Abstract
P-glycoprotein (P-gp) is present in various tissue cells, required for the pumping of lipophilic drugs (including glucocorticoids) out of cells. We hypothesized that polymorphisms in the P-gp encoding gene (multidrug-resistant transporter-1 [MDR1]) are related to individual differences in glucocorticoid sensitivity and the development of glucocorticoid-induced avascular necrosis of the femoral head (GANFH). In this case–control study, we genotyped three known single-nucleotide polymorphisms (SNPs: C1236T, G2677T/A, and C3435T) within the MDR1 gene in 662 Chinese subjects. Statistically significant differences between GANFH patients and either healthy controls or glucocorticoid-resistant patients (non-GANFH) were found for the T allele or TT genotype of C3435T. The haplotype TTT, composed of these three SNPs, exhibited a significant association with the disease. No associations were identified between C1236T or G2677T/A and GANFH. Our results suggest that the C3435T polymorphism of the MDR1 gene is associated with susceptibility to GANFH in a Chinese population.
Introduction
Avascular necrosis of the femoral head (ANFH) occurs when the bone tissue undergoes necrosis and the bone collapses, occurring in a particular anatomical site of the femoral head. ANFH may induce hip joint dysfunction and partial or complete loss of the ability to walk. Glucocorticoid therapy is considered as one of the most common risk factors of ANFH. However, it cannot be ignored that, while some people develop osteonecrosis others do not, under the same conditions. This phenomenon suggests that susceptibility to glucocorticoid-induced avascular necrosis of the femoral head (GANFH) and individual genetic factors are closely related. Specific single-nucleotide polymorphisms (SNPs) have previously been considered accountable for the susceptibility to ANFH (Jones and Hungerford, 2007). These SNPs are located in the genes encoding for plasminogen activator inhibitor-1 (PAI-1), methylenetetrahydrofolate reductase (MTHFR), apolipoprotein B (APOB), collagen type II alpha 1 (COL2A1), among others (Glueck et al., 1999; Zalavms et al., 2002; Liu et al., 2005; Hirata et al., 2007). However, it still remains unclear exactly which gene polymorphisms are responsible for GANFH.
Multidrug-resistant transporter-1 (MDR1) is a housekeeping gene, located on human chromosome 7 band q21.1. This gene spans 4.5 kb encoding a 1280 amino acid (aa) polypeptide. A 170-kDa of protein named P-glycoprotein (P-gp) can be formed after glycosylation of the amino acid polypeptide. P-gp is present in various tissue cells, required for pumping lipophilic drugs (including glucocorticoids) out of the cells. This causes the intracellular drug concentration to decrease and cytotoxic damage is subsequently reduced. In addition, P-gp can indirectly regulate other genes such as cytochrome p450 CYP450, which also has an important role in drug absorption, distribution, and metabolism (Pal and Mitra, 2006).
Almost 30 SNPs have been identified in the gene region of MDR1. The most widely studied SNPs are C1236T (exon 12, rs1128503), G2677T/A (exon 21, rs2032582), and C3435T (exon 26, rs1045642). The polymorphisms C1236T and C3435T do not change the amino acid, while G2677T/A induces the replacement of amino acid residues (alanine 893–serine 893 or alanine 893–threonine 893). These three SNPs have previously been shown to be associated with both disease development and drug response in different populations (Balcerczak et al., 2010; Ozen et al., 2011; Chen et al., 2012).
Chinese osteonecrosis cases account for more than half of the new cases in the world (Pei, 2010). Whether or not genetic factors increase GANFH disease susceptibility in Chinese is unclear, with controversy surrounding some reported results (Gong et al., 2013). The present study aimed to investigate the frequency of the C1236T, G2677T/A, and C3435T polymorphisms of the MDR1 gene, and furthermore, their association with GANFH disease susceptibility in a Chinese population.
Materials and Methods
Subjects
In total, 105 unrelated patients who had been diagnosed with GANFH were enrolled into the GANFH group. GANFH patients were undergoing GANFH treatment in the Bone-setting Hospital of Luoyang and Taizhou Hospital of Zhejiang Province between October 2010 and March 2013. These patients had received standard glucocorticoid therapy after being diagnosed with systemic lupus erythematosus or acute lymphoblastic leukemia or having had organ transplantation (average doses of gulucocorticoid were 1400±200, 1500±400, and 1300±500 mg of prednisone, respectively). The development of GANFH was diagnosed according to glucocorticoid therapy history; assessment by X-rays, magnetic resonance imaging (MRI), and bone scans (Sugano et al., 2002; Zhang and Li, 2007). Two hundred seventeen patients who were confirmed to have no osteonecrosis in the hip, knee, shoulder, or ankle joints more than 1 year after using >2000 mg of prednisone were considered as the steroid-resistant group. Three hundred forty healthy blood donors were considered as the healthy control group. All individuals from the steroid-resistant and control groups were of the same ethnicity (Han) and geographical origin, and were living in the same region as the patients with GANFH (Central and Southeast China). All enrolled individuals in these three groups were aged between 18 and 48 years, and individuals who had a history of heavy drinking (alcohol intake >1000 mL/week) were excluded. This study was approved by the Ethics Committees of the Faculty of Medicine (Bone-setting Hospital of Luoyang and Taizhou Hospital of Zhejiang Province, China), and informed consent was obtained from all subjects before blood sampling. The informed consent obtained from patients and controls also collected basic personal data, for example, name, age, sex, race, place of origin, telephone number, and address.
Genotyping
Polymorphisms were detected by polymerase chain reaction (PCR) followed by direct sequencing. Genomic DNA was extracted from blood samples using the salting-out method, as previously described (Rousseau et al., 1994). PCR primers for individual SNPs are shown in Table 1. PCR amplifications were carried out in 25 mL volumes containing ∼30 ng genomic DNA, 0.3 mM upstream and downstream primers, 200 mM dNTPs, and 0.5 units Taq polymerase (Sangon Biotech, Shanghai, China). PCR conditions were as follows: 5 min at 94°C followed by 30 cycles of 45 s at 94°C, 45 s at 57–59°C, and 30 s at 72°C (Table 1). Amplified products were purified with the EZ-10 Spin Column PCR Product Purification Kit (Sangon Biotech, Shanghai, China). Sequencing reactions were performed using the BIG Dye Terminator Cycle Sequencing Ready Reaction Kit (ABI, Foster City, CA). The sequenced products were purified by ethanol precipitation. The DNA pellet obtained was then dissolved in 4 mL of loading dye (deionized formamide; Applied Biosystems, Foster city, CA) and analyzed on an Applied Biosystems Genetic Analyzer 3100 (PE Applied Biosystems).
Table 1.
Sequences of Polymerase Chain Reaction Primers
| SNPs | Primers (5′-3′) | Annealing Temp. (°C) | Extension time (s) | Size (bp) |
|---|---|---|---|---|
| C1236 T | CACTTCAGTTACCCATCTCG | 58 | 30 | 316 |
| GGTCATAGAGCCTCTGCATC | ||||
| G2677 T/A | AGCAAATCTTGGGACAGGA | 57 | 30 | 352 |
| GTCCAAGAACTGGCTTTGC | ||||
| C3435 T | TGTTTTCAGCTGCTTGATGG | 59 | 30 | 196 |
| AGGCATGTATGTTGGCCTC |
GenBank accession numbers were NC_000007.13 used as the reference sequence.
SNP, single-nucleotide polymorphism.
Statistical analysis
Chi-square (χ2) tests were used to compare allele and genotype distribution of the GANFH patients against their respective controls. Odds ratios (ORs) and 95% confidence intervals (CIs) were also calculated. p-Values<0.05 were considered statistically significant. Hardy–Weinberg equilibrium (HWE) was assessed using a χ2-test in each control group and r2 values were calculated to evaluate the magnitude of linkage disequilibrium (LD). SNPstats software (http://bioinfo.iconcologia.net/SNPstats) and SPSS 15.0 (SPSS, Chicago, IL) software were used for all statistical analyses.
Results
Clinical characteristics of patients and control subjects are illustrated in Table 2. Analysis included 340 healthy controls, 217 patients who were receiving glucocorticoid therapy, but did not develop GANFH (glucocorticoid resistant) and 105 GANFH patients (glucocorticoid sensitive). The mean age was 35.0±10.4 years (range 18–48 years) in the healthy control group, 38.9±8.4 years (range 20–48 years) in the glucocorticoid-resistant control group, and 36.3±9.1 years (range 18–48 years) in the GANFH group. Females constituted 49.1% of the healthy control group, 51.6% of the glucocorticoid-resistant control group, and 41.9% of the GANFH group. Mean age and gender did not differ among these three groups (p>0.05).
Table 2.
Characteristics of GANFH Patients and Controls
| GANFH group (n=105) | GC-resistant group (n=217) | Healthy control group (n=340) | p | |
|---|---|---|---|---|
| Age, years range (mean±SD) | 18–48 (36.3±9.1) | 20–48 (38.9±8.4) | 18–48 (35.0±10.4) | 0.298a |
| Gender: female, no. (%) | 44 (41.9) | 112 (51.6) | 167 (49.1) | 0.259b |
| Body mass index (mean±SD) | 22.5±3.1 | 23.8±3.0 | 23.7±3.6 | 0.247a |
| Type of underlying disease | ||||
| Organ transplantation, no. (%) | 27 (25.7) | 72 (33.2) | ND | 0.174c |
| Systemic lupus erythematosus, no. (%) | 33 (31.4) | 50 (23.0) | ND | 0.107c |
| Acute lymphoblastic leukemia, no. (%) | 18 (17.1) | 52 (24.0) | ND | 0.164c |
| Others, no. (%) | 27 (25.8) | 43 (19.8) | ND | 0.229c |
p-Value among three groups, for ANOVA test.
p-Value among three groups, for χ2-test.
p-Value between GANFH group and glucocorticoid-resistant group, for χ2-test.
GANFH, glucocorticoid-induced avascular necrosis of the femoral head; GC, glucocorticoid; ND, not determined; χ,2 chi-square.
Genotypic and allelic frequencies of SNPs in GANFH patients, glucocorticoid-resistant patients, and healthy controls are shown in Table 3. The genotyping failure rate was 2.4–6.5% across the SNPs. The genotypes of each respective SNP in healthy control individuals were all in HWE (p>0.05). The T allele of C3435T was significantly associated with GANFH (OR=0.70; 95% CI, 0.50–0.98, GANFH group vs. healthy control group, OR=0.56; 95% CI, 0.39–0.80, GANFH group vs. glucocorticoid-resistant group, respectively) (Table 3). Moreover, significant associations were observed between the TT genotype of C3435T and GANFH (OR=0.36; 95% CI, 0.15–0.85, the GANFH group vs. healthy control group, OR=0.23; 95% CI, 0.09–0.54, the GANFH group vs. glucocorticoid-resistant group, respectively) (Table 3). However, no significant differences between patients and control groups were observed in either allele or genotype distribution of C1236T and G2677T/A (p>0.05) (Table 3).
Table 3.
Allele and Genotype Frequencies of Polymorphic Variants of the MDR1 Gene in GANFH Patients and Controls
| Frequency | GANFH vs. healthy control group | GANFH vs. GC-resistant group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SNP ID | Position | Allele and genotype | GANFH group, n (Freq) | Healthy control group, n (Freq) | GC-resistant group, n (Freq) | p-Value | OR (95% CI) | p-Value | OR (95% CI) |
| rs1128503 | C1236 T | CC | 15 (0.16) | 48 (0.15) | 21 (0.10) | 1 | 1 | ||
| CT | 44 (0.46) | 144 (0.44) | 92 (0.44) | 0.95 | 0.98 (0.50–1.91) | 0.30 | 0.67 (0.32–1.42) | ||
| TT | 37 (0.38) | 135 (0.41) | 97 (0.46) | 0.71 | 0.88 (0.44–1.74) | 0.10 | 0.53 (0.25–1.15) | ||
| T | 118 (0.61) | 414 (0.63) | 286 (0.68) | 0.64 | 0.92 (0.66–1.29) | 0.11 | 0.75 (0.52–1.07) | ||
| rs2032582 | G2677 T/A | GG | 24 (0.24) | 63 (0.19) | 36 (0.17) | 1 | 1 | ||
| GT/A | 49 (0.49) | 170 (0.51) | 109 (0.52) | 0.34 | 0.76 (0.43–1.34) | 0.21 | 0.67 (0.36–1.25) | ||
| T/AT/A | 27 (0.27) | 99 (0.30) | 64 (0.31) | 0.30 | 0.72 (0.38–1.35) | 0.19 | 0.63 (0.32–1.26) | ||
| T/A | 103 (0.52) | 368 (0.55) | 237 (0.57) | 0.33 | 0.85 (0.62–1.17) | 0.22 | 0.81 (0.58–1.14) | ||
| rs1045642 | C3435 T | CC | 43 (0.43) | 121 (0.37) | 65 (0.32) | 1 | 1 | ||
| C/T | 49 (0.50) | 154 (0.46) | 91 (0.45) | 0.65 | 0.90 (0.56–1.44) | 0.44 | 0.81 (0.49–1.37) | ||
| T/T | 7 (0.07) | 55 (0.17) | 47 (0.23) | 0.016a | 0.36 (0.15–0.85) | <0.001a | 0.23 (0.09–0.54) | ||
| T | 63 (0.32) | 264 (0.40) | 185 (0.46) | 0.038a | 0.70 (0.50–0.98) | 0.001 | 0.56 (0.39–0.80) | ||
For χ2-tests, p<0.05.
OR, odds ratios; CI, confidence intervals; n, number of alleles or genotypes; Freq, frequency; MDR1, multidrug-resistant transporter-1.
SNP loci 1236, 2677, and 3435 were found to be in LD. The strength of pairwise LD between the three SNPs was as follows: 1236 and 2677, r2=0.42; 1236 and 3435, r2=0.67; 2677 and 3435, r2=0.73. (r2>0.33 was considered as thresholds of strong LD). A significantly higher occurrence of the haplotype TTT was found in the GANFH group than in the glucocorticoid-resistant group or healthy control group (OR=0.72; 95% CI, 0.43–0.94, GANFH group vs. glucocorticoid-resistant group, OR=0.67; 95% CI, 0.30–0.89, GANFH group vs. healthy control group, respectively) (Table 4).
Table 4.
Haplotype of Polymorphic Variants of the MDR1 Gene in GANFH Patients and Controls
| Frequency | GANFH vs. healthy control group | GANFH vs. GC-resistant group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C1236 T | G2677 T/A | C3435 T | GANFH group, Freq | Healthy control group, Freq | GC-resistant group, Freq | p-Value | OR (95% CI) | p-Value | OR (95% CI) |
| T | T | T | 0.26 | 0.35 | 0.41 | 0.03a | 0.72 (0.43–0.94) | 0.007a | 0.67 (0.30–0.89) |
| T | G | C | 0.23 | 0.21 | 0.19 | 0.86 | 1.03 (0.54–1.57) | 0.55 | 1.14 (0.61–1.45) |
| C | G | C | 0.24 | 0.17 | 0.15 | 0.24 | 1.18 (0.68–1.59) | 0.10 | 1.23 (0.74–1.67) |
| C | A | C | 0.15 | 0.12 | 0.10 | 1 | 1 | ||
For χ2-tests, p<0.05.
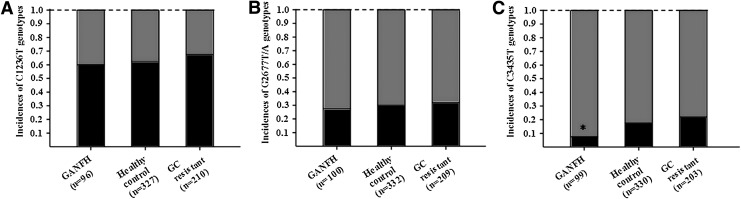
The allele distribution in the three groups showed a linear trend (Fig. 1). The frequency of the T allele of C3435T was significantly lower in the GANFH group than both the healthy control group and glucocorticoid-resistant group (p<0.05) (Fig. 1). Although not statistically significant, the same trends were found for the T allele of C1236T and G2677T/A (Fig. 1).
FIG. 1.
Incidences of C1236T, G2677T/A, and C3435T genotypes in the glucocorticoid-induced avascular necrosis of the femoral head (GANFH) patients and control groups. (A) C1236T, black indicates TT genotype and gray indicates CC and CT genotypes; (B) G2677T/A, black indicates T/AT/A genotype and gray indicates GG and GT/A genotypes; (C) C3435T, black indicates TT genotype and gray indicates CC and CT genotypes; * for chi-square (χ2) tests, p=0.016, GANFH versus healthy control group; p<0.001, GANFH group versus GC-resistant group.
Discussion
Here we performed a case–control study to investigate the role of MDR1 SNPs C1236T, G2677T/A, and C3435T in GANFH susceptibility. Our results suggest that SNP C3435T may be a risk factor for the development of GANFH in the Chinese population. Whereas no significant associations of SNPs C1236T and G2677T/A with GANFH susceptibility were found, loci C1236T, G2677T/A, and C3435T were in LD. The TTT haplotype, composed of these loci, may decrease the susceptibility to GANFH when compared with other haplotypes.
In the present study, the homozygosity of both the wild-type and variant alleles of MDR1 (C1236 T, G2677T/A, and C3435T) in healthy control subjects was similar to that reported by other Asian studies, which were performed with healthy Chinese (Zhang et al., 2008; Qiu et al., 2012), Japanese (Komoto et al., 2006), and Korean subjects (Choi et al., 2007). However, the allele frequencies were significantly higher than those observed in European ethnicities, such as German (Cascorbi et al., 2001), Russian (Gaikovitch et al., 2003), and Serbian (Milojkovic et al., 2011). MDR1 C3435T has previously been reported to be associated with susceptibility to GANFH in a Chinese population with systemic lupus erythematosus (Yang et al., 2007) and a Japanese population with kidney transplantation (Asano et al., 2003). The current study verifies this finding in a larger Chinese population with underlying conditions that had not previously been studied in this context. Therefore, MDR1 C3435T polymorphism and its respective association to susceptibility to GANFH may be consistent among different populations. However, further studies are required to confirm possible associations in other populations, especially non-Asians, since the allele distributions seem to vary significantly between Oriental and non-Oriental groups.
In our study, MDR1 C1236T was not found to be associated with GANFH susceptibility. SNP G2677T/A was once found to have a weak association with GANFH in a Chinese population with systemic lupus erythematosus (Yang and Xu, 2007) and a Japanese population with kidney transplantation (Asano et al., 2003). However, despite our larger sample size, we found no association (p=0.15). These results suggest that C1236T and G2677T/A may have less functional influence on the MDR1 gene than C3435T on GANFH susceptibility.
It has been widely accepted that glucocorticoids may induce blood to enter a hypercoagulable hypofibrinolysis state. This blood state mediates femoral vein thrombosis, resulting in bone ischemia, necrosis of the bone tissue structure, and function damage. However, individuals differ in their abilities to absorb and metabolize glucocorticoids, which may affect GANFH development. The membrane transporter protein P-gp, encoded by the MDR1 gene, plays an important role in drug absorption and distribution. Therefore, we selected MDR1 C1236T, G2677T/A, and C3435T, the most widely studied functional SNPs, to investigate a possible association with femoral head necrosis. Our results showed a significant association of C3435T with GANFH in a Chinese population. We speculate that the 3435TT genotype reduces the incidence of GANFH by enhancing the pump activity of P-gp to clear the excess glucocorticoid out of cells. Our speculation is partially supported by Asano et al. (2003) who reported that patients with 3435TT genotype have a significantly higher dose/concentration ratio of tacrolimus than the 3435CC genotype, indicating enhanced P-gp pump activity of the 3435TT genotype in a Japanese population.
To obtain a more exact conclusion of the study, our experiments were somewhat different from others. First, subject to difficulty in acquiring the experimental samples, the previous studies only contained a small number of GANFH samples (no more than 30 cases), which may have some influence to statistical conclusions. We therefore obtained >100 GANFH cases by using the plentiful case resources in China, which we believe has allowed us to conduct more conclusive analyses. Second, the previous studies often contain only a single underlying disease (such as systemic lupus erythematosus) with femoral head necrosis posthormone treatment. This situation presents a risk in terms of genetic association: is the genetic association related to GANFH or to the pathogenesis and special treatment of the underlying disease itself? Our study encompassed multiple underlying diseases in the hope of eliminating the influence of a single underlying disease on the result of any genetic association with GANFH.
In addition, age across both our cases and control subjects ranged from 18 to 48 years, ruling out factors such as senile osteoporosis (naturally increased post-middle age) and postmenopausal osteoporosis in females, which can both affect femoral head necrosis. Patients with a history of heavy drinking were also excluded to eliminate any effects due to alcohol. These efforts are useful in eliminating any background noise in our GANFH cases. Previous research has only contained the glucocorticoid-sensitive (GANFH) and glucocorticoid-resistant (no GANFH) groups, whereas we added the random healthy control group whose glucocorticoid sensitivity may lie between the levels of the other two groups. Our results showed that the T allele of the C3435T showed a linear trend increase in the glucocorticoid-sensitive group (GANFH), healthy control group, and glucocorticoid-resistant group (no GANFH). Therefore, we have confirmed from a unique angle that the MDR1 gene polymorphisms effect glucocorticoid sensitivity, with downstream effects on GANFH diseases.
Due to difficulties and challenges in defining the history of glucocorticoid treatments for underlying disease, especially when patients discharged from hospitals, exact doses of glucocorticoid used for some patients were not identified in this study. However, we obtained an average dose of glucocorticoid used in the patients according to hospital records and careful consultation with the patients. Another limitation was that complex phenotypes, polygenic diseases, or both could not be completely explained by SNPs in one single gene, as the SNPs in other genes were possibly involved in the availability of glucocorticoid metabolism.
In short, MDR1 C3435T polymorphism may be a risk factor for susceptibility to GANFH in a Chinese population and could potentially be used to predict the development of GANFH.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (NSFC, No. 81171748, 81271971, and 31001051) and the Zhejiang Science fund Y2111236.
Author Disclosure Statement
No competing financial interests exist.
References
- Asano T, Takahashi KA, Fujioka M, et al. (2003) ABCB1 C3435T and G2677T/A polymorphism decreased the risk for steroid-induced osteonecrosis of the femoral head after kidney transplantation. Pharmacogenetics 13:675–682 [DOI] [PubMed] [Google Scholar]
- Balcerczak E, Panczyk M, Piaskowski S, et al. (2010) ABCB1/MDR1 gene polymorphisms as a prognostic factor in colorectal cancer. Int J Colorectal Dis 25:1167–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascorbi I, Gerloff T, Johne A, et al. (2001) Frequency of single nucleotide polymorphisms in the P-glycoprotein drug transporter MDR1 gene in white subjects. Clin Pharmacol Ther 69:169–174 [DOI] [PubMed] [Google Scholar]
- Chen J, Chen L, Mao N, et al. (2012) Association of the MDR1 3435 polymorphism in patients with refractory rheumatoid arthritis in a Chinese population. Rheumatol Int 32:3127–3130 [DOI] [PubMed] [Google Scholar]
- Choi JH, Lee YJ, Jang SB, et al. (2007) Influence of the CYP3A5 and MDR1 genetic polymorphisms on the pharmacokinetics of tacrolimus in healthy Korean subjects. Br J Clin Pharmacol 64:185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaikovitch EA, Cascorbi I, Mrozikiewicz PM, et al. (2003) Polymorphisms of drug-metabolizing enzymes CYP2C9, CYP2C19, CYP2D6, CYP1A1, NAT2 and of P-glycoprotein in a Russian population. Eur J Clin Pharmacol 59:303–312 [DOI] [PubMed] [Google Scholar]
- Glueck CJ, Fontaine RN, Gruppo R, et al. (1999) The plasminogen activator inhibitor-1 gene, hypofibrinolysis, and osteonecrosis. Clin Orthop Relat Res 366:133–146 [DOI] [PubMed] [Google Scholar]
- Gong LL, Fang LH, Wang HY, et al. (2013) Genetic risk factors for glucocorticoid-induced osteonecrosis: a meta-analysis. Steroids 78:401–408 [DOI] [PubMed] [Google Scholar]
- Hirata T, Fujioka M, Takahashi KA, et al. (2007) ApoB C7623T polymorphism predicts risk for steroid-induced osteonecrosis of the femoral head after renal transplantation. J Orthop Sei 12:199–206 [DOI] [PubMed] [Google Scholar]
- Jones LC, Hungerford DS. (2007) The pathogenesis of osteonecrosis. Instr Course Lect 56:179–196 [PubMed] [Google Scholar]
- Komoto C, Nakamura T, Sakaeda T, et al. (2006) MDR1 haplotype frequencies in Japanese and Caucasian, and in Japanese patients with colorectal and esophageal cancer. Drug Metab Pharmacokinet 21:126–132 [DOI] [PubMed] [Google Scholar]
- Liu YF, Chen WM, Lin YF, et al. (2005) TypeII collagen gene varimlts and inherited osteonecrosis of the femoral head. N Engl J Med 352:2294–2301 [DOI] [PubMed] [Google Scholar]
- Milojkovic M, Stojnev S, Jovanovic I, et al. (2011) Frequency of the C1236T, G2677T/A and C3435T MDR1 gene polymorphisms in the Serbian population. Pharmacol Rep 63:808–814 [DOI] [PubMed] [Google Scholar]
- Ozen F, Silan C, Uludag A, et al. (2011) Association between ABCB1 (MDR1) gene 3435 C>T polymorphism and colchicine unresponsiveness of FMF patients. Ren Fail 33:899–903 [DOI] [PubMed] [Google Scholar]
- Pal D, Mitra AK. (2006) MDR- and CYP3A4-mediated drug-drug interactions. J Neuroimmune Pharmacol 1:323–339 [DOI] [PubMed] [Google Scholar]
- Pei FX. (2010) Strengthen basic and clinical research, and improve the diagnosis and treatment of osteonecrosis. Chin J Orthop 30:3 [Google Scholar]
- Qiu H, Dong H, Pan S, et al. (2012) The single nucleotide polymorphism and haplotype analysis of MDR1 in Jiangsu Han population of China. Biomed Pharmacother 66:459–463 [DOI] [PubMed] [Google Scholar]
- Rousseau F, Rehel R, Rouillard P, et al. (1994) High throughput and economical mutation detection and RFLP analysis using a minimethod for DNA preparation from whole blood and acrylamide gel electrophoresis. Hum Mutat 4:51–54 [DOI] [PubMed] [Google Scholar]
- Sugano N, Atsumi T, Ohzono K, Kubo T, Hotokebuchi T, Takaoka K. (2002) The 2001 revised criteria for diagnosis, classification, and staging of idiopathic osteonecrosis of the femoral head. J Orthop Sci 7:601–605 [DOI] [PubMed] [Google Scholar]
- Yang XY, Xu DH. (2007) MDR1(ABCB1) gene polymorphisms associated with steroidinduced osteonecrosis of femoral head in systemic lupus erythematosus. Pharmazie 62:930–932 [PubMed] [Google Scholar]
- Zalavms CG, Malizos KN, Dokou E, et al. (2002) The 677c-+T mutation of the methylene-tetrahydrofolate reductase gene in the pathogenesis of osteonecrosis of the femoral head. Haematologica 87:1111–1112 [PubMed] [Google Scholar]
- Zhang HS, Li ZR. (2007) Expert advices on diagnosis and treatment of osteonecrosis. Chin J Orthop 27:146–148 [Google Scholar]
- Zhang Y, Jiang XH, Hu YQ, et al. (2008) MDR1 genotypes do not influence the absorption of a single oral dose of 600 mg valacyclovir in healthy Chinese Han ethnic males. Br J Clin Pharmacol 66:247–254 [DOI] [PMC free article] [PubMed] [Google Scholar]