Abstract
We consider a population subdivided into two demes connected by migration in which selection acts in opposite direction. We explore the effects of recombination and migration on the maintenance of multilocus polymorphism, on local adaptation, and on differentiation by employing a deterministic model with genic selection on two linked diallelic loci (i.e., no dominance or epistasis). For the following cases, we characterize explicitly the possible equilibrium configurations: weak, strong, highly asymmetric, and super-symmetric migration, no or weak recombination, and independent or strongly recombining loci. For independent loci (linkage equilibrium) and for completely linked loci, we derive the possible bifurcation patterns as functions of the total migration rate, assuming all other parameters are fixed but arbitrary. For these and other cases, we determine analytically the maximum migration rate below which a stable fully polymorphic equilibrium exists. In this case, differentiation and local adaptation are maintained. Their degree is quantified by a new multilocus version of  and by the migration load, respectively. In addition, we investigate the invasion conditions of locally beneficial mutants and show that linkage to a locus that is already in migration-selection balance facilitates invasion. Hence, loci of much smaller effect can invade than predicted by one-locus theory if linkage is sufficiently tight. We study how this minimum amount of linkage admitting invasion depends on the migration pattern. This suggests the emergence of clusters of locally beneficial mutations, which may form ‘genomic islands of divergence’. Finally, the influence of linkage and two-way migration on the effective migration rate at a linked neutral locus is explored. Numerical work complements our analytical results.
and by the migration load, respectively. In addition, we investigate the invasion conditions of locally beneficial mutants and show that linkage to a locus that is already in migration-selection balance facilitates invasion. Hence, loci of much smaller effect can invade than predicted by one-locus theory if linkage is sufficiently tight. We study how this minimum amount of linkage admitting invasion depends on the migration pattern. This suggests the emergence of clusters of locally beneficial mutations, which may form ‘genomic islands of divergence’. Finally, the influence of linkage and two-way migration on the effective migration rate at a linked neutral locus is explored. Numerical work complements our analytical results.
Keywords: Selection, Migration, Recombination, Population subdivision, Genetic architecture, Multilocus polymorphism, Fixation index
Introduction
Migration in a geographically structured population may have opposing effects on the genetic composition of that population and, hence, on its evolutionary potential. On the one hand, gene flow caused by migration may be so strong that it not only limits but hinders local adaptation by swamping the whole population with a genotype that has high fitness in only one or a few demes. On the other hand, if migration is sufficiently weak, gene flow may replenish local populations with genetic variation and contribute to future adaptation. In this case, locally adapted genotypes may coexist in the population and maintain high levels of genetic variation as well as differentiation between subpopulations. For reviews of the corresponding, well developed one-locus theory, see Karlin (1982), Lenormand (2002), and Nagylaki and Lou (2008).
If selection acts on more than one locus, additional questions arise immediately. For instance, what are the consequences of the genetic architecture, such as linkage between loci, relative magnitude of locus effects, or epistasis, on the degree of local adaptation and of differentiation achieved for a given amount of gene flow? What are the consequences for genetic variation at linked neutral sites? What genetic architectures can be expected to evolve under various forms of spatially heterogeneous selection?
For selection acting on multiple loci, the available theory is much less well developed than for a single locus. One of the main reasons is that the interaction of migration and selection, even if the latter is nonepistatic, leads to linkage disequilibrium (LD) between loci (Li and Nei 1974; Christiansen and Feldman 1975; Slatkin 1975; Barton 1983). LD causes substantial, often insurmountable, complications in the analysis of multilocus models. Therefore, many multilocus studies are primarily numerical and focus on quite specific situations or problems. For instance, Spichtig and Kawecki (2004) investigated numerically the influence of the number of loci and of epistasis on the degree of polymorphism if selection acts antagonistically in two demes. Yeaman and Whitlock (2011) showed that concentrated genetic architecture, i.e., clusters of linked, locally beneficial alleles, evolve if stabilizing selection acts on a trait such that the fitness optima in two demes differ.
Linkage disequilibrium is also essential for the evolution of recombination. The evolution of recombination in heterogeneous environments has been studied by a number of authors (e.g., Charlesworth and Charlesworth 1979; Pylkov et al. 1998; Lenormand and Otto 2000), and the results depend strongly on the kind of variability of selection across environments, the magnitude of migration, and the sign and strength of epistasis.
Recent years have seen some advances in developing general theory for multilocus migration-selection models. The focus of this work was on the properties of the evolutionary dynamics and the conditions for the maintenance of multilocus polymorphism in limiting or special cases, such as weak or strong migration (Bürger 2009a; Bürger 2009b), or in the Levene model (Nagylaki 2009; Bürger 2009c; Bürger 2010; Barton 2010; Chasnov 2012). This progress was facilitated by the fact that in each case, LD is weak or absent.
Using a continent-island-model framework, Bürger and Akerman (2011) and Bank et al. (2012) analyzed the effects of gene flow on local adaptation, differentiation, the emergence of Dobzhansky-Muller incompatibilities, and the maintenance of polymorphism at two linked diallelic loci. They obtained analytical characterizations of the possible equilibrium configurations and bifurcation patterns for wide ranges of parameter combinations. In these models, typically high LD is maintained. In particular, explicit formulas were derived for the maximum migration rate below which a fully polymorphic equilibrium can be maintained, as well as for the minimum migration rate above which the island is swamped by the continental haplotype.
Here, we explore the robustness of some of these results by admitting arbitrary (forward and backward) migration between two demes. This generalization leads to substantial mathematical complications, but also to new biological insight. Because our focus is on the consequences of gene flow for local adaptation and differentiation, we assume divergent selection among the demes, i.e., alleles  and
and  are favored in deme 1, and
are favored in deme 1, and  and
and  are favored in deme 2. The loci may recombine at an arbitrary rate. By ignoring epistasis and dominance, we assume genic selection. Mutation and random drift are neglected. Because we assume evolution in continuous time, our model also describes selection on haploids.
are favored in deme 2. The loci may recombine at an arbitrary rate. By ignoring epistasis and dominance, we assume genic selection. Mutation and random drift are neglected. Because we assume evolution in continuous time, our model also describes selection on haploids.
The model is set up in Sect. 2. In Sect. 3, we derive the equilibrium and stability structure for several important special cases. These include weak, strong, highly asymmetric, and super-symmetric migration, no or weak recombination, independent or strongly recombining loci, and absence of genotype-environment interaction. In Sect. 4, we study the dependence of the equilibrium and stability patterns on the total migration rate while keeping the ratio of migration rates, the recombination rate, and the selection coefficients constant (but arbitrary). In particular, we derive the possible bifurcation patterns for the cases of independent loci (linkage equilibrium) and for completely linked loci. With the help of perturbation theory, we obtain the equilibrium and stability configurations for weak or strong migration, highly asymmetric migration, and weak or strong recombination. For these cases, we determine the maximum migration rate below which a stable, fully polymorphic equilibrium is maintained, and the minimum migration rate above which the population is monomorphic. Numerical work complements our analytical results.
The next four sections are devoted to applications of the theory developed in Sects. 3 and 4. In Sects. 5 and 6, we use the migration load and a new, genuine multilocus, fixation index ( ), respectively, to quantify the dependence of local adaptation and of differentiation on various parameters, especially, on the migration and the recombination rate. In Sect. 7, we investigate the invasion conditions for a mutant of small effect (
), respectively, to quantify the dependence of local adaptation and of differentiation on various parameters, especially, on the migration and the recombination rate. In Sect. 7, we investigate the invasion conditions for a mutant of small effect ( ) that is beneficial in one deme but disadvantageous in the other deme. We assume that the mutant is linked to a polymorphic locus which is in selection-migration balance. We show that linkage between the loci facilitates invasion. Therefore, in such a scenario, clusters of locally adapted alleles are expected to emerge (cf. Yeaman and Whitlock 2011; Bürger and Akerman 2011). In Sect. 8, we study the strength of barriers to gene flow at neutral sites linked to the selected loci by deriving an explicit approximation for the effective migration rate at a linked neutral site. Our results are summarized and discussed in Sect. 9. Several purely technical proofs are relegated to the Appendix.
) that is beneficial in one deme but disadvantageous in the other deme. We assume that the mutant is linked to a polymorphic locus which is in selection-migration balance. We show that linkage between the loci facilitates invasion. Therefore, in such a scenario, clusters of locally adapted alleles are expected to emerge (cf. Yeaman and Whitlock 2011; Bürger and Akerman 2011). In Sect. 8, we study the strength of barriers to gene flow at neutral sites linked to the selected loci by deriving an explicit approximation for the effective migration rate at a linked neutral site. Our results are summarized and discussed in Sect. 9. Several purely technical proofs are relegated to the Appendix.
The model
We consider a sexually reproducing population of monoecious, diploid individuals that is subdivided into two demes connected by genotype-independent migration. Within each deme, there is random mating. We assume that two diallelic loci are under genic selection, i.e., there is no dominance or epistasis, and different alleles are favored in different demes. We assume soft selection, i.e., population regulation occurs within each deme. We ignore random genetic drift and mutation and employ a deterministic continuous-time model to describe evolution. A continuous-time model is obtained from the corresponding discrete-time model in the limit of weak evolutionary forces (here, selection, recombination, and migration).
We denote the rate at which individuals in deme 1 (deme 2) are replaced by immigrants from the other deme by  (
( ). Then
). Then  is the total migration rate. The recombination rate between the two loci is designated by
is the total migration rate. The recombination rate between the two loci is designated by  .
.
Alleles at locus  are denoted by
are denoted by  and
and  , at locus
, at locus  by
by  and
and  . We posit that
. We posit that  and
and  are favored in deme 1, whereas
are favored in deme 1, whereas  and
and  are favored in deme 2. In deme
are favored in deme 2. In deme  (
( ), we assign the Malthusian parameters
), we assign the Malthusian parameters  and
and  to
to  and
and  , and
, and  and
and  to
to  and
and  . Because we assume absence of dominance and of epistasis, the resulting fitness matrix for the genotypes reads
. Because we assume absence of dominance and of epistasis, the resulting fitness matrix for the genotypes reads
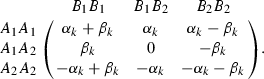 |
2.1 |
By relabeling alleles, we can assume without loss of generality  and
and  . Hence,
. Hence,  and
and  may be called the locally adapted haplotypes in deme 1 and deme 2, respectively. By relabeling loci, we can assume
may be called the locally adapted haplotypes in deme 1 and deme 2, respectively. By relabeling loci, we can assume  . We define
. We define
 |
2.2 |
By exchanging demes, i.e., by the transformation  and
and  (where
(where  denotes the deme
denotes the deme  ), or by exchanging loci, i.e., by the transformation
), or by exchanging loci, i.e., by the transformation  and
and  , we can further assume
, we can further assume  without loss of generality, cf. Appendix A.1.
without loss of generality, cf. Appendix A.1.
The fitness matrix (2.1) is also obtained if the two loci contribute additively to a quantitative trait that is under linear directional selection in each deme (Bürger 2009c). Then  if the genotypic values are deme independent, i.e., if there is no genotype-environment interaction on the trait level.
if the genotypic values are deme independent, i.e., if there is no genotype-environment interaction on the trait level.
Because in the case  degenerate features can occur, it will be treated separately (Sects. 3.9 and 3.10). Therefore, unless stated otherwise, we always impose the following assumptions on our parameters:
degenerate features can occur, it will be treated separately (Sects. 3.9 and 3.10). Therefore, unless stated otherwise, we always impose the following assumptions on our parameters:
 |
2.3a |
and
 |
2.3b |
and
 |
2.3c |
From (2.3a) and (2.3c), we infer
 |
2.4 |
Therefore, locus  is under weaker selection than locus
is under weaker selection than locus  in both demes, i.e.,
in both demes, i.e.,  for
for  , if and only if
, if and only if  holds.
holds.
The population can be described by the gamete frequencies in each of the demes. We denote the frequencies of the four possible gametes  ,
,  ,
,  , and
, and  in deme
in deme  by
by  ,
,  ,
,  , and
, and  . Then the state space is
. Then the state space is  , where
, where  is the simplex.
is the simplex.
The following differential equations for the evolution of gamete frequencies in deme  can be derived straightforwardly:
can be derived straightforwardly:
 |
2.5 |
Here the marginal fitness  of gamete
of gamete  and the mean fitness
and the mean fitness  in deme
in deme  are calculated from (2.1),
are calculated from (2.1),  , and
, and  is the linkage-disequilibrium (LD) measure. We note that
is the linkage-disequilibrium (LD) measure. We note that  corresponds to an excess of the locally adapted haplotypes in deme
corresponds to an excess of the locally adapted haplotypes in deme  . The equations (2.5) also describe the dynamics of a haploid population if in deme
. The equations (2.5) also describe the dynamics of a haploid population if in deme  we assign the fitnesses
we assign the fitnesses  ,
,  ,
,  ,
,  to the alleles
to the alleles  ,
,  ,
,  ,
,  , respectively.
, respectively.
Instead of gamete frequencies it is often more convenient to work with allele frequencies and the LD measures  . We write
. We write  and
and  for the frequencies of
for the frequencies of  and
and  in deme
in deme  . Then the gamete frequencies
. Then the gamete frequencies  are calculated from the
are calculated from the  ,
,  , and
, and  by
by
 |
2.6a |
 |
2.6b |
The constraints  and
and  for
for  and
and  transform into
transform into  and
and  . It follows that
. It follows that  ,
,  , and
, and  evolve according to
evolve according to
 |
2.7a |
 |
2.7b |
 |
2.7c |
We emphasize that, because we are treating a continuous-time model, the parameters  ,
,  ,
,  , and
, and  are rates (of recombination, migration, growth), whence they can be arbitrarily large. Their magnitude is determined by the time scale. By rescaling time, for instance to units of
are rates (of recombination, migration, growth), whence they can be arbitrarily large. Their magnitude is determined by the time scale. By rescaling time, for instance to units of  or
or  , the number of independent parameters could be reduced by one without changing the equilibrium properties.
, the number of independent parameters could be reduced by one without changing the equilibrium properties.
Equilibria and their stability
We distinguish three types of equilibria: (i) monomorphic equilibria (ME), (ii) single-locus polymorphisms (SLPs), and (iii) full (two-locus) polymorphisms (FPs). The first two types are boundary equilibria, whereas FPs are internal equilibria (except when  ). The stability properties of the ME and the coordinates and conditions for admissibility of the SLPs can be derived explicitly. However, the stability conditions for the SLPs and the conditions for existence or stability of FPs could be derived only for a number of limiting cases. These include strong recombination, weak or no recombination, weak, strong, or highly asymmetric migration.
). The stability properties of the ME and the coordinates and conditions for admissibility of the SLPs can be derived explicitly. However, the stability conditions for the SLPs and the conditions for existence or stability of FPs could be derived only for a number of limiting cases. These include strong recombination, weak or no recombination, weak, strong, or highly asymmetric migration.
Existence of boundary equilibria
The four ME, corresponding to fixation of one of the gametes, exist always. Their coordinates are as follows:
 |
where a  signifies an equilibrium. There are up to four SLPs, one in each marginal one-locus system. We denote the SLPs where
signifies an equilibrium. There are up to four SLPs, one in each marginal one-locus system. We denote the SLPs where  or
or  is fixed by
is fixed by  or
or  , respectively, and the SLPs where
, respectively, and the SLPs where  or
or  is fixed by
is fixed by  or
or  . Their coordinates and the conditions for their admissibility can be calculated explicitly (Eyland 1971). We define
. Their coordinates and the conditions for their admissibility can be calculated explicitly (Eyland 1971). We define
 |
3.1 |
By (2.3a), we have
 |
3.2 |
In addition, it is easy to show that the assumptions (2.3) imply:
 |
3.3a |
 |
3.3b |
If locus  is fixed (for
is fixed (for  or
or  ), the equilibrium allele frequencies at locus
), the equilibrium allele frequencies at locus  are
are
 |
3.4 |
If locus  is fixed, the equilibrium allele frequencies at locus
is fixed, the equilibrium allele frequencies at locus  are given by
are given by
 |
3.5 |
Thus, the four SLPs have the following coordinates:
 |
3.6a |
 |
3.6b |
 |
3.6c |
 |
3.6d |
The equilibria  and
and  are admissible if and only if
are admissible if and only if
 |
3.7 |
and the equilibria  and
and  are admissible if and only if
are admissible if and only if
 |
3.8 |
The SLPs leave the state space through one of their ‘neighboring’ ME if  or
or  increases above 1. In particular, we find
increases above 1. In particular, we find
 |
3.9a |
 |
3.9b |
 |
3.9c |
 |
3.9d |
Throughout, we use  to indicated convergence from above and
to indicated convergence from above and  to indicate convergence from below. Figure 1 illustrates the location of the possible equilibria.
to indicate convergence from below. Figure 1 illustrates the location of the possible equilibria.
Fig. 1.
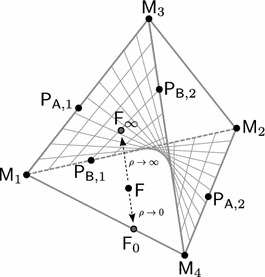
Location of equilibria. In terms of gamete frequencies, the state space is  , where each
, where each  corresponds to one deme. This figure shows (schematically) the location in
corresponds to one deme. This figure shows (schematically) the location in  of all boundary equilibria and of the stable internal equilibrium
of all boundary equilibria and of the stable internal equilibrium  .
.  converges to
converges to  if
if  and to
and to  if
if  . The LE manifold is indicated by hatching
. The LE manifold is indicated by hatching
The SLPs are asymptotically stable within their marginal one-locus system if and only if they are admissible. Then they are also globally asymptotically stable within their marginal system (Eyland 1971). (We use globally stable in the sense that at least all trajectories from the interior of the designated set converge to the equilibrium.) The reader may notice that (3.7) and (3.8) are precisely the conditions for maintaining a protected polymorphism at locus  and
and  , respectively.
, respectively.
Stability of monomorphic equilibria
At each monomorphic equilibrium, the characteristic polynomial factors into three quadratic polynomials. Two of them determine stability with respect to the marginal one-locus systems, whereas the third determines stability with respect to the interior of the state space. The stability properties of the monomorphic equilibria are as follows. The proof is given in Appendix A.2.
Proposition 3.1
 is asymptotically stable if
is asymptotically stable if
 |
3.10 |
and one of the following conditions hold:
 |
3.11a |
or
 |
3.11b |
 is always unstable.
is always unstable.
 is asymptotically stable if
is asymptotically stable if
 |
3.12 |
 is asymptotically stable if
is asymptotically stable if
 |
3.13 |
and one of the following conditions hold:
 |
3.14a |
or
 |
3.14b |
If one of the inequalities in (3.10), (3.12), or (3.13), or one of the inequalities for  in (3.11b) or (3.14b) is reversed, the respective equilibrium is unstable.
in (3.11b) or (3.14b) is reversed, the respective equilibrium is unstable.
If we assumed  , then
, then  would always be unstable and
would always be unstable and  would be stable if
would be stable if  and
and  .
.
The above result shows that each of  ,
,  , or
, or  can be stable, but never simultaneously. For sufficiently loose linkage, the stability of a ME is determined solely by its stability within the two marginal one-locus systems in which it occurs. Stability of
can be stable, but never simultaneously. For sufficiently loose linkage, the stability of a ME is determined solely by its stability within the two marginal one-locus systems in which it occurs. Stability of  is independent of the recombination rate. For given migration rates, the equilibria
is independent of the recombination rate. For given migration rates, the equilibria  and
and  may be stable for high recombination rates but unstable for low ones. For a low total migration rate (
may be stable for high recombination rates but unstable for low ones. For a low total migration rate ( ), no ME is stable. For a sufficiently high total migration rate, there is a globally asymptotically stable ME (Sect. 4.5).
), no ME is stable. For a sufficiently high total migration rate, there is a globally asymptotically stable ME (Sect. 4.5).
Stability of single-locus polymorphisms
As already mentioned, a single-locus polymorphism is globally attracting within its marginal one-locus system whenever it is admissible. Although the coordinates of the SLPs are given explicitly, the conditions for stability within the full, six-dimensional system on  are uninformative because the four eigenvalues that determine stability transversal to the marginal one-locus system are solutions of a complicated quartic equation.
are uninformative because the four eigenvalues that determine stability transversal to the marginal one-locus system are solutions of a complicated quartic equation.
In the following we treat several limiting cases in which the conditions for stability of the SLPs and for existence and stability of FPs can be obtained explicitly.
Weak migration
The equilibrium and stability structure for weak migration can be deduced from the model with no migration by perturbation theory. In the absence of migration ( ), the two subpopulations evolve independently. Because selection is nonepistatic and there is no dominance, in each deme the fittest haplotype becomes eventually fixed. In fact, mean fitness is nondecreasing (Ewens 1969). Our assumptions about fitness, i.e., (2.1) and (2.3a), imply that in deme 1 the equilibrium with
), the two subpopulations evolve independently. Because selection is nonepistatic and there is no dominance, in each deme the fittest haplotype becomes eventually fixed. In fact, mean fitness is nondecreasing (Ewens 1969). Our assumptions about fitness, i.e., (2.1) and (2.3a), imply that in deme 1 the equilibrium with  and
and  is globally attracting, and in deme 2 the equilibrium with
is globally attracting, and in deme 2 the equilibrium with  and
and  is globally attracting. Therefore, in the combined system, i.e., on
is globally attracting. Therefore, in the combined system, i.e., on  , but still with
, but still with  , the (unique) globally attracting equilibrium is given by
, the (unique) globally attracting equilibrium is given by
 |
3.15 |
All other equilibria are on the boundary and unstable.
Because, generically, all equilibria in the system without migration are hyperbolic and it is a gradient system (Shahshahani 1979; Bürger 2000, p. 42), Theorem 5.4 in Bürger (2009a) applies and shows that the perturbation  of the equilibrium (3.15) is globally asymptotically stable for sufficiently small migration rates
of the equilibrium (3.15) is globally asymptotically stable for sufficiently small migration rates  and
and  . Boundary equilibria remain unstable for sufficiently small migration rates. It is straightforward to calculate the coordinates of the perturbed equilibrium to leading order in
. Boundary equilibria remain unstable for sufficiently small migration rates. It is straightforward to calculate the coordinates of the perturbed equilibrium to leading order in  and
and  . They are given by
. They are given by
 |
3.16a |
 |
3.16b |
Therefore, we conclude
Proposition 3.2
For sufficiently weak migration, there is a unique, globally attracting, fully polymorphic equilibrium  . To leading order in
. To leading order in  and
and  , its coordinates are given by (3.16).
, its coordinates are given by (3.16).
Proposition 3.2 remains valid if the assumptions (2.3b) and (2.3c) are dropped. Apart from the obvious fact that migration reduces differences between subpopulations, the above approximations show that the lower the recombination rate, the smaller is this reduction. Thus, for given (small) migration rates, differentiation between subpopulations is always enhanced by reduced recombination. Linkage disequilibria within subpopulations are always positive.
Linkage equilibrium
If recombination is so strong relative to selection and migration that linkage equilibrium (LE) can be assumed, i.e., if  , the dynamics (2.7) simplifies to
, the dynamics (2.7) simplifies to
 |
3.17a |
 |
3.17b |
 |
3.17c |
 |
3.17d |
which is defined on  .
.
In (3.17), the differential equations for the two loci are decoupled, i.e., (3.17a) and (3.17b) as well as (3.17c) and (3.17d) form closed systems. Thus, the dynamics of the full system is a Cartesian product of the two one-locus dynamics. Therefore, in addition to the ME and to the SLPs determined above, the following internal equilibrium, denoted by  , may exist
, may exist
 |
3.18 |
where the  and
and  are given by (3.4) and (3.5), respectively. No other internal equilibrium can exist. This equilibrium is admissible if and only if (3.7) and (3.8) are satisfied, i.e., if and only if all four SLPs are admissible.
are given by (3.4) and (3.5), respectively. No other internal equilibrium can exist. This equilibrium is admissible if and only if (3.7) and (3.8) are satisfied, i.e., if and only if all four SLPs are admissible.
Because in the one-locus model the FP is globally asymptotically stable (hence, it attracts all trajectories from the interior of the state space) whenever it is admissible (Eyland 1971; Hadeler and Glas 1983, Theorem 2; Nagylaki and Lou 2008, Section 4.3.2), and because the full dynamics is the Cartesian product of the one-locus dynamics, the fully polymorphic equilibrium  is globally asymptotically stable whenever it is admissible. Similarly, we conclude that a boundary equilibrium is globally asymptotically stable whenever it is asymptotically stable in the full system. These results in combination with those in Sects. 3.1 and 3.2 yield the following proposition.
is globally asymptotically stable whenever it is admissible. Similarly, we conclude that a boundary equilibrium is globally asymptotically stable whenever it is asymptotically stable in the full system. These results in combination with those in Sects. 3.1 and 3.2 yield the following proposition.
Proposition 3.3
Assume (3.17). Then a globally asymptotically stable equilibrium exists always. This equilibrium is internal, hence equals  (3.18), if and only if (3.7) and (3.8) hold. It is a SLP if one of (3.7) or (3.8) is violated, and a ME if both (3.7) and (3.8) are violated.
(3.18), if and only if (3.7) and (3.8) hold. It is a SLP if one of (3.7) or (3.8) is violated, and a ME if both (3.7) and (3.8) are violated.
If, by variation of parameters, the internal equilibrium leaves (or enters) the state space, generically, it does so through one of the SLPs. The precise conditions are:
 |
3.19a |
 |
3.19b |
 |
3.19c |
 |
3.19d |
When, upon leaving the state space,  collides with a boundary equilibrium (SLP or ME), the respective boundary equilibrium becomes globally asymptotically stable.
collides with a boundary equilibrium (SLP or ME), the respective boundary equilibrium becomes globally asymptotically stable.
We note that  does not occur because it requires
does not occur because it requires  and
and  , which is impossible by (3.3). We leave the simple determination of the conditions for bifurcations of
, which is impossible by (3.3). We leave the simple determination of the conditions for bifurcations of  with one of the ME to the interested reader.
with one of the ME to the interested reader.
Proposition 3.3 can be extended straightforwardly to an arbitrary number of loci because the dynamics at each locus is independent of that at the other loci. This decoupling of loci occurs because there is no epistasis.
Strong recombination: quasi-linkage equilibrium
If recombination is strong, a regular perturbation analysis of the internal equilibrium  of (3.17) can be performed. The allele frequencies and linkage disequilibria can be calculated to order
of (3.17) can be performed. The allele frequencies and linkage disequilibria can be calculated to order  . Formally, we set
. Formally, we set
 |
3.20 |
keep  and
and  constant, and let
constant, and let  . Then, we obtain
. Then, we obtain
 |
3.21a |
 |
3.21b |
 |
3.21c |
and analogous formulas hold for the second deme. Because LD is of order  , this approximation may be called the quasi-linkage equilibrium approximation of the fully polymorphic equilibrium (Kimura 1965; Turelli and Barton 1990; Nagylaki et al. 1999). We note that LD is positive in both demes and increases with increasing differentiation between the demes, increasing migration, or decreasing recombination.
, this approximation may be called the quasi-linkage equilibrium approximation of the fully polymorphic equilibrium (Kimura 1965; Turelli and Barton 1990; Nagylaki et al. 1999). We note that LD is positive in both demes and increases with increasing differentiation between the demes, increasing migration, or decreasing recombination.
Proposition 5.1 in Bürger (2009a) shows that in every small neighborhood of an equilibrium of the model with LE (3.17), there is one equilibrium of the perturbed system, and it has the same stability properties as the unperturbed equilibrium. Because of the simple structure of (3.17), a stronger result can be obtained. In an isolated one-locus system on  (e.g., (3.17a) and (3.17b)), every trajectory from the interior converges to the unique asymptotically stable equilibrium (Sect. 3.5), and the chain-recurrent points (Conley 1978) are the equilibria. Therefore, the same holds for the LE dynamics (3.17), and the regular global perturbation result of Nagylaki et al. (1999) (the proof of their Theorem 2.3) applies for large
(e.g., (3.17a) and (3.17b)), every trajectory from the interior converges to the unique asymptotically stable equilibrium (Sect. 3.5), and the chain-recurrent points (Conley 1978) are the equilibria. Therefore, the same holds for the LE dynamics (3.17), and the regular global perturbation result of Nagylaki et al. (1999) (the proof of their Theorem 2.3) applies for large  . Hence the dynamical behavior with strong recombination is qualitatively the same as that under LE. We conclude that for sufficiently strong recombination every asymptotically stable equilibrium is globally asymptotically stable.
. Hence the dynamical behavior with strong recombination is qualitatively the same as that under LE. We conclude that for sufficiently strong recombination every asymptotically stable equilibrium is globally asymptotically stable.
No recombination
Let recombination be absent, i.e.,  . Then, effectively, we have a one-locus model in which the four alleles correspond to the four gametes
. Then, effectively, we have a one-locus model in which the four alleles correspond to the four gametes  ,
,  ,
,  ,
,  . In deme
. In deme  , they have the selection coefficients
, they have the selection coefficients  ,
,  ,
,  ,
,  , respectively. According to Theorem 2.4 of Nagylaki and Lou (2001), generically, no more than two gametes can be present at an equilibrium. We will prove a stronger result and characterize all possible equilibria and their local stability.
, respectively. According to Theorem 2.4 of Nagylaki and Lou (2001), generically, no more than two gametes can be present at an equilibrium. We will prove a stronger result and characterize all possible equilibria and their local stability.
Because  , there may be a polymorphic equilibrium at which only the gametes
, there may be a polymorphic equilibrium at which only the gametes  and
and  are present. We call it
are present. We call it  and set
and set
 |
3.22 |
Then one-locus theory (Sect. 3.1) informs us that  is admissible if and only if
is admissible if and only if
 |
3.23 |
Its coordinates are given by
 |
3.24a |
 |
3.24b |
 |
3.24c |
where  ,
,  , and
, and  (
( ). Within the subsystem in which only the gametes
). Within the subsystem in which only the gametes  and
and  are present,
are present,  is asymptotically stable whenever it is admissible. One-locus theory implies that
is asymptotically stable whenever it is admissible. One-locus theory implies that
 |
3.25a |
 |
3.25b |
A simple application of Corollary 3.9 of Nagylaki and Lou (2007) shows that the gamete  will always be lost (to apply their result, recall assumptions (2.3) and use
will always be lost (to apply their result, recall assumptions (2.3) and use  ,
,  ,
,  ). This strengthens the result in Sect. 3.2 that
). This strengthens the result in Sect. 3.2 that  is always unstable. Thus, we are left with the analysis of the tri-gametic system consisting of
is always unstable. Thus, we are left with the analysis of the tri-gametic system consisting of  ,
,  , and
, and  . (If
. (If  , then gamete
, then gamete  is lost.)
is lost.)
In Appendix A.3 it is proved that  is the only equilibrium at which both loci are polymorphic except when
is the only equilibrium at which both loci are polymorphic except when
 |
3.26 |
holds, where
 |
3.27 |
If (3.26) holds, then there is a line of internal equilibria connecting  with
with  or
or  (or
(or  ); see Appendix A.3.
); see Appendix A.3.
We find that  is asymptotically stable if
is asymptotically stable if
 |
3.28 |
and unstable if the inequality is reversed (Appendix A.4). For sufficiently small migration rates, Proposition 3.2 implies that  and
and  is globally asymptotically stable. If the inequality in (3.28) is reversed,
is globally asymptotically stable. If the inequality in (3.28) is reversed,  may or may not be admissible.
may or may not be admissible.
Of course, if  is asymptotically stable, then the equilibria
is asymptotically stable, then the equilibria  and
and  are unstable; cf. (3.25). The following argument shows that
are unstable; cf. (3.25). The following argument shows that  cannot be simultaneously stable with
cannot be simultaneously stable with  . We rewrite (3.28) as
. We rewrite (3.28) as
 |
3.29 |
Because
 |
3.30 |
(3.29) becomes
 |
3.31 |
Since  is asymptotically stable if (3.12) holds and because, as is easy to show, (3.12) and (3.31) are incompatible, the assertion follows. It can also be shown from (3.12) and (3.31) that
is asymptotically stable if (3.12) holds and because, as is easy to show, (3.12) and (3.31) are incompatible, the assertion follows. It can also be shown from (3.12) and (3.31) that  cannot become stable when
cannot become stable when  loses its stability except in the degenerate case when
loses its stability except in the degenerate case when  and
and  .
.
In our tri-gametic system,  and
and  are the only possible SLPs. They may exist simultaneously with
are the only possible SLPs. They may exist simultaneously with  if (3.28) holds, i.e., if
if (3.28) holds, i.e., if  is stable, but not otherwise (Appendix A.5). If (3.28) holds, both are unstable (if admissible).
is stable, but not otherwise (Appendix A.5). If (3.28) holds, both are unstable (if admissible).  or
or  have an eigenvalue 0 if and only if (3.26) holds or if they leave or enter the state space through a ME. In Appendix A.5 it is shown that
have an eigenvalue 0 if and only if (3.26) holds or if they leave or enter the state space through a ME. In Appendix A.5 it is shown that  is asymptotically stable if and only if
is asymptotically stable if and only if
 |
3.32 |
and  is asymptotically stable if and only if
is asymptotically stable if and only if
 |
3.33 |
Hence, if  increases above
increases above  , the SLP that is admissible becomes asymptotically stable. Upon collision of the stable SLP with one of the adjacent ME, the corresponding ME becomes stable and remains so for all higher migration rates. We summarize these findings as follows:
, the SLP that is admissible becomes asymptotically stable. Upon collision of the stable SLP with one of the adjacent ME, the corresponding ME becomes stable and remains so for all higher migration rates. We summarize these findings as follows:
Proposition 3.4
Except in the degenerate case when (3.26) holds, only equilibria with at most two gametes present exist. If (3.23) is satisfied, the equilibrium  given by (3.24) is admissible. If, in addition, (3.28) is fulfilled, then
given by (3.24) is admissible. If, in addition, (3.28) is fulfilled, then  is asymptotically stable. For sufficiently small migration rates, it is globally asymptotically stable. If
is asymptotically stable. For sufficiently small migration rates, it is globally asymptotically stable. If  is unstable or not admissible, then one of the ME
is unstable or not admissible, then one of the ME  ,
,  ,
,  or one of the SLPs
or one of the SLPs  ,
,  is asymptotically stable. If (3.26) holds, then there is a line of equilibria with three gametes present.
is asymptotically stable. If (3.26) holds, then there is a line of equilibria with three gametes present.
The proposition shows that, except for the nongeneric case when (3.26) holds, there is always precisely one stable equilibrium point. Numerical results support the conjecture that the stable equilibrium is globally asymptotically stable. Bifurcation patterns as functions of  are derived in Sect. 4.8.
are derived in Sect. 4.8.
In addition to  , there exists a second FP on the edge connecting
, there exists a second FP on the edge connecting  and
and  . Although its coordinates can be calculated easily, it is not of interest here as it is unstable for every choice of selection and migration parameters. This unstable equilibrium leaves the state space under small perturbations, i.e., if
. Although its coordinates can be calculated easily, it is not of interest here as it is unstable for every choice of selection and migration parameters. This unstable equilibrium leaves the state space under small perturbations, i.e., if  .
.
Highly asymmetric migration
All special cases treated above suggest that there always exists a globally asymptotically stable equilibrium. This, however, is generally not true as was demonstrated by the analysis of the two-locus continent-island (CI) model in Bürger and Akerman (2011). There, all possible bifurcation patterns were derived and it was shown that the fully polymorphic equilibrium can be simultaneously stable with a boundary equilibrium. For highly asymmetric migration rates, the equilibrium and stability structure can be obtained by a perturbation analysis of this CI model.
Therefore, we first summarize the most relevant features of the analysis in Bürger and Akerman (2011). Because in that analysis the haplotype  is fixed on the continent (here, deme 2) and there is no back migration (
is fixed on the continent (here, deme 2) and there is no back migration ( ), it is sufficient to treat the dynamics on the island (here, deme 1) where immigration of
), it is sufficient to treat the dynamics on the island (here, deme 1) where immigration of  occurs at rate
occurs at rate  . Thus, the state space is
. Thus, the state space is  .
.
It was shown that up to two internal (fully polymorphic) equilibria, denoted by  and
and  , may exist. Only one (
, may exist. Only one ( ) can be stable. Two SLPs,
) can be stable. Two SLPs,  and
and  , may exist. At
, may exist. At  , locus
, locus  is polymorphic and allele
is polymorphic and allele  is fixed; at
is fixed; at  , locus
, locus  is polymorphic and allele
is polymorphic and allele  is fixed.
is fixed.  (
( ) is admissible if and only if
) is admissible if and only if  (
( ).
).  is always unstable. Finally, there always exists the monomorphic equilibrium
is always unstable. Finally, there always exists the monomorphic equilibrium  at which the haplotype
at which the haplotype  is fixed on the island. The equilibrium coordinates of all equilibria were obtained explicitly. In addition, it was proved (see also Bank et al. 2012, Supporting Information, Theorem S.4) that precisely the following two types of bifurcation patterns can occur:
is fixed on the island. The equilibrium coordinates of all equilibria were obtained explicitly. In addition, it was proved (see also Bank et al. 2012, Supporting Information, Theorem S.4) that precisely the following two types of bifurcation patterns can occur:
Type 1 There exists a critical migration rate  such that:
such that:
If
 , a unique internal equilibrium,
, a unique internal equilibrium,  , exists. It is asymptotically stable and, presumably, globally asymptotically stable.
, exists. It is asymptotically stable and, presumably, globally asymptotically stable.At
 ,
,  leaves the state space through a boundary equilibrium (
leaves the state space through a boundary equilibrium ( or
or  ) by an exchange-of-stability bifurcation.
) by an exchange-of-stability bifurcation.If
 , a boundary equilibrium (
, a boundary equilibrium ( or
or  ) is asymptotically stable and, presumably, globally stable.
) is asymptotically stable and, presumably, globally stable.
Type 2 There exist critical migration rates  and
and  satisfying
satisfying  such that:
such that:
If
 , there is a unique internal equilibrium
, there is a unique internal equilibrium  . It is asymptotically stable and, presumably, globally stable.
. It is asymptotically stable and, presumably, globally stable.At
 , an unstable equilibrium
, an unstable equilibrium  enters the state space by an exchange-of-stability bifurcation with a boundary equilibrium (
enters the state space by an exchange-of-stability bifurcation with a boundary equilibrium ( or
or  ).
).If
 , there are two internal equilibria, one asymptotically stable
, there are two internal equilibria, one asymptotically stable  , the other unstable
, the other unstable  , and one of the boundary equilibria (
, and one of the boundary equilibria ( or
or  ) is asymptotically stable.
) is asymptotically stable.At
 , the two internal equilibria merge and annihilate each other by a saddle-node bifurcation.
, the two internal equilibria merge and annihilate each other by a saddle-node bifurcation.If
 , a boundary equilibrium (
, a boundary equilibrium ( or
or  ) is asymptotically stable and, presumably, globally stable.
) is asymptotically stable and, presumably, globally stable.
For sufficiently large migration rates ( ),
),  is globally asymptotically stable in both cases. Bifurcation patterns of Type 2 occur only if the recombination rate is intermediate, i.e., if
is globally asymptotically stable in both cases. Bifurcation patterns of Type 2 occur only if the recombination rate is intermediate, i.e., if  is about as large as
is about as large as  .
.
By imbedding the CI model into the two-deme dynamics, (2.5) or (2.7), perturbation theory can be applied to obtain analogous results for highly asymmetric migration, i.e., for sufficiently small  (Karlin and McGregor 1972). This is so because all equilibria in the CI model are hyperbolic except when collisions between equilibria occur (Bürger and Akerman 2011). Since the coordinates of the internal equilibria
(Karlin and McGregor 1972). This is so because all equilibria in the CI model are hyperbolic except when collisions between equilibria occur (Bürger and Akerman 2011). Since the coordinates of the internal equilibria  and
and  were derived, the perturbed equilibrium frequencies can be obtained. Because they are too complicated to be informative, we do not present them. The perturbation of
were derived, the perturbed equilibrium frequencies can be obtained. Because they are too complicated to be informative, we do not present them. The perturbation of  , denoted by
, denoted by  , is asymptotically stable. As
, is asymptotically stable. As  is internal, it cannot be lost by a small perturbation. Also the boundary equilibria and their stability properties are preserved under small perturbations. In particular,
is internal, it cannot be lost by a small perturbation. Also the boundary equilibria and their stability properties are preserved under small perturbations. In particular,  gives rise to
gives rise to  , and the SLPs
, and the SLPs  and
and  give rise to
give rise to  and
and  , respectively,
, respectively,
If recombination is intermediate, (at least) under highly asymmetric two-way migration, one stable and one unstable FP can coexist. In this case the stable FP,  , is simultaneously stable with either
, is simultaneously stable with either  or
or  . Although there is precisely one (perturbed) equilibrium in a small neighborhood of every equilibrium of the CI model, we can not exclude that other internal equilibria or limit sets are generated by perturbation.
. Although there is precisely one (perturbed) equilibrium in a small neighborhood of every equilibrium of the CI model, we can not exclude that other internal equilibria or limit sets are generated by perturbation.
The case 
The analyses in the previous sections are based on the assumptions (2.3), in particular, on  . However, many of the results obtained above remain valid if
. However, many of the results obtained above remain valid if  . Here, we point out the necessary adjustments.
. Here, we point out the necessary adjustments.
Without loss of generality, we can assume
 |
3.34 |
in addition to  and (2.3a). Then we observe that
and (2.3a). Then we observe that
 |
3.35 |
Therefore, either
 |
3.36a |
or
 |
3.36b |
or
 |
3.36c |
applies, where equality in (3.36a) and (3.36b) holds if  (
( ). In addition,
). In addition,
 |
3.37 |
With these preliminaries, we can treat the changes required in the above propositions if  .
.
From (3.36) we infer that in Proposition 3.1 not only  but also
but also  is always unstable. In addition, if
is always unstable. In addition, if  , then
, then  is asymptotically stable for sufficiently strong migration, whereas
is asymptotically stable for sufficiently strong migration, whereas  is stable for sufficiently strong migration if
is stable for sufficiently strong migration if  holds.
holds.
As already noted, Proposition 3.2 remains valid independently of the value of  .
.
In Proposition 3.3, the only SLPs through which the internal equilibrium  can leave the state space are
can leave the state space are  and
and  ; see (3.19c) and (3.19d). The reason is that, except when
; see (3.19c) and (3.19d). The reason is that, except when  (and (3.37) applies),
(and (3.37) applies),  and
and  are only admissible if
are only admissible if  and
and  are. Thus, the locus under weaker selection always becomes monomorphic at lower rates of gene flow than the locus under stronger selection.
are. Thus, the locus under weaker selection always becomes monomorphic at lower rates of gene flow than the locus under stronger selection.
If  (Proposition 3.4),
(Proposition 3.4),  is asymptotically stable whenever it is admissible because
is asymptotically stable whenever it is admissible because  as
as  ; see (3.28). In addition, (3.36) implies that
; see (3.28). In addition, (3.36) implies that  persists stronger gene flow than the SLPs, which are always unstable; see (3.32) and (3.33).
persists stronger gene flow than the SLPs, which are always unstable; see (3.32) and (3.33).
In the highly symmetric case of (3.36c), SLPs cannot be lost. Thus,  is always admissible and globally stable, cf. Proposition 3.3. If
is always admissible and globally stable, cf. Proposition 3.3. If  , (3.37) implies that
, (3.37) implies that  exists always (and is stable). In the next section we show that in this highly symmetric case the FP is always admissible for arbitrary recombination rates.
exists always (and is stable). In the next section we show that in this highly symmetric case the FP is always admissible for arbitrary recombination rates.
The super-symmetric case
In many, especially ecological, applications highly symmetric migration-selection models are studied. Frequently made assumptions are that the migration rates between the demes are identical ( ), selection in deme 2 mirrors that in deme 1 (
), selection in deme 2 mirrors that in deme 1 ( ), and the loci are equivalent (
), and the loci are equivalent ( ). Thus,
). Thus,  and (3.36c) holds, which we assume now.
and (3.36c) holds, which we assume now.
Conditions (3.7) and (3.8) imply that all four SLPs are admissible. Hence, all monomorphisms are unstable. In addition, it can be proved that all SLPs are unstable (Appendix A.6). If migration is weak, a globally asymptotically stable, fully polymorphic equilibrium ( ) exists (Proposition 3.2).
) exists (Proposition 3.2).
Because every boundary equilibrium is hyperbolic for every parameter choice, the index theorem of Hofbauer (1990) can be applied. Since none of the boundary equilibria is saturated, it follows that an internal equilibrium with index 1 exists. For small migration rates, this is  because it is unique. Since the boundary equilibria are always hyperbolic, no internal equilibrium can leave the state space through the boundary. However, we cannot exclude that the internal equilibrium undergoes a pitchfork or a Hopf bifurcation. Numerical results support the conjecture that the internal equilibrium is unique and globally attracting, independently of the strength of migration. This is a very special feature of this super-symmetric case; cf. Proposition 4.3.
because it is unique. Since the boundary equilibria are always hyperbolic, no internal equilibrium can leave the state space through the boundary. However, we cannot exclude that the internal equilibrium undergoes a pitchfork or a Hopf bifurcation. Numerical results support the conjecture that the internal equilibrium is unique and globally attracting, independently of the strength of migration. This is a very special feature of this super-symmetric case; cf. Proposition 4.3.
General case
Because a satisfactory analysis for general parameter choices seems out of reach, we performed extensive numerical work to determine the possible equilibrium structures. In no case did we find more complicated equilibrium structures than indicated above, i.e., apparently there are never more than two internal equilibria. If there is one internal equilibrium, it appears to be globally asymptotically stable. If there are two internal equilibria, then one is unstable and the other is simultaneously stable with one boundary equilibrium (as in the CI model). Apparently, two internal equilibria occur only for sufficiently asymmetric migration rates and only if the recombination rate is of similar magnitude as the selection coefficients.
A glance at the dynamical equations (2.7) reveals that an internal equilibrium can be in LE only if  or
or  . From (3.18), (3.4) and (3.5), we find that this can occur only if
. From (3.18), (3.4) and (3.5), we find that this can occur only if  or
or  , i.e., for a boundary equilibrium. Thus, internal equilibria always exhibit LD.
, i.e., for a boundary equilibrium. Thus, internal equilibria always exhibit LD.
For low migration rates as well as for high recombination rates, there is a unique, fully polymorphic equilibrium which is globally asymptotically stable and exhibits positive LD (Sects. 3.4 or 3.6). We denote the (presumably unique) asymptotically stable, fully polymorphic equilibrium by  . If migration is weak, or recombination is weak, or recombination is strong, we have proved that
. If migration is weak, or recombination is weak, or recombination is strong, we have proved that  is unique. Useful approximations are available for weak migration or strong recombination; see (3.16) or (3.21). Finally, for sufficiently high migration rates one of the monomorphic equilibria is globally asymptotically stable.
is unique. Useful approximations are available for weak migration or strong recombination; see (3.16) or (3.21). Finally, for sufficiently high migration rates one of the monomorphic equilibria is globally asymptotically stable.
Bifurcation patterns and maintenance of polymorphism
Here we study how genetic variation and polymorphism depend on the strength and pattern of migration. In particular, we are interested in determining how the maximum migration rate that permits genetic polymorphism depends on the other parameters. For this end, we explore properties of our model, such as the possible bifurcation patterns, as functions of the total migration rate  . We do this by assuming that
. We do this by assuming that  ,
,  ,
,  ,
,  ,
,  , and the migration ratio
, and the migration ratio
 |
4.1 |
where  and
and  , are constant. The values
, are constant. The values  and
and  correspond to one-way migration, as in the CI model. If
correspond to one-way migration, as in the CI model. If  , migration between the demes is symmetric, an assumption made in many studies of migration-selection models. Fixing
, migration between the demes is symmetric, an assumption made in many studies of migration-selection models. Fixing  and treating
and treating  as the only migration parameter corresponds to the migration scheme introduced by Deakin (1966).
as the only migration parameter corresponds to the migration scheme introduced by Deakin (1966).
Important quantities
We define several important quantities that will be needed to describe our results and summarize the relevant relations between them. Let
 |
4.2a |
 |
4.2b |
 |
4.2c |
 |
4.2d |
 |
4.2e |
 |
4.2f |
 |
4.2g |
 |
4.2h |
 |
4.2i |
 |
4.2j |
 |
4.2k |
and
 |
4.3a |
 |
4.3b |
 |
4.3c |
 |
4.3d |
 |
4.3e |
 |
4.3f |
We set  ,
,  , and
, and  if
if  ,
,  , and
, and  , respectively. Similarly, we set
, respectively. Similarly, we set  if
if  ,
,  , or
, or  .
.
The quantities  ,
,  , and
, and  yield the bounds for the intervals of total migration rates
yield the bounds for the intervals of total migration rates  in which the SLPs at
in which the SLPs at  ,
,  , and the polymorphic equilibrium
, and the polymorphic equilibrium  , respectively, are admissible:
, respectively, are admissible:
 |
4.4a |
 |
4.4b |
 |
4.4c |
Here, the left and the right inequalities correspond, and we have
 |
4.5a |
 |
4.5b |
 |
4.5c |
From (2.3), we obtain
 |
4.6 |
 |
4.7 |
The quantities  and
and  occur in the stability conditions of the monomorphic equilibria
occur in the stability conditions of the monomorphic equilibria  and
and  (Proposition 4.1), and
(Proposition 4.1), and  determines the range of stability of
determines the range of stability of  ; see (4.56). They satisfy
; see (4.56). They satisfy
 |
4.8 |
We note that  ,
,  ,
,  , and
, and  assume their minima if
assume their minima if  and their maxima if
and their maxima if  , whereas
, whereas  assumes its minimum or maximum at
assumes its minimum or maximum at  or
or  , respectively.
, respectively.  is a convex function of
is a convex function of  , and symmetric around its minimum
, and symmetric around its minimum  .
.
The definitions of (several of) the quantities  are motivated by the following relations:
are motivated by the following relations:
 |
4.9a |
 |
4.9b |
 |
4.9c |
 |
4.9d |
 |
4.9e |
 |
4.9f |
 |
4.9g |
 |
4.9h |
where we have
 |
4.10 |
The following relations apply to  :
:
 |
4.11a |
 |
4.11b |
 |
4.11c |
where we derived (4.11b) and (4.11c) from (4.9c) and (4.9f) using (4.10).
In the following, we summarize the most important inequalities between the quantities  :
:
 |
4.12 |
 |
4.13 |
 |
4.14 |
They can be derived straightforwardly from their definitions and our general assumption (2.3). Finally, if  and
and  , the following relations hold:
, the following relations hold:
 |
4.15a |
 |
4.15b |
and
 |
4.15c |
Additional relations that are needed only in the proofs may be found in Appendix A.7.
Admissibility of SLPs
We begin by expressing the conditions for admissibility of the SLPs in terms of the total migration rate  and the migration ratio
and the migration ratio  . Since, by (3.7), (3.8), and (4.4), every SLP is admissible if
. Since, by (3.7), (3.8), and (4.4), every SLP is admissible if  is sufficiently small and leaves the state space at a uniquely defined critical migration rate, it is sufficient to determine this critical rate and the monomorphism through which it leaves the state space. Using (4.3a), (4.3b), (4.5), and (4.4), we infer from (3.9) that
is sufficiently small and leaves the state space at a uniquely defined critical migration rate, it is sufficient to determine this critical rate and the monomorphism through which it leaves the state space. Using (4.3a), (4.3b), (4.5), and (4.4), we infer from (3.9) that
 |
4.16a |
 |
4.16b |
 |
4.16c |
 |
4.16d |
In particular, no SLP is admissible if
 |
4.17 |
We observe that locus  is polymorphic and locus
is polymorphic and locus  is monomorphic if and only if
is monomorphic if and only if
 |
4.18 |
If  , we infer from (10.18c) and (10.18d) that (4.18) holds if and only if
, we infer from (10.18c) and (10.18d) that (4.18) holds if and only if
 |
4.19 |
Therefore, (2.4) implies that if locus  is under weaker selection than locus
is under weaker selection than locus  in both demes (
in both demes ( ), then there is a range of values
), then there is a range of values  and
and  such that locus
such that locus  is polymorphic whereas
is polymorphic whereas  is monomorphic. This is in contrast to the CI model or highly asymmetric migration rates or
is monomorphic. This is in contrast to the CI model or highly asymmetric migration rates or  , where it is always the locus under weaker selection that first loses its polymorphism while
, where it is always the locus under weaker selection that first loses its polymorphism while  increases. This is a pure one-locus result and a consequence of the classical condition for a protected polymorphism, e.g., (3.7). With two-way migration, a locus with alleles of small and similar (absolute) effects in the demes (
increases. This is a pure one-locus result and a consequence of the classical condition for a protected polymorphism, e.g., (3.7). With two-way migration, a locus with alleles of small and similar (absolute) effects in the demes ( ) may be maintained polymorphic for higher migration rates than a locus with alleles of large and very different (absolute) effects.
) may be maintained polymorphic for higher migration rates than a locus with alleles of large and very different (absolute) effects.
Stability of monomorphic equilibria
Here, we reformulate the stability conditions of the ME derived in Sect. 3.2 in terms of  and
and  .
.
Proposition 4.1
 is asymptotically stable if
is asymptotically stable if
 |
4.20 |
 is always unstable.
is always unstable.
 is asymptotically stable if
is asymptotically stable if
 |
4.21 |
 is asymptotically stable if
is asymptotically stable if
 |
4.22 |
If in these conditions one inequality is reversed, the corresponding equilibrium is unstable.
Proof
We prove only that the statement about  is equivalent to that in Proposition 3.1. The others follow analogously or are immediate.
is equivalent to that in Proposition 3.1. The others follow analogously or are immediate.
From Proposition 3.1 and (4.3a), (4.3b), (4.3d), and (4.4), we infer immediately that  is asymptotically stable if and only if
is asymptotically stable if and only if
 |
4.23 |
and
 |
4.24 |
The possible inequalities between  ,
,  , and
, and  are given in (10.32) and (10.33). By (4.5a), (4.5b), and (4.12), it follows that (4.23) is feasible if and only if
are given in (10.32) and (10.33). By (4.5a), (4.5b), and (4.12), it follows that (4.23) is feasible if and only if  . Thus if
. Thus if  ,
,  is unstable. Therefore, (4.23) and (4.24) are equivalent to (4.20).
is unstable. Therefore, (4.23) and (4.24) are equivalent to (4.20). 
Remark 4.2
- (i)
- (ii)
-
(iii)
An internal equilibrium in LD can leave or enter the state space through
 or
or  only if
only if  or
or  , respectively. If (4.25) or (4.26) holds, then
, respectively. If (4.25) or (4.26) holds, then  or
or  , respectively, become asymptotically stable by the bifurcation.
, respectively, become asymptotically stable by the bifurcation.
Proof of Remark 4.2
If  , statement (i) is an immediate consequence of (10.32a) and (10.32b) because
, statement (i) is an immediate consequence of (10.32a) and (10.32b) because  implies
implies  . If
. If  , then (10.32a), (10.32b), and (10.32c) show that
, then (10.32a), (10.32b), and (10.32c) show that  if (a)
if (a)  (10.23) and
(10.23) and  or (b)
or (b)  and
and  or (c)
or (c)  and
and  , where
, where  by (10.26a). Invoking (10.31), we can combine conditions (a), (b), and (c) to obtain (4.25a).
by (10.26a). Invoking (10.31), we can combine conditions (a), (b), and (c) to obtain (4.25a).
Statement (ii) follows directly from (10.18f) and (10.35a).
Statement (iii) follows by observing that only internal equilibria in LD will depend on  , the factor
, the factor  (10.4) in the characteristic polynomial at
(10.4) in the characteristic polynomial at  is the only one that depends on
is the only one that depends on  , and
, and  gives rise to an eigenvalue zero if and only if
gives rise to an eigenvalue zero if and only if  . An analogous argument holds for
. An analogous argument holds for  .
. 
The asymmetry between (4.25) and (4.26) results from the fact that  is assumed, whereas
is assumed, whereas  or
or  is possible. The reader may recall the comments made below Proposition 3.1. In addition, we note that if the fitness parameters and
is possible. The reader may recall the comments made below Proposition 3.1. In addition, we note that if the fitness parameters and  and
and  are fixed, a stable ME remains stable if
are fixed, a stable ME remains stable if  is increased. This is not necessarily so if
is increased. This is not necessarily so if  and
and  are varied simultaneously. For related phenomena in the one-locus case, see Karlin (1982) and Nagylaki (2012). In Sect. 4.5, we will prove global convergence to one of the asymptotically stable ME if
are varied simultaneously. For related phenomena in the one-locus case, see Karlin (1982) and Nagylaki (2012). In Sect. 4.5, we will prove global convergence to one of the asymptotically stable ME if  is sufficiently large.
is sufficiently large.
Weak migration
We recall from Proposition 3.2 that for sufficiently weak migration, there is a fully polymorphic equilibrium, it is globally asymptotically stable, and exhibits positive LD in both demes.
Strong migration
Proposition 4.3
For sufficiently large  , one of the monomorphic equilibria
, one of the monomorphic equilibria  ,
,  , or
, or  is globally attracting. This equilibrium is
is globally attracting. This equilibrium is  ,
,  , or
, or  if
if  ,
,  , or
, or  , respectively.
, respectively.
Proof
The proof is based on the perturbation results about the strong-migration limit in Section 4.2 of Bürger (2009a). The strong-migration limit is obtained if  . In this limit, the demes become homogeneous and the system of differential equations (2.7) converges to a system, where in each deme
. In this limit, the demes become homogeneous and the system of differential equations (2.7) converges to a system, where in each deme
 |
4.27a |
 |
4.27b |
 |
4.27c |
holds with  ,
,  ,
,  . Here,
. Here,
 |
4.28 |
are the spatially averaged selection coefficients and averaging is performed with respect to the Perron-Frobenius eigenvector  of the migration matrix (see Section 4.2 in Bürger 2009a for a much more general treatment starting with a multilocus model in discrete time). Therefore, Proposition 4.10 in Bürger (2009a) applies and, provided
of the migration matrix (see Section 4.2 in Bürger 2009a for a much more general treatment starting with a multilocus model in discrete time). Therefore, Proposition 4.10 in Bürger (2009a) applies and, provided  is sufficiently large, all trajectories of (2.7) converge to a manifold on which the allele frequencies and the linkage disequilibria in both demes are nearly identical. In addition, in the neighborhood of each hyperbolic equilibrium of (4.27) there is exactly one equilibrium of (2.7), and it has the same stability.
is sufficiently large, all trajectories of (2.7) converge to a manifold on which the allele frequencies and the linkage disequilibria in both demes are nearly identical. In addition, in the neighborhood of each hyperbolic equilibrium of (4.27) there is exactly one equilibrium of (2.7), and it has the same stability.
In the present case, the conclusion of Proposition 4.10 in Bürger (2009a) can be considerably strengthened. Because the system (4.27) describes evolution in an ordinary two-locus model under genic selection, the ME representing the gamete of highest fitness is globally asymptotically stable. In fact, (4.27) is also a generalized gradient system for which Lemma 2.2 of Nagylaki et al. (1999) holds. Therefore, the analog of statement (c) in Theorem 4.3 of Bürger (2009a) applies and yields global convergence to the unique stable equilibrium.
Finally, it is an easy exercise to show that, in the strong-migration limit, i.e., with fitnesses averaged according to (4.28), gamete  ,
,  , or
, or  has highest fitness if
has highest fitness if  ,
,  , or
, or  , respectively. Since there is no dominance, the corresponding ME is the unique stable equilibrium.
, respectively. Since there is no dominance, the corresponding ME is the unique stable equilibrium. 
Linkage equilibrium
We shall establish all possible equilibrium configurations and their dependence on the parameters under LE. In Fig. 2, the equilibrium configurations are displayed as schematic bifurcation diagrams with the total migration rate  as the bifurcation parameter. In Theorem 4.4, we assign to each diagram its pertinent parameter combinations.
as the bifurcation parameter. In Theorem 4.4, we assign to each diagram its pertinent parameter combinations.
Fig. 2.
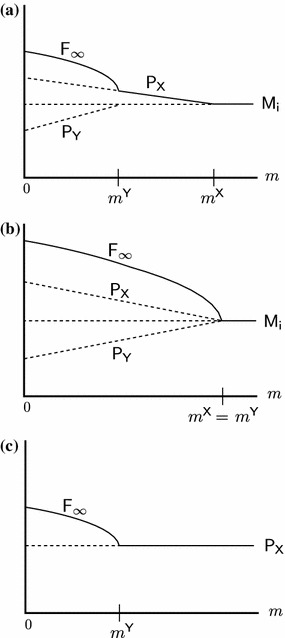
Bifurcation diagrams for LE. Diagrams (a)–(c) display the equilibrium configurations listed in Theorem 4.4. Each line indicates one admissible equilibrium as a function of the total migration rate  . Only equilibria are shown that can be stable or are involved in a bifurcation with an equilibrium that can be stable. Lines are drawn such that intersections occur if and only if the corresponding equilibria collide. Solid lines represent asymptotically stable equilibria, dashed lines unstable equilibria. The meaning of the superscripts
. Only equilibria are shown that can be stable or are involved in a bifurcation with an equilibrium that can be stable. Lines are drawn such that intersections occur if and only if the corresponding equilibria collide. Solid lines represent asymptotically stable equilibria, dashed lines unstable equilibria. The meaning of the superscripts  and
and  is given in (L1)–(L5)
is given in (L1)–(L5)
In order to have only one bifurcation diagram covering cases that can be obtained from each other by simple symmetry considerations but are structurally equivalent otherwise, we use the sub- and superscripts  and
and  in the labels of Fig. 2. For an efficient presentation of the results, we define
in the labels of Fig. 2. For an efficient presentation of the results, we define
 |
L1 |
 |
L2 |
 |
L3 |
 |
L4 |
 |
L5 |
Theorem 4.4
Assume LE, i.e., (3.17). Figure 2 shows all possible bifurcation diagrams that involve bifurcations with equilibria that can be stable for some  given the other parameters.
given the other parameters.
A. Diagram (a) in Fig. 2 occurs generically. It occurs if and only if one of the following cases applies:
 |
4.29 |
or
 |
4.30 |
or
 |
4.31 |
or
 |
4.32 |
or
 |
4.33 |
or
 |
4.34 |
B. The following two diagrams occur only if the parameters satisfy particular relations.
Diagram (b) in Fig. 2 applies if one of the following two cases holds:
 |
4.35 |
or
 |
4.36 |
Diagram (c) in Fig. 2 applies if one of the following two cases holds:
 |
4.37 |
or
 |
4.38 |
C. Figure 3 shows the order in which the bifurcation diagrams of Fig. 2 arise if  is increased from 0 to 1.
is increased from 0 to 1.
Fig. 3.
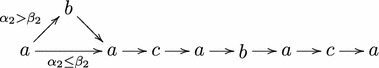
Order in which the bifurcation diagrams of Fig. 2 occur as  increases from 0 to 1
increases from 0 to 1
Proof
We prove parts A and B simultaneously, essentially by rewriting the conditions in Proposition 3.3 on admissibility and stability of the equilibria in terms of  ,
,  , and
, and  (4.3).
(4.3).
From (3.19) and (4.4), we infer easily:
 |
4.39a |
 |
4.39b |
 |
4.39c |
 |
4.39d |
 |
4.39e |
 |
4.39f |
 |
4.39g |
Invoking the relations (10.18), we can rewrite conditions (4.39a), (4.39c)–(4.39f) in the form
 |
4.40a |
 |
4.40b |
 |
4.40c |
 |
4.40d |
 |
4.40e |
We conclude immediately that (4.40b) applies in case (4.29) (Part A), (4.40d) in case (4.35) (Part B), and (4.40e) in case (4.36) (Part B). From (4.12) and (4.13) we conclude that (4.40a) applies in the following cases: (4.30)–(4.32) (Part A), or (4.37) (Part B). Analogously we conclude that (4.40c) applies in the following cases: (4.33), (4.34) (Part A), or (4.38) (Part B).
From Proposition 3.3 and (4.16) we obtain:
 |
4.41a |
 |
4.41b |
 |
4.41c |
As  , the stable SLP leaves the state space according to (4.16), which gives precisely the cases corresponding to diagrams (a) and (c). If
, the stable SLP leaves the state space according to (4.16), which gives precisely the cases corresponding to diagrams (a) and (c). If  (
( ),
),  is always admissible, cf. (4.37). If
is always admissible, cf. (4.37). If  (
( ),
),  is always admissible, cf. (4.38).
is always admissible, cf. (4.38).
A ME is globally asymptotically stable and only if
 |
4.42 |
By Proposition 4.1 and Remark 4.2 this equilibrium is  if
if  (cases (4.29)-(4.31), (4.35)), or
(cases (4.29)-(4.31), (4.35)), or  if
if  (cases (4.32), (4.33), (4.36)), or
(cases (4.32), (4.33), (4.36)), or  if
if  (4.34).
(4.34). 
The bifurcations of equilibria that cannot be stable can be derived easily from Sects. 4.2 and 4.3 and the above theorem by noting that these are boundary equilibria and corresponding pairs of SLPs are admissible for the same parameters; see (3.7) and (3.8). Inclusion of these bifurcations would require the introduction of subcases.
Corollary 4.5
Under the assumption of LE, the maximum migration rate, below which a stable two-locus polymorphism exists, is given by
 |
4.43 |
The corollary is a simple consequence of Proposition 3.3 and (4.40).
Strong recombination: quasi-linkage equilibrium
We recall from Sect. 3.6 that for sufficiently strong recombination, global convergence to the unique stable equilibrium occurs. From the coordinates (3.21) of the perturbed internal equilibrium, which is in quasi-linkage equilibrium, approximations could be derived for the critical migration rates at which the internal equilibrium collides with a boundary equilibrium and leaves the state space. It is not difficult to check with Mathematica that for large  ,
,  collides with
collides with  if
if  , where
, where
 |
4.44 |
We note that  if and only if
if and only if  , as is expected from (4.40c). Closer examination of (4.44) reveals that both
, as is expected from (4.40c). Closer examination of (4.44) reveals that both  and
and  may hold.
may hold.
Thus, the fully polymorphic equilibrium may be maintained for higher or lower migration rates than in the case of LE. This does not conform with the intuitive expectation that for reduced recombination  should hold because the locally adapted haplotypes (
should hold because the locally adapted haplotypes ( in deme
in deme  ) are less frequently broken apart. However, numerical evaluation of (4.44) shows that
) are less frequently broken apart. However, numerical evaluation of (4.44) shows that  occurs only for about 3% of the admissible parameter combinations and if it holds,
occurs only for about 3% of the admissible parameter combinations and if it holds,  is only very slightly less than
is only very slightly less than  (results not shown). If
(results not shown). If  is about as large as the largest selection coefficient or smaller,
is about as large as the largest selection coefficient or smaller,  increases with decreasing
increases with decreasing  . Expressions analogous to (4.44) can be obtained for collisions of
. Expressions analogous to (4.44) can be obtained for collisions of  with the other equilibria.
with the other equilibria.
No recombination
Our aim is to establish all possible equilibrium configurations and their dependence on the parameters if recombination is absent. In Fig. 4, the equilibrium configurations are displayed as schematic bifurcation diagrams with the total migration rate  as the bifurcation parameter. In Theorem 4.6, we assign to each diagram its pertinent parameter combinations.
as the bifurcation parameter. In Theorem 4.6, we assign to each diagram its pertinent parameter combinations.
Fig. 4.
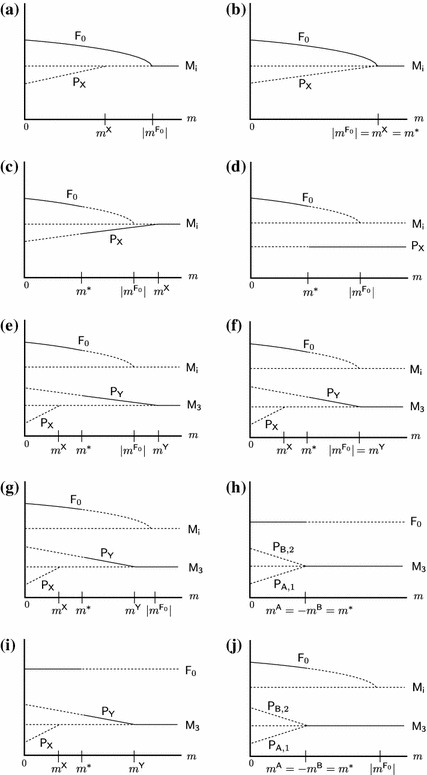
Bifurcation diagrams for  . Diagrams (a)–(j) represent all equilibrium and stability configurations listed in Theorem 4.6. Each diagram displays the possible equilibria as a function of the total migration rate
. Diagrams (a)–(j) represent all equilibrium and stability configurations listed in Theorem 4.6. Each diagram displays the possible equilibria as a function of the total migration rate  . Each line indicates one admissible equilibrium, drawn if and only if it is admissible. Only equilibria are shown that can be stable or are involved in a bifurcation with an equilibrium that can be stable. Lines are drawn such that intersections occur if and only if the corresponding equilibria collide. Solid lines represent asymptotically stable equilibria, dashed lines unstable equilibria
. Each line indicates one admissible equilibrium, drawn if and only if it is admissible. Only equilibria are shown that can be stable or are involved in a bifurcation with an equilibrium that can be stable. Lines are drawn such that intersections occur if and only if the corresponding equilibria collide. Solid lines represent asymptotically stable equilibria, dashed lines unstable equilibria
In order to have only one bifurcation diagram covering cases that can be obtained from each other by simple symmetry considerations but are structurally equivalent otherwise, we use the sub- and superscripts  and
and  in the labels of Fig. 4. For an efficient presentation of the results, we define
in the labels of Fig. 4. For an efficient presentation of the results, we define
 |
R1 |
 |
R2 |
 |
R3 |
 |
R4 |
 |
R1′ |
 |
R2′ |
 |
R3′ |
 |
R4′ |
Theorem 4.6
Let  . Figure 4 shows all possible bifurcation diagrams that involve bifurcations with equilibria that can be stable for some
. Figure 4 shows all possible bifurcation diagrams that involve bifurcations with equilibria that can be stable for some  given the other parameters.
given the other parameters.
A. The following diagrams occur for an open set of parameters:
- Diagram (g) in Fig. 4 applies if one of the following four cases holds:
or
4.48a
or
4.48b
or
4.48c
B. The following diagrams are degenerate, i.e., occur only if the parameters satisfy particular relations.
4.48d - Diagram (h) in Fig. 4 applies if

4.52
C. Figure 5 shows the order in which the bifurcation diagrams of Fig. 4 arise if  is increased from 0 to 1.
is increased from 0 to 1.
Fig. 5.
Order in which the bifurcation diagrams of Fig. 4 occur as  increases from 0 to 1, where
increases from 0 to 1, where 
Proof
We prove parts A and B simultaneously and derive the statements about admissibility and stability of the equilibria by rewriting the conditions in Sect. 3.7 in terms of  ,
,  ,
,  ,
,  , and
, and  (4.3). These critical migration rates satisfy the relations given in (4.9), (4.11), (10.19) and (10.37).
(4.3). These critical migration rates satisfy the relations given in (4.9), (4.11), (10.19) and (10.37).
We start by treating the bifurcations and stability of  . Using (4.5c) and (4.4c), we infer from (3.25):
. Using (4.5c) and (4.4c), we infer from (3.25):
 |
4.55a |
 |
4.55b |
From (4.15) we conclude that (4.55a) applies precisely in the following cases: (4.45a), (4.46a), (4.47a), (4.48a), (4.48c) (Part A), or (4.49a), (4.50a), (4.51a), (4.54a) (Part B). Similarly, (4.55b) applies in precisely the following cases: (4.45b), (4.46b), (4.47b), (4.48b), (4.48d) (Part A) or (4.49b), (4.50b), (4.51b), (4.54b) (Part B).  is admissible for every
is admissible for every  if and only if
if and only if  , which corresponds to the remaining three cases (4.52) and (4.53b), (4.53a).
, which corresponds to the remaining three cases (4.52) and (4.53b), (4.53a).
Condition (3.26), which determines when  changes stability, is equivalent to
changes stability, is equivalent to  . Therefore, Proposition 3.4 and the definitions of
. Therefore, Proposition 3.4 and the definitions of  and
and  imply that
imply that  is asymptotically stable if and only if either
is asymptotically stable if and only if either
 |
4.56a |
or
 |
4.56b |
holds, where
 |
4.57a |
 |
4.57b |
If (4.56a) applies, according to (4.55),  leaves the state space at
leaves the state space at  or
or  and exchanges stability with the respective monomorphism. By (4.57a), this occurs in the cases (4.45) or (4.49) of the theorem.
and exchanges stability with the respective monomorphism. By (4.57a), this occurs in the cases (4.45) or (4.49) of the theorem.
If (4.56b) applies,  loses stability at
loses stability at  and, generically, either
and, generically, either  or
or  is asymptotically stable if
is asymptotically stable if  (see below).
(see below).  remains admissible up to
remains admissible up to  , when it collides with
, when it collides with  or
or  . By (4.15) and (4.57b), this occurs in the cases (4.46)–(4.48), (4.50), (4.51), (4.53), and (4.54).
. By (4.15) and (4.57b), this occurs in the cases (4.46)–(4.48), (4.50), (4.51), (4.53), and (4.54).
Finally, if  (cases (4.52) and (4.54) in the theorem),
(cases (4.52) and (4.54) in the theorem),  becomes stable. This follows from the statement below (3.31) together with (4.11a).
becomes stable. This follows from the statement below (3.31) together with (4.11a).
Next, we treat the bifurcations of the SLPs. The SLPs are admissible in intervals of the form  or
or  and leave the state space upon collision with a ME (Sect. 4.2). From (3.32) we conclude by simple calculations that
and leave the state space upon collision with a ME (Sect. 4.2). From (3.32) we conclude by simple calculations that  is asymptotically stable if and only if
is asymptotically stable if and only if
 |
4.58 |
as is the case in (4.46a), (4.47a), (4.48a) (if  ), and (4.48b), as well as in (4.50a), (4.51a), and (4.53b).
), and (4.48b), as well as in (4.50a), (4.51a), and (4.53b).
From (3.33), we conclude that  is asymptotically stable if and only if
is asymptotically stable if and only if
 |
4.59 |
as is the case in (4.46b), (4.47b), (4.48c), and (4.48d) (if  ), as well as in (4.50b), (4.51b), and (4.53a).
), as well as in (4.50b), (4.51b), and (4.53a).
It remains to study the stability of the ME. For  , we infer from Sect. 4.1 and Proposition 4.1:
, we infer from Sect. 4.1 and Proposition 4.1:
 |
4.60a |
 |
4.60b |
 |
4.60c |
In conjunction with the above results on  and the SLPs, this shows that, except in the degenerate cases (4.49), (4.50), (4.52), and (4.54), a ME becomes stable through a transcritical bifurcation with either
and the SLPs, this shows that, except in the degenerate cases (4.49), (4.50), (4.52), and (4.54), a ME becomes stable through a transcritical bifurcation with either  ,
,  , or
, or  . In particular,
. In particular,  becomes asymptotically stable for large
becomes asymptotically stable for large  if (4.45a), (4.46a), or (4.49a) applies,
if (4.45a), (4.46a), or (4.49a) applies,  becomes asymptotically stable if one of (4.47), (4.48), (4.51), (4.52), (4.53), or (4.54) applies, and
becomes asymptotically stable if one of (4.47), (4.48), (4.51), (4.52), (4.53), or (4.54) applies, and  becomes asymptotically stable if (4.45b), (4.46b), or (4.49b) applies. If
becomes asymptotically stable if (4.45b), (4.46b), or (4.49b) applies. If  or
or  (4.50), then
(4.50), then  or
or  , respectively, is admissible and asymptotically stable for every
, respectively, is admissible and asymptotically stable for every  , and every ME is unstable. This finishes the proof of parts A and B.
, and every ME is unstable. This finishes the proof of parts A and B.
Part C of Theorem 4.6 follows immediately from parts A and B by applying the relations in (4.15). 
This theorem demonstrates that, for given selection parameters, the equilibrium structure, hence also the evolutionary dynamics, depends strongly on the degree  of asymmetry of the migration rates. However, it is also important to note (and maybe counter intuitive) that for symmetric migration (
of asymmetry of the migration rates. However, it is also important to note (and maybe counter intuitive) that for symmetric migration ( ) any of the ten possible bifurcation diagrams may apply, simply by choosing the selection parameters accordingly.
) any of the ten possible bifurcation diagrams may apply, simply by choosing the selection parameters accordingly.
The bifurcations of equilibria that cannot be stable can be derived easily from Sects. 4.2, 4.3, 3.7, and the above theorem by noting that these are boundary equilibria and corresponding pairs of SLPs are admissible for the same parameters. Inclusion of these bifurcations would require the introduction of subcases. In particular,  ,
,  , and
, and  are always unstable because gamete
are always unstable because gamete  is eventually lost. We observe from (10.20) and (10.38) that at most one pair of SLPs can be admissible if
is eventually lost. We observe from (10.20) and (10.38) that at most one pair of SLPs can be admissible if  is either unstable or not admissible. If this is the case, then one of these SLPs is asymptotically stable (Fig. 4).
is either unstable or not admissible. If this is the case, then one of these SLPs is asymptotically stable (Fig. 4).
Corollary 4.7
If  , the maximum migration rate, below which a stable two-locus polymorphism exists, is given by
, the maximum migration rate, below which a stable two-locus polymorphism exists, is given by
 |
4.61 |
The corollary follows from the arguments surrounding (4.56) and (4.57).
Weak recombination
If  , a regular perturbation analysis of
, a regular perturbation analysis of  yields the coordinates of a fully polymorphic (internal) equilibrium to leading order in
yields the coordinates of a fully polymorphic (internal) equilibrium to leading order in  . This equilibrium,
. This equilibrium,  , is asymptotically stable (Karlin and McGregor 1972). We denote the first-order approximation of
, is asymptotically stable (Karlin and McGregor 1972). We denote the first-order approximation of  by
by  . Therefore, we have
. Therefore, we have  and
and  as
as  . Because the coordinates of
. Because the coordinates of  are much too complicated to be informative, we refrain from presenting them.
are much too complicated to be informative, we refrain from presenting them.
For sufficiently small  , the following properties of
, the following properties of  (hence, of
(hence, of  ) can be inferred from Proposition 4.1, Remark 4.2, and Theorem 4.6, Part A.1:
) can be inferred from Proposition 4.1, Remark 4.2, and Theorem 4.6, Part A.1:
 |
4.62a |
 |
4.62b |
The above perturbation analysis can not be used to investigate the properties of the internal equilibrium  for given small positive
for given small positive  when
when  is varied in the proximity of
is varied in the proximity of  . Therefore, we performed numerical calculations to study the fate of
. Therefore, we performed numerical calculations to study the fate of  when
when  is small and fixed, and
is small and fixed, and  increases. It suggests the following:
increases. It suggests the following:
 |
4.63a |
 |
4.63b |
 |
4.63c |
where  and
and  are close to
are close to  . Thus, if
. Thus, if  is small,
is small,  stays close to
stays close to  as
as  increases from 0 until a value close to
increases from 0 until a value close to  is reached. Then, within a very short interval of
is reached. Then, within a very short interval of  ,
,  moves ‘quickly’ along the manifold given by (10.8) and (10.11) to one of the boundary equilibria (
moves ‘quickly’ along the manifold given by (10.8) and (10.11) to one of the boundary equilibria ( ,
,  , or
, or  ) on the ‘opposite’ side of the state space, where it exchanges stability upon collision with the respective equilibrium (at
) on the ‘opposite’ side of the state space, where it exchanges stability upon collision with the respective equilibrium (at  ,
,  , or
, or  ).
).  appears to be asymptotically stable whenever it is admissible.
appears to be asymptotically stable whenever it is admissible.
If one of the cases in (4.62) applies, then  can be maintained for higher migration rates than
can be maintained for higher migration rates than  because
because  and
and  are decreasing functions in
are decreasing functions in  . Numerical investigations support the conjecture that
. Numerical investigations support the conjecture that  can be maintained for higher migration rates than
can be maintained for higher migration rates than  whenever recombination is weak but positive. Thus, when recombination is weak, decreasing
whenever recombination is weak but positive. Thus, when recombination is weak, decreasing  increases the maximum migration rate below which a stable, fully polymorphic equilibrium can be maintained.
increases the maximum migration rate below which a stable, fully polymorphic equilibrium can be maintained.
Highly asymmetric migration
As already discussed in Sect. 3.8, by introducing weak back migration (i.e.,  close to 0 or 1) to the CI model, every equilibrium in the CI model gives rise to a unique equilibrium in a small neighborhood. This (perturbed) equilibrium has the same stability as the unperturbed. For weak or strong recombination, we can strengthen this conclusion. Because the CI model with
close to 0 or 1) to the CI model, every equilibrium in the CI model gives rise to a unique equilibrium in a small neighborhood. This (perturbed) equilibrium has the same stability as the unperturbed. For weak or strong recombination, we can strengthen this conclusion. Because the CI model with  is a generalized gradient system (Bürger and Akerman 2011, Section 3.4.4) and the LE dynamics (3.17) has a globally asymptotically stable equilibrium (Theorem 4.4), the proof of Theorem 2.3 of Nagylaki et al. (1999) applies and shows that in both cases the global dynamics remains qualitatively unchanged under small perturbations. In particular, no new equilibria or limit sets are generated by a small perturbation.
is a generalized gradient system (Bürger and Akerman 2011, Section 3.4.4) and the LE dynamics (3.17) has a globally asymptotically stable equilibrium (Theorem 4.4), the proof of Theorem 2.3 of Nagylaki et al. (1999) applies and shows that in both cases the global dynamics remains qualitatively unchanged under small perturbations. In particular, no new equilibria or limit sets are generated by a small perturbation.
Therefore, if  is sufficiently small and
is sufficiently small and  is sufficiently close to 0 or 1, we infer from Section 3.8 and Theorem 2 in Bürger and Akerman (2011) that the following bifurcation pattern applies (where
is sufficiently close to 0 or 1, we infer from Section 3.8 and Theorem 2 in Bürger and Akerman (2011) that the following bifurcation pattern applies (where  or 4):
or 4):
If
 , a unique internal equilibrium,
, a unique internal equilibrium,  , exists. It is globally asymptotically stable.
, exists. It is globally asymptotically stable.At
 ,
,  leaves the state space through the ME
leaves the state space through the ME  by an exchange-of-stability bifurcation.
by an exchange-of-stability bifurcation.If
 ,
,  is globally asymptotically stable.
is globally asymptotically stable.
This pattern is displayed in diagram (a) of Fig. 4, where  needs to be substituted by
needs to be substituted by  . We conjecture that it applies whenever
. We conjecture that it applies whenever  is sufficiently small and either
is sufficiently small and either  or
or  holds. The bounds
holds. The bounds  and
and  follow from Remark 4.2 because
follow from Remark 4.2 because  is not needed if
is not needed if  is sufficiently small; see (10.32b). However, the upper bounds for
is sufficiently small; see (10.32b). However, the upper bounds for  given in Remark 4.2 are, in general, too large to guarantee the above bifurcation pattern. This is known from the CI model in which the monomorphic equilibrium (
given in Remark 4.2 are, in general, too large to guarantee the above bifurcation pattern. This is known from the CI model in which the monomorphic equilibrium ( ) may be simultaneously stable with the internal equilibrium
) may be simultaneously stable with the internal equilibrium  because an unstable internal equilibrium enters the state space at
because an unstable internal equilibrium enters the state space at  through
through  . If
. If  , this may occur if
, this may occur if  , cf. (4.65b). For
, cf. (4.65b). For  or
or  , we have not been able to determine the upper bound for
, we have not been able to determine the upper bound for  below which
below which  indeed leaves the state space through
indeed leaves the state space through  .
.
Now we treat large  . Proposition 4.1 and Remark 4.2 show that if
. Proposition 4.1 and Remark 4.2 show that if  , then
, then  is asymptotically stable if and only if
is asymptotically stable if and only if  and
and  , and if
, and if  , then
, then  is asymptotically stable if and only if
is asymptotically stable if and only if  and
and  .
.
If, in addition to  being sufficiently large,
being sufficiently large,  is small or large, then Theorem 4.4 implies that the internal equilibrium (
is small or large, then Theorem 4.4 implies that the internal equilibrium ( ) leaves the state space through
) leaves the state space through  ,
,  , or
, or  . The respective conditions are small perturbations of those given in (4.40a), (4.40b), of (4.40c), respectively. Combining theses conditions with those for the stability of the ME and observing (4.12) and (4.13), we conclude that the following bifurcation pattern applies if one of the conditions (a)
. The respective conditions are small perturbations of those given in (4.40a), (4.40b), of (4.40c), respectively. Combining theses conditions with those for the stability of the ME and observing (4.12) and (4.13), we conclude that the following bifurcation pattern applies if one of the conditions (a)  and
and  , or (b)
, or (b)  and
and  , or (c)
, or (c)  holds approximately:
holds approximately:
If
 , a unique internal equilibrium,
, a unique internal equilibrium,  , exists. It is asymptotically stable.
, exists. It is asymptotically stable.At
 ,
,  leaves the state space through a SLP by an exchange-of-stability bifurcation.
leaves the state space through a SLP by an exchange-of-stability bifurcation.If
 , this SLP is asymptotically stable.
, this SLP is asymptotically stable.If
 , then a ME is asymptotically stable.
, then a ME is asymptotically stable.
If (a) holds, then  and the SLP and the ME are
and the SLP and the ME are  and
and  , respectively; if (b) holds, then
, respectively; if (b) holds, then  and the SLP and the ME are
and the SLP and the ME are  and
and  , respectively; if (c) holds, then
, respectively; if (c) holds, then  and the SLP and the ME are
and the SLP and the ME are  and
and  , respectively. Finally,
, respectively. Finally,  in (a) and (b), and
in (a) and (b), and  in (c).
in (c).
Maintenance of polymorphism
As already noted in Sect. 3.11, for general parameters the equilibrium configurations could not be determined analytically. To explore the potential of spatially heterogeneous selection in maintaining genetic variation in the presence of gene flow, we investigate the maximum total migration rate,  , that admits a stable, fully polymorphic equilibrium. We have already shown that
, that admits a stable, fully polymorphic equilibrium. We have already shown that  holds in the LE approximation (Corollary 4.5), and
holds in the LE approximation (Corollary 4.5), and  holds if
holds if  (Corollary 4.7). From (10.20) and (10.38) we conclude that
(Corollary 4.7). From (10.20) and (10.38) we conclude that
 |
4.64 |
where, as is not difficult to show, equality holds if and only if  .
.
For the CI model with  , Proposition 1 in Bürger and Akerman (2011) yields
, Proposition 1 in Bürger and Akerman (2011) yields
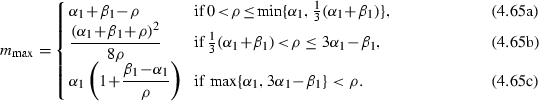 |
In this case, the fully polymorphic equilibrium is globally asymptotically stable if (4.65a) or (4.65c) apply, but only locally stable if (4.65b) holds and  is close to
is close to  . A formula analogous to (4.65), but with
. A formula analogous to (4.65), but with  and
and  instead of
instead of  and
and  , holds if
, holds if  .
.
In general, we have no explicit formula for  . However, extensive numerical work, as well as (4.65) and the considerations in Sect. 4.9 suggest that
. However, extensive numerical work, as well as (4.65) and the considerations in Sect. 4.9 suggest that
 |
4.66 |
holds always. This is illustrated by Fig. 6, which displays the dependence of  on the migration ratio
on the migration ratio  (Fig. 6a, c) and on the recombination rate
(Fig. 6a, c) and on the recombination rate  (Fig. 6b, d) for two selection regimes. In Fig. 6a, b, locus
(Fig. 6b, d) for two selection regimes. In Fig. 6a, b, locus  is under stronger selection in both demes. In Fig. 6c, d, each locus is under stronger selection in one deme.
is under stronger selection in both demes. In Fig. 6c, d, each locus is under stronger selection in one deme.
Fig. 6.
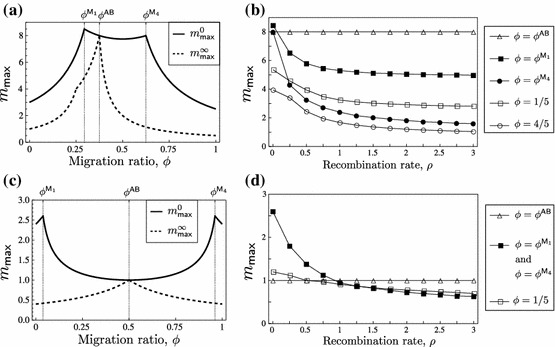
The maximum amount of gene flow,  , admitting an asymptotically stable two-locus polymorphism as a function of
, admitting an asymptotically stable two-locus polymorphism as a function of  or
or  . In panels a and b, locus
. In panels a and b, locus  is under stronger selection than locus
is under stronger selection than locus  in both demes (
in both demes ( ,
,  ,
,  ). In c and d, different loci are under stronger selection in the two demes (
). In c and d, different loci are under stronger selection in the two demes ( ,
,  ,
,  ). Panels a and c show
). Panels a and c show  as a function of
as a function of  for complete linkage (
for complete linkage ( , (4.61)) and under linkage equilibrium (
, (4.61)) and under linkage equilibrium ( , (4.43)). Panels b and d display
, (4.43)). Panels b and d display  for the indicated values of
for the indicated values of  as a function of
as a function of  . Here,
. Here,  is obtained by determining numerically the critical migration rate when the stable internal equilibrium hits the boundary. This is done by computing when the leading eigenvalue at the boundary equilibrium is zero and by calculating the coordinates of the fully polymorphic equilibrium in a small neighborhood. In a and b, we have
is obtained by determining numerically the critical migration rate when the stable internal equilibrium hits the boundary. This is done by computing when the leading eigenvalue at the boundary equilibrium is zero and by calculating the coordinates of the fully polymorphic equilibrium in a small neighborhood. In a and b, we have  (indicated by the kink in the dashed line in a),
(indicated by the kink in the dashed line in a),  ,
,  ,
,  ,
,  ,
,  . In c and d, we have
. In c and d, we have  ,
,  ,
,  ,
,  ,
, 
In Fig. 6a, c,  and
and  are shown as functions of
are shown as functions of  . The inequality (4.64) is a conspicuous feature in both cases. Also the shapes of
. The inequality (4.64) is a conspicuous feature in both cases. Also the shapes of  and
and  are conspicuous. The following properties are easy to prove:
are conspicuous. The following properties are easy to prove:  is not differentiable at
is not differentiable at  and
and  , and
, and  is not differentiable at
is not differentiable at  and
and  .
.  and
and  are piecewise convex functions in
are piecewise convex functions in  . If
. If  ,
,  increases in
increases in  ; if
; if  ,
,  decreases in
decreases in  ; if
; if  ,
,  assumes its minimum at
assumes its minimum at  provided
provided  . Therefore,
. Therefore,  attains its maximum at
attains its maximum at  or
or  .
.  increases if
increases if  and decreases if
and decreases if  . It assumes its maximum at
. It assumes its maximum at  .
.
Notably,  holds if
holds if  . Numerical work suggests that indeed
. Numerical work suggests that indeed  holds independently of
holds independently of  if
if  . If
. If  , then
, then  and
and  if
if  . The latter condition is equivalent to (3.36c). Therefore, the analysis in Sect. 3.10 applies and shows that an internal equilibrium, which presumably is globally asymptotically stable, exists always, i.e.,
. The latter condition is equivalent to (3.36c). Therefore, the analysis in Sect. 3.10 applies and shows that an internal equilibrium, which presumably is globally asymptotically stable, exists always, i.e.,  .
.
Figure 6b, d illustrates the effect of recombination on  for different values of
for different values of  . In all cases investigated,
. In all cases investigated,  decreased monotonically with increasing
decreased monotonically with increasing  . These findings support the conjecture that (4.66) is always valid. Therefore,
. These findings support the conjecture that (4.66) is always valid. Therefore,  serves as a useful estimate for the sensitivity of
serves as a useful estimate for the sensitivity of  to variation in
to variation in  . We can prove that
. We can prove that  is maximized at
is maximized at  , or at
, or at  , or at
, or at  if
if  .
.
Although we proved that  can occur (Sect. 4.7), all numerical examples showed that
can occur (Sect. 4.7), all numerical examples showed that  is only very slightly smaller than
is only very slightly smaller than  in this case (results not shown). Therefore, our results suggest that the cases of LE (infinitely strong recombination) and of no recombination ‘essentially’ bracket the range of parameters for which both loci can be maintained polymorphic.
in this case (results not shown). Therefore, our results suggest that the cases of LE (infinitely strong recombination) and of no recombination ‘essentially’ bracket the range of parameters for which both loci can be maintained polymorphic.
As Fig. 6a, c shows, the range of values  for which the equilibrium structure can be expected to be similar to the CI model, i.e.,
for which the equilibrium structure can be expected to be similar to the CI model, i.e.,  or
or  (Sect. 4.10), can vary considerably.
(Sect. 4.10), can vary considerably.
Finally, we infer from Proposition 4.1 that none of the ME is stable if  . Hence, in this case at least one locus is maintained polymorphic. By contrast, we have shown in Sect. 4.2 that no SLP is admissible if
. Hence, in this case at least one locus is maintained polymorphic. By contrast, we have shown in Sect. 4.2 that no SLP is admissible if  . However, as demonstrated by our results for
. However, as demonstrated by our results for  , an internal equilibrium may be asymptotically stable if
, an internal equilibrium may be asymptotically stable if  . These results suggest that no genetic variability can be maintained if
. These results suggest that no genetic variability can be maintained if
 |
4.67 |
This bound is best possible if  . For sufficiently large
. For sufficiently large  , the corresponding bound is
, the corresponding bound is  .
.
Migration load and local adaptation
Here, we briefly investigate some properties of the migration load of the subpopulations and of the total population. We use these migration loads as simple measures for local adaptation (but see Blanquart et al. 2012). Mean fitness in deme  is given by
is given by  , with its maximum at
, with its maximum at  . Therefore, the migration loads in demes 1 and 2, defined as the deviation of
. Therefore, the migration loads in demes 1 and 2, defined as the deviation of  from its maximum, are given by
from its maximum, are given by
 |
5.1 |
Assuming that the subpopulations are of equal size, we define the load of the total population by  .
.
If migration is weak, we can calculate the migration load in each deme at the fully polymorphic equilibrium  (Proposition 3.2) to leading order in
(Proposition 3.2) to leading order in  and
and  . For deme 1, we obtain
. For deme 1, we obtain
 |
5.2 |
and an analogous formula holds for deme 2. Obviously, the migration load increases with increasing migration rates  or
or  , hence with
, hence with  , in each of the demes and in the total population. Simple calculations show that each of the loads also increases with increasing recombination rate
, in each of the demes and in the total population. Simple calculations show that each of the loads also increases with increasing recombination rate  if migration is weak.
if migration is weak.
In general, however, the load in each deme does not always increase with increasing  . The reason is that for sufficiently strong migration, generically, first one locus, then one of the haplotypes becomes fixed (Proposition 4.3). If this is either
. The reason is that for sufficiently strong migration, generically, first one locus, then one of the haplotypes becomes fixed (Proposition 4.3). If this is either  or
or  , then the load in the corresponding deme will vanish for high migration rates, whereas that in the other deme will be very high. In such a case, the load of the total population may also decrease with increasing
, then the load in the corresponding deme will vanish for high migration rates, whereas that in the other deme will be very high. In such a case, the load of the total population may also decrease with increasing  . This occurs for large migration rates (not far below
. This occurs for large migration rates (not far below  ) and it can occur for completely linked loci as well as for loci in LE. In the CI model, the load always increases with the migration rate (Bürger and Akerman 2011)
) and it can occur for completely linked loci as well as for loci in LE. In the CI model, the load always increases with the migration rate (Bürger and Akerman 2011)
Finally, although  is increasing in
is increasing in  if migration is weak, this is not necessarily so if migration is strong. By using a grid of parameter combinations, we showed numerically that in about 0.34% of more than
if migration is weak, this is not necessarily so if migration is strong. By using a grid of parameter combinations, we showed numerically that in about 0.34% of more than  combinations of
combinations of  , and
, and  , the total load
, the total load  at the equilibrium
at the equilibrium  is lower than that at
is lower than that at  (results not shown). Again, this occurs for high migration rates, not far below the value
(results not shown). Again, this occurs for high migration rates, not far below the value  at which
at which  leaves the state space. Then a population maintained fully polymorphic by tight linkage may have a higher total load than a population in which fixation of a locus or a haplotype is facilitated by high recombination. In all such cases, selection in one deme was (considerably) stronger than in the other, and in more than 70
leaves the state space. Then a population maintained fully polymorphic by tight linkage may have a higher total load than a population in which fixation of a locus or a haplotype is facilitated by high recombination. In all such cases, selection in one deme was (considerably) stronger than in the other, and in more than 70 of the cases, a specialist haplotype became fixed at very high migration rates. In summary, under a wide range of conditions in this model, reduced recombination is favored, but there are instances where increased recombination is favored (cf. Pylkov et al. 1998; Lenormand and Otto 2000).
of the cases, a specialist haplotype became fixed at very high migration rates. In summary, under a wide range of conditions in this model, reduced recombination is favored, but there are instances where increased recombination is favored (cf. Pylkov et al. 1998; Lenormand and Otto 2000).
 and differentiation
and differentiation
The most commonly used measure for quantifying differentiation in spatially structured populations is  . For diallelic loci,
. For diallelic loci,  can be defined as
can be defined as  , where
, where  is the variance of the allele frequencies in the total population and
is the variance of the allele frequencies in the total population and  is the allele frequency averaged over the demes. Estimators of multilocus
is the allele frequency averaged over the demes. Estimators of multilocus  are usually defined as weighted averages of one-locus
are usually defined as weighted averages of one-locus  estimators (e.g., Weir and Cockerham 1984; Leviyang and Hamilton 2011). Here, we extend Nagylaki’s (1998) approach and define a genuine multilocus version of
estimators (e.g., Weir and Cockerham 1984; Leviyang and Hamilton 2011). Here, we extend Nagylaki’s (1998) approach and define a genuine multilocus version of  that measures the covariance of the frequencies of (multilocus) haplotypes. We restrict attention to the diallelic two-locus case, but the extension to multiple multiallelic loci is evident. A general multilocus theory of fixation indices will be developed elsewhere.
that measures the covariance of the frequencies of (multilocus) haplotypes. We restrict attention to the diallelic two-locus case, but the extension to multiple multiallelic loci is evident. A general multilocus theory of fixation indices will be developed elsewhere.
Let  denote the proportion of the population in deme
denote the proportion of the population in deme  , so that
, so that  . Then the frequency of haplotype
. Then the frequency of haplotype  in the entire population is
in the entire population is  . Because our subpopulations are randomly mating, the frequency of genotype
. Because our subpopulations are randomly mating, the frequency of genotype  in the entire population is given by
in the entire population is given by  . Following eqs. (6a) and (6b) in Nagylaki (1998), we define
. Following eqs. (6a) and (6b) in Nagylaki (1998), we define  as a standardized measure of the covariance of the frequencies of haplotypes
as a standardized measure of the covariance of the frequencies of haplotypes  and
and  :
:
 |
6.1a |
 |
6.1b |
The multilocus, or haplotype, heterozygosity in the entire population can be defined as
 |
6.2 |
where  runs over all haplotypes. If the entire population were panmictic, its multilocus heterozygosity would be
runs over all haplotypes. If the entire population were panmictic, its multilocus heterozygosity would be
 |
6.3 |
Thus,  is the probability that two gametes chosen at random from the entire population are the same haplotype.
is the probability that two gametes chosen at random from the entire population are the same haplotype.
Following eq. (32) in Nagylaki (1998), we define  by
by
 |
6.4 |
Then  can be written as
can be written as
 |
6.5 |
in direct generalization of the classical formula given above.
We focus on the dependence of the equilibrium value of  on the migration parameters
on the migration parameters  and
and  and on the recombination rate
and on the recombination rate  . Because we obtained the coordinates of the stable, fully polymorphic equilibrium equilibrium
. Because we obtained the coordinates of the stable, fully polymorphic equilibrium equilibrium  explicitly only in special or limiting cases, explicit formulas for
explicitly only in special or limiting cases, explicit formulas for  can be derived only in these cases. For instance, if migration is weak, we obtain from (3.16) that, to leading order in
can be derived only in these cases. For instance, if migration is weak, we obtain from (3.16) that, to leading order in  ,
,
 |
6.6 |
Here,  increases with decreasing
increases with decreasing  , and decreases with increasing
, and decreases with increasing  . Thus, stronger linkage leads to increased differentiation if migration is weak.
. Thus, stronger linkage leads to increased differentiation if migration is weak.
Figure 7 illustrates for two selection scenarios how  , evaluated at the stable, fully polymorphic equilibrium
, evaluated at the stable, fully polymorphic equilibrium  , depends on the total migration rate
, depends on the total migration rate  and the recombination rate
and the recombination rate  . In diagrams (a) and (c) of Fig. 7, it is assumed that locus
. In diagrams (a) and (c) of Fig. 7, it is assumed that locus  is under stronger selection than locus
is under stronger selection than locus  in both demes. It shows that
in both demes. It shows that  usually declines with increasing migration rate. However, there are a few instances, where
usually declines with increasing migration rate. However, there are a few instances, where  increases if
increases if  is slightly below the migration rate at which the fully polymorphic equilibrium loses admissibility. In diagrams (a) and (c) of Fig. 7, differentiation between the populations experiences the fastest decline for weak migration (relative to the selection parameters), whereas this is not necessarily so in diagrams (b) and (d). There,
is slightly below the migration rate at which the fully polymorphic equilibrium loses admissibility. In diagrams (a) and (c) of Fig. 7, differentiation between the populations experiences the fastest decline for weak migration (relative to the selection parameters), whereas this is not necessarily so in diagrams (b) and (d). There,  may experience its strongest decrease if migration is strong.
may experience its strongest decrease if migration is strong.
Fig. 7.
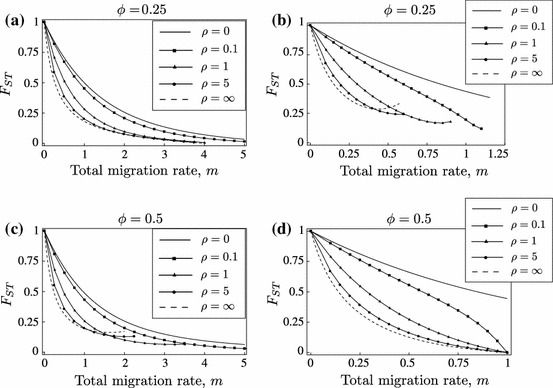
 as a function of the total migration rate
as a function of the total migration rate  . In panels a and c, locus
. In panels a and c, locus  is under stronger selection in both demes (
is under stronger selection in both demes ( ,
,  ,
,  ,
,  ). In panels b and d, locus
). In panels b and d, locus  is under stronger selection than
is under stronger selection than  in deme 2, and locus
in deme 2, and locus  is under stronger selection than
is under stronger selection than  in deme 1 (
in deme 1 ( ,
,  ,
,  ). Note that in all cases,
). Note that in all cases,  is also monotone decreasing in
is also monotone decreasing in  . For
. For  and
and  (LE), the lines are from numerical evaluation of (6.5) by substitution of the coordinates of
(LE), the lines are from numerical evaluation of (6.5) by substitution of the coordinates of  (3.24) or
(3.24) or  (3.18). For other values of
(3.18). For other values of  , the numerically determined coordinates of the internal equilibrium are used
, the numerically determined coordinates of the internal equilibrium are used
Figure 7 also shows that at large migration rates,  may increase if the recombination rate increases, i.e.,
may increase if the recombination rate increases, i.e.,  is not minimized under linkage equilibrium. However, this occurs only for large recombination rates, i.e., larger than the largest selection coefficient. This is compatible with the finding in Sect. 4.11 that at high recombination rates,
is not minimized under linkage equilibrium. However, this occurs only for large recombination rates, i.e., larger than the largest selection coefficient. This is compatible with the finding in Sect. 4.11 that at high recombination rates,  may (slightly) increase in
may (slightly) increase in  , and the finding in Sect. 5 that the load
, and the finding in Sect. 5 that the load  may decrease with increasing
may decrease with increasing  . We note that this ‘aberrant’ behavior of
. We note that this ‘aberrant’ behavior of  ,
,  , and
, and  does not necessarily occur for the same parameter combinations. Among more than
does not necessarily occur for the same parameter combinations. Among more than  parameter combinations of
parameter combinations of  , and
, and  , we found no instance where
, we found no instance where  evaluated at the equilibrium
evaluated at the equilibrium  was higher than that at
was higher than that at  (results not shown). Importantly, if recombination is weak or migration is weak then
(results not shown). Importantly, if recombination is weak or migration is weak then  apparently always increases with tighter linkage.
apparently always increases with tighter linkage.
Comparison of our multilocus  with averages of single-locus
with averages of single-locus  values showed that the multilocus
values showed that the multilocus  declines somwehat faster at small migration rates than the averaged single-locus
declines somwehat faster at small migration rates than the averaged single-locus  . For large parameter regions, the qualitative behavior of these measures of differentiation is the same. Differences occur only for a subset of selection coefficients at high migration rates and high recombination rates. Finally, we mention that our multilocus
. For large parameter regions, the qualitative behavior of these measures of differentiation is the same. Differences occur only for a subset of selection coefficients at high migration rates and high recombination rates. Finally, we mention that our multilocus  is a sensitive measure of differentiation only if the effective number of haplotypes is low. This parallels the well known fact that the classical
is a sensitive measure of differentiation only if the effective number of haplotypes is low. This parallels the well known fact that the classical  is a sensitive measure of differentiation only if the effective number of alleles is low (e.g., Nagylaki 1998, 2011). Thus, our multilocus
is a sensitive measure of differentiation only if the effective number of alleles is low (e.g., Nagylaki 1998, 2011). Thus, our multilocus  may be most useful if applied to short sequences of DNA. A thorough and more general study is in preparation.
may be most useful if applied to short sequences of DNA. A thorough and more general study is in preparation.
Invasion of a locally beneficial mutant
Differentiation between subpopulations can be increased by the invasion of mutants that establish a stable polymorphism at their locus. Therefore, we consider a locus ( ) at which a new mutant
) at which a new mutant  arises that is advantageous relative to the wild type
arises that is advantageous relative to the wild type  in deme 1, but disadvantageous in deme 2. In terms of our model, we assume
in deme 1, but disadvantageous in deme 2. In terms of our model, we assume  . If locus
. If locus  is isolated, this mutant can invade and become established in a stable polymorphism if and only if
is isolated, this mutant can invade and become established in a stable polymorphism if and only if  ; cf. (3.7) and (3.9). Using
; cf. (3.7) and (3.9). Using  and
and  , this condition can be rewritten as
, this condition can be rewritten as
 |
7.1 |
 |
7.2 |
We restrict attention to the case  (4.2a) when the influx of the deleterious allele
(4.2a) when the influx of the deleterious allele  into deme 1 is sufficiently strong such that
into deme 1 is sufficiently strong such that  is protected. (The case
is protected. (The case  is symmetric and more suitable to study invasion of
is symmetric and more suitable to study invasion of  under influx into deme 2 of
under influx into deme 2 of  which is deleterious there.) Then the mutant
which is deleterious there.) Then the mutant  can invade if any of the following equivalent conditions hold:
can invade if any of the following equivalent conditions hold:
 |
7.3a |
 |
7.3b |
or
 |
7.3c |
where  if and only if
if and only if  . Thus,
. Thus,  can always invade if
can always invade if  . For the CI model (
. For the CI model ( ), each of the conditions in (7.3) simplifies to the well known invasion condition
), each of the conditions in (7.3) simplifies to the well known invasion condition  (Haldane 1930). The conditions (7.3) show that invasion is facilitated whenever back migration is increased, either by keeping
(Haldane 1930). The conditions (7.3) show that invasion is facilitated whenever back migration is increased, either by keeping  constant and increasing
constant and increasing  , or by fixing
, or by fixing  and decreasing
and decreasing  .
.
For the CI model it was proved that invasion of a locally beneficial mutant is always facilitated by increased linkage to a locus in migration-selection balance (Bürger and Akerman 2011). In fact, mutants of arbitrarily small effect can invade provided they are sufficiently tightly linked to this polymorphic locus which may be considered as the background in which the new mutant appears.
Here, we investigate whether this is also the case with two-way migration. Thus, we assume that locus  is in migration-selection balance (which requires that analogs of (7.3) are satisfied for
is in migration-selection balance (which requires that analogs of (7.3) are satisfied for  and
and  ) and a locally beneficial mutant
) and a locally beneficial mutant  arises at the linked locus
arises at the linked locus  . Hence, the model in Sect. 2 applies and we assume (2.3).
. Hence, the model in Sect. 2 applies and we assume (2.3).
Because we are mainly interested in the invasion properties of mutants of small effect, we assume that locus  is under stronger selection than
is under stronger selection than  , i.e.,
, i.e.,  in deme
in deme  . Before the mutant
. Before the mutant  arises, the population is at the equilibrium
arises, the population is at the equilibrium  (where
(where  must hold for admissibility; see Sect. 3).
must hold for admissibility; see Sect. 3).  can invade if
can invade if  is unstable. Since the eigenvalues determining external stability are zeros of a complicated quartic equation, the stability of
is unstable. Since the eigenvalues determining external stability are zeros of a complicated quartic equation, the stability of  cannot be determined analytically. We expect that the new stable equilibrium that will be reached is the fully polymorphic equilibrium
cannot be determined analytically. We expect that the new stable equilibrium that will be reached is the fully polymorphic equilibrium  . For the CI model, this was be proved in (Bürger and Akerman 2011). For the case of LE, it follows from Theorem 4.4.
. For the CI model, this was be proved in (Bürger and Akerman 2011). For the case of LE, it follows from Theorem 4.4.
Figure 8 displays typical results about the invasion of the mutant  . In Fig. 8a, the maximum recombination rate admitting invasion, denoted by
. In Fig. 8a, the maximum recombination rate admitting invasion, denoted by  , is shown as a function of
, is shown as a function of  . In the shaded region,
. In the shaded region,  can invade. If
can invade. If  , (7.3c) implies that
, (7.3c) implies that  can always invade. If
can always invade. If  , there exists
, there exists  , such that
, such that  can invade only if
can invade only if  , i.e., if
, i.e., if  is sufficiently tightly linked to locus
is sufficiently tightly linked to locus  . In Fig. 8b, the minimum selection coefficient
. In Fig. 8b, the minimum selection coefficient  necessary for invasion of
necessary for invasion of  is shown as a function of
is shown as a function of  for various values of
for various values of  . These values are obtained by computing when the leading eigenvalue that determines external stability of
. These values are obtained by computing when the leading eigenvalue that determines external stability of  equals zero.
equals zero.
Fig. 8.
Invasion properties of locally beneficial alleles. In a, the maximum recombination rate between loci  and
and  , below which invasion of
, below which invasion of  can occur, is displayed as a function of
can occur, is displayed as a function of  . The parameters
. The parameters  ,
,  , and
, and  are fixed. Therefore,
are fixed. Therefore,  and
and  . In b, the minimum selective advantage
. In b, the minimum selective advantage  required for invasion of
required for invasion of  is shown as a function of
is shown as a function of  for different values of
for different values of  . The parameters
. The parameters  ,
,  , and
, and  are fixed
are fixed
We conclude that, as in the CI model, mutants of arbitrarily small effect can invade provided they are sufficiently tightly linked to a locus that is already maintained in migration-selection balance. In addition, as shown by both panels in Fig. 8, increasingly symmetric migration facilitates the invasion and establishment of locally beneficial alleles.
The effective migration rate at a linked neutral site
Linkage to loci under selection may impede or enhance gene flow at a neutral marker locus. In the first case, linkage may act as a barrier to gene flow. This was shown by the work of Petry (1983), Bengtsson (1985), Barton and Bengtsson (1986), and Charlesworth et al. (1997), who developed and studied the concept of the effective migration rate as a measure of the ‘effective’ gene flow at a neutral site. More recently, the effective migration rate was studied for CI models with selection on a single locus in a class-structured population (Kobayashi et al. 2008) or with selection on two linked loci (Bürger and Akerman 2011). Fusco and Uyenoyama (2011) investigated the consequences of a selectively maintained polymorphism on the rate of introgression at a linked neutral site under symmetric migration between two demes.
Here, we derive an explicit expression for the effective migration rate at a neutral locus ( ) that is located between the two selected loci,
) that is located between the two selected loci,  and
and  . Recombination between locus
. Recombination between locus  (
( ) and the neutral locus occurs with rate
) and the neutral locus occurs with rate  (
( ) such that
) such that  . Thus, only one crossover event occurs in a sufficiently small time interval. We assume that
. Thus, only one crossover event occurs in a sufficiently small time interval. We assume that  and
and  are positive, i.e., the neutral locus is not completely linked to a selected site. We consider two variants at the neutral locus,
are positive, i.e., the neutral locus is not completely linked to a selected site. We consider two variants at the neutral locus,  and
and  , each with arbitrary, positive initial frequency in at least one deme. The frequency of
, each with arbitrary, positive initial frequency in at least one deme. The frequency of  in deme
in deme  is denoted by
is denoted by  . We model evolution at the three loci by a system of
. We model evolution at the three loci by a system of  ordinary differential equations for the allele frequencies and linkage disequilibria (
ordinary differential equations for the allele frequencies and linkage disequilibria ( ,
,  ,
,  ,
,  ,
,  ,
,  ,
,  ,
,  ,
,  ,
,  ,
,  ,
,  ,
,  ,
,  ). We refrain from presenting the equations for the allele frequencies at the neutral locus and the associated linkage disequilibria because they are a straightforward extension of those in Section 4.6 of Bürger and Akerman (2011).
). We refrain from presenting the equations for the allele frequencies at the neutral locus and the associated linkage disequilibria because they are a straightforward extension of those in Section 4.6 of Bürger and Akerman (2011).
Obviously, the equilibrium allele frequencies at the neutral locus are the same in each deme and given by the initial allele frequencies averaged over the two demes:
 |
8.1 |
The equilibrium frequencies at the two selected loci are independent of the neutral locus and, thus, the same as in the two-locus model treated above. The linkage disequilibria involving the neutral locus ( ,
,  , and
, and  ) are zero at equilibrium. By (8.1), there is a one-dimensional manifold of equilibria resulting from the absence of selection at the neutral locus.
) are zero at equilibrium. By (8.1), there is a one-dimensional manifold of equilibria resulting from the absence of selection at the neutral locus.
We assume that parameters are such that the fully polymorphic equilibrium  is admissible and globally asymptotically stable. Using the above order for the allele frequencies and linkage equilibria, the Jacobian at the equilibrium
is admissible and globally asymptotically stable. Using the above order for the allele frequencies and linkage equilibria, the Jacobian at the equilibrium  has block structure,
has block structure,
 |
8.2 |
where  is the Jacobian describing convergence of
is the Jacobian describing convergence of  to
to  , and
, and  is the Jacobian describing convergence of
is the Jacobian describing convergence of  to
to  .
.
Because zero is the leading eigenvalue of  , the rate of convergence to equilibrium at the neutral locus is determined by the second largest eigenvalue of
, the rate of convergence to equilibrium at the neutral locus is determined by the second largest eigenvalue of  , which we denote by
, which we denote by  . We define the effective (total) migration rate by
. We define the effective (total) migration rate by  (Bengtsson 1985; Kobayashi et al. 2008; Bürger and Akerman 2011). It can be checked that under weak migration, i.e., to leading order in
(Bengtsson 1985; Kobayashi et al. 2008; Bürger and Akerman 2011). It can be checked that under weak migration, i.e., to leading order in  and
and  , one obtains
, one obtains
 |
8.3 |
(a Mathematica notebook is available on request). If the neutral site is linked only to one selected locus (e.g., because  ), then
), then
 |
8.4 |
is obtained. Thus, two linked selected loci act as a much stronger barrier to gene flow than a single selected locus, especially if the recombination rate between the two loci is not much larger than the selective coefficients. In Fig. 9, the approximation (8.3) of the effective migration rate  is displayed as a function of
is displayed as a function of  for various parameter combinations and compared with the exact value obtained by numerical evaluation of
for various parameter combinations and compared with the exact value obtained by numerical evaluation of  .
.
Fig. 9.
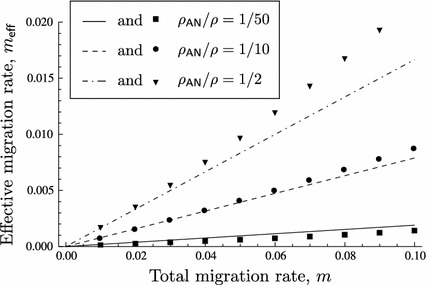
The effective migration rate  as a function of
as a function of  for
for  ,
,  ,
,  , and
, and  . Recall that
. Recall that  . Lines show the weak-migration approximation of
. Lines show the weak-migration approximation of  (8.3). Symbols give the exact numerical value of
(8.3). Symbols give the exact numerical value of 
We note that  is (approximately) the sum of the two effective one-way migration rates (Bürger and Akerman 2011) and closely related to Kobayashi and Telschow’s (2011) effective recombination rate. Our result complements their explicit example on two-locus incompatibilities. We refer to their paper for the discussion of the relation of this concept of an effective migration rate to that of Bengtsson (1985) and for applications in the context of speciation theory.
is (approximately) the sum of the two effective one-way migration rates (Bürger and Akerman 2011) and closely related to Kobayashi and Telschow’s (2011) effective recombination rate. Our result complements their explicit example on two-locus incompatibilities. We refer to their paper for the discussion of the relation of this concept of an effective migration rate to that of Bengtsson (1985) and for applications in the context of speciation theory.
Discussion
The purpose of this investigation was to improve our understanding of how genetic architecture, in particular recombination and locus effects, as well as the pattern and amount of migration determine polymorphism, local adaptation, and differentiation in a subdivided population inhabiting a heterogeneous environment. For simplicity, we restricted attention to two linked, diallelic loci and to migration between two demes. The study of diversifying selection in just two demes may also shape our intuition about clinal variation if the two subpopulations are from different ends of the cline. If alleles are beneficial in only one environment and detrimental in the other, local adaptation of subpopulations and differentiation between them can be obtained only if a (multilocus) polymorphism is maintained. Therefore, most of our mathematical results focus on existence and stability of polymorphic equilibria and on the dependence of the equilibrium configurations on the model parameters (migration rates, selection coefficients, recombination rate).
The model is introduced in Sect. 2. Sections 3 and 4 are devoted to the derivation of the possible equilibrium configurations and bifurcation patterns. They contain our main mathematical results. Explicit analytical results about existence and stability of equilibria were obtained for several limiting or special cases and are complemented by numerical work.
The conditions for admissibility of all single-locus polymorphisms (SLPs) are given in Sect. 3.1, those for asymptotic stability of the monomorphic equilibria (ME) in Proposition 3.1 in Sect. 3.2. The stability of SLPs could not generally be determined (Sect. 3.3). Weak migration is treated by perturbation methods in Sect. 3.4. For sufficiently weak migration, there exists a globally attracting fully polymorphic equilibrium,  (Proposition 3.2). Its approximate coordinates are given by (3.16).
(Proposition 3.2). Its approximate coordinates are given by (3.16).
The complete equilibrium and stability structure could be derived under the assumption of linkage equilibrium (Sect. 3.5). The unique, fully polymorphic equilibrium  is admissible and globally attracting if and only if all four SLPs are admissible. Otherwise, one boundary equilibrium (SLP or ME) is globally asymptotically stable (Proposition 3.3). These results extend straightforwardly to an arbitrary number of diallelic loci. Based on these results, nonlinear perturbation theory establishes the existence of a globally stable, fully polymorphic equilibrium in a perturbed parameter range if recombination is sufficiently strong (Sect. 3.6). This equilibrium is in quasi-linkage equilibrium and given by (3.21).
is admissible and globally attracting if and only if all four SLPs are admissible. Otherwise, one boundary equilibrium (SLP or ME) is globally asymptotically stable (Proposition 3.3). These results extend straightforwardly to an arbitrary number of diallelic loci. Based on these results, nonlinear perturbation theory establishes the existence of a globally stable, fully polymorphic equilibrium in a perturbed parameter range if recombination is sufficiently strong (Sect. 3.6). This equilibrium is in quasi-linkage equilibrium and given by (3.21).
Also for completely linked loci all equilibria and their local stability properties could be derived (Sect. 3.7). In this case, the fully polymorphic equilibrium  (3.24) may lose stability while it is admissible (3.28). At this threshold a boundary equilibrium becomes stable by a ‘jump bifurcation’ (Proposition 3.4). In general, however, more complicated equilibrium patterns than determined by Propositions 3.3 and 3.4 can occur, in particular, multiple stable equilibria.
(3.24) may lose stability while it is admissible (3.28). At this threshold a boundary equilibrium becomes stable by a ‘jump bifurcation’ (Proposition 3.4). In general, however, more complicated equilibrium patterns than determined by Propositions 3.3 and 3.4 can occur, in particular, multiple stable equilibria.
In Sect. 3.8, we apply perturbation theory to infer the equilibrium properties under highly asymmetric migration from those derived for the continent-island model in Bürger and Akerman (2011) and Bank et al. (2012). There, a stable ( ) and an unstable fully polymorphic equilibrium may exist if recombination is intermediate, and
) and an unstable fully polymorphic equilibrium may exist if recombination is intermediate, and  is simultaneously stable with a boundary equilibrium. In general (Sect. 3.11), we cannot exclude the existence of more than two internal equilibria or complicated dynamical behavior. Numerical searches produced no such instances. What can be shown easily is that, if
is simultaneously stable with a boundary equilibrium. In general (Sect. 3.11), we cannot exclude the existence of more than two internal equilibria or complicated dynamical behavior. Numerical searches produced no such instances. What can be shown easily is that, if  , any fully polymorphic equilibrium exhibits LD. In all cases, where an internal equilibrium was calculated (numerically or analytically), it exhibited positive LD.
, any fully polymorphic equilibrium exhibits LD. In all cases, where an internal equilibrium was calculated (numerically or analytically), it exhibited positive LD.
In the super-symmetric case, in which selection in deme 2 mirrors that in deme 1 and migration is symmetric, an assumption made in several applications, a fully polymorphic equilibrium exists always and, presumably, is stable (Sect. 3.10). This is a highly degenerate situation because if  , only a monomorphic equilibrium can be stable for sufficiently large migration rates (Proposition 4.3). If
, only a monomorphic equilibrium can be stable for sufficiently large migration rates (Proposition 4.3). If  (Sect. 3.9), then a fully polymorphic equilibrium can exist for arbitrarily large migration rates if
(Sect. 3.9), then a fully polymorphic equilibrium can exist for arbitrarily large migration rates if  (see also Sect. 4.11).
(see also Sect. 4.11).
Whereas in Sect. 3 the focus was on the efficient presentation of the existence and stability results of equilibria, in Sect. 4 these results are used to derive the possible bifurcation patterns with the total migration rate  as the bifurcation parameter. All possible bifurcation patterns could be derived under the assumption of LE (Theorem 4.4, Figs. 2, 3), and under the assumption of complete linkage (Theorem 4.6, Figs. 4, 5). The latter case is considerably more complex. Interestingly, in each case, every bifurcation pattern can occur for every ratio
as the bifurcation parameter. All possible bifurcation patterns could be derived under the assumption of LE (Theorem 4.4, Figs. 2, 3), and under the assumption of complete linkage (Theorem 4.6, Figs. 4, 5). The latter case is considerably more complex. Interestingly, in each case, every bifurcation pattern can occur for every ratio  of migration rates by choosing the selection coefficients appropriately. Hence, the assumption of symmetric migration does not yield simpler equilibrium configurations than general migration if arbitrary selection coefficients are admitted.
of migration rates by choosing the selection coefficients appropriately. Hence, the assumption of symmetric migration does not yield simpler equilibrium configurations than general migration if arbitrary selection coefficients are admitted.
In each of these cases (LE or  ), we determined the maximum migration rate
), we determined the maximum migration rate  admitting an asymptotically stable, fully polymorphic equilibrium (Corollaries 4.5 and 4.7). The maximum migration rate
admitting an asymptotically stable, fully polymorphic equilibrium (Corollaries 4.5 and 4.7). The maximum migration rate  for
for  always exceeds or equals that (
always exceeds or equals that ( ) for LE, i.e.,
) for LE, i.e.,  . Although for strong recombination,
. Although for strong recombination,  can be very slightly smaller than
can be very slightly smaller than  (Sect. 4.7), in the vast majority of investigated cases,
(Sect. 4.7), in the vast majority of investigated cases,  is bracketed by
is bracketed by  and
and  (Fig. 6, Sect. 4.11).
(Fig. 6, Sect. 4.11).
Proposition 4.3 demonstrates that a ME is globally attracting if migration is sufficiently strong (except in the degenerate case noted above). If we interpret the equilibria  and
and  as fixation of a generalist (
as fixation of a generalist ( and
and  are haplotypes of intermediate fitness), and
are haplotypes of intermediate fitness), and  and
and  as fixation of a specialist (
as fixation of a specialist ( and
and  are the locally adapted haplotypes), then depending on the sign of
are the locally adapted haplotypes), then depending on the sign of  one of the generalists becomes fixed for high
one of the generalists becomes fixed for high  if
if  is intermediate (i.e.,
is intermediate (i.e.,  if
if  ,
,  if
if  ; but note that, depending on the selection coefficients, both
; but note that, depending on the selection coefficients, both  and
and  can be arbitrarily close to 0 or 1.). The critical value
can be arbitrarily close to 0 or 1.). The critical value  as well as
as well as  and
and  are independent of
are independent of  . Otherwise, one of the specialists becomes fixed for large
. Otherwise, one of the specialists becomes fixed for large  .
.
The fact that a generalist becomes fixed for strong migration is a distinct feature of (balanced) two-way migration: in the CI model or if migration is sufficiently asymmetric ( or
or  if
if  ), one of the specialist haplotypes swamps the populations and becomes fixed. Another difference between highly asymmetric and more symmetric migration patterns is that in the first case, it is always the locus under weaker selection that first loses its polymorphism while
), one of the specialist haplotypes swamps the populations and becomes fixed. Another difference between highly asymmetric and more symmetric migration patterns is that in the first case, it is always the locus under weaker selection that first loses its polymorphism while  increases, whereas this not necessarily so in the latter case (see Sect. 4.2 and Theorem 4.6, cases A3 and A4).
increases, whereas this not necessarily so in the latter case (see Sect. 4.2 and Theorem 4.6, cases A3 and A4).
In summary, we determined quantitatively when the following three evolutionarily stable states discussed by Kawecki and Ebert (2004) occur: (i) existence of a single specialist optimally adapted to one deme and poorly to the other, (ii) existence of a single generalist type which has higher average fitness in the whole population than than any of the specialists, and (iii) existence of a set of specialists each adapted to its deme, i.e., coexistence in a polymorphism. Local adaptation and differentiation occur only in case (iii).
In Sect. 5, we used the migration load in each deme to quantify the degree of local adaptation. In Sect. 6 we introduced a new multilocus version of  to measure differentiation. If migration is weak, then local adaptation and differentiation decrease with increasing migration rate and increase with increasing linkage between the loci (Fig. 7). In particular, for given (small) migration rate, local adaptation and differentiation are maximized if the fitness effects are concentrated on a single locus (corresponding to
to measure differentiation. If migration is weak, then local adaptation and differentiation decrease with increasing migration rate and increase with increasing linkage between the loci (Fig. 7). In particular, for given (small) migration rate, local adaptation and differentiation are maximized if the fitness effects are concentrated on a single locus (corresponding to  in our model). However, as discussed in Sect. 5, for high migration rates, the migration load of the total population can decrease with increasing recombination or migration rate. Similarly, at high recombination and migration rates,
in our model). However, as discussed in Sect. 5, for high migration rates, the migration load of the total population can decrease with increasing recombination or migration rate. Similarly, at high recombination and migration rates,  can increase with increasing migration or recombination rate. Thus, for given, relatively high migration rate,
can increase with increasing migration or recombination rate. Thus, for given, relatively high migration rate,  may be minimized at intermediate recombination rates. Apparently, it is always maximized in the absence of recombination.
may be minimized at intermediate recombination rates. Apparently, it is always maximized in the absence of recombination.
In Sect. 7, we investigated the conditions for invasion of locally beneficial mutants. At an isolated locus, such a mutant can invade and become established in a migration-selection equilibrium if and only if its advantage exceeds a threshold that increases with the immigration rate of the wild type; see (7.3b). If, however, this mutant occurs at a locus that is linked to a locus that is already in migration-selection balance, then its invasion is facilitated, i.e., its local selective advantage can be smaller (Fig. 8b). Equivalently, for given selection coefficients and total migration rate, the minimum recombination rate needed for invasion increases if  , or the influx of the (deleterious) wild type relative to the efflux of the new mutant, increases (Fig. 8a). For the extreme case of one-way migration from a ‘continental’ population to an ‘island’ population that is adapting to a new environment, Bürger and Akerman (2011) proved that invasion of a locally beneficial mutant is always facilitated by increased linkage to a locus in migration-selection balance.
, or the influx of the (deleterious) wild type relative to the efflux of the new mutant, increases (Fig. 8a). For the extreme case of one-way migration from a ‘continental’ population to an ‘island’ population that is adapting to a new environment, Bürger and Akerman (2011) proved that invasion of a locally beneficial mutant is always facilitated by increased linkage to a locus in migration-selection balance.
Thus, our results complement the numerical finding by Yeaman and Whitlock (2011) for a multilocus quantitative-genetic model that clusters of locally adaptive mutations, or concentrated genetic architectures, build up in spatially structured populations with opposing selection pressures in two demes. Because tighter linkage is required for invasion under increasingly asymmetric migration rates, more concentrated architectures and a greater advantage for recombination-reducing mechanisms (such as chromosome inversions) should be expected for highly asymmetric migration. In finite populations, invasion of new mutants occurs only with a certain probability, and genetic drift may erase polymorphism. Numerical work, supported by analytical methods, has already shed some light on the dependence of the probability of establishment of new, locally adaptive mutations on the recombination rate and other factors (Yeaman and Otto 2011; Feder et al. 2012). Analytical work on the role of genetic drift and finite population size on these issues is in progress.
Our results also show that, in the absence of epistasis and under the present form of balancing selection, reduced recombination between selected loci is favored, except when migration rates are sufficiently symmetric and high (Sect. 5). Selection inducing certain forms of epistasis may favor high recombination in structured populations more easily (Pylkov et al. 1998; Lenormand and Otto 2000; Bank et al. 2012). Therefore, general predictions about the emergence of clusters of locally adaptive mutations in regions of reduced recombination, or of genomic islands of speciation (Wu and Ting 2004) or of differentiation (Feder et al. 2012), can not be made in the absence of detailed information about epistasis and the spatial pattern of selection and migration. At least in the absence of epistasis, the most favorable situation for the emergence of such clusters should occur in populations that are adapting to a new environment, still receiving maladaptive gene flow but sending out only very few or no migrants (corresponding to a continent-island model).
In Sect. 8, we derived the approximation (8.3) for the effective migration rate at a linked neutral locus that is located between the selected loci. This approximation is simply the sum of the two effective migration rates under one-way migration (Bürger and Akerman 2011). Because in the present model, polymorphism at the selected loci is maintained by balancing selection, the effective migration rate may be greatly reduced compared with the actual migration rate (see Fig. 9). Thus, strong barriers against gene flow may build up at such neutral sites and enhance (neutral) differentiation (see Charlesworth and Charlesworth 2010, Chap. 8.3). Future work will have to study the actual amount and pattern of neutral diversity at such sites in finite populations.
Acknowledgments
We are grateful for useful comments on the manuscript by two anonymous referees. One of them inspired us to introduce the new multilocus fixation index. This work was supported by grants P21305 and P25188 of the Austrian Science Fund FWF. Support by the Vienna Graduate School of Population Genetics funded by the Austrian Science Fund (FWF, W1225) is also acknowledged.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Appendix A
Sufficiency of the assumptions (2.3)
By relabeling alleles, we can assume without loss of generality (2.3a). Generically, one of the following six parameter sets applies:
 |
10.1a |
 |
10.1b |
 |
10.1c |
 |
10.1d |
 |
10.1e |
 |
10.1f |
In addition, there are the following three parameter sets:
 |
10.1g |
 |
10.1h |
 |
10.1i |
The sets (10.1a)–(10.1i) yield the complete parameter space of the selection coefficients.
We show that the parameter sets (10.1c)–(10.1f) can be derived from (10.1a) and (10.1b) by simple transformations. Let  denote the exchange of loci, i.e.,
denote the exchange of loci, i.e.,  and
and  , and
, and  the exchange of demes, i.e.,
the exchange of demes, i.e.,  and
and  . We observe that
. We observe that  and
and
 |
10.2a |
 |
10.2b |
hold. Therefore, (2.3) is sufficient to describe the (generic) parameter region where  . Since
. Since
 |
10.3 |
(3.34) is sufficient to describe the degenerate cases  .
.
Proof of Proposition 3.1
At each monomorphic equilibrium, the characteristic polynomial factors into three quadratic polynomials,  . Two of them,
. Two of them,  and
and  , determine stability with respect to the adjacent marginal one-locus systems. The corresponding conditions are already known from one-locus theory. The third,
, determine stability with respect to the adjacent marginal one-locus systems. The corresponding conditions are already known from one-locus theory. The third,  , determines stability with respect to the interior of the state space.
, determines stability with respect to the interior of the state space.
In the following, we derive the stability conditions (3.10) and (3.11) for  . Those for
. Those for  can be deduced analogously or by symmetry considerations by taking into account that (2.3b) implies
can be deduced analogously or by symmetry considerations by taking into account that (2.3b) implies  . The stability analysis of
. The stability analysis of  and
and  is much simpler and left to the reader.
is much simpler and left to the reader.
For  , it is straightforward to show that
, it is straightforward to show that
 |
10.4a |
 |
10.4b |
 |
10.4c |
Because  for every
for every  ,
,  if
if  ,
,  if and only if
if and only if  , and
, and  , we conclude that the two eigenvalues emanating from
, we conclude that the two eigenvalues emanating from  are negative if and only if
are negative if and only if
 |
10.5a |
Analogously, the two eigenvalues emanating from  are negative if and only if
are negative if and only if
 |
10.5b |
and those originating from  are negative if and only if
are negative if and only if
 |
10.5c |
Conditions (10.5a) and (10.5b) yield (3.10).
Concerning (10.5c), we observe that it is always satisfied if  because then the right-hand side is negative. Next we show, that (10.5c) is also satisfied if
because then the right-hand side is negative. Next we show, that (10.5c) is also satisfied if  . Because the right-hand side of (10.5c) is strictly monotone decreasing in
. Because the right-hand side of (10.5c) is strictly monotone decreasing in  , it is sufficient to prove that (10.5c) holds if
, it is sufficient to prove that (10.5c) holds if  . Then simple rearrangement of (10.5c) leads to the condition
. Then simple rearrangement of (10.5c) leads to the condition
 |
10.6 |
which can be rewritten as
 |
10.7 |
This is satisfied if (10.5b) holds because this also implies  . One shows similarly that (10.5c) is satisfied if
. One shows similarly that (10.5c) is satisfied if  . Therefore, we have proved that
. Therefore, we have proved that  is asymptotically stable if (3.10) and (3.11) hold.
is asymptotically stable if (3.10) and (3.11) hold.
Calculation of equilibria with two polymorphic loci if 
As shown in the main text, by Corollary 3.9 of Nagylaki and Lou (2007) it is sufficient to assume that  is absent, which implies
is absent, which implies  and
and  . Setting
. Setting  , we find from the equations
, we find from the equations  and
and  (2.7) that
(2.7) that
 |
10.8a |
 |
10.8b |
holds at equilibrium. Substituting (10.8) into  and
and  , we obtain at equilibrium,
, we obtain at equilibrium,
 |
10.9a |
 |
10.9b |
where  and
and  are quadratic polynomials in
are quadratic polynomials in  . The obvious substitution results in the equilibrium condition
. The obvious substitution results in the equilibrium condition
 |
10.10a |
It is easy to check that  always fulfills this condition and it is the only solution satisfying
always fulfills this condition and it is the only solution satisfying  . Hence, unless there is curve
. Hence, unless there is curve  of solutions of (10.10) that passes through
of solutions of (10.10) that passes through  and through either a point on
and through either a point on  with
with  or on
or on  with
with  ,
,  is the unique admissible solution of (10.10).
is the unique admissible solution of (10.10).
Because  has an eigenvalue 0 only if either (3.26) is satisfied or if
has an eigenvalue 0 only if either (3.26) is satisfied or if  (which occurs if and only if
(which occurs if and only if  collides with either
collides with either  or
or  ),
),  is the only equilibrium with both loci polymorphic, except when (3.26) is satisfied. In the latter case, a line of equilibria exists, as we show now.
is the only equilibrium with both loci polymorphic, except when (3.26) is satisfied. In the latter case, a line of equilibria exists, as we show now.
We calculate  from (3.26) and substitute into (10.10). The right-hand side factorizes into two linear terms. Only one of them gives rise to admissible equilibria and, in fact, yields the manifold:
from (3.26) and substitute into (10.10). The right-hand side factorizes into two linear terms. Only one of them gives rise to admissible equilibria and, in fact, yields the manifold:
 |
10.11 |
where  . The allele frequencies in the other deme are obtained from (10.8). It is straightforward to check that not only
. The allele frequencies in the other deme are obtained from (10.8). It is straightforward to check that not only  , but also the equilibria
, but also the equilibria  and
and  lie on this manifold. In terms of the gamete frequencies, this manifold is a straight line.
lie on this manifold. In terms of the gamete frequencies, this manifold is a straight line.
Stability of 
In this section we derive the stability of  .
.
As  is lost if
is lost if  and (2.3) hold, it is sufficient to consider the dynamics (2.5) in
and (2.3) hold, it is sufficient to consider the dynamics (2.5) in  . In this case, the characteristic polynomial at
. In this case, the characteristic polynomial at  factors into two quadratic polynomials,
factors into two quadratic polynomials,  . These are given by
. These are given by
 |
10.12a |
 |
10.12b |
The polynomial  determines the stability with respect to the (effectively one-locus) system where only ’alleles’
determines the stability with respect to the (effectively one-locus) system where only ’alleles’  and
and  are present. It is convex with
are present. It is convex with  if and only if
if and only if  (where the equalities correspond), i.e., whenever
(where the equalities correspond), i.e., whenever  is admissible, cf. (3.23). If
is admissible, cf. (3.23). If  ,
,  and
and  attains a negative value at its minimum (as can be shown easily). Therefore, all eigenvalues emanating from
attains a negative value at its minimum (as can be shown easily). Therefore, all eigenvalues emanating from  are real and negative whenever
are real and negative whenever  is admissible.
is admissible.
The polynomial  determines stability with respect to the interior of
determines stability with respect to the interior of  . It is convex and attains its minimum at
. It is convex and attains its minimum at
 |
10.13 |
where  by (2.3a) and (3.22). As
by (2.3a) and (3.22). As  , the eigenvalues emanating from
, the eigenvalues emanating from  are real. As
are real. As
 |
10.14 |
where the equalities correspond and  is defined in (3.27), and because
is defined in (3.27), and because  , we conclude that the two eigenvalues emanating from
, we conclude that the two eigenvalues emanating from  are negative if and only if (3.28) holds.
are negative if and only if (3.28) holds.
Stability of SLPs if 
For  it is sufficient to study the dynamics (2.5) in
it is sufficient to study the dynamics (2.5) in  . SLPs where
. SLPs where  (
( ), i.e.,
), i.e.,  and
and  , are unstable. It remains to study the stability of
, are unstable. It remains to study the stability of  and
and  .
.
We present the analysis for  in detail, as results for
in detail, as results for  follow analogously.
follow analogously.
At  the characteristic polynomial factors into two quadratic polynomials,
the characteristic polynomial factors into two quadratic polynomials,  , given by
, given by
 |
10.15a |
 |
10.15b |
 determines stability with respect to the one-locus system where
determines stability with respect to the one-locus system where  is fixed.
is fixed.  if and only if
if and only if  , i.e., whenever
, i.e., whenever  collides with a ME according to (3.9a) and (3.9b). Whenever
collides with a ME according to (3.9a) and (3.9b). Whenever  , i.e.,
, i.e.,  is admissible (3.7),
is admissible (3.7),  and
and  . As
. As  for every
for every  ,
,  attains a minimum, where it is straightforward to show that
attains a minimum, where it is straightforward to show that  takes a negative value at its minimum. Thus, all eigenvalues emanating from
takes a negative value at its minimum. Thus, all eigenvalues emanating from  are real and negative whenever
are real and negative whenever  is admissible.
is admissible.
 determines stability with respect to the interior of
determines stability with respect to the interior of  .
.  , if and only if
, if and only if  , cf. (3.27), where the equalities correspond. Whenever
, cf. (3.27), where the equalities correspond. Whenever  ,
,  . As
. As  for every
for every  ,
,  attains a minimum, where it is straightforward to show that
attains a minimum, where it is straightforward to show that  takes a negative value at its minimum. Thus, all eigenvalues emanating from
takes a negative value at its minimum. Thus, all eigenvalues emanating from  are real and negative whenever
are real and negative whenever  holds. Otherwise, at least one eigenvalue is positive.
holds. Otherwise, at least one eigenvalue is positive.
Combining the results obtained for  and
and  it follows that
it follows that  is asymptotically stable if and only if
is asymptotically stable if and only if
 |
10.16 |
hold. We note that  is equivalent to
is equivalent to  , and our general assumption (2.3) implies
, and our general assumption (2.3) implies  and
and  . Using these relations we can show with the help of Mathematica that (10.16) is incompatible with
. Using these relations we can show with the help of Mathematica that (10.16) is incompatible with  . Consequently,
. Consequently,  is not admissible if
is not admissible if  is asymptotically stable.
is asymptotically stable.
The super-symmetric case
We prove that in the super-symmetric case of Sect. 3.10, all SLPs are unstable.
We assume symmetric migration rates ( ), equivalent loci (
), equivalent loci ( ), and selection in deme 2 mirrors that in deme 1 (
), and selection in deme 2 mirrors that in deme 1 ( ). Thus,
). Thus,  . Equilibria may collide (thus leave or enter the state space) if and only if at least one of their eigenvalues is zero. Eigenvalues are zeros of the characteristic polynomial, which has the form
. Equilibria may collide (thus leave or enter the state space) if and only if at least one of their eigenvalues is zero. Eigenvalues are zeros of the characteristic polynomial, which has the form  . If zero is an eigenvalue at an equilibrium, i.e.,
. If zero is an eigenvalue at an equilibrium, i.e.,  , the constant term
, the constant term  must vanish. In the super-symmetric case every characteristic polynomials at an SLP has the same constant term
must vanish. In the super-symmetric case every characteristic polynomials at an SLP has the same constant term
 |
10.17 |
One can show that  is impossible if
is impossible if  .
.
Important quantities and relations
The following section complements Sect. 4.1. Here, we derive all relations of  (4.2) and
(4.2) and  (4.3) needed inSects. 4.2 to 4.8 and in the proofs of the theorems there.
(4.3) needed inSects. 4.2 to 4.8 and in the proofs of the theorems there.
Using (4.9a), (4.9b), (4.5a), (4.5b), (4.12) and (4.13), we derive all possible inequalities between  and
and  :
:
 |
10.18a |
 |
10.18b |
 |
10.18c |
 |
10.18d |
 |
10.18e |
where
 |
10.18f |
Using (4.6), (4.5), and (4.12)–(4.15) we obtain the following inequalities for  :
:
 |
10.19a |
 |
10.19b |
 |
10.19c |
 |
10.19d |
 |
10.19e |
 |
10.19f |
 |
10.19g |
 |
10.19h |
 |
10.19i |
 |
10.19j |
 |
10.19k |
 |
10.19l |
From (4.12), (4.13), (10.18), and (10.19e)–(10.19l) we infer
 |
10.20 |
Next, we derive the relations between  and
and  or
or  needed in the proof of Theorem 4.6. As their derivation is lengthy, the reader may wish to skip the proof and go immediately to the results given by (10.37) and (10.38).
needed in the proof of Theorem 4.6. As their derivation is lengthy, the reader may wish to skip the proof and go immediately to the results given by (10.37) and (10.38).
Our approach to derive the possible relations between  and
and  or
or  is as follows: First, we derive all relevant relations of
is as follows: First, we derive all relevant relations of  (4.2) for arbitrary recombination
(4.2) for arbitrary recombination  . We use these relations to determine the required relations between
. We use these relations to determine the required relations between  ,
,  ,
,  and
and  for arbitrary
for arbitrary  . By setting
. By setting  in the results obtained and by the equivalence given in (4.10), the possible relations between
in the results obtained and by the equivalence given in (4.10), the possible relations between  and
and  or
or  follow immediately.
follow immediately.
By definition, the values  ,
,  ,
,  ,
,  ,
,  ,
,  , and
, and  (4.2) are independent of the recombination rate
(4.2) are independent of the recombination rate  . Their relations under (2.3) are given in (4.12), (4.13), and (4.14).
. Their relations under (2.3) are given in (4.12), (4.13), and (4.14).
The values  ,
,  ,
,  , and
, and  (4.2) depend on
(4.2) depend on  , and we analyze this dependence in the following. The conditions which determine the admissibility of
, and we analyze this dependence in the following. The conditions which determine the admissibility of  and
and  (
( ) are:
) are:
 |
10.21a |
 |
10.21b |
 |
10.21c |
 |
10.21d |
with the relations
 |
10.22a |
 |
10.22b |
To determine further relations of  ,
,  ,
,  , and
, and  , we define the following critical recombination rates:
, we define the following critical recombination rates:
 |
10.23a |
 |
10.23b |
 |
10.23c |
 |
10.23d |
Next, we determine the admissibility of  and
and  defined in (10.23). Therefore, we partition the selection parameters satisfying (2.3) and
defined in (10.23). Therefore, we partition the selection parameters satisfying (2.3) and  according to
according to
 |
10.24a |
and
 |
10.24b |
Analogously, the selection parameters satisfying (2.3) and  can be partitioned according to
can be partitioned according to
 |
10.25a |
and
 |
10.25b |
Using these partitions, we obtain that  and
and  satisfy the following relations (as can be checked with Mathematica):
satisfy the following relations (as can be checked with Mathematica):
 |
10.26a |
 |
10.26b |
 |
10.26c |
 |
10.26d |
 |
10.26e |
 |
10.26f |
As  and (2.3) hold, we obtain that
and (2.3) hold, we obtain that
 |
10.27a |
 |
10.27b |
 |
10.27c |
and
 |
10.28a |
 |
10.28b |
where
 |
10.28c |
We derive the following relations additional to (10.22a) and (10.22b), using (10.26) and (10.27):
 |
10.29a |
 |
10.29b |
and
 |
10.30a |
 |
10.30b |
and by recalling (4.13):
 |
10.31a |
 |
10.31b |
Now we derived all relations between  necessary to deduce the relevant relations between
necessary to deduce the relevant relations between  (
( ) and
) and  ,
,  .
.
First, we note that under (2.3),  if
if  and
and  if
if  .
.
In the following, we derive all possible relations between  ,
,  , and
, and  where we assume that
where we assume that  (otherwise
(otherwise  is unstable, cf. Proposition 4.1, and
is unstable, cf. Proposition 4.1, and  is not of particular interest). By (4.5a), (4.5b), (4.12), (4.13), (10.22a), (10.27), (10.29), and (10.31), we obtain that
is not of particular interest). By (4.5a), (4.5b), (4.12), (4.13), (10.22a), (10.27), (10.29), and (10.31), we obtain that
 |
10.32a |
 |
10.32b |
 |
10.32c |
and
 |
10.33a |
 |
10.33b |
 |
10.33c |
 |
10.33d |
From (10.18f) it follows that
 |
10.34a |
 |
10.34b |
 |
10.34c |
To derive all possible relations between  ,
,  , and
, and  we assume
we assume  (cf. Proposition 4.1). By (4.5a), (4.5b), (4.12), (4.13), (10.22b), (10.28), and (10.30), we obtain that
(cf. Proposition 4.1). By (4.5a), (4.5b), (4.12), (4.13), (10.22b), (10.28), and (10.30), we obtain that
 |
10.35a |
 |
10.35b |
 |
10.35c |
From (10.18f) it follows that
 |
10.36a |
 |
10.36b |
 |
10.36c |
If  or
or  , the relations involving
, the relations involving  ,
,  and
and  follow immediately by (4.10), i.e., by setting
follow immediately by (4.10), i.e., by setting  in the relevant formulas in (10.32)–(10.35). The remaining cases where
in the relevant formulas in (10.32)–(10.35). The remaining cases where  can be calculated easily using
can be calculated easily using  , (4.5), (4.15), and (10.18). Then, all admissible relations are:
, (4.5), (4.15), and (10.18). Then, all admissible relations are:
 |
10.37a |
 |
10.37b |
 |
10.37c |
 |
10.37d |
 |
10.37e |
 |
10.37f |
 |
10.37g |
 |
10.37h |
 |
10.37i |
 |
10.37j |
 |
10.37k |
Because other strict inequalities between  ,
,  , and
, and  do not occur, we infer
do not occur, we infer
 |
10.38 |
References
- Bank C, Bürger R, Hermisson J. The limits to parapatric speciation: Dobzhansky–Muller incompatibilities in a continent-island model. Genetics. 2012;191:845–865. doi: 10.1534/genetics.111.137513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton NH. Multilocus clines. Evolution. 1983;37:454–471. doi: 10.2307/2408260. [DOI] [PubMed] [Google Scholar]
- Barton NH. What role does natural selection play in speciation? Phil Trans R Soc B. 2010;365:1825–1840. doi: 10.1098/rstb.2010.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton NH, Bengtsson BO. The barrier to genetic exchange between hybridising populations. Heredity. 1986;56:357–376. doi: 10.1038/hdy.1986.135. [DOI] [PubMed] [Google Scholar]
- Bengtsson BO. The flow of genes through a genetic barrier. In: Greenwood JJ, Harvey PH, Slatkin M, editors. Evolution essays in honour of John Maynard Smith. Cambridge: University Press; 1985. pp. 31–42. [Google Scholar]
- Blanquart F, Gandon S, Nuismer SL. The effects of migration and drift on local adaptation to a heterogeneous environment. J Evol Biol. 2012;25:1351–1363. doi: 10.1111/j.1420-9101.2012.02524.x. [DOI] [PubMed] [Google Scholar]
- Bürger R. The mathematical theory of selection, recombination, and mutation. Chichester: Wiley; 2000. [Google Scholar]
- Bürger R (2009) Multilocus selection in subdivided populations I. Convergence properties for weak or strong migration. J Math Biol 58:939–978 [DOI] [PubMed]
- Bürger R. Multilocus selection in subdivided populations II. Maintenance of polymorphism under weak or strong migration. J Math Biol. 2009;58:979–997. doi: 10.1007/s00285-008-0237-4. [DOI] [PubMed] [Google Scholar]
- Bürger R. Polymorphism in the two-locus Levene model with nonepistatic directional selection. Theor Popul Biol. 2009;76:214–228. doi: 10.1016/j.tpb.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Bürger R. Evolution and polymorphism in the multilocus Levene model with no or weak epistasis. Theor Popul Biol. 2010;78:123–138. doi: 10.1016/j.tpb.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürger R, Akerman A. The effects of linkage and gene flow on local adaptation: a two-locus continent-island model. Theor Popul Biol. 2011;80:272–288. doi: 10.1016/j.tpb.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth B, Nordborg M, Charlesworth D. The effects of local selection, balanced polymorphism and background selection on equilibrium patterns of genetic diversity in subdivided populations. Genet Res. 1997;70:155–174. doi: 10.1017/S0016672397002954. [DOI] [PubMed] [Google Scholar]
- Charlesworth B, Charlesworth D. Elements of evolutionary genetics. Greenwood Village: Roberts & Co.; 2010. [Google Scholar]
- Charlesworth D, Charlesworth B. Selection on recombination in clines. Genetics. 1979;91:581–589. doi: 10.1093/genetics/91.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasnov JR. Equilibrium properties of a multi-locus, haploid-selection, symmetric-viability model. Theor Popul Biol. 2012;81:119–130. doi: 10.1016/j.tpb.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Christiansen FB, Feldman M. Subdivided populations: a review of the one- and two-locus deterministic theory. Theor Popul Biol. 1975;7:13–38. doi: 10.1016/0040-5809(75)90003-9. [DOI] [PubMed] [Google Scholar]
- Conley C (1978) Isolated invariant sets and the Morse index. NSF CBMS Lecture Notes, vol 38. American Mathematical Society, Providence
- Deakin MAB. Sufficient conditions for genetic polymorphism. Am Nat. 1966;100:690–692. doi: 10.1086/282462. [DOI] [Google Scholar]
- Ewens WJ. Mean fitness increases when fitnesses are additive. Nature. 1969;221:1076. doi: 10.1038/2211076a0. [DOI] [PubMed] [Google Scholar]
- Eyland EA. Moran’s island migration model. Genetics. 1971;69:399–403. doi: 10.1093/genetics/69.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder JL, Gejji R, Yeaman S, Nosil P. Establishment of new mutations under divergence and genome hitchhiking. Phil Trans R Soc B. 2012;367:461–474. doi: 10.1098/rstb.2011.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco D, Uyenoyama M. Effects of polymorphism for locally adapted genes on rates of neutral introgression in structured populations. Theor Popul Biol. 2011;80:121–131. doi: 10.1016/j.tpb.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadeler KP, Glas D. Quasimonotone systems and convergence to equilibrium in a population genetic model. J Math Anal Appl. 1983;95:297–303. doi: 10.1016/0022-247X(83)90108-7. [DOI] [Google Scholar]
- Haldane JBS. A mathematical theory of natural and artificial selection. Part VI. Isolation. Proc Camb Phil Soc. 1930;26:220–230. doi: 10.1017/S0305004100015450. [DOI] [Google Scholar]
- Hofbauer J (1990) An index theorem for dissipative semiflows. Rocky Mt J Math 20:1017–1031
- Karlin S. Classification of selection-migration structures and conditions for a protected polymorphism. Evol Biol. 1982;14:61–204. [Google Scholar]
- Karlin S, McGregor J. Polymorphism for genetic and ecological systems with weak coupling. Theor Popul Biol. 1972;3:210–238. doi: 10.1016/0040-5809(72)90027-5. [DOI] [PubMed] [Google Scholar]
- Kawecki TJ, Ebert D. Conceptual issues in local adaptation. Ecol Lett. 2004;7:1225–1241. doi: 10.1111/j.1461-0248.2004.00684.x. [DOI] [Google Scholar]
- Kimura M. Attainment of quasi linkage equilibrium when gene frequencies are changing by natural selection. Genetics. 1965;52:875–890. doi: 10.1093/genetics/52.5.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Hammerstein P, Telschow A. The neutral effective migration rate in a mainland-island context. Theor Popul Biol. 2008;74:84–92. doi: 10.1016/j.tpb.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Telschow A. The concept of effective recombination rate and its application in speciation theory. Evolution. 2011;65:617–628. doi: 10.1111/j.1558-5646.2010.01156.x. [DOI] [PubMed] [Google Scholar]
- Lenormand T. Gene flow and the limits to natural selection. Trends Ecol Evol. 2002;17:183–189. doi: 10.1016/S0169-5347(02)02497-7. [DOI] [Google Scholar]
- Lenormand T, Otto SP. The evolution of recombination in a heterogeneous environment. Genetics. 2000;156:423–438. doi: 10.1093/genetics/156.1.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
Leviyang S, Hamilton MB. Properties of Weir and Cockerham’s
 estimators and associated bootstrap confidence intervals. Theor Popul Biol. 2011;79:39–52. doi: 10.1016/j.tpb.2010.11.001. [DOI] [PubMed] [Google Scholar]
estimators and associated bootstrap confidence intervals. Theor Popul Biol. 2011;79:39–52. doi: 10.1016/j.tpb.2010.11.001. [DOI] [PubMed] [Google Scholar] - Li W-H, Nei M. Stable linkage disequilibrium without epistasis in subdivided populations. Theor Popul Biol. 1974;6:173–183. doi: 10.1016/0040-5809(74)90022-7. [DOI] [PubMed] [Google Scholar]
- Nagylkaki T. Fixation indices in subdivided populations. Genetics. 1998;148:1325–1332. doi: 10.1093/genetics/148.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagylaki T. Evolution under the multilocus Levene model. Theor Popul Biol. 2009;76:197–213. doi: 10.1016/j.tpb.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Nagylaki T. The influence of partial panmixia on neutral models of spatial variation. Theor Popul Biol. 2011;79:19–38. doi: 10.1016/j.tpb.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Nagylaki T. Clines with partial panmixia in an unbounded unidimensional habitat. Theor Popul Biol. 2012;82:22–28. doi: 10.1016/j.tpb.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Nagylaki T, Hofbauer J, Brunovský P (1999) Convergence of multilocus systems under weak epistasis or weak selection. J Math Biol 38:103–133 [DOI] [PubMed]
- Nagylaki T, Lou Y. Patterns of multiallelic polymorphism maintained by migration and selection. Theor Popul Biol. 2001;59:297–313. doi: 10.1006/tpbi.2001.1526. [DOI] [PubMed] [Google Scholar]
- Nagylaki T, Lou Y. Evolution under multiallelic migration-selection models. Theor Popul Biol. 2007;72:21–40. doi: 10.1016/j.tpb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Nagylaki T, Lou Y (2008) The dynamics of migration-selection models. In: Friedman A (ed) Tutorials in mathematical biosciences IV. Lecture Notes in Mathematics, vol 1922. Springer, Berlin, pp. 119–172
- Petry D. The effect on neutral gene flow of selection at a linked locus. Theor Popul Biol. 1983;23:300–313. doi: 10.1016/0040-5809(83)90020-5. [DOI] [PubMed] [Google Scholar]
- Pylkov KV, Zhivotovsky LA, Feldman MW. Migration versus mutation in the evolution of recombination under multilocus selection. Genet Res. 1998;71:247–256. doi: 10.1017/S0016672398003243. [DOI] [PubMed] [Google Scholar]
- Shahshahani S (1979) A new mathematical framework for the study of linkage and selection. Memoirs of the American Mathematical Society, vol 211. American Mathematical Society, Providence
- Slatkin M. Gene flow and selection in two-locus systems. Genetics. 1975;81:787–802. doi: 10.1093/genetics/81.4.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spichtig M, Kawecki TJ. The maintenance (or not) of polygenic variation by soft selection in heterogeneous environments. Am Nat. 2004;164:70–84. doi: 10.1086/421335. [DOI] [PubMed] [Google Scholar]
- Turelli M, Barton NH. Dynamics of polygenic characters under selection. Theor Popul Biol. 1990;38:1–57. doi: 10.1016/0040-5809(90)90002-D. [DOI] [Google Scholar]
-
Weir B, Cockerham C. Estimating
 statistics for the analysis of population structure. Evolution. 1984;38:1358–1370. doi: 10.2307/2408641. [DOI] [PubMed] [Google Scholar]
statistics for the analysis of population structure. Evolution. 1984;38:1358–1370. doi: 10.2307/2408641. [DOI] [PubMed] [Google Scholar] - Wu C-I, Ting C-T. Genes and speciation. Nat Rev Genet. 2004;5:114–122. doi: 10.1038/nrg1269. [DOI] [PubMed] [Google Scholar]
- Yeaman S, Otto SP. Establishment and maintenance of adaptive genetic divergence under migration, selection, and drift. Evolution. 2011;65:2123–2129. doi: 10.1111/j.1558-5646.2011.01277.x. [DOI] [PubMed] [Google Scholar]
- Yeaman S, Whitlock M. The genetic architecture of adaptation under migration-selection balance. Evolution. 2011;65:1897–1911. doi: 10.1111/j.1558-5646.2011.01269.x. [DOI] [PubMed] [Google Scholar]























