Abstract
The display of repertoires of antibody fragments on the surface of filamentous bacteriophage offers a new way of making antibodies with predefined binding specificities. Here we explored the use of this technology to make immunochemical reagents to a range of antigens by selection from a repertoire of > 10(8) clones made in vitro from human V gene segments. From the same 'single pot' repertoire, phage were isolated with binding activities to each of 18 antigens, including the intracellular proteins p53, elongation factor EF-1 alpha, immunoglobulin binding protein, rhombotin-2 oncogene protein and sex determining region Y protein. Both phage and scFv fragments secreted from infected bacteria were used as monoclonal and polyclonal reagents in Western blots. Furthermore the monoclonal reagents were used for epitope mapping (a new epitope of p53 was identified) and for staining of cells. This shows that antibody reagents for research can be readily derived from 'single pot' phage display libraries.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
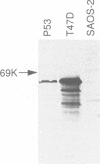
- Bartek J., Iggo R., Gannon J., Lane D. P. Genetic and immunochemical analysis of mutant p53 in human breast cancer cell lines. Oncogene. 1990 Jun;5(6):893–899. [PubMed] [Google Scholar]
- Bass S., Greene R., Wells J. A. Hormone phage: an enrichment method for variant proteins with altered binding properties. Proteins. 1990;8(4):309–314. doi: 10.1002/prot.340080405. [DOI] [PubMed] [Google Scholar]
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton D. R. Human and mouse monoclonal antibodies by repertoire cloning. Trends Biotechnol. 1991 May;9(5):169–175. doi: 10.1016/0167-7799(91)90055-m. [DOI] [PubMed] [Google Scholar]
- Bye J. M., Carter C., Cui Y., Gorick B. D., Songsivilai S., Winter G., Hughes-Jones N. C., Marks J. D. Germline variable region gene segment derivation of human monoclonal anti-Rh(D) antibodies. Evidence for affinity maturation by somatic hypermutation and repertoire shift. J Clin Invest. 1992 Dec;90(6):2481–2490. doi: 10.1172/JCI116140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn G. C., Chappell T. G., Rothman J. E. Peptide binding and release by proteins implicated as catalysts of protein assembly. Science. 1989 Jul 28;245(4916):385–390. doi: 10.1126/science.2756425. [DOI] [PubMed] [Google Scholar]
- Foroni L., Boehm T., White L., Forster A., Sherrington P., Liao X. B., Brannan C. I., Jenkins N. A., Copeland N. G., Rabbitts T. H. The rhombotin gene family encode related LIM-domain proteins whose differing expression suggests multiple roles in mouse development. J Mol Biol. 1992 Aug 5;226(3):747–761. doi: 10.1016/0022-2836(92)90630-3. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Griffiths A. D., Malmqvist M., Marks J. D., Bye J. M., Embleton M. J., McCafferty J., Baier M., Holliger K. P., Gorick B. D., Hughes-Jones N. C. Human anti-self antibodies with high specificity from phage display libraries. EMBO J. 1993 Feb;12(2):725–734. doi: 10.1002/j.1460-2075.1993.tb05706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley V. R., Jackson D. I., Hextall P. J., Hawkins J. R., Berkovitz G. D., Sockanathan S., Lovell-Badge R., Goodfellow P. N. DNA binding activity of recombinant SRY from normal males and XY females. Science. 1992 Jan 24;255(5043):453–456. doi: 10.1126/science.1734522. [DOI] [PubMed] [Google Scholar]
- Hawkins R. E., Russell S. J., Winter G. Selection of phage antibodies by binding affinity. Mimicking affinity maturation. J Mol Biol. 1992 Aug 5;226(3):889–896. doi: 10.1016/0022-2836(92)90639-2. [DOI] [PubMed] [Google Scholar]
- Holliger P., Prospero T., Winter G. "Diabodies": small bivalent and bispecific antibody fragments. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6444–6448. doi: 10.1073/pnas.90.14.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenboom H. R., Griffiths A. D., Johnson K. S., Chiswell D. J., Hudson P., Winter G. Multi-subunit proteins on the surface of filamentous phage: methodologies for displaying antibody (Fab) heavy and light chains. Nucleic Acids Res. 1991 Aug 11;19(15):4133–4137. doi: 10.1093/nar/19.15.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenboom H. R., Marks J. D., Griffiths A. D., Winter G. Building antibodies from their genes. Immunol Rev. 1992 Dec;130:41–68. doi: 10.1111/j.1600-065x.1992.tb01520.x. [DOI] [PubMed] [Google Scholar]
- Hoogenboom H. R., Winter G. By-passing immunisation. Human antibodies from synthetic repertoires of germline VH gene segments rearranged in vitro. J Mol Biol. 1992 Sep 20;227(2):381–388. doi: 10.1016/0022-2836(92)90894-p. [DOI] [PubMed] [Google Scholar]
- Huse W. D., Sastry L., Iverson S. A., Kang A. S., Alting-Mees M., Burton D. R., Benkovic S. J., Lerner R. A. Generation of a large combinatorial library of the immunoglobulin repertoire in phage lambda. Science. 1989 Dec 8;246(4935):1275–1281. doi: 10.1126/science.2531466. [DOI] [PubMed] [Google Scholar]
- Johnsson B., Löfås S., Lindquist G. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Anal Biochem. 1991 Nov 1;198(2):268–277. doi: 10.1016/0003-2697(91)90424-r. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marks J. D., Griffiths A. D., Malmqvist M., Clackson T. P., Bye J. M., Winter G. By-passing immunization: building high affinity human antibodies by chain shuffling. Biotechnology (N Y) 1992 Jul;10(7):779–783. doi: 10.1038/nbt0792-779. [DOI] [PubMed] [Google Scholar]
- Marks J. D., Hoogenboom H. R., Bonnert T. P., McCafferty J., Griffiths A. D., Winter G. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J Mol Biol. 1991 Dec 5;222(3):581–597. doi: 10.1016/0022-2836(91)90498-u. [DOI] [PubMed] [Google Scholar]
- Marks J. D., Hoogenboom H. R., Griffiths A. D., Winter G. Molecular evolution of proteins on filamentous phage. Mimicking the strategy of the immune system. J Biol Chem. 1992 Aug 15;267(23):16007–16010. [PubMed] [Google Scholar]
- Marks J. D., Tristem M., Karpas A., Winter G. Oligonucleotide primers for polymerase chain reaction amplification of human immunoglobulin variable genes and design of family-specific oligonucleotide probes. Eur J Immunol. 1991 Apr;21(4):985–991. doi: 10.1002/eji.1830210419. [DOI] [PubMed] [Google Scholar]
- Masuda H., Miller C., Koeffler H. P., Battifora H., Cline M. J. Rearrangement of the p53 gene in human osteogenic sarcomas. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7716–7719. doi: 10.1073/pnas.84.21.7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCafferty J., Griffiths A. D., Winter G., Chiswell D. J. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990 Dec 6;348(6301):552–554. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- Merrick W. C. Assays for eukaryotic protein synthesis. Methods Enzymol. 1979;60:108–123. doi: 10.1016/s0076-6879(79)60011-3. [DOI] [PubMed] [Google Scholar]
- Midgley C. A., Fisher C. J., Bártek J., Vojtesek B., Lane D., Barnes D. M. Analysis of p53 expression in human tumours: an antibody raised against human p53 expressed in Escherichia coli. J Cell Sci. 1992 Jan;101(Pt 1):183–189. doi: 10.1242/jcs.101.1.183. [DOI] [PubMed] [Google Scholar]
- Milstein C. The Croonian lecture, 1989. Antibodies: a paradigm for the biology of molecular recognition. Proc R Soc Lond B Biol Sci. 1990 Feb 22;239(1294):1–16. doi: 10.1098/rspb.1990.0006. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Napier R. M., Fowke L. C., Hawes C., Lewis M., Pelham H. R. Immunological evidence that plants use both HDEL and KDEL for targeting proteins to the endoplasmic reticulum. J Cell Sci. 1992 Jun;102(Pt 2):261–271. doi: 10.1242/jcs.102.2.261. [DOI] [PubMed] [Google Scholar]
- Olden K., Pratt R. M., Jaworski C., Yamada K. M. Evidence for role of glycoprotein carbohydrates in membrane transport: specific inhibition by tunicamycin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):791–795. doi: 10.1073/pnas.76.2.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi R., Güssow D. H., Jones P. T., Winter G. Cloning immunoglobulin variable domains for expression by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 May;86(10):3833–3837. doi: 10.1073/pnas.86.10.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack P., Plückthun A. Miniantibodies: use of amphipathic helices to produce functional, flexibly linked dimeric FV fragments with high avidity in Escherichia coli. Biochemistry. 1992 Feb 18;31(6):1579–1584. doi: 10.1021/bi00121a001. [DOI] [PubMed] [Google Scholar]
- Palter K. B., Watanabe M., Stinson L., Mahowald A. P., Craig E. A. Expression and localization of Drosophila melanogaster hsp70 cognate proteins. Mol Cell Biol. 1986 Apr;6(4):1187–1203. doi: 10.1128/mcb.6.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Perelson A. S., Oster G. F. Theoretical studies of clonal selection: minimal antibody repertoire size and reliability of self-non-self discrimination. J Theor Biol. 1979 Dec 21;81(4):645–670. doi: 10.1016/0022-5193(79)90275-3. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Shiu R. P., Pastan I. Induction of two transformation-sensitive membrane polypeptides in normal fibroblasts by a block in glycoprotein synthesis or glucose deprivation. Cell. 1977 Aug;11(4):941–947. doi: 10.1016/0092-8674(77)90305-1. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasso E. H., Silverman G. J., Mannik M. Human IgA and IgG F(ab')2 that bind to staphylococcal protein A belong to the VHIII subgroup. J Immunol. 1991 Sep 15;147(6):1877–1883. [PubMed] [Google Scholar]
- Sastry L., Alting-Mees M., Huse W. D., Short J. M., Sorge J. A., Hay B. N., Janda K. D., Benkovic S. J., Lerner R. A. Cloning of the immunological repertoire in Escherichia coli for generation of monoclonal catalytic antibodies: construction of a heavy chain variable region-specific cDNA library. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5728–5732. doi: 10.1073/pnas.86.15.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichtholz B., Legros Y., Gillet D., Gaillard C., Marty M., Lane D., Calvo F., Soussi T. The immune response to p53 in breast cancer patients is directed against immunodominant epitopes unrelated to the mutational hot spot. Cancer Res. 1992 Nov 15;52(22):6380–6384. [PubMed] [Google Scholar]
- Smith G. P. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985 Jun 14;228(4705):1315–1317. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- Tomlinson I. M., Walter G., Marks J. D., Llewelyn M. B., Winter G. The repertoire of human germline VH sequences reveals about fifty groups of VH segments with different hypervariable loops. J Mol Biol. 1992 Oct 5;227(3):776–798. doi: 10.1016/0022-2836(92)90223-7. [DOI] [PubMed] [Google Scholar]
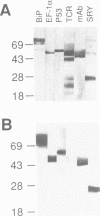
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtesek B., Bártek J., Midgley C. A., Lane D. P. An immunochemical analysis of the human nuclear phosphoprotein p53. New monoclonal antibodies and epitope mapping using recombinant p53. J Immunol Methods. 1992 Jul 6;151(1-2):237–244. doi: 10.1016/0022-1759(92)90122-a. [DOI] [PubMed] [Google Scholar]
- Ward E. S., Güssow D., Griffiths A. D., Jones P. T., Winter G. Binding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coli. Nature. 1989 Oct 12;341(6242):544–546. doi: 10.1038/341544a0. [DOI] [PubMed] [Google Scholar]
- Waterhouse P., Griffiths A. D., Johnson K. S., Winter G. Combinatorial infection and in vivo recombination: a strategy for making large phage antibody repertoires. Nucleic Acids Res. 1993 May 11;21(9):2265–2266. doi: 10.1093/nar/21.9.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber S., Traunecker A., Oliveri F., Gerhard W., Karjalainen K. Specific low-affinity recognition of major histocompatibility complex plus peptide by soluble T-cell receptor. Nature. 1992 Apr 30;356(6372):793–796. doi: 10.1038/356793a0. [DOI] [PubMed] [Google Scholar]
- Winter G., Milstein C. Man-made antibodies. Nature. 1991 Jan 24;349(6307):293–299. doi: 10.1038/349293a0. [DOI] [PubMed] [Google Scholar]
- Zouali M., Theze J. Probing VH gene-family utilization in human peripheral B cells by in situ hybridization. J Immunol. 1991 Apr 15;146(8):2855–2864. [PubMed] [Google Scholar]