of gas exchange (Ergostik, Geratherm Respiratory, Bad Kissingen, Germany) during an incremental exercise test (Lode-Corival cycle ergometer, Lode B.V., Groningen, The Netherlands) and expressed relative to lean body mass. The intensity of aerobic exercises was monitored and evaluated by a Polar RS300X heart rate monitor (Polar, Finland) during each session. Intensity was maintained at 70–85% of maximal heart rate. After initial strength testing with the computer-controlled dynamometer (horizontal leg-press developed at the Faculty of Physical Education and Sports, Comenius University), training was initiated by using 50–60% of the 1RM. Load increased in the course of the training study progressively and according to the individual initial performance level, approximately 2.5% 1RM per week. Adherence to the training programme was closely monitored and regularly encouraged. Clinical phenotyping was performed before and after training intervention. The dynamic response of sedentary and trained individuals to a single exercise bout (1 h 75% of maximal capacity) was determined. Participants were instructed not to change their eating habits in the course of the study. The individual food preference was evaluated by a validated questionnaire (Geiselman et al. 1998).
) was calculated from the continuous measurement of gas exchange (Ergostik, Geratherm Respiratory, Bad Kissingen, Germany) during an incremental exercise test (Lode-Corival cycle ergometer, Lode B.V., Groningen, The Netherlands) and expressed relative to lean body mass. The intensity of aerobic exercises was monitored and evaluated by a Polar RS300X heart rate monitor (Polar, Finland) during each session. Intensity was maintained at 70–85% of maximal heart rate. After initial strength testing with the computer-controlled dynamometer (horizontal leg-press developed at the Faculty of Physical Education and Sports, Comenius University), training was initiated by using 50–60% of the 1RM. Load increased in the course of the training study progressively and according to the individual initial performance level, approximately 2.5% 1RM per week. Adherence to the training programme was closely monitored and regularly encouraged. Clinical phenotyping was performed before and after training intervention. The dynamic response of sedentary and trained individuals to a single exercise bout (1 h 75% of maximal capacity) was determined. Participants were instructed not to change their eating habits in the course of the study. The individual food preference was evaluated by a validated questionnaire (Geiselman et al. 1998).
Clinical phenotyping
Body weight and height were used to calculate BMI (kg. m−2). Waist circumference was measured at the midpoint between the lower border of the rib cage and the iliac crest. Bioelectric impedance was used to evaluate total and visceral adiposity and to estimate lean body mass (Omron BF511, Omron Healthcare Ltd, Matsusaka, Japan). Resting energy expenditure was measured after an overnight fast and following 30 min bed rest with the Ergostik for a period of 30 min. Volume and intensity of daily ambulatory activity were determined with accelerometers (Lifecorder PLUS) used during 3 consecutive working days (Study 1) or continually within the period of the 3 month exercise intervention (Study 2). Medium and high intensity habitual free-living ambulatory activity was defined as the sum of the daily life activities requiring more energy than 3 times the resting metabolic rate (>3METs).
Magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS) studies
Abdominal fat distribution was measured in all individuals (both studies) by MRI using GRE sequence, TR 134 ms, TE 2.38/5.24 ms, on a 1.5 T Magnetom Symphony MRI scanner (Siemens, Germany). The volume of visceral and subcutaneous abdominal adipose tissue depots was evaluated from five consecutive slices centred between the L4 and L5 vertebrae. Imaging of the calf muscle enabled the exact calculation of muscle volume and spectra from tibialis anterior (postural oxidative) muscle acquired by using a point-resolved 1H-MRS (PRESS) sequence with TR/TE/ACQ = 2000 ms/270 ms/256. Volumes of interest (VOI) = 1.5 cm × 1.5 cm × 1.5 cm were used to assess the content of intra-and extracellular lipids. Hepatic lipid content was determined by a similar 1H-MRS technique: TR/TE/ACQ = 4000 ms/30 ms/4; VOI = 3 cm × 3 cm × 3 cm. The amount of lipid is expressed as a ratio to creatine signal in the case of muscle spectra or as a ratio to whole MRS signal in the case of liver. Spectra were processed by jMRUI 3.0 software.
In 10 individuals from Study 2, a 31P-MRS ‘magnetization transfer experiment’ was performed on a 7T scanner (Magnetom, Siemens Healthcare, Erlangen, Germany) before and after the 3 month training. Resting-state inorganic phosphate (Pi)-to-ATP flux was measured using a magnetization transfer experiment as described in (Valkovic et al. 2013). Baseline intramyocellular concentrations of phosphorous metabolites were assessed. The unidirectional flux between phosphocreatine (PCr) and γ-ATP (i.e. creatine kinase reaction) and between Pi and γ-ATP (i.e. ATP flux) was investigated (Brindle et al. 1989; Lebon et al. 2001).
Euglycaemic–hyperinsulinaemic clamp and oral glucose tolerance test
Insulin sensitivity was determined by the euglycaemic–hyperinsulinaemic clamp (clamp) in the morning after an overnight fast and after having abstained from alcohol and moderate/vigorous physical activity for at least 48 h. Two intravenous cannulas were inserted into the antecubital veins of both arms, one for administration of insulin and 20% glucose with KCl and the other (contralateral arm) for frequent blood sampling. The hand was kept warm to ‘arterialize’ blood samples. A primed (80 mU m−2 min−1) continuous (40 mU m−2 min−1) insulin (Actrapid, 100 IU ml−1; Novo Nordisk, Denmark) infusion was used to achieve hyperinsulinaemia. Blood glucose was measured in 5 min intervals and maintained at euglycaemia (5 ± 0.25 mmol l–1) using a variable infusion rate of 20% glucose. The whole body insulin sensitivity index (M-value/insulin) was calculated from the steady-state plasma glucose infusion rate required to maintain euglycaemia, expressed per kilogram body weight per minute and normalized to the average steady-state insulinaemia (μU ml−1) of four samples taken during the last 60 min of the 150 min clamp.
For the oral glucose tolerance test, an indwelling catheter (Surflo-W, Therumo, Belgium) was placed into the antecubital vein after an overnight fast. Blood samples were drawn before (fasted state, 0 min) and 30, 60, 90 and 120 min after ingestion of 75 g glucose and used to determine levels of circulating glucose (all samples), insulin, total and high-density lipoprotein (HDL) cholesterol, triglycerides and free fatty acids (in the fasted state).
Indirect calorimetry
Resting metabolic rate as well as the insulin-induced change in metabolic substrate preference (Δ respiratory quotient (RQ)) were calculated from the two 25 min measurements of O2 and CO2 in exhaled air with the aid of the Ergostik. A first measurement in the fasting state was followed by a second measurement in the steady state of euglycaemic hyperinsulinaemia. Basal metabolic rate was expressed relative to lean body mass.
Adipose tissue collection and isolation of adipocytes
Subcutaneous adipose tissue (AT) samples were taken by needle biopsy from the abdominal region in the fasted state and during steady state of the clamp as described previously (Ukropec et al. 2008). Samples were immediately cleaned of blood and connective tissue and (i) frozen in liquid nitrogen or (ii) used to isolate adipocytes and stromal fraction by collagenase digestion. Adipocyte diameter was determined by analysis of images obtained by light microscopy with the aid of ImageTool software (UTHSCSA, USA). The mean diameter of at least 100 cells from each adipocyte suspension was calculated. Tissue samples and freshly isolated adipocytes were immediately frozen in liquid nitrogen and stored at –80°C.
Skeletal muscle biopsy
Samples of skeletal muscle (vastus lateralis) were taken by Bergström needle biopsy under local anaesthesia in the fasted state (Study 1). In the exercise intervention study (Study 2), the sampling was performed in the fasted state before and after the 3 month training. Eight out of 16 participants also provided muscle samples after an acute exercise bout, with the second biopsy performed within the 60–70 min interval post-exercise. Muscle samples were immediately blotted from excessive blood, frozen in liquid nitrogen and stored at −80°C. A small piece of muscle (approx. 80 mg) was used to establish human primary skeletal muscle cultures (Study 1).
Primary human skeletal muscle cell culture
Satellite cells (quiescent mononuclear muscle cells) were isolated by trypsin digestion of the freshly obtained muscle, pre-plated on an uncoated Petri dish for 1 h to remove adherent cells (fibroblasts), and subsequently transferred to T-25 collagen-coated flasks with growth medium composed of Dulbecco's modified Eagle's medium (DMEM) with 15% fetal bovine serum (FBS), human epidermal growth factor, bovine serum albumin (BSA), dexamethasone, gentamycin, fungizone and fetuin, as previously described (Ukropcova et al. 2005). Cells were passaged once, harvested and stored frozen in liquid nitrogen. For Experiment 2, cells were grown, harvested and immunopurified with the aid of the mouse monoclonal anti-CD56 (Neural Cell Adhesion Molecule 1) antibody (clone 5.1H11, Developmental Studies Hybridoma Bank, The University of Iowa, Iowa City, USA) and magnetic beads attached secondary antibody (Miltenyi Biotech, Germany) as previously described (Sparks et al. 2011).
Experiment 1. Saturated fatty acid and glucose treatments of human primary muscle cells
Muscle cells from lean (n = 6) and age-matched overweight/obese (n = 6), prediabetic (n = 6) and type 2 diabetic (n = 6) individuals were seeded at a density of 4 × 103 cells cm–2. Cells were grown and differentiated at 37°C in a humidified atmosphere of 5% CO2. Differentiation of myoblasts into myotubes was initiated at approximately 90% confluence by switching from the growth medium (as specified above) to the α modification of Eagle's minimum essential medium (α-MEM) with penicillin/streptomycin, 2% FBS and fetuin. The differentiating cells were treated with (i) palmitate (100 μm), coupled to BSA (in a molar ratio 5:1), and (ii) glucose in three different concentrations: 5.55 mm (baseline, low glucose DMEM), 10 mm, 20 mm. Treatment with fatty acid was initiated 48 h after the induction of differentiation, lasted for 4 days and was compared to control cells treated with BSA. Glucose treatments were initiated with the differentiation and lasted for 6 days. Media were changed every other day. Both of these treatment strategies have been previously optimized and shown to modulate muscle oxidative metabolism (Ukropcova et al. 2005; Sparks et al. 2011). The effects of palmitate or glucose are expressed as a percentage of gene expression in control cells.
Experiment 2. Treatment of immunopurified human primary muscle cells with saturated fatty acid
Immunopurified CD56+ muscle satellite cells from lean (n = 8) and age-matched overweight/obese (n = 7), prediabetic (n = 8) and type 2 diabetic (n = 9) individuals were grown and differentiated as described above. After 2 days of differentiation, cells were treated with 80 μm palmitate (Sigma-Aldrich, St Louis, MO, USA) coupled to BSA in a molar ratio 5:1 or with equivalent BSA alone. In contrast to Experiment 1, cells and media were harvested after 3 days of treatment (5 days of differentiation in total), when about 80–90% of mononuclear myoblasts had fused to form multinuclear elongated myotubes. Irisin concentration was determined in serum-and fetuin-free conditioned media after 6 h of incubation.
RNA isolation and real-time PCR
Total RNA and DNA from skeletal muscle, adipose tissue, differentiated and undifferentiated muscle cells were isolated using TriReagent (Molecular Research Center, Inc., USA). Purified (RNeasy mini Kit, Qiagen, USA), DNase-treated (Qiagen, USA) RNA was used for gene expression studies and DNA to determine the relative mitochondrial DNA content (expression of the NADH dehydrogenase subunit 1 (MT-ND1) relative to 18S rRNA). RNA was quantified spectrophotometrically with the aid of a NanoPhotometer (NanoDrop 2000, Implen, Germany). cDNA was produced with the aid of a High Capacity RNA to cDNA kit (Applied Biosystems, USA). Gene expression was measured by qRT-PCR (ABI7900HT, Applied Biosystems, USA) using either pre-designed TaqMan gene expression assays (Ppargc1A, Hs01016719_m1; MT-ND1, Hs02596873_s1; 18S rRNA Hs99999901_s1, Applied Biosystems, USA) or set of primers designed with the PrimerExpress software (Applied Biosystems, USA); Fndc5 (fwd: TGAGGTTGTCATCGGATTTGC; rev: GCGGGTGGTGGTGTTCAC); Rpl13a (fwd: GGACCGTGCGAGGTATGCT; rev: ATGCCGTCAAACACCTTGAGA); and B2m (fwd: CGCTCCGTGGCCTTAGC; rev: AATCTTTGGAGTACGCTGGATAGC). Ribosomal protein L13a (Rpl13a) and β-2-microglobulin (B2m) were used as the internal reference genes to calculate ddCt expression values. The PCR efficiency for Fndc5, Pgc1α, Rpl13a and B2m was between 1.9 and 2.0 in skeletal muscle and adipose tissue and between 1.9 and 2.1 in muscle cells. The approximate Ct values for different genes with 5 ng cDNA per reaction were as follows. Muscle: Fndc5 (23.9), Rpl13a (20.5), B2m (21.4). Adipose tissue: Fndc5 (28.1), RPl13a (18.7), B2m (17.7). Muscle cells: Fndc5 (22.7), RPl13a (20.2), B2m (21.3).
Biochemical assays
Circulating irisin was determined in samples of EDTA plasma taken (i) after an overnight fast (n = 95) and during the steady state of the clamp (n = 38) into the precooled tubes containing aprotinin (Study 1), and (ii) before and after 3 months training (n = 16); on both occasions blood was taken before, and immediately and 60 min after, completing the exercise session (Study 2). Irisin concentration in both plasma and conditioned media was determined using Irisin/FNDC5 RIA kit (cat. no. RK-067-16, Phoenix Pharmaceuticals, USA) with the range of 0.78–100.00 ng ml−1. The manufacturer provided us with the information that the rabbit polyclonal antibody used in this kit recognizes the 8 and 12 kDa proteins corresponding to C-terminal fragment of irisin (57–127) and supposedly full length irisin (16–127) as well as the 22 kDa protein corresponding to the FNDC5-irisin precursor protein (<9%). All samples were measured in one batch and intra-assay variability was < 5%. A subset of the 21 plasma samples from Study 2 was also measured with the more recently developed Irisin, Recombinant Enzyme Immunoassay Kit (cat. no. EK-067-29, Phoenix Pharmaceuticals, USA) with the range 0.1–1000 ng ml−1. This assay provided us with identical results but revealed that previously measured plasma concentrations might be underestimated by a factor of approximately 25 (Supplemental Fig. S2 available online). Glucose was measured using the glucose oxidase method (Beckman, USA), insulin levels with IRMA (Immunotech, France), total cholesterol, HDL-cholesterol and triglycerides with diagnostic kits from Roche (Germany). Free fatty acids were measured by a colorimetric assay kit (Randox, UK) and high-sensitivity C-reactive protein (hsCRP) with an immunoturbidimetric method (Randox, UK).
Statistical analyses
Differences between two groups were analysed by Student's t test, more than two groups differences were evaluated with a 2-way ANOVA followed by Tukey's post hoc test. Normality of the data distribution was tested and non-parametric tests were used where appropriate. Associations between variables were analysed by Pearson's bivariate correlation (r) and by multiple linear regression. Statistical analysis was performed in JMP (version 4.0.4 academic; SAS Institute, USA). The data are reported as mean ± SEM with P < 0.05 indicating statistical significance.
Results
Fndc5/irisin in obesity and metabolic disease (Study 1)
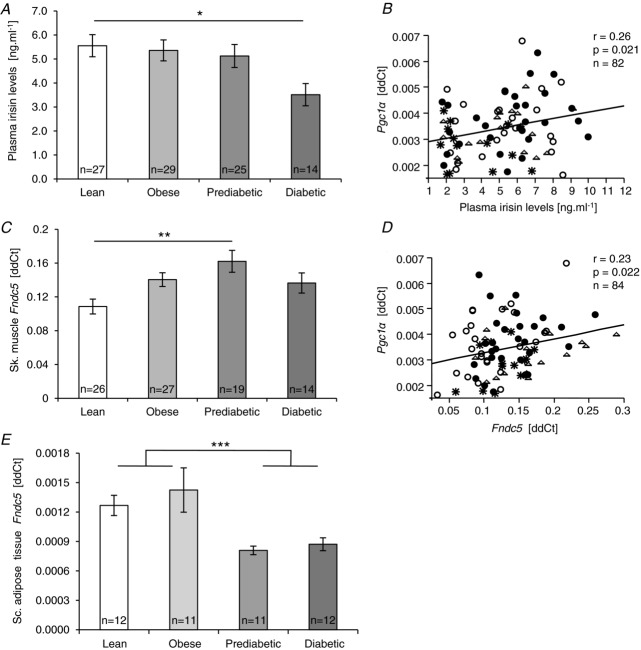
Fndc5 gene expression in muscle and subcutaneous adipose tissue and circulating irisin were measured in sedentary lean, obese, prediabetic and type 2 diabetic (T2D) men. Circulating irisin levels were highest in lean, not significantly changed in obese and prediabetic individuals, but almost 40% reduced in patients with T2D (P < 0.05; Fig. 1A). This pattern of lowered plasma irisin in T2D was preserved in a state of euglycaemic hyperinsulinaemia (clamp) (P < 0.05), with levels virtually identical to those found in the fasted state (r = 0.909; P < 0.0001; n = 38). This indicates that insulin had no effect on circulating irisin in vivo, irrespective of the level of obesity or insulin resistance. Circulating irisin was negatively associated with age (P < 0.0001), fasting glycaemia (P < 0.0308), area under the glycaemic curve (P < 0.0056), visceral adiposity (P < 0.0264) and extramyocellular lipid content (P < 0.0020) and positively with percentage of daily moderate and high intensity ambulatory activity (>3METs) (P < 0.0118) and with muscle Pgc1a expression (Table 2, Fig. 1B). After adjusting for age and BMI, circulating irisin was positively associated with the moderate and high intensity ambulatory activity level (P < 0.027), resting metabolic rate (P < 0.027), and negatively with waist circumference (P < 0.033) (Table 3).
Figure 1.
Plasma irisin concentration (A), and Fndc5 gene expression in skeletal muscle (C) and subcutaneous adipose tissue (E) in individuals with obesity, prediabetes and type 2 diabetes. B and D, associations of plasma irisin and muscle Fndc5 mRNA with muscle Pgc1α mRNA; ○ lean, • obese, Δ prediabetic, type 2 diabetic. Data are shown as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.005.
type 2 diabetic. Data are shown as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.005.
Table 2.
Bivariate correlation analysis of Fndc5/irisin with clinical metabolic phenotypes (Study 1)
| Irisin | Fndc5 mRNA Sc. | Fndc5 mRNA | |
|---|---|---|---|
| circulation | adipose tissue | skeletal muscle | |
| Age (years) | r = −0.399 | — | — |
| P < 0.0001 | |||
| n = 96 | |||
| Fasting plasma glucose (mmol l–1) | r = −0.221 | — | — |
| P = 0.0308 | |||
| n = 96 | |||
| 2 h glucose (mmol l–1) | — | r = −0.494 | — |
| P = 0.0016 | |||
| n = 46 | |||
| Area under the curve glucose oGTT | r = −0.293 | r = −0.468 | r = 0.253 |
| P = 0.0056 | P = 0.0030 | P = 0.0267 | |
| n = 88 | n = 46 | n = 78 | |
| Fasting plasma insulin (μU ml–1) | — | r = −0.295 | r = 0.242 |
| P = 0.0718 | P = 0.0260 | ||
| n = 46 | n = 85 | ||
| M-value/insulin (mg kg–1 min–1 μU–1 ml–1) | — | r = 0.362 | r = −0.223 |
| P = 0.0133 | P = 0.0454 | ||
| n = 46 | n = 84 | ||
| BMI (kg m–2) | — | r = −0.296 | r = 0.358 |
| P = 0.0702 | P = 0.0007 | ||
| n = 46 | n = 86 | ||
| Waist circumference (cm) | — | r = −0.347 | r = 0.350 |
| P = 0.0197 | P = 0.0011 | ||
| n = 46 | n = 84 | ||
| Body fat content (%) | — | r = −0.271 | r = 0.251 |
| P = 0.0073 | P = 0.0211 | ||
| n = 46 | n = 84 | ||
| Lean body mass (kg) | — | r = −0.299 | r = 0.300 |
| P = 0.0464 | P = 0.0055 | ||
| n = 46 | n = 84 | ||
| Fat cell size – diameter (μm) | — | r = −0.361 | r = 0.296 |
| P = 0.0162 | P = 0.0070 | ||
| n = 46 | n = 82 | ||
| Sc. AT cross-sectional area (cm2) | — | r = −0.371 | r = 0.269 |
| P = 0.00155 | P = 0.0160 | ||
| n = 46 | n = 80 | ||
| Visceral AT cross-sectional area (cm2) | r = −0.237 | r = −0.268 | — |
| P = 0.0264 | P = 0.0856 | ||
| n = 88 | n = 46 | ||
| IMCL (relative to creatine) | — | — | r = 0.280 |
| P = 0.0100 | |||
| n = 81 | |||
| EMCL (relative to creatine) | r = −0.320 | r = −0.264 | — |
| P = 0.0020 | P = 0.099 | ||
| n = 86 | n = 46 | ||
| ΔRQ | — | r = −0.320 | |
| P = 0.0265 | |||
| n = 48 | |||
| Triglycerides (mmol l–1) | — | r = −0.280 | r = 0.235 |
| P = 0.0443 | P = 0.0307 | ||
| n = 46 | n = 85 | ||
| HDL cholesterol (mmol l–1) | — | — | r = −0.252 |
| P = 0.0206, | |||
| n = 84 | |||
| Atherogenic index (T.chol. – HDL chol.)/HDL chol.) | — | — | r = 0.309 |
| P = 0.0042 | |||
| n = 84 | |||
| FFAs during the steady state of clamp (mmol l–1) | — | — | r = 0.246 |
| P = 0.0241 | |||
| n = 84 | |||
| hsCRP (mg l–1) | — | — | r = 0.233 |
| P = 0.0318 | |||
| n = 85 | |||
| Moderate and high intensity ambulatory activity (%) | r = 0.262 | r = 0.260 | — |
| P = 0.0118 | P = 0.1002 | ||
| n = 92 | n = 46 |
AT, adipose tissue; EMCL, extramyocellular lipid content; hsCRP, high-sensitivity C-reactive protein; IMCL, intramyocellular lipid content; oGTT, oral glucose tolerance test; Sc. subcutaneous; T. chol, total cholesterol.
Table 3.
Associations of Fndc5/irisin with parameters of metabolic phenotype: multiple linear regression model
|
Fndc5 mRNA skeletal muscle |
Fndc5 mRNA adipose tissue |
Irisin circulation |
Pgc1α mRNA skeletal muscle |
|||||
|---|---|---|---|---|---|---|---|---|
| β | P | β | P | β | P | β | P | |
| Parameter | coeff. | value | coeff. | value | coeff. | value | coeff. | value |
| M–value/insulin (mg kg–1 min–1 μU–1 ml–1) | — | — | 0.754 | 0.006 | — | — | 0.571 | 0.019 |
| Lean body mass (kg) | 0.470 | 0.085 | — | — | — | — | –0.657 | 0.002 |
| Waist circumference (cm) | — | — | — | — | –0.473 | 0.033 | –0.602 | 0.018 |
| Triglycerides (mmol l–1) | — | — | –0.615 | 0.046 | — | — | — | — |
| Moderate and high intensity activity (%) | — | — | — | — | 0.480 | 0.027 | — | — |
| RQ fasted state | — | — | — | — | –0.365 | 0.138 | –0.515 | 0.058 |
| ΔRQ (clamp-fasted) | — | — | — | — | — | — | 0.557 | 0.047 |
| Resting metabolic rate (kcal (24 h)–1 (kg LBM)–1) | — | — | — | — | 0.479 | 0.027 | — | — |
Adjusted for age and BMI. β coeff., β coefficient; clamp, euglycaemic–hyperinuslinaemic clamp; LBM, lean body mass; RQ, respiratory quotient.
Fndc5 expression in skeletal muscle
The skeletal muscle of prediabetic individuals displayed a 45% (P < 0.01) increase in Fndc5 mRNA when compared to lean. However, in T2D, mRNA levels were similar to those found in healthy obese individuals (Fig. 1C). Fndc5 in muscle was positively associated with many obesity-and metabolic disease-related phenotypes such as BMI (P < 0.0007), fat cell size (P < 0.0070), subcutaneous adiposity (P < 0.0160), intramyocellular lipid content (P < 0.0100), glucose tolerance (area under the glycaemic curve; P < 0.0267), the whole-body as well as adipose tissue insulin sensitivity (M-value/insulin, P < 0.0454; FFAs during the clamp, P < 0.0241; Table 2). Skeletal muscle Fndc5 expression was positively, albeit weakly, associated with muscle Pgc1α mRNA (Fig. 1D) and with relative mitochondrial DNA content expressed as ND1/18S rRNA (r = 0.237; n = 83; P = 0.032). More importantly, when adjusted for age and BMI, the amount of lean body mass remained the only independent predictor of Fndc5 expression in muscle (Table 3).
Fndc5 expression in adipose tissue
Expression of Fndc5 in subcutaneous adipose tissue was approximately 100 times lower than in muscle (Fig. 1C and E) as determined within the single real-time PCR assay. Interestingly, prediabetic and diabetic patients expressed 40–45% less Fndc5 in subcutaneous adipose tissue compared to lean and healthy obese men (Fig. 1E), which paralleled the reduction of circulating irisin observed in T2D (Fig. 1A and E). The discordant expression of Fndc5 in muscle and adipose tissue was reflected in the virtually opposite association pattern with obesity and metabolic phenotypes (Table 2), adipose tissue Fndc5 mRNA being negatively related to many metabolic disease-related phenotypes such as waist circumference (P < 0.0197), glucose tolerance (area under the glycaemic curve; P < 0.0030) and whole-body insulin sensitivity (M-value; P < 0.0133; Table 2). More importantly, adipose tissue Fndc5 gene expression was negatively associated with both fat cell size (P < 0.0162) and subcutaneous adiposity (P < 0.0015; Table 2). Similarly to circulating irisin, adipose tissue Fndc5 expression was not regulated by in vivo hyperinsulinaemia during the steady state of the clamp (P > 0.05). Adjustment for age and BMI revealed that the insulin sensitivity index (M-value/insulin) and plasma triglycerides were the only independent predictors of the adipose tissue Fndc5 mRNA (Table 3).
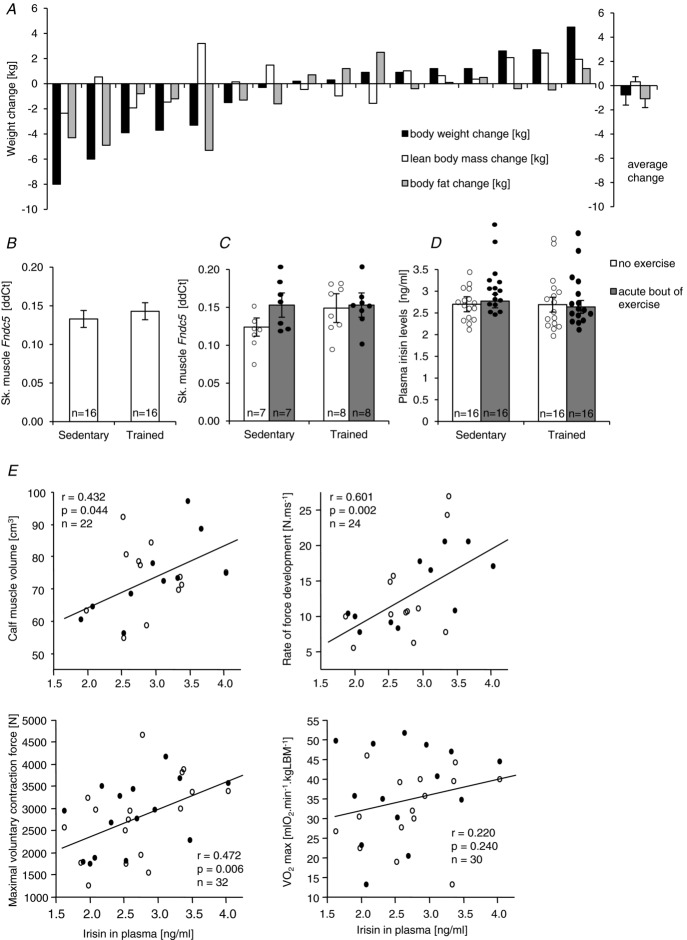
Neither acute nor regular exercise alters Fndc5/irisin levels (Study 2)
In the presented study, the 3 month exercise intervention resulted in variable changes in body weight, lean body mass, and fat mass (Fig. 2A). It is, however, important to note that increased body weight in an exercising individual was always associated with increased lean body mass and that exercise unequivocally improved endurance performance (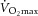 ; P < 0.003) and reduced visceral adiposity (P < 0.05). We showed that neither 3 months exercise training nor the acute bout of exercise (sample taken 60–70 min post-exercise) in sedentary or trained individuals affected the skeletal muscle Fndc5 expression or circulating irisin levels in 16 healthy overweight/obese individuals (Fig. 2B, C and D). It is important to note that the acute bout of exercise massively (>6-fold) induced expression of Pgc1α in both sedentary and trained individuals (Supplemental Fig. S1A and B). In addition, skeletal muscle Fndc5 mRNA was positively correlated with Pgc1α mRNA in muscle of sedentary and trained individuals not affected by an acute exercise bout (Supplemental Fig. S1C). Circulating irisin was positively associated with calf muscle volume, maximal voluntary contraction force, rate of force development (Fig. 2E) and with the high dietary protein score (inferred from the food preference questionnaire, r = 0.39; P < 0.02; n = 32) but not with the maximal aerobic capacity (
; P < 0.003) and reduced visceral adiposity (P < 0.05). We showed that neither 3 months exercise training nor the acute bout of exercise (sample taken 60–70 min post-exercise) in sedentary or trained individuals affected the skeletal muscle Fndc5 expression or circulating irisin levels in 16 healthy overweight/obese individuals (Fig. 2B, C and D). It is important to note that the acute bout of exercise massively (>6-fold) induced expression of Pgc1α in both sedentary and trained individuals (Supplemental Fig. S1A and B). In addition, skeletal muscle Fndc5 mRNA was positively correlated with Pgc1α mRNA in muscle of sedentary and trained individuals not affected by an acute exercise bout (Supplemental Fig. S1C). Circulating irisin was positively associated with calf muscle volume, maximal voluntary contraction force, rate of force development (Fig. 2E) and with the high dietary protein score (inferred from the food preference questionnaire, r = 0.39; P < 0.02; n = 32) but not with the maximal aerobic capacity (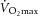 ) (Fig. 2E). Positive association with muscle strength (maximal voluntary contraction force) was retained after adjustment for age and BMI. More importantly, circulating irisin was negatively associated with fasting glycaemia (r = −0.52; P < 0.05; n = 32).
) (Fig. 2E). Positive association with muscle strength (maximal voluntary contraction force) was retained after adjustment for age and BMI. More importantly, circulating irisin was negatively associated with fasting glycaemia (r = −0.52; P < 0.05; n = 32).
Figure 2.
A, effect of the 3 month exercise intervention on body weight, lean body mass and fat mass in all 16 participants. Effect of the 3 month exercise intervention and an acute bout of exercise (sampled 60–90 min post exercise) on skeletal muscle Fndc5 mRNA (B and C) and on the circulating irisin levels (D). E, associations of circulating irisin with muscle mass (calf volume), strength (maximal voluntary contraction force) and contractility (rate of force development); ○ before training, • after training. Data are shown as means ± SEM.
Irisin and muscle metabolism in vivo
The 31P-MRS magnetization transfer experiment revealed that the circulating irisin level measured in plasma taken in the fasted, resting state was positively associated with the kATP, the rate constant for the Pi-to-ATP conversion reflecting the resting Pi-to-ATP flux (r = 0.903; P < 0.00001; n = 10), but the association with the creatine kinase reaction rate did not reach the appropriate level of statistical significance. The association between circulating irisin and kATP in resting muscle remained significant when adjusted for age and BMI.
Fndc5/irisin expression and secretion in vitro and their regulation by palmitate and glucose in myotubes originating from lean, obese, prediabetic and diabetic individuals
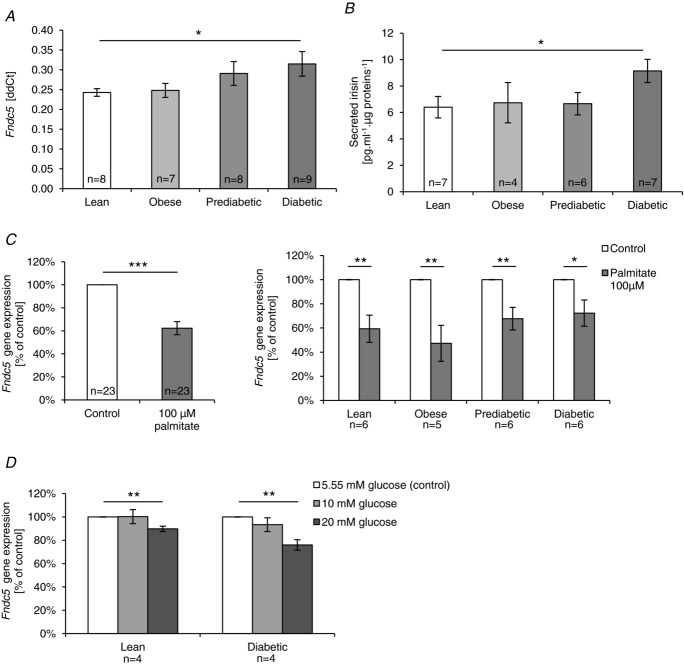
Human primary muscle cell culture was used to investigate skeletal muscle Fndc5/irisin expression/secretion in an in vitro system devoid of the neurohumoral milieu of an integral human body. Fndc5 mRNA was detected in human primary myotubes and irisin levels were determined in the conditioned cell culture media. Fndc5 expression was measured in two sets of experiments, both using cells originating from donors with different obesity and metabolic disease phenotypes. The reason for performing the second experiment was to replicate the observations under different experimental conditions: (i) more individuals (n = 8 per group); (ii) an additional immunopurification of the founder satellite cells with the CD56 antibody (which after sorting represented over 95% of the cell population, as assessed by FACS (fluorescence-activated cell sorting)), thus excluding the putative contribution of other cell types to irisin production.
Fndc5 expression was significantly higher in myotubes from patients with T2D as compared to that of lean donors (Fig. 3A). A similar trend was observed in myotubes from overweight/obese and prediabetic individuals (Fig. 3A). The evidence of increased Fndc5 mRNA in myotubes derived from diabetic vs. lean donors produced in both in vitro experiments is based on two replicates per individual, in cultures derived from more than 13 individuals per group. Moreover, we have observed that cells derived from T2D patients, expressing the highest levels of Fncd5 mRNA, displayed the highest irisin levels in the media (Fig. 3B).
Figure 3.
A, Fndc5 mRNA in differentiated human primary muscle cells. B, irisin in the conditioned media from human primary muscle cells. C, effect of palmitate on Fndc5 gene expression. D, effect of glucose on Fndc5 gene expression. Data are shown as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
It is important to point out a clear dissociation between the in vivo and in vitro situation. While circulating irisin levels were down-regulated in diabetes and levels of Fndc5 mRNA in muscle of T2D individuals were not significantly higher than those in lean healthy controls (Fig. 1A and C), both Fndc5 expression in myotubes and irisin levels in conditioned media were highest in myotubes originating from individuals with T2D. This indicated that Fndc5 expression and/or irisin secretion in vivo was probably regulated by an endogenous signal associated with type 2 diabetes. To shed more light on this interesting phenomenon, in our subsequent in vitro experiment we clearly demonstrated that saturated fatty acid palmitate as well as glucose treatment decreased the expression of the Fndc5 gene in cultured cells by 40 and 20%, respectively. While the lowering effect of palmitate seems to be slightly less pronounced in individuals with type 2 diabetes, the effect of glucose might be more significant in the diabetic state (Fig. 3C and D).
In addition, we have found that the expression of Fndc5 in undifferentiated muscle cells (n = 4 independent experiments) is over 20-fold lower than that in differentiated myotubes.
Discussion
Fndc5 and irisin in obesity and type 2 diabetes
One of the greatest assets of the work presented here is the complexity of in vivo and in vitro studies which enabled us to discern the specific regulation patterns of Fndc5/irisin in muscle, myotubes, adipose tissue and circulation in obesity, prediabetes and type 2 diabetes. This was performed in a well-phenotyped population of drug-naive sedentary men. Highlights include (i) the consistent T2D-related down-regulation of Fndc5/irisin in adipose tissue and circulation; (ii) the opposing expression pattern of Fndc5 in muscle and adipose tissue, as well as (iii) discordance between the muscle in vivo and in vitro Fndc5/irisin expression pattern related specifically to T2D, which together with results from in vitro experiments on human myotubes suggest the regulatory role for glucose and fatty acids. They all point to complex and tissue-specific mechanisms, leading to timely regulation of local and systemic irisin content, which might be important in determining metabolic health and disease.
Furthemore, we have observed that muscle expression of Fndc5, the precursor of the putative fat browning agent produced by contracting muscle, irisin, was gradually upregulated with increasing obesity and glucose intolerance in non-diabetic individuals, thus compromising the proposed positive role of irisin in metabolic disease. Another option to explain this observation would be the desensitization to irisin effects or ‘irisin resistance’ (Polyzos et al. 2013), which might represent part of a rescue setting, active in the preclinical phase of diabetes. Increased irisin levels could therefore potentially modulate deteriorated
insulin sensitivity. Interestingly, the presence of newly diagnosed, yet untreated type 2 diabetes was not associated with a further increase in Fndc5, but levels of expression were comparable to those found in healthy obese individuals. In addition, diabetes led to a 40% decrease in circulating irisin. This is in accordance with the previous recent reports indicating that circulating irisin is significantly lower in patients with type 2 diabetes (Liu et al. 2013; Moreno-Navarrete et al. 2013), the advantage of our study being the lack of potentially confounding pharmacotherapy.
Reported results on the association of irisin with obesity and metabolic phenotypes are quite controversial. A recent report showed that circulating irisin correlated positively with BMI, total cholesterol and triglycerides, as well as with the fasting blood glucose in non-diabetic, non-obese individuals (not excluding those with impaired glucose tolerance; Liu et al. 2013). The positive association of circulating irisin with BMI was also reported in two other, mostly female, non-diabetic populations (Huh et al. 2012; Stengel et al. 2013) while negative associations, similar to our findings, were described elsewhere (Choi et al. 2013; Moreno-Navarrete et al. 2013). The discrepancy might be related to the frequent (poly)pharmacotherapy and other confounding variables such as age, sex, race or the level of physical activity, contributing considerably to the heterogeneity of the study populations.
Two recent reports indicated that muscle mass was a good predictor of circulating irisin in humans (Huh et al. 2012; Stengel et al. 2013), which could be important, especially in light of the age-related decline of muscle mass, strength and oxidative capacity, particularly in obese sedentary individuals (Irving et al. 2012). Further exploring the link between irisin and muscle, we demonstrated that circulating irisin was positively associated with calf muscle volume, muscle strength and contractility in overweight/obese sedentary individuals. Moreover, the 31P-MRS magnetization transfer experiment revealed a positive association of plasma irisin with resting muscle Pi-to-ATP flux. Importantly, associations between plasma irisin and muscle phenotypes were independent of age and BMI (Study 2). Circulating irisin was also positively associated with high dietary protein preference (data not shown), a factor possibly supporting muscle growth, as well as with the muscle metabolic regulator Pgc1α and mitochondrial DNA content. It is plausible to speculate that the link between plasma irisin and muscle characteristics points at possible common underlying regulatory mechanisms. The positive role for this myokine in modulating the skeletal muscle functional and metabolic phenotype also cannot be completely ruled out. The original observations by Boström et al. that irisin enhances oxygen consumption and improves glucose tolerance in mice with high-fat-diet-induced obesity (Boström et al. 2012) could be, in a limited fashion and perhaps by different mechanisms, translated to men. This notion is further supported by the observation that the level of medium and high intensity habitual free-living ambulatory activity and resting metabolic rate are age-and BMI-independent predictors of circulating irisin in the population of lean, obese, prediabetic and diabetic men. A positive association between circulating irisin levels and 24 h energy expenditure (metabolic chamber) has recently been observed in hypermetabolic individuals with 24 h energy expenditure exceeding the value predicted by fat-free mass (Swick et al. 2013). Although, limited by the correlative nature of our findings, we can conclude that positive metabolic outcomes of higher intensity habitual physical activity could be related to circulating irisin.
Nevertheless, our results on muscle Fndc5 expression are not in a conflict with the observations by Timmons et al. who reported in a cohort of over 200 individuals with a large BMI range that Fndc5 gene expression is unrelated to the exercise-induced improvement in insulin sensitivity, fasting glucose or blood pressure (Timmons et al. 2012). Similar to other recent reports (Huh et al. 2012) we showed that muscle Fndc5 is positively associated with body mass index, lean body mass, waist circumference, accumulation of subcutaneous adipose tissue, as well as circulating triglycerides and insulin. Moreover, muscle Fndc5 was, in our studies, positively associated with fat cell size, intramyocellular lipid content, as well as with markers of whole body inflammation, and negatively associated with insulin sensitivity (M-value), metabolic flexibility (ΔRQ) and HDL-cholesterol. The fact that circulating irisin and muscle Fndc5 are unrelated and even have opposing relations with many clinical markers of metabolic health/disease might point to a distinct pattern of regulation and function of irisin in muscle and circulation and/or the regulation at a post-transcriptional/post-translational level. Other resource(s) of circulating irisin such as adipose tissue cannot be excluded. What regulates muscle Fndc5 expression is uncertain but increased amount of lean body mass seemed to be the only age-and BMI-independent predictor of muscle Fndc5 expression in this cohort. It is plausible to speculate that an increase in muscle Fndc5 in obese prediabetic individuals might reflect the need for local muscle irisin actions. The data on the direct effect of irisin on muscle is very scarce. The single report by Boström showed that irisin had no direct effect on mitochondrial gene expression in murine muscle or in primary myotubes (Boström et al. 2012).
It is important to emphasize that the production of irisin is not limited to skeletal muscle. In humans, Fndc5 mRNA is also abundant in pericardium (Bordicchia et al. 2012; Huh et al. 2012) and a low level of Fndc5 was detected in kidney, liver, lung, neurons (Huh et al. 2012; Dun et al. 2013) and in adipose tissue (Huh et al. 2012; Moreno-Navarrete et al. 2013; Roca-Rivada et al. 2013). We confirmed the observations of the previous human (Huh et al. 2012; Moreno-Navarrete et al. 2013) and animal studies (Roca-Rivada et al. 2013) indicating that Fndc5 is expressed in adipose tissue albeit in much lower amounts than in skeletal muscle. In our studies, subcutaneous abdominal adipose tissue expressed approximately 100 times less Fndc5 than skeletal muscle. Interestingly, our preliminary observations indicate that levels of irisin/FNDC5 protein detected by EIA assay in human subcutaneous adipose tissue might be comparable to that in muscle, supporting the idea that the regulation of irisin production/release in/from adipose tissue might contribute to irisin circulating levels (preliminary data not shown).
In contrast to muscle, adipose tissue Fndc5 mRNA was decreased in prediabetic and diabetic individuals but not affected by obesity. The only available report in this respect showed reduced adipose tissue Fndc5 mRNA in morbid obesity and T2D (Moreno-Navarrete et al. 2013). Our study also identified many metabolic disease-related phenotypes being negatively associated with the adipose tissue Fndc5 mRNA. However, we showed that the whole body insulin sensitivity, and fasting triglyceridaemia, are the only identified age-and BMI-independent predictors of adipose tissue Fndc5.
Next, we aimed to explore the effect of acute exercise and 3 months supervised exercise training on irisin in muscle and circulation. The effectiveness of the exercise intervention was documented by an almost 20% increase in maximal aerobic capacity (P < 0.05) and a similar reduction in visceral adiposity (P < 0.05). Nevertheless, the exercise training failed to affect circulating irisin levels as well as muscle Fndc5 expression. Moreover, muscle Fndc5 mRNA, as well as circulating irisin (60 min post-exercise), were unaffected by an acute bout of exercise in both sedentary and trained individuals, indicating that muscle contraction is perhaps not the main switch of Fndc5/irisin production. In contrast to our results, Boström et al. reported an almost 2–fold increase in skeletal muscle Fndc5 mRNA and circulating irisin levels in a small cohort of elderly obese individuals after 10 weeks of endurance training (Boström et al. 2012). However, Timmons et al. failed to detect any significant effects of exercise on Fndc5 muscle expression in a larger cohort, despite improvements in physical fitness and insulin sensitivity (Timmons et al. 2012), observing some 30% up-regulation of the Fndc5 gene only in skeletal muscle of highly active elderly subjects (Timmons et al. 2012). Lecker et al. have shown that Fndc5 expression was higher in the group of individuals with a higher aerobic performance (Lecker et al. 2012) and Huh et al. reported a mild increase in circulating irisin levels 30 min after sprint in 15 moderately trained healthy young men, but failed to detect an effect of 8 weeks exercise training intervention (Huh et al. 2012). The large inconsistency could be related to the differences in the study design and populations.
We and others have previously shown that human primary muscle cells retain specific metabolic characteristics of their donors (Ukropcova et al. 2005; Bouzakri et al. 2011; Jiang et al. 2013). In the presented work we clearly showed that Fndc5 expression and irisin secretion was highest in human myotubes derived from type 2 diabetic donors compared to lean, obese and prediabetic individuals. Based on our work as well as others (Ukropcova et al. 2005; Bouzakri et al. 2011; Jiang et al. 2013; Staiger et al. 2013) it is plausible to think that muscle secretory activity, mostly believed to be under the direct control of behavioural factors such as physical activity, can also be finely tuned by muscle genetic/epigenetic make-up related to metabolic disease. It could also be speculated that a distinct myokine spectrum is being produced by insulin-sensitive and insulin-resistant muscle.
Notably, in T2D, opposite effects on Fndc5/irisin were observed in vivo and in vitro. Fndc5 expression in diabetic muscle in vivo was not different from healthy obese muscle, and irisin in circulation was significantly reduced in individuals with T2D. In contrast, myotubes derived from patients with T2D expressed the most Fndc5 and produced the most irisin into the media. This has been reproduced in several independent in vitro experiments with almost identical results. The only published information on Fndc5 expression in human primary muscle cells shows a similar positive association between myotube Fndc5 mRNA and fasting insulin and insulin sensitivity (HOMA-IR) in 37 young healthy individuals and 14 elderly men (Staiger et al. 2013). We think that our observations provide indirect evidence that there is a diabetes-associated factor inhibiting muscle Fndc5 expression in vivo. These factor(s) could potentially affect Fndc5 expression, irisin release, secretion or biodegradation. The euglycaemic–hyperinsulinaemic clamp study clearly showed that insulin is not the factor acutely regulating adipose tissue or circulating Fndc5/irisin levels in vivo. On the other hand, correlative evidence indicated that glycaemia, circulating triglycerides, visceral adiposity or extramyocelluar lipid deposition were associated with either adipose tissue Fndc5 mRNA or with circulating irisin levels. It is therefore plausible to speculate that glucose or lipids could represent/trigger the missing regulatory factor associated with lower irisin levels in T2D. Our in vitro experiments clearly support this notion by showing a 40 and 20% reduction in Fndc5 gene expression in muscle cells treated with palmitate and glucose, respectively.
The limitation of Study 1 is its cross-sectional design and a lack of specific information on irisin protein levels (a largely understudied parameter in all existing reports on irisin). This might be outbalanced by the size of the cohort, detailed clinical phenotyping, use of the newly diagnosed pharmacotherapy-naive patients with type 2 diabetes, or by the parallel investigation of muscle, adipose tissue, primary muscle cells and plasma of the same well-phenotyped patient. The small sample size is the obvious major limitation of Study 2, which we think could be outbalanced by the effectiveness of the complex and motivating training regimen, and the design enabling the effects of both 3 months training and an acute bout of exercise in both a sedentary and trained state to be determined.
In conclusion, our studies clearly showed consistent T2D-related down-regulation of Fndc5/irisin in adipose tissue and circulation, opposed by positive associations of the muscle Fndc5 with BMI and metabolic disease-related phenotypes, suggesting the tissue-specific regulatory mechanisms associated with Fndc5 expression, irisin release and bioavailability. The absence of a diabetes-associated lowering effect in muscle cells in vitro advocates the presence of an as yet unknown irisin-regulating factor in vivo, and our results point to a potential regulatory role for glucose and saturated fatty acids.
Exercise, in a chronic or acute form applied to either sedentary or trained individuals was not effective in modulating the irisin circulating levels and skeletal muscle Fndc5 gene expression. However, the positive metabolic outcomes of a higher free-living ambulatory activity (Study 1) together with the muscle mass and strength gained by the exercise training (Study 2) were related to the levels of circulating irisin and could therefore represent the link between irisin and metabolic health.
Key points
Considerable controversy exists regarding the role of irisin, a putative exercise-induced myokine, in human metabolism.
We therefore studied irisin and its precursor Fndc5 in obesity, type 2 diabetes and exercise.
Complex clinical studies combined with cell culture work revealed that Fndc5/irisin was decreased in type 2 diabetes in vivo, but not in muscle cells in vitro, indicating that diabetes-related factor(s) regulate Fndc5/irisin in vivo.
Several attributes of type 2 diabetes, such as hyperglycaemia, triglyceridaemia, visceral adiposity and extramyocellular lipid deposition were negatively associated with adipose tissue Fndc5 mRNA and circulating irisin. Moreover, mimicking diabetic status in vitro by treating muscle cells with palmitate and glucose lowered Fndc5 mRNA.
Neither exercise training nor an acute exercise bout modulated circulating irisin or muscle Fndc5 expression. However, the associations between intensity of habitual physical activity, muscle volume, strength, contractility and circulating irisin provide a link between irisin and positive outcomes of increased physical activity.
Acknowledgments
The authors would like to express their gratitude to all those who contributed to this work (in alphabetical order): K. Babinska, A. Banarova, M. Chmelik, P. Danco, A. Dlesk, L. Fabryova, D. Gajdosova, K. Giertlova, L. Gogova, D. Hamar, T. Hantabal, E. Hessova, Z. Janakova, M. Jelen, M. Kuzma, A. Mitkova, G. Ole, J. Olejnik, J. Payer, A. Penesova, K. Raslova, S. Stankovicova and S. Trattnig. We would like to thank R. Cerny (CEO of MR Diagnostics) for his perpetual enthusiasm and help with establishing the indirect calorimetry, K. Skybova (Kompliment – the institute for overweight prevention and reduction, Bratislava) and L. Grancic (The Body Energy Club, Bratislava), as well as all the study volunteers for their cooperative attitude and genuine interest in our work and in their metabolic health.
Glossary
- 1RM
1 repetition maximum
- AT
adipose tissue
- BMI
body mass index
- clamp
euglycaemic–hyperinsulinaemic clamp
- EMCL
extramyocellular lipids
- FFAs
free fatty acids
- Fndc5 gene for
fibronectin type III domain-containing protein 5
- HDL
high-density lipoprotein
- HOMA-IR
homeostasis model of assessment, insulin resistance
- IMCL
intramyocellular lipids
- LBM
lean body mass
- MET
metabolic equivalent = resting metabolic rate
- MRI
magnetic resonance imaging
- MRS
magnetic resonance spectroscopy
- M-value
insulin sensitivity index
- oGTT
oral glucose tolerance test
- PGC1α
peroxisome proliferator-activated receptor-γ coactivator 1α
- Pi
inorganic phosphate
- qRT-PCR
quantitative reverse transcriptase PCR
- RPL13A
ribosomal protein L13a
- Sc.
subcutaneous
- T2D
type 2 diabetes
Additional information
Competing interests
None declared.
Author contributions
T.K.: data generation, analysis and interpretation, drafting the manuscript. M.B: data analysis, interpretation and critical reading of the manuscript. M.Vician: muscle sample collection, data analysis. D.M.: data generation and analysis. M.Vlcek: data analysis, critical reading of the manuscript. L.V: 31P-MRS data generation, analysis. M.S.: 1H-MRS and MRI data generation, analysis. R.I.: data analysis and interpretation. O.K.: training programme conception, data analysis. V.B.: 1HMRS and MRI data interpretation. I.J.: training status assessment, bicycle spiro-ergometry, data generation and interpretation. C.W.: data interpretation, critical reading of the manuscript. I.K.: data interpretation; critical reading of the manuscript. M.K.: conception of the 31P-MRS experiment, data interpretation, analysis, critical reading of the manuscript. E.Z.: kinesiology data analysis and interpretation. D.G.: data interpretation. J.U.: study conception and design, data interpretation, drafting and critical reading of the manuscript. B.U.: study conception and design, data generation, analysis and interpretation, drafting and critically revising the manuscript. All authors have read and approved the final version of the manuscript. Experiments were carried out at the Institute of Experimental Endocrinology Slovak Academy of Sciences in Bratislava, Slovakia with a few exceptions: 31P-magnetic resonance spectroscopy studies were performed at the MR Centre of Excellence, Department of Radiology, Medical University of Vienna, Austria; (ii) cell culture experiment with sorted human muscle cells was carried out in a frame of the the short therm scientific mission of TK in Institute of Food Nutrition and Health, ETH Zürich, Schwerzenbach, Switzerland.
Funding
This work was supported by…. The EFSD New Horizons grant (JU) and The EFSD Lilly Research Fellowship (BU), 7FP-EC-LipidomicNET #202272 (DG), Scientific Grant Agency of the Slovak Academy of Sciences (VEGA) 2/0198/11 (JU) and 2/0174/12 (BU) and The National Scholarship Programme of the Slovak Republic (TK).
References
- Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM. A PGC1–α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzakri K, Plomgaard P, Berney T, Donath MY, Pedersen BK, Halban PA. Bimodal effect on pancreatic β-cells of secretory products from normal or insulin-resistant human skeletal muscle. Diabetes. 2011;60:1111–1121. doi: 10.2337/db10-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindle KM, Blackledge MJ, Challiss RA, Radda GK. 31P NMR magnetization-transfer measurements of ATP turnover during steady-state isometric muscle contraction in the rat hind limb in vivo. Biochemistry. 1989;28:4887–4893. doi: 10.1021/bi00437a054. [DOI] [PubMed] [Google Scholar]
- Choi YK, Kim MK, Bae KH, Seo HA, Jeong JY, Lee WK, Kim JG, Lee IK, Park KG. Serum irisin levels in new-onset type 2 diabetes. Diabetes Res Clin Pract. 2013;100:96–101. doi: 10.1016/j.diabres.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Dun SL, Lyu RM, Chen YH, Chang JK, Luo JJ, Dun NJ. Irisin-immunoreactivity in neural and non-neural cells of the rodent. Neuroscience. 2013;240:155–162. doi: 10.1016/j.neuroscience.2013.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiselman PJ, Anderson AM, Dowdy ML, West DB, Redmann SM, Smith SR. Reliability and validity of a macronutrient self-selection paradigm and a food preference questionnaire. Physiol Behav. 1998;63:919–928. doi: 10.1016/s0031-9384(97)00542-8. [DOI] [PubMed] [Google Scholar]
- Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE, Mantzoros CS. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61:1725–1738. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving BA, Robinson MM, Nair KS. Age effect on myocellular remodeling: response to exercise and nutrition in humans. Ageing Res Rev. 2012;11:374–389. doi: 10.1016/j.arr.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang LQ, Duque-Guimaraes DE, Machado UF, Zierath JR, Krook A. Altered response of skeletal muscle to IL–6 in type 2 diabetic patients. Diabetes. 2013;62:355–361. doi: 10.2337/db11-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebon V, Dufour S, Petersen KF, Ren J, Jucker BM, Slezak LA, Cline GW, Rothman DL, Shulman GI. Effect of triiodothyronine on mitochondrial energy coupling in human skeletal muscle. J Clin Invest. 2001;108:733–737. doi: 10.1172/JCI11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecker SH, Zavin A, Cao P, Arena R, Allsup K, Daniels KM, Joseph J, Schulze PC, Forman DE. Expression of the irisin precursor FNDC5 in skeletal muscle correlates with aerobic exercise performance in patients with heart failure. Circ Heart Fail. 2012;5:812–818. doi: 10.1161/CIRCHEARTFAILURE.112.969543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J-J, Wong M, Toy W, Tan C, Liu S, Ng X, Tavintharan S, Sum C, Lim S. Lower circulating irisin is associated with type 2 diabetes mellitus. J Diabetes Complications. 2013;27:365–369. doi: 10.1016/j.jdiacomp.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Moreno-Navarrete JM, Ortega F, Serrano M, Guerra E, Pardo G, Tinahones F, Ricart W, Fernández-Real JM. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab. 2013;98:E769–E778. doi: 10.1210/jc.2012-2749. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin–6. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- Polyzos SA, Kountouras J, Shields K, Mantzoros CS. Irisin: A renaissance in metabolism? Metabolism. 2013;62:1037–1044. doi: 10.1016/j.metabol.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Roca-Rivada A, Castelao C, Senin LL, Landrove MO, Baltar J, Belén Crujeiras A, Seoane LM, Casanueva FF, Pardo M. FNDC5/irisin is not only a myokine but also an adipokine. Plos One. 2013;8:e60563. doi: 10.1371/journal.pone.0060563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks LM, Moro C, Ukropcova B, Bajpeyi S, Civitarese AE, Hulver MW, Thoresen GH, Rustan AC, Smith SR. Remodeling lipid metabolism and improving insulin responsiveness in human primary myotubes. PLoS One. 2011;6:e21068. doi: 10.1371/journal.pone.0021068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman BM. Banting Lecture 2012: Regulation of adipogenesis: toward new therapeutics for metabolic disease. Diabetes. 2013;62:1774–1782. doi: 10.2337/db12-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger H, Böhm A, Scheler M, Berti L, Machann J, Schick F, Machicao F, Fritsche A, Stefan N, Weigert C, Krook A, Häring HU, de Angelis MH. Common genetic variation in the human FNDC5 locus, encoding the novel muscle-derived ‘browning’ factor irisin, determines insulin sensitivity. PLoS One. 2013;8:e61903. doi: 10.1371/journal.pone.0061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Hofmann T, Goebel-Stengel M, Elbelt U, Kobelt P, Klapp BF. Circulating levels of irisin in patients with anorexia nervosa and different stages of obesity – Correlation with body mass index. Peptides. 2013;39:125–130. doi: 10.1016/j.peptides.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Swick AG, Orena S, O'Connor A. Irisin levels correlate with energy expenditure in a subgroup of humans with energy expenditure greater than predicted by fat free mass. Metabolism. 2013;62:1070–1073. doi: 10.1016/j.metabol.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Teufel A, Malik N, Mukhopadhyay M, Westphal H. Frcp1 and Frcp2, two novel fibronectin type III repeat containing genes. Gene. 2002;297:79–83. doi: 10.1016/s0378-1119(02)00828-4. [DOI] [PubMed] [Google Scholar]
- Timmons JA, Baar K, Davidsen PK, Atherton PJ. Is irisin a human exercise gene. Nature. 2012;488:E9–E10. doi: 10.1038/nature11364. [DOI] [PubMed] [Google Scholar]
- Ukropcova B, McNeil M, Sereda O, de Jonge L, Xie H, Bray GA, Smith SR. Dynamic changes in fat oxidation in human primary myocytes mirror metabolic characteristics of the donor. J Clin Invest. 2005;115:1934–1941. doi: 10.1172/JCI24332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukropec J, Penesova A, Skopkova M, Pura M, Vlcek M, Radikova Z, Imrich R, Ukropcova B, Tajtakova M, Koska J, Zorad S, Belan V, Vanuga P, Payer J, Eckel J, Klimes I, Gasperikova D. Adipokine protein expression pattern in growth hormone deficiency predisposes to the increased fat cell size and the whole body metabolic derangements. J Clin Endocrinol Metab. 2008;93:2255–2262. doi: 10.1210/jc.2007-2188. [DOI] [PubMed] [Google Scholar]
- Valkovic L, Ukropcova B, Chmelik M, Balaz M, Bogner W, Schmid AI, Frollo I, Zemkova E, Klimes I, Ukropec J, Trattnig S, Krssak M. Interrelation of 31P-MRS metabolism measurements in resting and exercised quadriceps muscle of overweight-to-obese sedentary individuals. NMR Biomed. 2013;26:1714–1722. doi: 10.1002/nbm.3008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.