Abstract
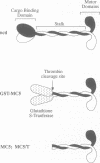
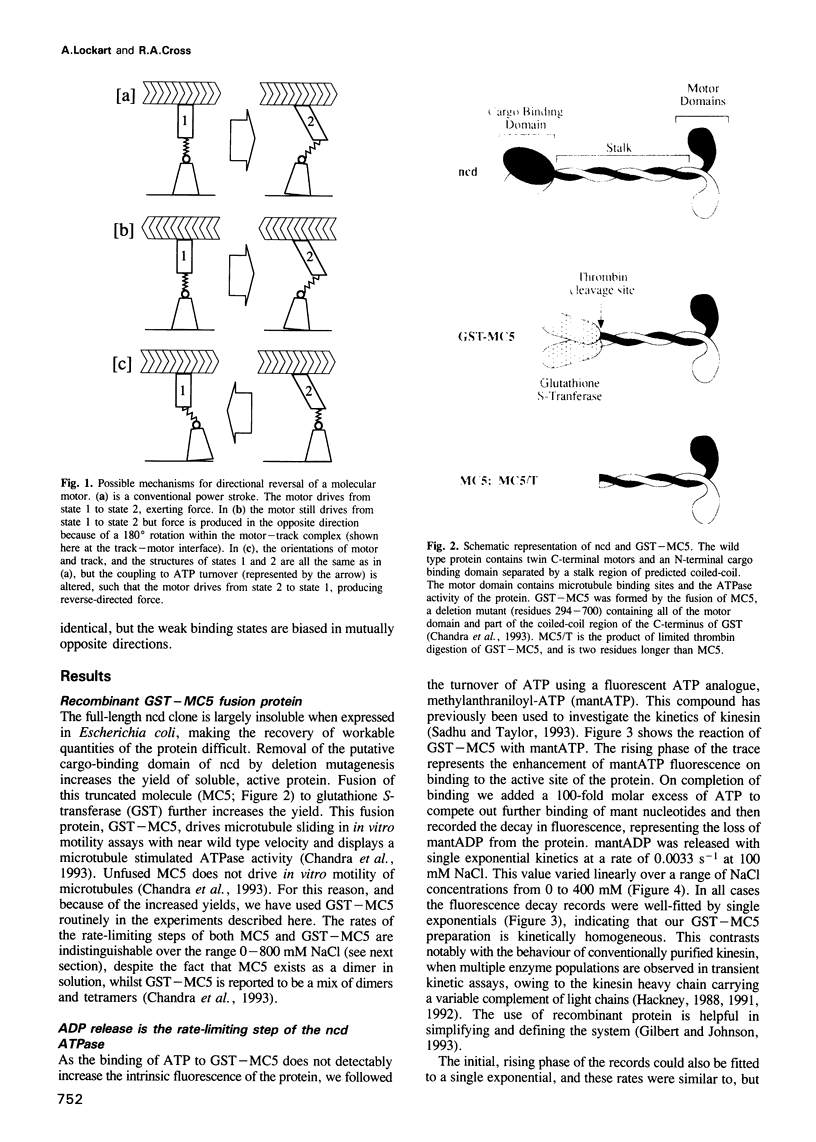
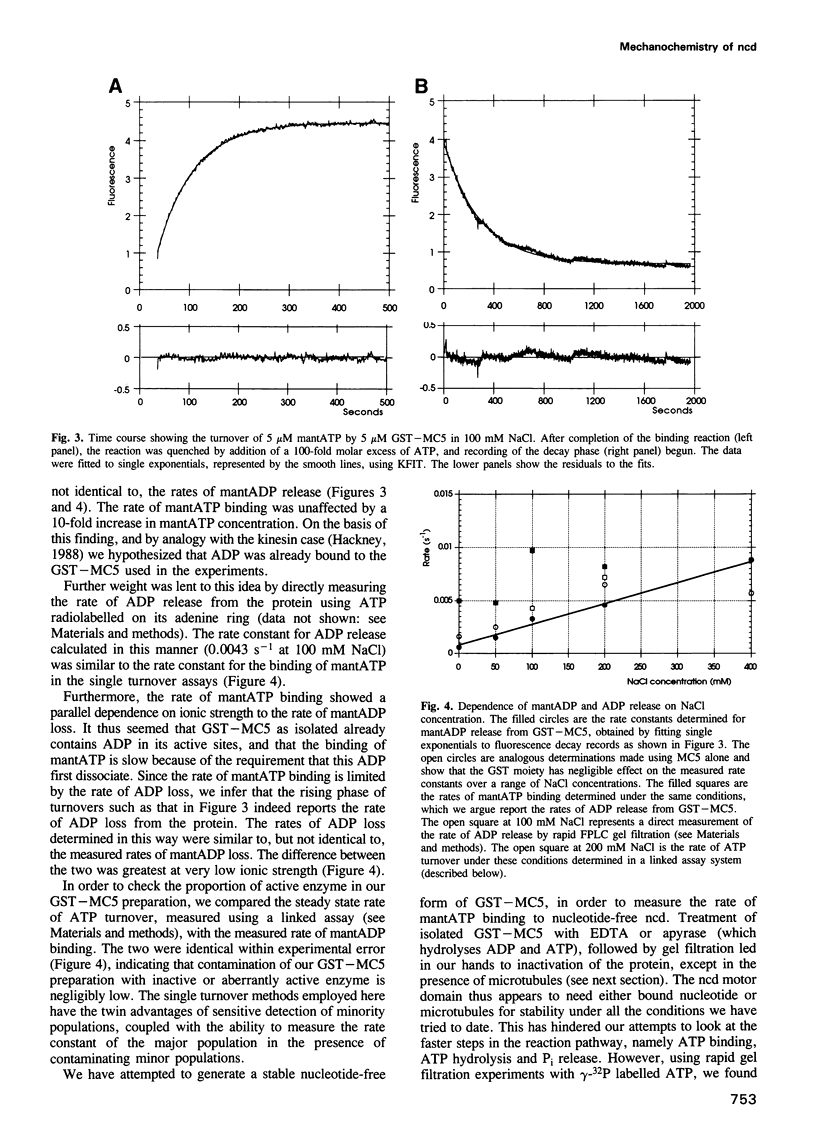
The head or motor domain of the ncd (non-claret disjunctional) molecular motor is 41% identical to that of kinesin, yet moves along microtubules in the opposite direction to kinesin. We show here that despite the reversed directionality of ncd, its kinetics in solution are homologous in key respects to those of kinesin. The rate limiting step, ADP release, occurs at 0.0033 s-1 at 100 mM NaCl and is accelerated approximately 1000-fold when the motor binds to microtubules. Other reaction steps are all very fast (> 0.1 s-1) compared with ADP release, and the motor is consequently paused in the ncd.ADP state until microtubule binding occurs (Kd = 2 microM), at which point ADP release is triggered and the motor locks onto the microtubule in a rigor-like state. These data identify close functional homology between the strong binding states of kinesin and ncd, and in view of this we discuss a possible mechanism for directional reversal, in which the strong binding states of ncd and kinesin are functionally identical, but the weak binding states are biased in opposite directions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chandra R., Salmon E. D., Erickson H. P., Lockhart A., Endow S. A. Structural and functional domains of the Drosophila ncd microtubule motor protein. J Biol Chem. 1993 Apr 25;268(12):9005–9013. [PubMed] [Google Scholar]
- Endow S. A., Henikoff S., Soler-Niedziela L. Mediation of meiotic and early mitotic chromosome segregation in Drosophila by a protein related to kinesin. Nature. 1990 May 3;345(6270):81–83. doi: 10.1038/345081a0. [DOI] [PubMed] [Google Scholar]
- Endow S. A. The emerging kinesin family of microtubule motor proteins. Trends Biochem Sci. 1991 Jun;16(6):221–225. doi: 10.1016/0968-0004(91)90089-e. [DOI] [PubMed] [Google Scholar]
- Endow S. A., Titus M. A. Genetic approaches to molecular motors. Annu Rev Cell Biol. 1992;8:29–66. doi: 10.1146/annurev.cb.08.110192.000333. [DOI] [PubMed] [Google Scholar]
- Geeves M. A. Dynamic interaction between actin and myosin subfragment 1 in the presence of ADP. Biochemistry. 1989 Jul 11;28(14):5864–5871. doi: 10.1021/bi00440a024. [DOI] [PubMed] [Google Scholar]
- Gilbert S. P., Johnson K. A. Expression, purification, and characterization of the Drosophila kinesin motor domain produced in Escherichia coli. Biochemistry. 1993 May 4;32(17):4677–4684. doi: 10.1021/bi00068a028. [DOI] [PubMed] [Google Scholar]
- Goldstein L. S. The kinesin superfamily: tails of functional redundancy. Trends Cell Biol. 1991 Oct;1(4):93–98. doi: 10.1016/0962-8924(91)90036-9. [DOI] [PubMed] [Google Scholar]
- Hackney D. D. Kinesin ATPase: rate-limiting ADP release. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6314–6318. doi: 10.1073/pnas.85.17.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney D. D., Levitt J. D., Wagner D. D. Characterization of alpha 2 beta 2 and alpha 2 forms of kinesin. Biochem Biophys Res Commun. 1991 Jan 31;174(2):810–815. doi: 10.1016/0006-291x(91)91490-4. [DOI] [PubMed] [Google Scholar]
- Hatsumi M., Endow S. A. Mutants of the microtubule motor protein, nonclaret disjunctional, affect spindle structure and chromosome movement in meiosis and mitosis. J Cell Sci. 1992 Mar;101(Pt 3):547–559. doi: 10.1242/jcs.101.3.547. [DOI] [PubMed] [Google Scholar]
- Hiratsuka T. New ribose-modified fluorescent analogs of adenine and guanine nucleotides available as substrates for various enzymes. Biochim Biophys Acta. 1983 Feb 15;742(3):496–508. doi: 10.1016/0167-4838(83)90267-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McDonald H. B., Goldstein L. S. Identification and characterization of a gene encoding a kinesin-like protein in Drosophila. Cell. 1990 Jun 15;61(6):991–1000. doi: 10.1016/0092-8674(90)90064-l. [DOI] [PubMed] [Google Scholar]
- Romberg L., Vale R. D. Chemomechanical cycle of kinesin differs from that of myosin. Nature. 1993 Jan 14;361(6408):168–170. doi: 10.1038/361168a0. [DOI] [PubMed] [Google Scholar]
- Sadhu A., Taylor E. W. A kinetic study of the kinesin ATPase. J Biol Chem. 1992 Jun 5;267(16):11352–11359. [PubMed] [Google Scholar]
- Scholey J. M., Heuser J., Yang J. T., Goldstein L. S. Identification of globular mechanochemical heads of kinesin. Nature. 1989 Mar 23;338(6213):355–357. doi: 10.1038/338355a0. [DOI] [PubMed] [Google Scholar]
- Stewart R. J., Thaler J. P., Goldstein L. S. Direction of microtubule movement is an intrinsic property of the motor domains of kinesin heavy chain and Drosophila ncd protein. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5209–5213. doi: 10.1073/pnas.90.11.5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor E. W. Cell motility. Variations on the theme of movement. Nature. 1993 Jan 14;361(6408):115–116. doi: 10.1038/361115a0. [DOI] [PubMed] [Google Scholar]
- Trentham D. R., Bardsley R. G., Eccleston J. F., Weeds A. G. Elementary processes of the magnesium ion-dependent adenosine triphosphatase activity of heavy meromyosin. A transient kinetic approach to the study of kinases and adenosine triphosphatases and a colorimetric inorganic phosphate assay in situ. Biochem J. 1972 Feb;126(3):635–644. doi: 10.1042/bj1260635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Goldstein L. S. One motor, many tails: an expanding repertoire of force-generating enzymes. Cell. 1990 Mar 23;60(6):883–885. doi: 10.1016/0092-8674(90)90334-b. [DOI] [PubMed] [Google Scholar]
- Walker R. A., Salmon E. D., Endow S. A. The Drosophila claret segregation protein is a minus-end directed motor molecule. Nature. 1990 Oct 25;347(6295):780–782. doi: 10.1038/347780a0. [DOI] [PubMed] [Google Scholar]