Abstract
Voltage-gated ClC chloride channels play important roles in cell volume regulation, control of muscle excitability, and probably transepithelial transport. ClC channels can be functionally expressed without other subunits, but it is unknown whether they function as monomers. We now exploit the properties of human mutations in the muscle chloride channel, ClC-1, to explore its multimeric structure. This is based on analysis of the dominant negative effects of ClC-1 mutations causing myotonia congenita (MC, Thomsen's disease), including a newly identified mutation (P480L) in Thomsen's own family. In a co-expression assay, Thomsen's mutation dramatically inhibits normal ClC-1 function. A mutation found in Canadian MC families (G230E) has a less pronounced dominant negative effect, which can be explained by functional WT/G230E heterooligomeric channels with altered kinetics and selectivity. Analysis of both mutants shows independently that ClC-1 functions as a homooligomer with most likely four subunits.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdalla J. A., Casley W. L., Cousin H. K., Hudson A. J., Murphy E. G., Cornélis F. C., Hashimoto L., Ebers G. C. Linkage of Thomsen disease to the T-cell-receptor beta (TCRB) locus on chromosome 7q35. Am J Hum Genet. 1992 Sep;51(3):579–584. [PMC free article] [PubMed] [Google Scholar]
- Attali B., Guillemare E., Lesage F., Honoré E., Romey G., Lazdunski M., Barhanin J. The protein IsK is a dual activator of K+ and Cl- channels. Nature. 1993 Oct 28;365(6449):850–852. doi: 10.1038/365850a0. [DOI] [PubMed] [Google Scholar]
- Bauer C. K., Steinmeyer K., Schwarz J. R., Jentsch T. J. Completely functional double-barreled chloride channel expressed from a single Torpedo cDNA. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11052–11056. doi: 10.1073/pnas.88.24.11052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker P. E. Heterozygote manifestation in recessive generalized myotonia. Hum Genet. 1979 Feb 15;46(3):325–329. doi: 10.1007/BF00273316. [DOI] [PubMed] [Google Scholar]
- Bretag A. H. Muscle chloride channels. Physiol Rev. 1987 Apr;67(2):618–724. doi: 10.1152/physrev.1987.67.2.618. [DOI] [PubMed] [Google Scholar]
- Bryant S. H., Morales-Aguilera A. Chloride conductance in normal and myotonic muscle fibres and the action of monocarboxylic aromatic acids. J Physiol. 1971 Dec;219(2):367–383. doi: 10.1113/jphysiol.1971.sp009667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon S. C., Strittmatter S. M. Functional expression of sodium channel mutations identified in families with periodic paralysis. Neuron. 1993 Feb;10(2):317–326. doi: 10.1016/0896-6273(93)90321-h. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper E., Couturier S., Ballivet M. Pentameric structure and subunit stoichiometry of a neuronal nicotinic acetylcholine receptor. Nature. 1991 Mar 21;350(6315):235–238. doi: 10.1038/350235a0. [DOI] [PubMed] [Google Scholar]
- Cummins T. R., Zhou J., Sigworth F. J., Ukomadu C., Stephan M., Ptácek L. J., Agnew W. S. Functional consequences of a Na+ channel mutation causing hyperkalemic periodic paralysis. Neuron. 1993 Apr;10(4):667–678. doi: 10.1016/0896-6273(93)90168-q. [DOI] [PubMed] [Google Scholar]
- Fahlke C., Zachar E., Rüdel R. Chloride channels with reduced single-channel conductance in recessive myotonia congenita. Neuron. 1993 Feb;10(2):225–232. doi: 10.1016/0896-6273(93)90313-g. [DOI] [PubMed] [Google Scholar]
- Franke C., Iaizzo P. A., Hatt H., Spittelmeister W., Ricker K., Lehmann-Horn F. Altered Na+ channel activity and reduced Cl- conductance cause hyperexcitability in recessive generalized myotonia (Becker). Muscle Nerve. 1991 Aug;14(8):762–770. doi: 10.1002/mus.880140811. [DOI] [PubMed] [Google Scholar]
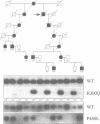
- George A. L., Jr, Crackower M. A., Abdalla J. A., Hudson A. J., Ebers G. C. Molecular basis of Thomsen's disease (autosomal dominant myotonia congenita). Nat Genet. 1993 Apr;3(4):305–310. doi: 10.1038/ng0493-305. [DOI] [PubMed] [Google Scholar]
- Gisselmann G., Sewing S., Madsen B. W., Mallart A., Angaut-Petit D., Müller-Holtkamp F., Ferrus A., Pongs O. The interference of truncated with normal potassium channel subunits leads to abnormal behaviour in transgenic Drosophila melanogaster. EMBO J. 1989 Aug;8(8):2359–2364. doi: 10.1002/j.1460-2075.1989.tb08364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gründer S., Thiemann A., Pusch M., Jentsch T. J. Regions involved in the opening of CIC-2 chloride channel by voltage and cell volume. Nature. 1992 Dec 24;360(6406):759–762. doi: 10.1038/360759a0. [DOI] [PubMed] [Google Scholar]
- Hanke W., Miller C. Single chloride channels from Torpedo electroplax. Activation by protons. J Gen Physiol. 1983 Jul;82(1):25–45. doi: 10.1085/jgp.82.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch T. J., Steinmeyer K., Schwarz G. Primary structure of Torpedo marmorata chloride channel isolated by expression cloning in Xenopus oocytes. Nature. 1990 Dec 6;348(6301):510–514. doi: 10.1038/348510a0. [DOI] [PubMed] [Google Scholar]
- Koch M. C., Steinmeyer K., Lorenz C., Ricker K., Wolf F., Otto M., Zoll B., Lehmann-Horn F., Grzeschik K. H., Jentsch T. J. The skeletal muscle chloride channel in dominant and recessive human myotonia. Science. 1992 Aug 7;257(5071):797–800. doi: 10.1126/science.1379744. [DOI] [PubMed] [Google Scholar]
- Kwieciński H., Lehmann-Horn F., Rüdel R. Drug-induced myotonia in human intercostal muscle. Muscle Nerve. 1988 Jun;11(6):576–581. doi: 10.1002/mus.880110609. [DOI] [PubMed] [Google Scholar]
- Langosch D., Thomas L., Betz H. Conserved quaternary structure of ligand-gated ion channels: the postsynaptic glycine receptor is a pentamer. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7394–7398. doi: 10.1073/pnas.85.19.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipicky R. J., Bryant S. H., Salmon J. H. Cable parameters, sodium, potassium, chloride, and water content, and potassium efflux in isolated external intercostal muscle of normal volunteers and patients with myotonia congenita. J Clin Invest. 1971 Oct;50(10):2091–2103. doi: 10.1172/JCI106703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991 Mar 21;350(6315):232–235. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- McClatchey A. I., Van den Bergh P., Pericak-Vance M. A., Raskind W., Verellen C., McKenna-Yasek D., Rao K., Haines J. L., Bird T., Brown R. H., Jr Temperature-sensitive mutations in the III-IV cytoplasmic loop region of the skeletal muscle sodium channel gene in paramyotonia congenita. Cell. 1992 Feb 21;68(4):769–774. doi: 10.1016/0092-8674(92)90151-2. [DOI] [PubMed] [Google Scholar]
- Mehrke G., Brinkmeier H., Jockusch H. The myotonic mouse mutant ADR: electrophysiology of the muscle fiber. Muscle Nerve. 1988 May;11(5):440–446. doi: 10.1002/mus.880110505. [DOI] [PubMed] [Google Scholar]
- Moorman J. R., Palmer C. J., John J. E., 3rd, Durieux M. E., Jones L. R. Phospholemman expression induces a hyperpolarization-activated chloride current in Xenopus oocytes. J Biol Chem. 1992 Jul 25;267(21):14551–14554. [PubMed] [Google Scholar]
- Müller-Hill B., Crapo L., Gilbert W. Mutants that make more lac repressor. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1259–1264. doi: 10.1073/pnas.59.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptácek L. J., George A. L., Jr, Griggs R. C., Tawil R., Kallen R. G., Barchi R. L., Robertson M., Leppert M. F. Identification of a mutation in the gene causing hyperkalemic periodic paralysis. Cell. 1991 Nov 29;67(5):1021–1027. doi: 10.1016/0092-8674(91)90374-8. [DOI] [PubMed] [Google Scholar]
- Pusch M., Steinmeyer K., Jentsch T. J. Low single channel conductance of the major skeletal muscle chloride channel, ClC-1. Biophys J. 1994 Jan;66(1):149–152. doi: 10.1016/S0006-3495(94)80753-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989 Sep 8;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Rojas C. V., Wang J. Z., Schwartz L. S., Hoffman E. P., Powell B. R., Brown R. H., Jr A Met-to-Val mutation in the skeletal muscle Na+ channel alpha-subunit in hyperkalaemic periodic paralysis. Nature. 1991 Dec 5;354(6352):387–389. doi: 10.1038/354387a0. [DOI] [PubMed] [Google Scholar]
- Rüdel R., Lehmann-Horn F. Membrane changes in cells from myotonia patients. Physiol Rev. 1985 Apr;65(2):310–356. doi: 10.1152/physrev.1985.65.2.310. [DOI] [PubMed] [Google Scholar]
- Rüdel R., Ricker K., Lehmann-Horn F. Transient weakness and altered membrane characteristic in recessive generalized myotonia (Becker). Muscle Nerve. 1988 Mar;11(3):202–211. doi: 10.1002/mus.880110303. [DOI] [PubMed] [Google Scholar]
- Steinmeyer K., Klocke R., Ortland C., Gronemeier M., Jockusch H., Gründer S., Jentsch T. J. Inactivation of muscle chloride channel by transposon insertion in myotonic mice. Nature. 1991 Nov 28;354(6351):304–308. doi: 10.1038/354304a0. [DOI] [PubMed] [Google Scholar]
- Steinmeyer K., Ortland C., Jentsch T. J. Primary structure and functional expression of a developmentally regulated skeletal muscle chloride channel. Nature. 1991 Nov 28;354(6351):301–304. doi: 10.1038/354301a0. [DOI] [PubMed] [Google Scholar]
- Streib E. W., Sun S. F. EMG in detection of heterozygote carriers of recessive generalized myotonia. Muscle Nerve. 1982 Feb;5(2):179–181. [PubMed] [Google Scholar]
- Takumi T., Ohkubo H., Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science. 1988 Nov 18;242(4881):1042–1045. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]
- Thiemann A., Gründer S., Pusch M., Jentsch T. J. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature. 1992 Mar 5;356(6364):57–60. doi: 10.1038/356057a0. [DOI] [PubMed] [Google Scholar]
- Uchida S., Sasaki S., Furukawa T., Hiraoka M., Imai T., Hirata Y., Marumo F. Molecular cloning of a chloride channel that is regulated by dehydration and expressed predominantly in kidney medulla. J Biol Chem. 1993 Feb 25;268(6):3821–3824. [PubMed] [Google Scholar]
- Unwin N., Toyoshima C., Kubalek E. Arrangement of the acetylcholine receptor subunits in the resting and desensitized states, determined by cryoelectron microscopy of crystallized Torpedo postsynaptic membranes. J Cell Biol. 1988 Sep;107(3):1123–1138. doi: 10.1083/jcb.107.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Schürholz S., Wischmeyer E., Laurien M., Jockusch H., Schürholz T., Landry D. W., al-Awqati Q. Indanyloxyacetic acid-sensitive chloride channels from outer membranes of skeletal muscle. J Biol Chem. 1993 Jan 5;268(1):547–551. [PubMed] [Google Scholar]
- Wilson I. A., Niman H. L., Houghten R. A., Cherenson A. R., Connolly M. L., Lerner R. A. The structure of an antigenic determinant in a protein. Cell. 1984 Jul;37(3):767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- Zellweger H., Pavone L., Biondi A., Cimino V., Gullotta F., Hart M., Ionasescu V., Mollica F., Schieken R. Autosomal recessive generalized myotonia. Muscle Nerve. 1980 Mar-Apr;3(2):176–180. doi: 10.1002/mus.880030212. [DOI] [PubMed] [Google Scholar]