The MDASI “sadness” item has modest sensitivity and high negative predictive value and can serve as a useful initial screen for depressed mood, an approach that may improve the efficiency and acceptability of depression screening for both clinicians and patients.
Abstract
Purpose:
Depression is a significant concern in outpatient oncology care, yet clinicians face practical challenges in accurately and efficiently screening patients for it. This study investigated whether a single item or multiple items from an existing multisymptom scale, the MD Anderson Symptom Inventory (MDASI), might serve as effective initial screens for depressed mood.
Methods:
Data were collected from two cohorts of patients. Cohort 1 comprised 187 patients with non–small-cell lung cancer who completed the Beck Depression Inventory II; cohort 2 comprised 281 patients with renal cell carcinoma who completed the Center for Epidemiologic Studies Depression Scale. All patients completed the MDASI. Single-item and multiple-item MDASI solutions were identified using cohort 1 and validated in cohort 2. Sensitivity and specificity of the solutions were assessed through binary linear regression; cut points were identified using receiver operating characteristic analysis.
Results:
The MDASI single item “sadness” was the best solution identified in cohort 1 for screening for depressed mood relative to other affective items (distress, enjoyment of life, mood). At a cut point ≥ 4 (0 to 10 scale), the “sadness” item exhibited a clinically acceptable specificity of 81.5%, sensitivity of 72.0%, a negative predictive value of 95.0%, and a positive predictive value of 37.5%. This solution was successfully validated in cohort 2.
Conclusion:
The MDASI “sadness” item has modest sensitivity and high negative predictive value and can serve as a useful initial screen for depressed mood. This approach may improve the efficiency and acceptability of depression screening for both clinicians and patients.
Introduction
Depression frequently accompanies cancer and other serious diseases and, if recognized, is often treatable. Several questionnaires are available to assess depression; however, they are not routinely used in oncology practice because of time constraints1 and the discomfort patients experience at having to answer repeated questions about depression. Recent work2 indicates that although two thirds of cancer clinicians attempt to detect mood disorder as part of routine care, most rely on their clinical skills alone rather than on validated questionnaires, and only one third would be prepared to use short instruments such as the 14-item Hospital Anxiety and Depression Scale3 or the nine-item Patient Health Questionnaire.4 Unfortunately, an oncologist's perception of patients' depression and distress generally correlates poorly with results obtained through validated screening instruments.5,6 The consequences of poor initial screening for depression in oncology practice are substantial and include inappropriate risk stratification and resource allocation, errors in treatment decisions (eg, antidepressant prescriptions), and poorer health outcomes for patients.
To improve acceptability of depression screening, ultrashort tools with fewer than five items (eg, the Two-Question Depression Screen7 or the single-question Distress Thermometer8) have been developed. Although ultrashort methods for detecting mood disorders are reasonably sensitive and specific as initial screens for depression, they are independent instruments that are generally administered alongside a multisymptom scale that summarizes the patient's overall symptoms, such as the MD Anderson Symptom Inventory (MDASI),9 Edmonton Symptom Assessment Scale (ESAS),10 Symptom Distress Scale,11 or other similar instrument.12 Frequently used multisymptom scales such as the MDASI and the ESAS have been validated across multiple cancers, disease stages, and languages, and often use a simple 0 to 10 scale to rate symptom severity.9–11 These instruments themselves typically include one or more items assessing depression-related symptoms that could potentially be used to quickly screen patients for depressed mood. Using an already-available item or items in existing, widely used multisymptom scales may provide a practical and efficient initial depression screening method for cancer outpatients.
In the current study, we sought to determine the degree to which responses to individual items or a limited number of depression-related items from such scales might help to identify patients needing further evaluation for depression. Specifically, we sought to determine the efficacy of both single-item and multiple-item solutions from the MDASI to screen for depressed mood. We hypothesized that individual items from the MDASI (eg, “sadness” or “symptom interference with mood”) would adequately screen patients for depressed mood, and that a multiple-item solution derived from relevant items of the MDASI would be more likely to provide greater sensitivity in screening.
Methods
Study Participants
Data were retrospectively collected from two patient cohorts. Cohort 1 was derived from a multicenter trial of patients with advanced (stage IIIB or IV) non–small-cell lung cancer (NSCLC) who were recruited to evaluate symptom burden in late-stage disease.13 Patients were recruited from two public hospitals and one tertiary cancer center in Houston, TX and one public hospital in Miami, FL. Eligible patients were scheduled to receive chemotherapy, were at least 18 years of age, and were able to read and speak English.
Cohort 2 comprised patients with renal cell carcinoma (RCC) who were participating in a randomized trial evaluating the effects of expressive writing. All patients were recruited from a tertiary cancer center in Houston. Eligible patients were at least 18 years of age, had a Zubrod performance status of ≤ 2, and were able to write and speak English. Patients with a history of immunodeficiency, using immunosuppressive drugs, having a confirmed psychiatric diagnosis of depression, or receiving psychiatric services were excluded.
All patients provided written informed consent. All protocols were approved by the institutional review boards of the participating institutions.
Measures
All patients had completed the MDASI. Cohort 1 had also completed the Beck Depression Inventory-II (BDI-II),14 and cohort 2 had also completed the Center for Epidemiologic Studies Depression Scale (CES-D).15 These are established, well-validated instruments for assessing depressed mood, and each was used as a reference criterion.
The MDASI assesses patients' symptom burden within the previous 24 hours. Its 13 core cancer or treatment-related symptoms, including pain, fatigue, nausea, vomiting, dry mouth, shortness of breath, lack of appetite, difficulty remembering, drowsiness, disturbed sleep, sadness, distress, and numbness, are rated on a 0 (not present) to 10 (as bad as you can image) scale. The MDASI also contains six additional items that measure the degree to which symptoms interfere with daily activities. Interference items can be divided into affective (relations with others, enjoyment of life, and mood) and activity (walking ability, general activity, and normal work) dimensions and are scored on a 0 (did not interfere) to 10 (interfered completely) scale.9
One of the advantages of the MDASI is that it can be completed in less than 5 minutes with paper and pencil, digitally, or by a telephone-based interactive voice response system. It has also been validated across a variety of cancers9 and in numerous languages16–19 and can thus be administered to diverse patient populations.20
The BDI-II is a validated assessment of the severity of depressive symptoms that has high sensitivity and predictive value.14 It contains 21 items that are rated on a 0 to 3 scale, with a maximum attainable score of 63. Standard cut points for the BDI-II have been established: a score less than 14 indicates minimal symptoms of depression; a score of 14 to 19 indicates mild depressive symptoms; a score of 20 to 28 indicates moderate depressive symptoms; and a score ≥ 29 indicates severe depressive symptoms.21
The CES-D is another validated scale used to assess the severity of depressive symptoms.11,15 It contains 20 items that are scored on a 0 to 3 scale, with a maximum attainable score of 60. A score ≥ 16 has been established as the cut point for mild depressive symptoms warranting further screening, and a score ≥ 27 is the cut point for more-severe depressive symptoms.22–24
Statistical Analysis
Cohort 1 was used to identify optimal single-item and multiple-item solutions for screening for depressed mood. Solutions identified in cohort 1 were validated using cohort 2. We used data from patients' baseline assessments.
Cohort 1.
To derive single-item solutions to screen for depressed mood, we used Pearson correlations to examine the association between theoretically relevant individual MDASI items (eg, “sadness,” “distress,” “interference with mood,” and “interference with enjoyment of life”) and depressive symptom-severity scores derived from the BDI-II. Next, using binary linear regression models, we evaluated the cut points for each MDASI item that were best able to identify moderate-to-severe depressed mood.
To derive the optimal multiple-item solution, we conducted stepwise linear regression analysis to determine which combination of the 13 core and six interference MDASI items best predicted depression severity as determined by the BDI-II. A depressed-mood component score was derived by summing the scores of the MDASI items identified by the model. The ability of the multiple-item solution to discriminate between patients with and without depressed mood was examined using receiver operating characteristic (ROC) analysis. On such a curve, an area under the curve (AUC) of 0.5 indicates discrimination no better than chance; an AUC of 1.0 indicates perfect discrimination between cases and noncases. We identified the optimal cut points associated with moderate depressed mood using the Youden's J (sensitivity + specificity − 1), an index that summarizes diagnostic test accuracy. The positive and negative predictive value (PPV and NPV, respectively) associated with a specific cut point on a scale are good indicators of its clinical utility in a given population.25 Thus, we also calculated the PPV (the percentage of individuals who are true cases among those who are screened positive) and the NPV (the proportion of patients who are true noncases among those who are screened negative) corresponding to the individual, identified cut points on the MDASI.
Cohort 2.
To validate and assess the generalizability of the single-item solution identified in cohort 1, we evaluated the correlations between individual MDASI items and CES-D depressive symptom scores. We then examined the performance of the cut points for moderate-to-severe depressed mood identified in cohort 1 in terms of their sensitivity and specificity in cohort 2.
To validate the multiple-item solution obtained from cohort 1, we conducted a linear regression between the MDASI depressed-mood component score and the CES-D depression-severity score. The operating characteristics of the cut points for moderate-to-severe depressed mood identified in cohort 1 were then evaluated using cohort 2.
Results
Patient demographics are shown in Table 1. Cohort 1 had 187 patients with late-stage NSCLC; cohort 2 had 281 patients with RCC spanning all stages. In cohort 1, 13.4% of patients met the criteria for moderate-to-severe depressed mood (BDI-II score ≥ 20); in cohort 2, 8.9% of patients met the criteria for moderate-to-severe depressed mood (CES-D score ≥ 27) (Table 2).
Table 1.
Patient Characteristics
| Characteristic | Cohort 1 (n = 187): Public Hospitals (n = 85), Tertiary Center (n = 102), Non–Small-Cell Lung Cancer | Cohort 2 (n = 281): All Patients From Tertiary Center, Renal Cell Carcinoma |
|---|---|---|
| Age, years | ||
| Mean | 59.8 | 58.1 |
| SD | 9.0 | 9.9 |
| Range | 31-85 | 31-82 |
| No. | % | No. | % | |
|---|---|---|---|---|
| Sex | ||||
| Men | 116 | 62.0 | 165 | 58.7 |
| Women | 71 | 38.0 | 105 | 37.4 |
| Unknown | 0 | 0.0 | 11 | 3.9 |
| Race/ethnicity | ||||
| White | 117 | 62.6 | 226 | 80.4 |
| Hispanic | 25 | 13.4 | 29 | 10.3 |
| Black | 44 | 23.5 | 9 | 3.2 |
| Other | 1 | 0.5 | 15 | 5.3 |
| Unknown | 0 | 0.0 | 2 | 0.7 |
| Education | ||||
| ≤ High school | 105 | 56.1 | 70 | 24.9 |
| > High school | 81 | 43.3 | 207 | 73.7 |
| Unknown | 1 | 0.5 | 4 | 1.4 |
| Employment | ||||
| Employed | 36 | 19.3 | 176 | 62.6 |
| Retired | 56 | 29.9 | 92 | 32.7 |
| Unemployed | 93 | 49.7 | 9 | 3.2 |
| Unknown | 2 | 1.1 | 4 | 1.4 |
| Marital status | ||||
| Married | 117 | 62.6 | 227 | 76.5 |
| Unmarried | 70 | 37.4 | 50 | 17.8 |
| Unknown | 0 | 0.0 | 4 | 1.4 |
| Cancer stage | ||||
| I | 0 | 0.0 | 103 | 36.7 |
| II | 0 | 0.0 | 35 | 12.5 |
| III | 39 | 20.9 | 53 | 19.9 |
| IV | 148 | 79.1 | 90 | 32.0 |
Abbreviation: SD, standard deviation.
Table 2.
Depressed Mood Scores and Severity As Assessed by the BDI-II in Cohort 1 and the CES-D in Cohort 2 and MDASI Individual Mood-Related Items
| Item | Cohort 1 (n = 187) | Cohort 2 (n = 281) | ||||
|---|---|---|---|---|---|---|
| Depressed-mood category* | ||||||
| None to minimal | ||||||
| No. | 128 | 220 | ||||
| % | 68.4 | 78.3 | ||||
| Mild | ||||||
| No. | 34 | 36 | ||||
| % | 18.2 | 12.8 | ||||
| Moderate to severe | ||||||
| No. | 25 | 25 | ||||
| % | 13.4 | 8.9 | ||||
| Mean | SD | Range | Mean | SD | Range | |
|---|---|---|---|---|---|---|
| Depressed-mood component score† | 11.2 | 7.9 | 0-36 | 10.7 | 9.3 | 0-45 |
| MDASI mood-related item scores | ||||||
| Sadness | 2.2 | 3.1 | 0-10 | 1.3 | 2.1 | 0-9 |
| Distress | 2.2 | 2.9 | 0-10 | 1.5 | 2.2 | 0-9 |
| Fatigue | 3.4 | 2.9 | 0-10 | 3.0 | 2.1 | 0-10 |
| Interference with enjoyment of life | 3.2 | 3.4 | 0-10 | 1.6 | 2.5 | 0-10 |
| Interference with mood | 2.3 | 2.8 | 0-10 | 1.4 | 2.1 | 0-10 |
| Interference with relations with others | 1.4 | 2.4 | 0-10 | 1.1 | 2.0 | 0-10 |
Abbreviations: BDI-II, Beck Depression Inventory-II; CES-D, Center for Epidemiologic Studies Depression Scale; MDASI, MD Anderson Symptom Inventory; SD, standard deviation.
For cohort 1: BDI-II score < 14 = minimal depressed mood; 14 to 19 = mild depressed mood; ≥ 20 = moderate-to-severe depressed mood. For cohort 2: CES-D score < 16 = no or minimal depressed mood; 16 to 26 = mild depressed mood; ≥ 27 = moderate-to-severe depressed mood.
Depressed-mood component score = sum score of MDASI sadness, fatigue, interference with mood, relations with others, and enjoyment of life.
MDASI Single-Item Solutions for Screening for Depressed Mood
Correlations between relevant MDASI single items and BDI-II depressive symptom scores (analyzed continuously) were as follows: “interference with mood” (r = .540), “interference with enjoyment of life” (r = .536), “sadness” (r = .491), and “distress” (r = 0.427; all P < .001).
To maximize sensitivity and specificity, we identified a score of 4 or greater on the individual MDASI items as the best general cut point for assessing moderately depressed mood (BDI-II ≥ 20). The MDASI item “sadness” was most indicative of moderate-to-severe depressed mood (Table 3). At a cut point of ≥ 4, it exhibited a sensitivity of 72.0% and specificity of 81.5%. The tradeoffs between sensitivity and specificity, as well as the PPVs and NPVs at other cut points for “sadness,” are shown in Table 4.
Table 3.
Sensitivity and Specificity of Individual MDASI Items and the Depressed-Mood Component Score at Various Cut Points for Assessing Depressed Mood
| Assessment | Moderate-to-Severe Depressive Symptoms* |
|
|---|---|---|
| Specificity (%) | Sensitivity (%) | |
| Individual Item Score ≥4 | ||
| Single item | ||
| Cohort 1 | ||
| Sadness | 81.5 | 72.0 |
| Distress | 78.4 | 56.0 |
| Interference with enjoyment of life | 66.5 | 76.0 |
| Interference with mood | 78.4 | 68.0 |
| Cohort 2 | ||
| Sadness | 91.0 | 68.0 |
| Distress | 89.5 | 72.0 |
| Interference with enjoyment of life | 85.9 | 64.0 |
| Interference with mood | 87.4 | 72.0 |
| Component Score ≥19 | ||
| Multiple item | ||
| Depressed-mood component score† | ||
| Cohort 1 | 83.0 | 84.0 |
| Cohort 2 | 88.9 | 76.0 |
Abbreviations: BDI-II, Beck Depression Inventory-II; CES-D, Center for Epidemiologic Studies Depression Scale; MDASI, MD Anderson Symptom Inventory.
For cohort 1, BDI-II score ≥ 20 = moderate-to-severe depressed mood; for cohort 2, CES-D score ≥ 27 = moderate-to-severe depressed mood.
Depressed-mood component score = sum score of MDASI items: sadness, fatigue, interference with mood, relations with others, and enjoyment of life.
Table 4.
Operating Characteristics of the Individual MDASI Item “Sadness” and the Depressed-Mood Component Score; Positive and Negative Predictive Values Corresponding to Moderate-to-Severe Depressive Symptoms (Cohort 1)
| Cut Point | Prevalence (%) | Moderate-to-Severe Depressive Symptoms* |
||||
|---|---|---|---|---|---|---|
| Specificity (%) | Sensitivity (%) | PPV (%) | NPV (%) | |||
| Sadness | ≥ 2 | 32.6 | 64.8 | 80.0 | 25.9 | 95.5 |
| ≥ 3 | 25.7 | 73.5 | 72.0 | 29.5 | 94.4 | |
| ≥ 4 | 20.9 | 81.5 | 72.0 | 37.5 | 95.0 | |
| ≥ 5 | 16.0 | 85.2 | 60.0 | 38.5 | 93.2 | |
| Depressed-mood component score† | ≥ 14 | 35.9 | 67.9 | 88.0 | 30.1 | 97.3 |
| ≥ 15 | 32.6 | 72.3 | 88.0 | 33.3 | 97.5 | |
| ≥ 16 | 31.0 | 76.1 | 88.0 | 36.7 | 97.6 | |
| ≥ 17 | 28.8 | 78.0 | 88.0 | 38.6 | 97.6 | |
| ≥ 18 | 26.1 | 79.9 | 84.0 | 39.6 | 96.9 | |
| ≥ 19 | 22.3 | 83.0 | 84.0 | 43.8 | 97.1 | |
| ≥ 20 | 20.1 | 85.5 | 72.0 | 43.9 | 95.1 | |
Abbreviations: BDI-II, Beck Depression Inventory-II; MDASI, MD Anderson Symptom Inventory; NPV, negative predictive value; PPV, positive predictive value.
BDI-II score ≥20 for moderate-to-severe depressed mood.
Depressed-mood component score = sum score of MDASI sadness, fatigue, interference with mood, relations with others, and enjoyment of life.
MDASI Multiple-Item Solutions for Screening for Depressed Mood
Results from stepwise linear regression indicated that the MDASI model with the strongest association with depressive symptom severity as determined by the BDI-II included “sadness” (β = 0.239, SE = 0.183), “fatigue” (β = 0.153, SE = 0.177), “interference with relations with others” (β = 0.160, SE = 0.019), “interference with enjoyment of life” (β = 0.249, SE = 0.162), and “interference with mood” (β = 0.129, SE = 0.231). This model had an R2 of .673 and SE of 5.9 (P < .001). Although the final statistical model excluded the “interference with mood” variable, we retained the five-variable model as optimal, given the theoretical importance of mood. These variables were summed to produce the MDASI depressed-mood component score.
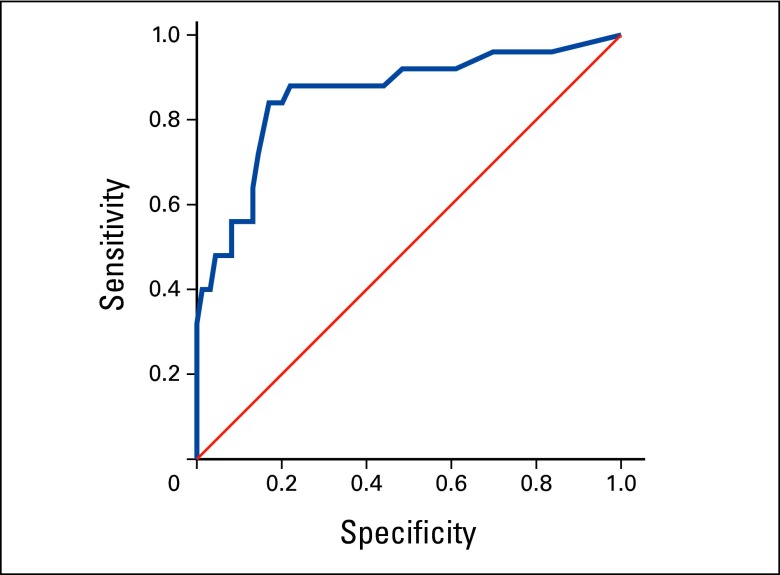
Next, we evaluated the ability of this MDASI component score to identify patients with moderate-to-severe depressed mood (BDI-II score ≥ 20). This analysis resulted in an AUC of 0.860 (95% CI, 0.770 to 0.949; Appendix Figure A1, online only). A depressed-mood component score of ≥ 19 (mean individual item score of 3.8 on a 0 to 10 scale) provided the optimal cut point for screening for moderate-to-severe depressed mood (sensitivity, 84.0%; specificity, 83.0%; Table 4). On the basis of this criterion, 22.3% of patients in cohort 1 had moderate-to-severe depressed mood. The tradeoffs between sensitivity and specificity, as well as the PPVs and NPVs at other cut points for the depressed-mood component score, are shown in Table 4.
Validation in Cohort 2 of Solutions Obtained From Cohort 1
Single-item solutions.
Correlations between relevant MDASI single items and CES-D depressive symptom severity were as follows: “sadness” (r = .688), “distress” (r = .666), “interference with mood” (r = .628), and “interference with enjoyment of life” (r = .514; all P < .001).
The cut points established in cohort 1 for these individual items provided comparable, or slightly better, sensitivity and specificity in cohort 2. The items “sadness” and “distress” were both equally indicative of moderate-to-severe depressed mood (CES-D score ≥ 27). A cut point of ≥ 4 yielded a sensitivity of 68.0% and specificity of 91.0% for “sadness” and a sensitivity of 72.0% and specificity of 89.5% for “distress” (Table 3).
Multiple-item solutions.
To validate the five-variable solution identified in cohort 1, a linear regression of the MDASI depressed-mood component score and the CES-D depressive symptom severity score was performed, resulting in an R2 value of .674 (P < .001). This score was comparable to that observed for the BDI-II (r = .673; P < .001).
An MDASI depressed-mood component score of ≥ 19 identified 16.9% of patients from cohort 2 as having moderate-to-severe depressed mood. When evaluated against the CES-D criterion for moderate-to-severe depression (CES-D score ≥ 27), this cut point had a specificity of 88.9% and a sensitivity of 76.0% (Table 3).
Discussion
Depression is common during the course of cancer, and a substantial body of research has established its association with patients' quality of life, satisfaction with and participation in medical treatment,2 and survival.26–29 Notwithstanding, the literature suggests that clinicians face practical challenges in accurately and efficiently screening for depression. In this study, we investigated whether individual depression-related items or a combination of items from an existing, widely used, multisymptom measure such as the MDASI could serve as effective initial screens for depressed mood. If so, they would provide a practical solution to the oncologist, replacing both disease-specific and ultrashort instruments.
Results from cohort 1 indicated that at a cut point of ≥ 4 to detect moderate-to-severe depressed mood, the MDASI item “sadness” demonstrated a sensitivity of 72.0%, acceptable specificity (81.5%), a high NPV of 95.0%, and a PPV of 37.5%. In practical terms, these operating characteristics would help a clinician rule out 95% of patients who screen negative at the defined cut point and are in fact not depressed. This solution was validated in cohort 2, demonstrating an acceptable but lower sensitivity of 68.0%, a specificity of 91.0%, an NPV of 97.0%, and a PPV of 42.5%. Using a data-driven approach, we also identified a five-variable multiple-item solution (summed score of MDASI items: “sadness,” “fatigue,” “interference with relations with others,” “interference with mood,” and “interference with enjoyment of life”). In cohort 1, it was determined that a cut point of ≥ 19 was optimal for identifying patients with moderate-to-severe depressed mood. When validated in cohort 2, this solution demonstrated acceptable sensitivity (76.0%) and specificity (88.9%).
Pooled results from 38 analyses of the accuracy of the Distress Thermometer and several other ultrashort methods of detecting cancer-related mood disorders indicate that, in general, these measures are good at excluding cases of depression but relatively poor at confirming diagnosis, as indicated by a sensitivity of 78.4% and a specificity of 66.8%.2 In addition, the Distress Thermometer has been reported to have a sensitivity of 77% to 88% and a specificity of 72% to 79% at a score of ≥ 3 for detecting anxiety, depression, and comorbid anxiety-depression.30 Compared with these criteria, as well as with commonly used measures such as the Two-Question Depression Screen (demonstrated sensitivity of 68% to 89% and specificity of 70% to 84% when compared with four criterion measures7), the single-item and multiple-item MDASI solutions for moderate-to-severe depressed mood derived from our study provide comparable, or somewhat better, sensitivity and specificity. Further, at a cut point of ≥ 4, the PPV of the MDASI single-item solution (37.5%) is acceptable when compared, for example, with the PPV of a widely-used depression-specific instrument (ie, 25% for the Patient Health Questionnaire25 at its suggested cutoff for depression screening in patients with cancer). A clinician might also use a different cut point to obtain a higher PPV (eg, select “sadness” ≥ 5 on the MDASI to obtain a PPV of nearly 39%, or a component score ≥ 20 to obtain a PPV of 44%; Table 4).
In light of the above, and on the basis of our analyses, we propose these two valid options for screening for moderate-to-severe depressed mood: (1) use the single-item MDASI solution of “sadness” ≥ 4; or (2) use the multiple-item solution of the MDASI depressed-mood component score ≥ 19. These cut points are recommended starting points and may not be optimal for every application; consequently, the sensitivity/specificity and PPV/NPV trade-offs (Table 4) would be valuable to the clinician. In addition, although the multiple-item solution demonstrated better sensitivity, it may be less practical in a clinical setting than the single-item solution. For efficiency in most current settings, the single-item solution provides reasonably good precision for identifying patients who may need referral for further evaluation.
Our study had some limitations that are essential to bear in mind while interpreting the presented results. Although cohort 2 was used to validate solutions obtained in cohort 1, it is important to note that some demographic and clinical differences existed between the two cohorts that may have had an influence on depressed mood prevalence levels in the two study samples. For example, cohort 1 was recruited from both public hospitals and a tertiary care center, whereas cohort 2 was recruited from only a tertiary care center. Cohort 1 also had a broader racial distribution (eg, 62.6% White patients v 80.4% in cohort 2; 23.5% Black patients v 3.2% in cohort 2), a lower percentage of employed patients (19.3% v 62.6% in cohort 2), and more patients with advanced disease relative to cohort 2. The demographic factors in particular (eg, being a member of a racial/ethnic minority and unemployed) are generally correlated with higher depression levels, and more advanced disease in cohort 1 patients may have contributed to the same. In addition, it is possible that depressed mood prevalence levels in cohort 2 were lower than expected (8.9% relative to 13.4% in cohort 1) as a result of the exclusion of patients with a confirmed psychiatric diagnosis. These sample-selection–related limitations were inherent to the nature of the retrospective design of our study. Future studies should examine our solutions in more comparable samples utilizing prospective designs.
To summarize, the main objective of this methodological paper was not to demonstrate that single-item or multiple-item solutions derived from the MDASI or similar measures provide better depression screening relative to established screening instruments. Indeed, more robust, validated, depression-specific tools (eg, the BDI-II) as well as ultrashort measures (eg, the Distress Thermometer) are available. However, the former tools are seldom in use in the practical clinical setting, and the latter may be replaceable by brief multisymptom scales that not only summarize a patient's affective symptoms, but also have the added salutary benefit of more fully assessing patients' functional status, as well as other critical symptoms of concern. Our results indicate that the solutions presented here provide a surprisingly good index of a patient's depression status, and although not intended as diagnostic tools, they can serve as adequate initial screens, thus facilitating the oncologist's decision to refer or not refer a patient for further evaluation. The implications inherent in effective depression screening are substantial and encompass appropriate risk stratification (ie, preventing under- or over-referral); decision support for resource allocation; and overall outcome improvement, including survival, for patients. Thus, all resources available to the clinician that may contribute to better initial case finding can be of value. Previous research suggests that numerous indices routinely collected in clinical practice, such as family history of depression, higher number of concurrent medications, and sedative use, are predictive of depressed status.31 Along with the use of the solutions presented here, these indices can serve as valuable auxiliary resources to the oncologist to assist in case finding.
In routine practice, it is neither feasible nor always desirable to add one or more specialized tools to screen for depression, anxiety, and so on. Our data provide the statistical foundation to equip the oncologist to screen reasonably accurately for depressed mood using a familiar and robust multisymptom instrument. As electronic health records evolve (coupled with future cognitive computing capabilities), key additional parameters predictive of depression can be added to instruments such as the MDASI to greatly augment both the quality and efficiency of depression screening.
Acknowledgment
Supported by grants from the National Cancer Institute (CA026582 to C.S.C., CA090966 to L.C.), MD Anderson Cancer Center Support Grant CA016672, and the Hawn Foundation. The content does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Appendix
Figure A1.
Receiver operating characteristic curves for the MD Anderson Symptom Inventory (MDASI) depressed-mood component score (sum score of MDASI items: sadness, fatigue, interference with mood, relations with others, and enjoyment of life) according to the reference criterion of the Beck Depression Inventory-II (score ≥ 20) for moderate-to-severe depressed mood.
Authors' Disclosures of Potential Conflicts of Interest
The author(s) indicated no potential conflicts of interest.
Author Contributions
Conception and design: Desiree Jones, Charles S. Cleeland, Seema M. Thekdi, Xin Shelley Wang
Provision of study materials or patients: Lorenzo Cohen
Data analysis and interpretation: Desiree Jones, Elisabeth G. Vichaya, Charles S. Cleeland, Lorenzo Cohen, Xin Shelley Wang, Michael J. Fisch
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.Mitchell AJ, Kaar S, Coggan C, et al. Acceptability of common screening methods used to detect distress and related mood disorders-preferences of cancer specialists and non-specialists. Psychooncology. 2008;17:226–236. doi: 10.1002/pon.1228. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell AJ. Pooled results from 38 analyses of the accuracy of distress thermometer and other ultra-short methods of detecting cancer-related mood disorders. J Clin Oncol. 2007;25:4670–4681. doi: 10.1200/JCO.2006.10.0438. [DOI] [PubMed] [Google Scholar]
- 3.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 4.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 5.Sollner W, DeVries A, Steixner E, et al. How successful are oncologists in identifying patient distress, perceived social support, and need for psychosocial counselling? Br J Cancer. 2001;84:179–185. doi: 10.1054/bjoc.2000.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fallowfield L, Ratcliffe D, Jenkins V, et al. Psychiatric morbidity and its recognition by doctors in patients with cancer. Br J Cancer. 2001;84:1011–1015. doi: 10.1054/bjoc.2001.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Low J, Gessler S, Williams R, et al. Screening for distress and depression in cancer patients: Is ultrashort depression screening a valid measure in the UK? A prospective validation study. J Pain Symptom Manage. 2009;38:234–243. doi: 10.1016/j.jpainsymman.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Roth AJ, Kornblith AB, Batel-Copel L, et al. Rapid screening for psychologic distress in men with prostate carcinoma: A pilot study. Cancer. 1998;82:1904–1908. doi: 10.1002/(sici)1097-0142(19980515)82:10<1904::aid-cncr13>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 9.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: The M.D. Anderson Symptom Inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 10.Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer. 2000;88:2164–2171. doi: 10.1002/(sici)1097-0142(20000501)88:9<2164::aid-cncr24>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 11.McCorkle R, Young K. Development of a symptom distress scale. Cancer Nurs. 1978;1:373–378. [PubMed] [Google Scholar]
- 12.Kim E, Jahan T, Aouizerat BE, et al. Differences in symptom clusters identified using occurrence rates versus symptom severity ratings in patients at the end of radiation therapy. Cancer Nurs. 2009;32:429–436. doi: 10.1097/NCC.0b013e3181b046ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cleeland CS, Mendoza TR, Wang XS, et al. Levels of symptom burden during chemotherapy for advanced lung cancer: Differences between public hospitals and a tertiary cancer center. J Clin Oncol. 2011;29:2859–2865. doi: 10.1200/JCO.2010.33.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck AT, Steer RA, Ball R, et al. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 15.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:285–401. [Google Scholar]
- 16.Guirimand F, Buyck JF, Lauwers-Allot E, et al. Cancer-related symptom assessment in France: Validation of the French M. D. Anderson Symptom Inventory. J Pain Symptom Manage. 2010;39:721–733. doi: 10.1016/j.jpainsymman.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Lin CC, Chang AP, Cleeland CS, et al. Taiwanese version of the M. D. Anderson Symptom Inventory: Symptom assessment in cancer patients. J Pain Symptom Manage. 2007;33:180–188. doi: 10.1016/j.jpainsymman.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Nejmi M, Wang XS, Mendoza TR, et al. Validation and application of the Arabic version of the M. D. Anderson Symptom Inventory in Moroccan patients with cancer. J Pain Symptom Manage. 2010;40:75–86. doi: 10.1016/j.jpainsymman.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Wang XS, Wang Y, Guo H, et al. Chinese version of the M. D. Anderson Symptom Inventory: Validation and application of symptom measurement in cancer patients. Cancer. 2004;101:1890–1901. doi: 10.1002/cncr.20448. [DOI] [PubMed] [Google Scholar]
- 20.Wang XS, Cleeland CS, Mendoza TR, et al. Impact of cultural and linguistic factors on symptom reporting by patients with cancer. J Natl Cancer Inst. 2010;102:732–738. doi: 10.1093/jnci/djq097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beck AT, Steer RA, Brown G. Manual for the Beck Depression Inventory II. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 22.Geisser ME, Roth RS, Robinson ME. Assessing depression among persons with chronic pain using the Center for Epidemiological Studies-Depression Scale and the Beck Depression Inventory: A comparative analysis. Clin J Pain. 1997;13:163–170. doi: 10.1097/00002508-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Logsdon MC, McBride AB, Birkimer JC. Social support and postpartum depression. Res Nurs Health. 1994;17:449–457. doi: 10.1002/nur.4770170608. [DOI] [PubMed] [Google Scholar]
- 24.Zich JM, Attkisson CC, Greenfield TK. Screening for depression in primary care clinics: The CES-D and the BDI. Int J Psychiatry Med. 1990;20:259–277. doi: 10.2190/LYKR-7VHP-YJEM-MKM2. [DOI] [PubMed] [Google Scholar]
- 25.Thekkumpurath P, Walker J, Butcher I, et al. Screening for major depression in cancer outpatients: The diagnostic accuracy of the 9-item patient health questionnaire. Cancer. 2011;117:218–227. doi: 10.1002/cncr.25514. [DOI] [PubMed] [Google Scholar]
- 26.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: A meta-analysis. Cancer. 2009;115:5349–5361. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 27.Cohen L, Cole SW, Sood AK, et al. Depressive symptoms and cortisol rhythmicity predict survival in patients with renal cell carcinoma: Role of inflammatory signaling. PLoS One. 2012;7:e42324. doi: 10.1371/journal.pone.0042324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pirl WF, Greer JA, Traeger L, et al. Depression and survival in metastatic non-small-cell lung cancer: Effects of early palliative care. J Clin Oncol. 2012;30:1310–1315. doi: 10.1200/JCO.2011.38.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giese-Davis J, Collie K, Rancourt KM, et al. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: A secondary analysis. J Clin Oncol. 2011;29:413–420. doi: 10.1200/JCO.2010.28.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyes A, D'Este C, Carey M, et al. How does the Distress Thermometer compare to the Hospital Anxiety and Depression Scale for detecting possible cases of psychological morbidity among cancer survivors? Support Care Cancer. 2013;21:119–127. doi: 10.1007/s00520-012-1499-3. [DOI] [PubMed] [Google Scholar]
- 31.Manola J, Zhao F, Miller AH, et al. Patterns of antidepressant use in cancer patients: An analysis form SOAPP (ECOG E2Z02: Symptom Outcomes and Practice Patterns) J Clin Oncol. 2012;30(suppl):571s. abstr 9016. [Google Scholar]