Abstract
The similarity between the structure and function of the breast and prostate has been known for a long time, but there are serious discrepancies in the terminology describing breast and prostate cancers. The use of the large, thick-section (3D) histology technique for both organs exposes the irrationality of the breast cancer terminology. Pathologists with expertise in diagnosing prostate cancer take the anatomic site of cancer origin into account when using the terms AAP (acinar adenocarcinoma of the prostate) and DAP (ductal adenocarcinoma of the prostate) to distinguish between the prostate cancers originating primarily from the fluid-producing acinar portion of the organ (AAP) and the tumors originating either purely from the larger ducts (DAP) or from both the acini and the main ducts combined (DAP and AAP). Long-term patient outcome is closely correlated with the terminology, because patients with DAP have a significantly poorer prognosis than patients with AAP.
The current breast cancer terminology could be improved by modeling it after the method of classifying prostate cancer to reflect the anatomic site of breast cancer origin and the patient outcome. The long-term survival curves of our consecutive breast cancer cases collected since 1977 clearly show that the non-palpable, screen-detected breast cancers originating from the milk-producing acini have excellent prognosis, irrespective of their histologic malignancy grade or biomarkers. Correspondingly, the breast cancer subtypes of truly ductal origin have a significantly poorer outcome, despite recent improvements in diagnosis and therapy. The mammographic appearance of breast cancers reflects the underlying tissue structure. Addition of these “mammographic tumor features” to the currently used histologic phenotypes makes it possible to distinguish the breast cancer cases of ductal origin with a poor outcome, termed DAB (ductal adenocarcinoma of the breast), from the more easily managed breast cancers of acinar origin, termed AAB (acinar adenocarcinoma of the breast), which have a significantly better outcome. This simple and easily communicable terminology could lead to better communication between the diagnostic and therapeutic team members and result in more rational treatment planning for the benefit of their patients.
Keywords: breast cancer terminology, prostate cancer terminology, ductal adenocarcinoma of the breast (DAB), acinar adenocarcinoma of the breast (AAB), subgross 3-D histology, neoductgenesis
Introduction
The prospective, population-based randomized-controlled breast cancer screening trial, which began in two counties (W & E) in Sweden in 1977, has provided the opportunity to correlate the entire spectrum of breast pathology with 29-year patient outcome.1 The radiologic–pathologic analyses included large thin-section and large thick-section (subgross, 3D) histopathology techniques.2 The recent application of the subgross (3D) technique to total prostatectomy specimens revealed striking structural similarities between prostate and breast cancers.3 Despite many dissimilarities, these two diseases have two basic features in common. At the one extreme, the majority of cases have a low fatality rate, when early detection is combined with appropriate treatment. At the other extreme, both organs exhibit aggressive tumor forms that have a high fatality rate, regardless of all attempted therapeutic regimens. The morphologic correlation of breast images with large thin- and thick- (subgross, 3D) section histology images has resulted in a highly specific prospective differentiation of breast cancer subtypes into those with a poor outcome and those with good to excellent long-term outcome even when detected at screening.2 This work has demonstrated that the breast cancer subtypes with a high fatality are the diffuse types that are already extensive at the time of detection and appear to originate primarily in the major ducts of the breast; on the other hand, the breast cancers that originate in the milk-forming portion of the breast (so-called terminal ductal lobular units (TDLUs)) are detectable in their early phase as small tumors and have a low fatality rate. Using the 3D histology technique on more than 100 total prostatectomy specimens, a similar observation was made: the prostate cancers of apparently ductal origin (pure ductal or mixed ductal–acinar type) are highly aggressive with a poor outcome, whereas the prostate cancers of acinar origin are the less aggressive forms with a considerably better outcome.3
Our purpose in this article is to propose a new classification system with anatomically consistent terminology for breast cancer similar to that currently in use for prostate cancer. Our proposed classification for breast cancer is based on the anatomic site of origin (duct vs. TDLU), because the long-term patient outcome appears to be largely determined by the ductal vs. acinar origin of both breast and prostate cancers.
Emphasis Should be Placed on Differentiating Between the Ductal and Acinar Origin of Adenocarcinoma of the Prostate and Breast
The current terminology for prostate cancer is straightforward. “Although most adenocarcinomas of the prostate are of acinar origin, between 0.4 and 0.8% of prostatic carcinomas arise from prostatic ducts. In about 5% of prostatic carcinomas, tumors showing both ductal and acinar differentiation are found.” “Most prostatic duct adenocarcinomas have a fairly aggressive course, approximating Gleason score 8 adenocarcinomas. Prostatic duct adenocarcinomas at radical prostatectomy tend to be large, of advanced pathologic stage, and have a high risk of early postoperative recurrence.”4
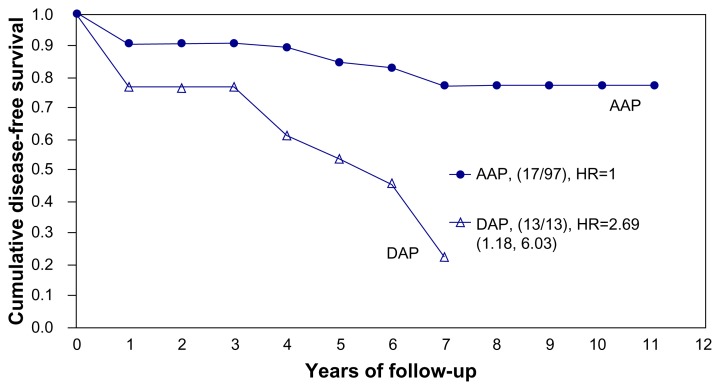
The acinar versus ductal site of origin of prostate cancer is thus reflected in the terminology and has also been recognized as being related to the outcome, in contrast to the current terminology of breast cancer. We consider the terminology used to describe prostate cancer (acinar adenocarcinoma of the prostate (AAP) and ductal adenocarcinoma of the prostate (DAP)) to be logical because it focuses on the site of origin of the disease, which also has prognostic relevance to long-term outcome.4 Our personal observations based on more than 100 consecutive prostatectomy specimens indicate that the use of subgross (3D) histology technique provides a reliable differentiation of the low-risk acinar-type from the high-risk ductal-type prostatic adenocarcinoma.3 We could also confirm that the prostate cancers of ductal origin had a significantly greater frequency of pT3a and more advanced cancers, largest tumor focus >20 mm and a high-grade PIN (prostate intraepithelial neoplasia), Gleason score ≥7, positive surgical margin, extracapsular extension, vascular invasion, seminal vesicle infiltration, biochemical/local recurrence, regional lymph node metastases, and distant metastases.3 Figure 1 shows a significantly poor disease-free survival for patients with DAP (RR 2.69, 95% CI 1.18–6.03).
Figure 1.
Cumulative disease-free survival of AAP and DAP in our series of 104 total prostatectomy patients.
The aggressive behavior of DAP could be explained by neoductgenesis occurring in the prostate, analogous to neoductgenesis in the breast.5 Although the majority of breast carcinomas are of acinar origin (developing from the TDLUs), these are still officially termed ductal carcinomas (invasive and/or in situ).
Suggestion for Improving the Current Classification of Breast Cancer
The striking similarities in the structural morphology of the breast and prostate and the similarities in outcome of the carcinomas of ductal and acinar origin in the breast and prostate, respectively, provide a rational basis for improving terminology, developing a consistent classification, fully exploiting preoperative imaging, and planning targeted therapy.
We consider the terminology used to describe prostate cancer (AAP and DAP) to be logical because it focuses on the site of origin of the disease, which also has prognostic relevance to long-term outcome.4 AAP is analogous to acinar adenocarcinoma of the breast (AAB), in which the cancer originates from the TDLUs. Similarly, prostate cancer originating from the major ducts (DAP) is analogous to the breast cancer subtypes originating from the major ducts. Our suggestion is that all forms of breast cancers originating from the TDLU should be termed AAB. Correspondingly, the breast cancer subtypes originating from the major ducts should be termed ductal adenocarcinoma of the breast (DAB). The significant difference between the long-term outcome of breast cancers of similar size (as defined by the current TNM classification) originating from the TDLUs (AAB) and the cancers originating from the major ducts (DAB) justifies this radical change in terminology.2,5–9
Inconsistencies in the Current Terminology Describing Breast Cancer
The current terminology for breast cancer has evolved from experience with large, advanced tumors having a poor overall outcome. Mammography screening has led to the detection of breast cancer at earlier phases of development, challenging physicians with an unprecedented, large number of non-palpable breast cancers. Many innovations have been made to appropriately diagnose and treat these early breast cancers, but few changes have been made in terminology. The current TNM classification system was created before the widespread use of mammography screening and understandably has shortcomings in describing these small, early-phase tumors. These limit its usefulness in predicting the long-term outcome of individual patients with screen-detected, non-palpable breast cancers.6,7,9 The following examples illustrate some of these shortcomings.
Example 1
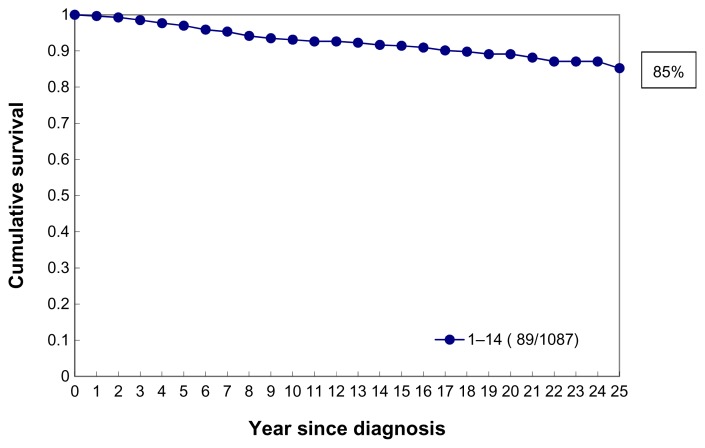
Demonstration of the limitations of the current TNM classification in predicting the long-term outcome of five early (T1ab) breast cancer cases. In the 1–14 mm tumor size range, which includes these five examples, the 25-year disease-specific survival curve (Fig. 2) will be an aggregate of five diverse survival curves, representing five separate breast cancer subtypes.
Figure 2.
Cumulative 25-year survival of 1,087 consecutive 40–69-year-old women diagnosed with 1–14 mm invasive breast cancer during 1977–2005 in Dalarna County, Sweden.
The outcome can be more specifically predicted with the inclusion of the following mammographic tumor features:
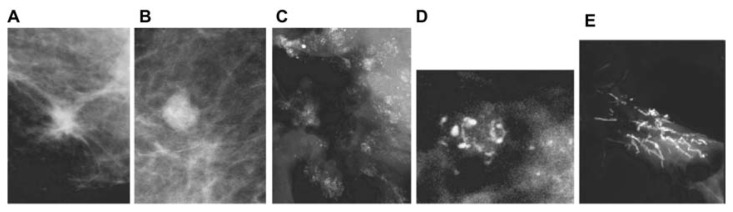
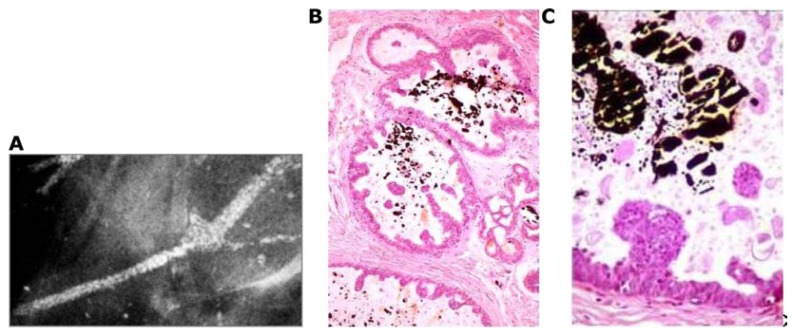
a solitary stellate lesion on the mammogram, diagnosed as a 9 × 9 mm solitary tubular carcinoma at histology (Fig. 3a);
a solitary circular lesion on the mammogram, diagnosed as a 9 × 9 mm solitary, moderately differentiated invasive “ductal” carcinoma at histology (Fig. 3b);
multiple clusters of powdery calcifications on the mammogram, diagnosed at histology as Grade 1 in situ cancer associated with a 9 × 10 mm well-differentiated invasive “ductal” carcinoma (Fig. 3c);
a cluster of crushed stone-like/pleomorphic calcifications on the mammogram, diagnosed as Grade 2 in situ cancer at histology, associated with a 8 × 10 mm moderately differentiated invasive “ductal” carcinoma (Fig. 3d); and
fragmented (Fig. 3e) or dotted casting-type calcifications on the mammogram, diagnosed as Grade 3 in situ cancer at histology, associated with a 9 × 9 mm moderately differentiated invasive “ductal” carcinoma.
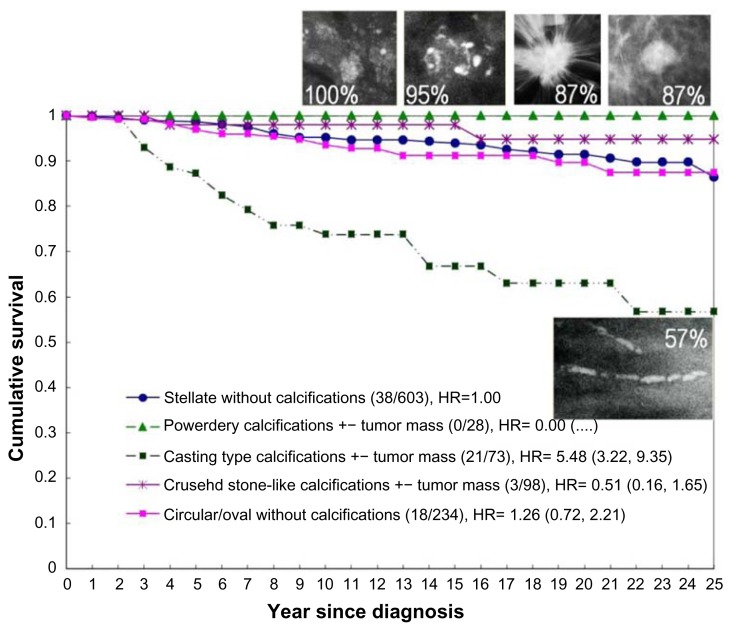
Figure 3.
The five basic mammography tumor features.
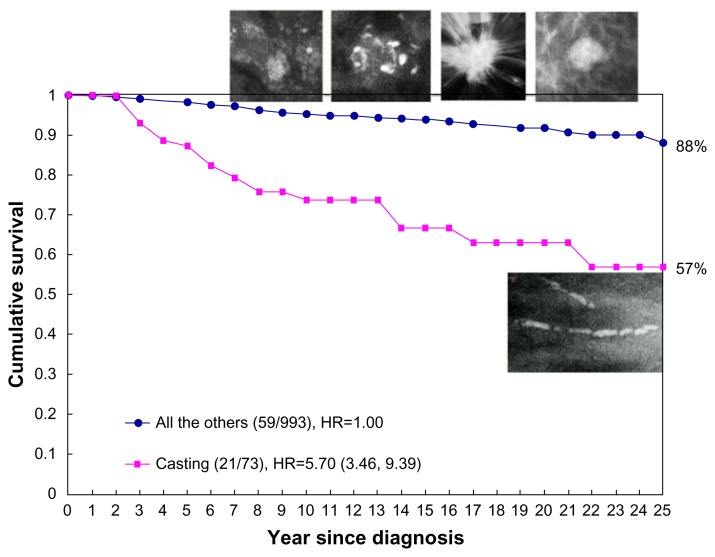
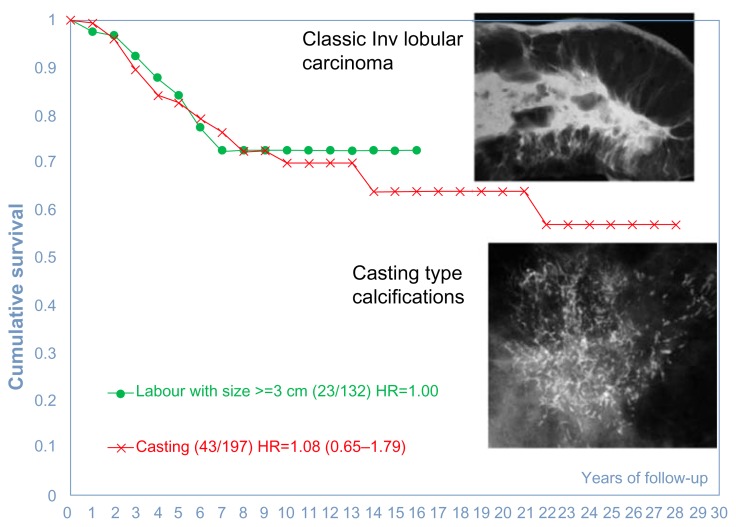
Because none of these five examples had lymph node metastases, the current TNM classification places each of the tumors in Figure 3a–e into the T1b category. Inclusion of the mammographic tumor features (illustrated in Figure 3a–e) considerably improves our ability to distinguish the breast cancer subtype with poor outcome (Fig. 3e, tumors with casting-type calcifications on the mammogram) from the remaining cancers with good long-term aggregate survival (Fig. 3a–d, tumors not having casting-type calcifications). The comparison of the significantly different outcome is shown in Figure 4.
Figure 4.
Cumulative 25-year survival of 1,087 consecutive 40–69 year old women diagnosed with 1–14 mm invasive breast cancers according to the presence or absence of casting-type calcifications on the mammogram during 1977–2005 in Dalarna County, Sweden.
The long-term outcome of each of the five breast cancer subtypes determined according to the mammographic tumor features is illustrated in Figure 5.
Figure 5.
Cumulative 25-year survival of 1,087 consecutive 40–69-year-old women having 1–14 mm invasive breast cancers according to the mammographic tumor features during 1977–2005 in Dalarna County, Sweden.
Breast Cancers of Acinar Origin (AAB)
In situ AAB
As has been pointed out by Jensen et al., “ductal carcinoma in situ of the human breast is of lobular origin. Progressive distension of ductules with dysplastic and anaplastic cellular elements leads to unfolding and coalescence of the ductules within the lobule to form larger ovoid structures. Such lesions falsely appear to be small ducts in conventional histology slides.”10 These observations were made with the help of subgross, 3D histology specimens. Using the same methodology, we concur that the majority of in situ carcinomas originate within the acini of the TDLUs, but are unfortunately still termed ductal carcinoma in situ (DCIS). This nomenclature is misleading and anatomically incorrect as noted above10; it is also an unfortunate misnomer because malignancies that actually originate within the major ducts have a much worse outcome (Figs. 4 and 5). Our suggested terminology for in situ carcinomas originating within the TDLUs is Grade 1 or 2 in situ AAB.
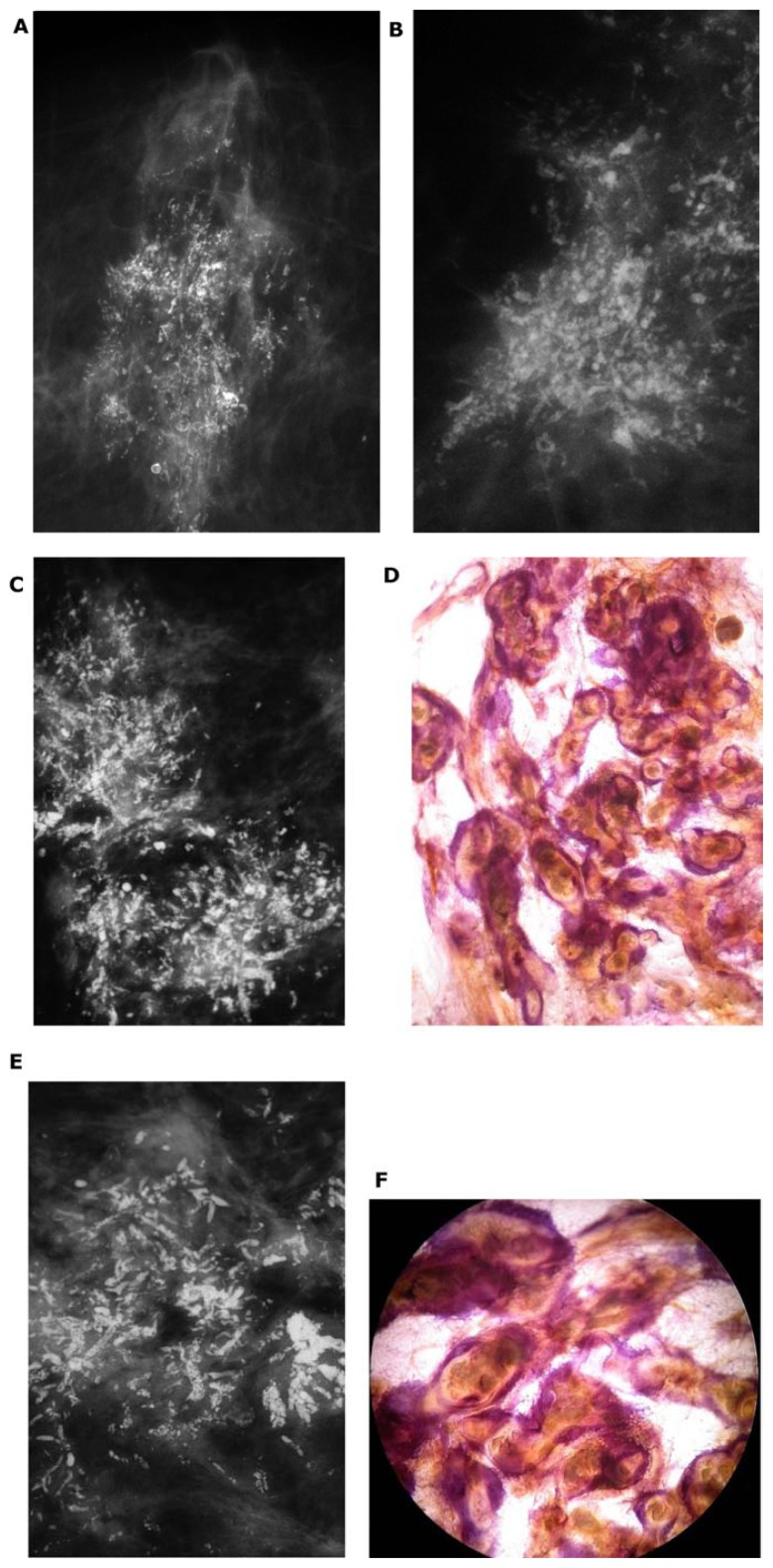
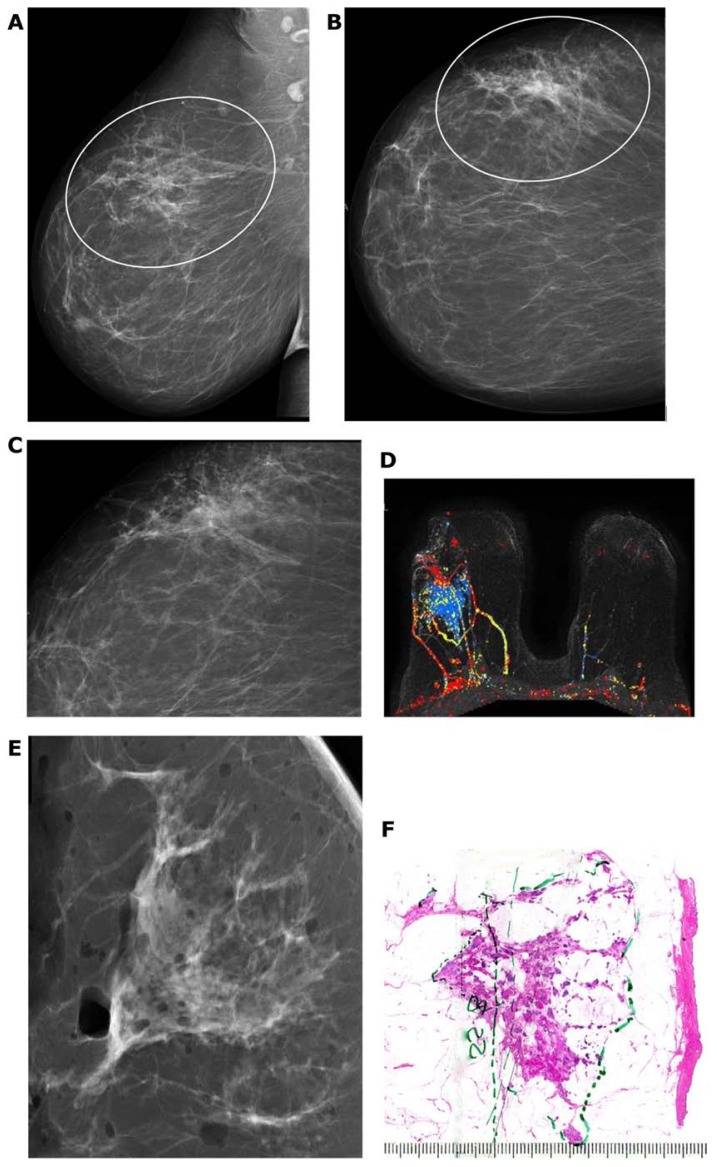
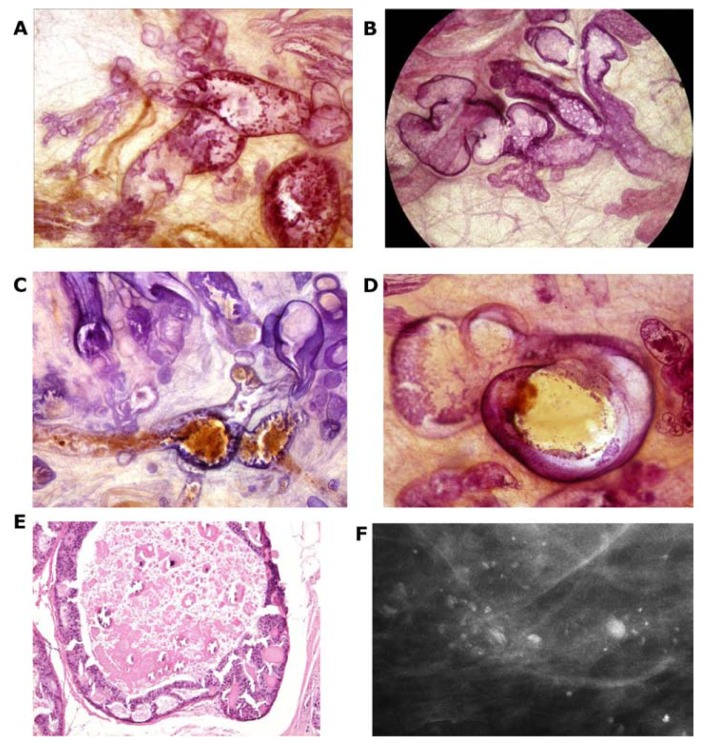
The development of Grade 2 carcinoma in situ within the TDLU, shown in Figure 6a–f, is reflected on the mammograms as single or multiple clusters of crushed stone-like microcalcifications (Figs. 7a,c and 8a). The mammographic—histologic correlation of such a case is presented in Figures 7 and 8.
Figure 6.
(A–F) Computer simulation images of the development of Grade 2 in situ carcinoma within the TDLU. The lobule gradually becomes distended and deformed. Calcifications are formed within the necrotic debris and are seen on the mammogram as crushed stone-like/pleomorphic calcifications. (Artwork from Ref. 8. Copyright by Georg Thieme Verlag. Used with permission).
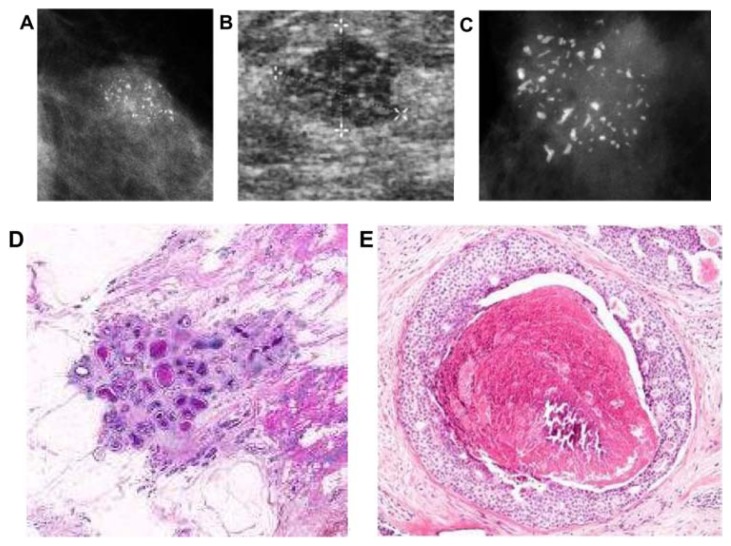
Figure 7.
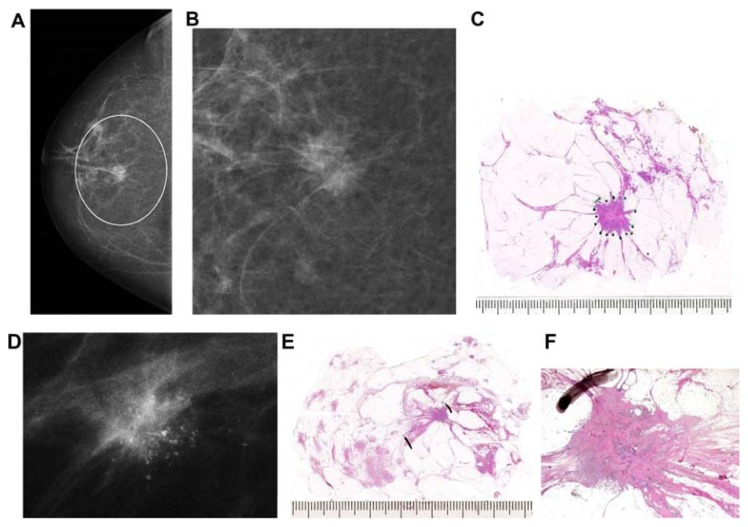
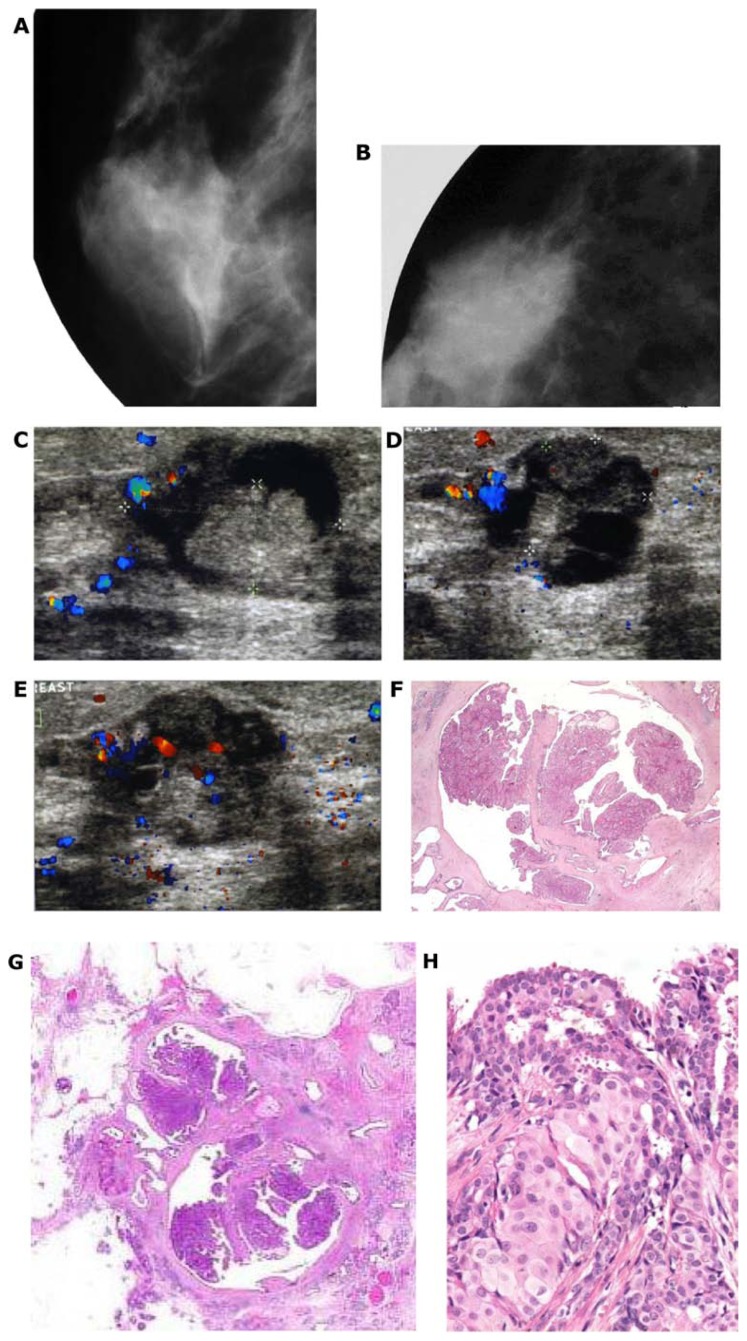
(A) A solitary cluster of crushed stone-like (pleomorphic) calcifications was detected at mammography screening. (B) Ultrasound examination: a 12-mm solitary hypoechogenic area with calcifications, corresponding to a distended TDLU. (C) Microfocus magnification specimen radiograph: malignant-type calcifications in a cluster. (D) Low power large section histology image of the solitary TDLU, distended and distorted by the Grade 2 in situ carcinoma. (E) Higher power magnification of a single acinus: solid and cribriform cancer in situ with central necrosis and amorphous calcification distending the acinus. Histologic diagnosis: Grade 2 in situ carcinoma within the TDLU (unifocal AAB).
Figure 8.
(A) Mammographically detected solitary cluster of crushed stone-like calcifications with no associated tumor mass. (B) Thick-section (3D) histology image: the individual acinus is distended and each measures approximately 1 mm (which corresponds to the size of an entire normal TDLU).This remarkable growth is the result of increased intraluminal pressure because of accumulation of cancer cells, intraluminal necrosis, and amorphous calcification. (C) Low power histology image of a segment of one distended acinus showing cribriform cell-architecture, intraluminal necrosis, and a central amorphous calcification. Histologic diagnosis: Grade 2 in situ carcinoma localized to a single TDLU. Note that the malignant process is confined to a TDLU and is not within the ducts, so that it is not “ductal” carcinoma in situ (DCIS).
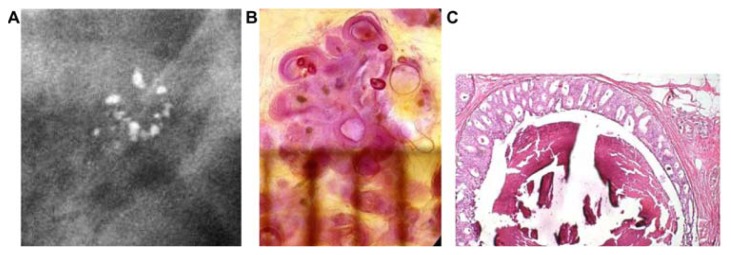
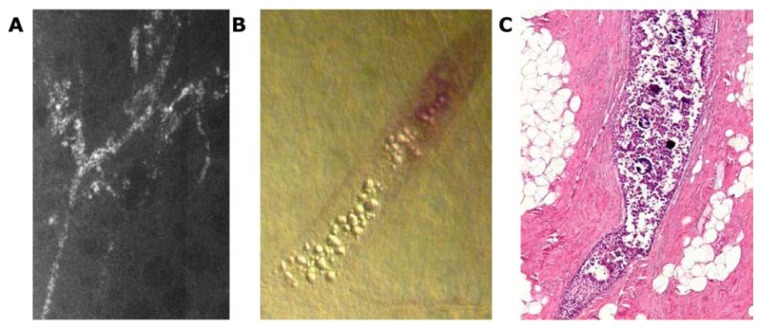
The development of Grade 1 carcinoma in situ within the TDLUs, shown in Figure 9a–h, is reflected on the mammograms as multiple clusters of powdery/cotton ball-like microcalcifications (Fig. 9a and b). The mammographic—histologic correlation of such a case is presented in Figure 9a–h.
Figure 9.
Screen-detected multiple clusters of powdery, dust-like calcifications; specimen radiograph with and without microfocus magnification (A,B). Thick-section (3D) histology images (C,D) demonstrate that the calcifications are localized within distended TDLUs. Low (E,G,H) and intermediate (F) power histology images: low-grade cancer in situ localized to the TDLUs. The powdery calcifications seen on the mammograms correspond to psammoma body-like calcifications. Histology: Grade 1 in situ carcinoma localized to the TDLU (not “ductal” carcinoma in situ).
Invasive AAB
The in situ tumors of acinar origin progress to unifocal or multifocal 1–9 mm stellate/spiculated or circular/oval-shaped invasive cancers. This group includes tumors conventionally termed tubular cancer, well/moderately/poorly differentiated invasive “ductal” carcinoma NOS (not otherwise specified), mucinous carcinoma, invasive cribriform carcinoma, basal-like breast carcinoma, solid form of invasive lobular carcinoma, tubulolobular cancer, etc. Because these cancers all originate from the TDLU, calling them “invasive ductal carcinoma” is misleading and may also lead to overtreatment of early stage breast cancer. Our suggested terminology is well/moderately/poorly differentiated invasive AAB. The combination of the mammographic tumor features, biomarkers, and histologic tumor distribution (unifocal, multifocal, diffuse) would considerably assist in further characterization of the malignant process.
Early detection of breast cancer at a small (1–14 mm) tumor size does not guarantee that the disease will be localized to a small tissue volume, because multifocal infiltrating breast cancers comprise a considerable portion of breast malignancies (invasive and in situ combined).11–13 The invasive breast cancers originating from the TDLUs can appear on the mammogram as one of the following:
a unifocal stellate or circular tumor without associated microcalcifications, corresponding to a unifocal stellate or circular/oval-shaped invasive breast cancer without associated extratumoral in situ carcinoma at histologic examination (Fig. 10a–c);
a unifocal stellate or circular tumor on the mammogram with associated crushed stone-like and/or powdery microcalcifications, representing the in situ AAB components, when the histologic examination reveals a solitary invasive breast cancer with associated extratumoral Grade 1 or 2 in situ carcinoma (Fig. 10d–f);
multiple stellate or circular tumors on the mammogram, corresponding to multifocal invasive breast cancer without associated extratumoral in situ carcinoma at histologic examination (Fig. 11a–l); and
multiple stellate or circular tumors on the mammogram with associated crushed stone-like and/or powdery microcalcifications, representing the in situ AAB components, when the histologic examination reveals multifocal invasive breast cancers with associated extratumoral Grade 1 or 2 in situ carcinoma (Fig. 12a–f).
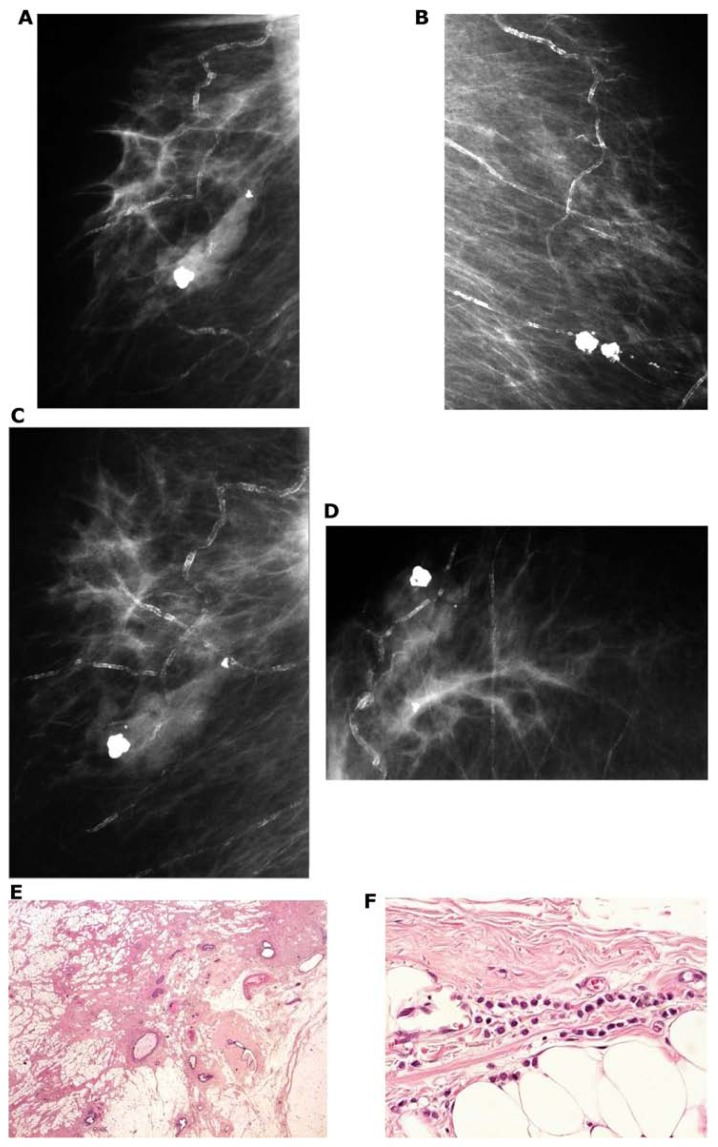
Figure 10.
Right breast, craniocaudal projection (A) and microfocus magnification (B). There is a centrally located solitary stellate malignant tumor with no associated microcalcifications. Large thin-section histology image (C) confirms the 15 × 13 mm unifocal invasive cancer with no extratumoral in situ components. A solitary <10 mm stellate tumor with associated malignant-type microcalcifications (D). Large section (E) and low power histology (F) images demonstrate the single focus invasive cancer associated with low-grade cancer in situ within and outside the invasive tumor.
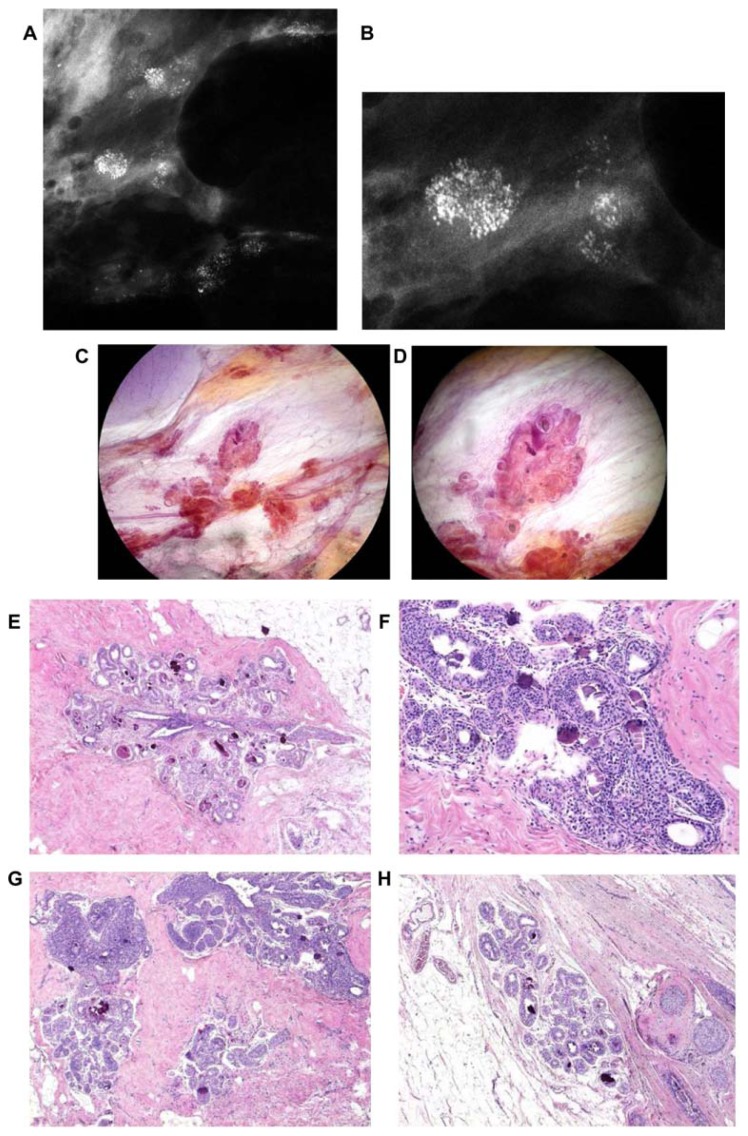
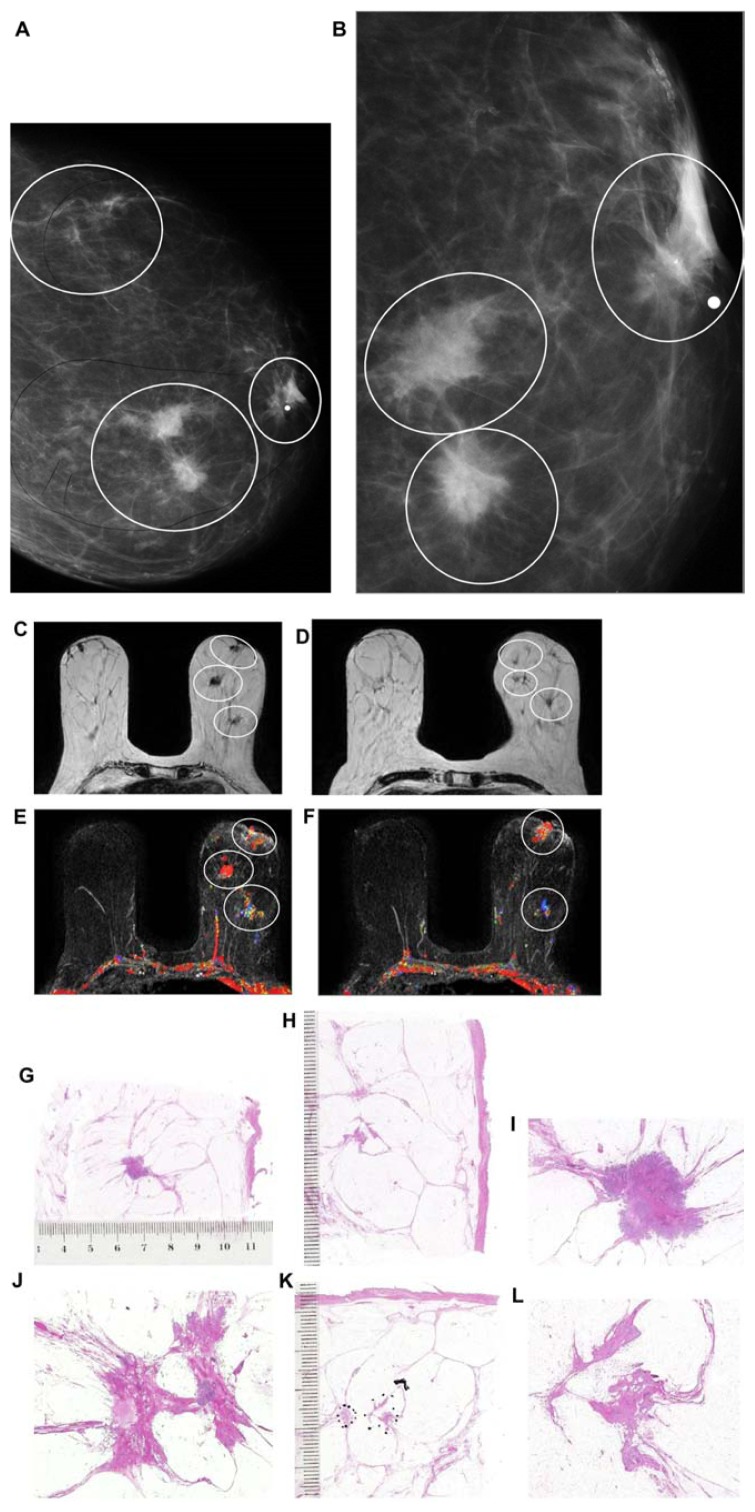
Figure 11.
Left breast mediolateral oblique projection (A) and microfocus magnification (B) shows multifocal stellate malignant tumors with no associated malignant-type calcifications. Breast MRI (C–F) demonstrates the presence of multifocal invasive carcinomas spread over a >10 cm area between the nipple and the chest wall. Multiple large thin-section histology images confirm multifocal (seven foci) invasive lobular carcinoma (the largest focus measuring 11 × 10 mm), ER/PR positive, HER2-negative invasive cancer with low proliferation index (Ki-67: 5%), and no lymph vessel invasion (LVI). Histologic examination of 16 surgically removed axillary lymph nodes showed no metastases (pN0/16).
Figure 12.
Microfocus magnification images on the craniocaudal and the lateromedial horizontal projections (A,B) demonstrating ill-defined circular lesions bridged together, associated with crushed stone-like, malignant-type calcifications. Mastectomy specimen slice radiographs (C,E) and large thin-section histology images confirm the presence of 20 invasive mucinous cancer foci (the largest focus measuring 15 × 15 mm) associated with 65 × 40 mm Grade 2 in situ carcinoma, pNX. LVI was present.
Large section histology examination of 1,000 consecutive breast cancers13 showed that approximately 40% of the early invasive breast cancer cases were unifocal and 34% multifocal. We consider these two groups, comprising 74% of the material, to have originated from the TDLUs, and propose that they be termed unifocal and multifocal AAB, respectively. The unifocal 1–9 mm stellate or circular invasive AAB cases are primarily surgical diseases with excellent 26-year survival, even without adjuvant therapy.14 The remaining 26% of invasive breast malignancies were diffusely invasive breast cancers, which we consider to have originated from the major ducts, DAB. Breast MRI is essential for the preoperative assessment of tumor distribution and disease extent.
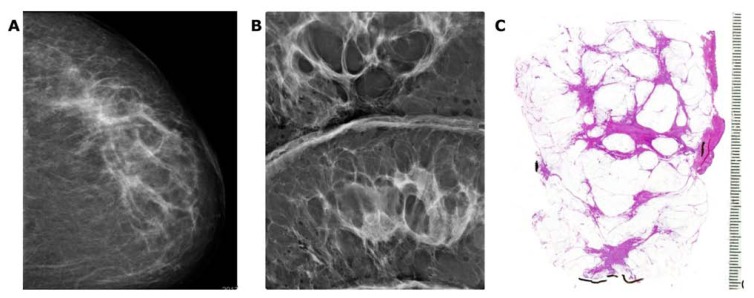
The TNM classification ignores the multifocal and diffusely infiltrating forms of breast cancer. Unfortunately, the TNM classification does not take multifocality into account; instead, it considers the diameter of only the largest invasive tumor focus. This omission results in, for example, a solitary, 9 × 9 mm invasive carcinoma being classified into the same size category as cases with multiple tumor foci having the largest focus of 9 × 9 mm, regardless of the number and extent of the other foci present. Because multifocal invasive cancers contain a considerably larger tumor burden, they have a significantly higher frequency of lymph node and lymph vessel involvement,15–18 an increased local and distant metastasis rate,19 and most importantly, a significantly higher disease specific fatality.20–24 Four recent studies have come to similar conclusions: lesion distribution and disease extent represent important independent survival-related prognostic parameters in breast carcinoma;21 multicentric/multifocal BC is an independent BC risk factor and should be included in the risk assessment by re-evaluating the current TNM classification by the UICC;22 and multifocality is a negative prognostic factor, independent of tumor size, although its effect becomes more significant with the increasing tumor size.22,23 Our data suggest that multifocal invasive breast cancer is associated with inferior survival versus survival in patients with unifocal breast cancer, stage by stage.24 Multifocal cancers originating from the TDLU are often separated from each other by intervening normal breast tissue. As can be seen in Figure 13a–e, the determination of a “free margin” at histologic examination does not guarantee that there will be no residual tumor foci remaining in the breast. Such residual foci could be responsible for “recurrence” at future follow-up showing poor outcome. The current practice for margin determination has considerable limitations, because <20% of the removed tissue margin is examined when using the small section histology technique.25 The presence of intervening normal tissue among the cancer foci in AAB cases and the obvious limitations of the small section histology technique make the margin determination in AAB cases at best unreliable. The use of large section histology technique, correlated with the preoperative breast MRI and specimen radiographs, greatly increases the description of the true extent of the disease and increases the reliability of the margin determination (Fig. 13a–c).
Figure 13.
Breast MRI showing a large number of individual foci within the right breast (A,B). Radiograph of a 5 mm thick mastectomy specimen slice (C) containing some of the invasive foci. Large thick-section histopathology slices (D,E) demonstrate the normal breast tissue surrounding the individual invasive breast cancer foci. The dashed lines indicate possible “clean” surgical margins.
Correlation with outcome
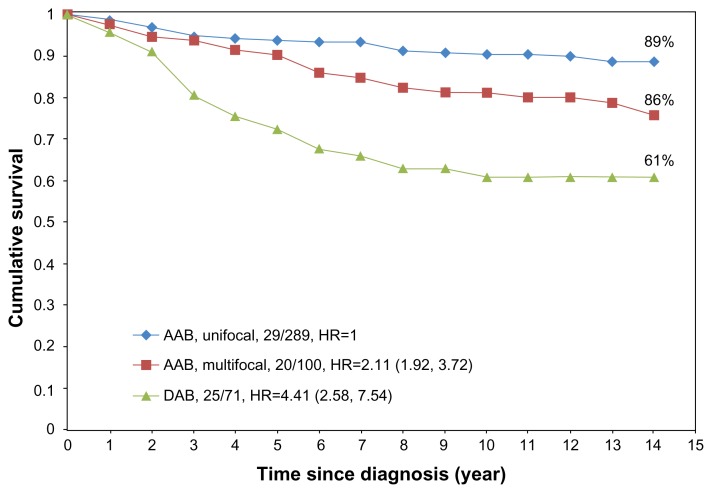
The failure to take multifocality into account places the unifocal tumors having excellent long-term prognosis into the same TNM category as the multifocal tumors having the same maximum individual tumor size, but with a considerably large tumor burden. The 10-year disease specific fatality rate of the multifocal AAB cases is 2.2 times greater than that of the unifocal cancers, presumably because of a larger tumor burden.6,12,21–23 The 10-year fatality rate of patients with diffusely invasive breast cancer is even worse, 2.9 times greater than that of unifocal invasive cancers.21,23
Further follow-up confirms these findings. The 14-year disease-specific survival of unifocal, multifocal, and diffuse breast cancer cases is shown in Figure 14.
Figure 14.
14-year disease-specific survival of breast cancer patients with unifocal (89%), multifocal (76%), and diffuse (61%) breast cancer diagnosed during 1996–1998 in Dalarna county, Sweden.
The four subgroups with good to excellent long-term survival (Figs. 4 and 5) share a common feature in that they all appear to originate from the acini of the TDLU23 and do not originate from the major ducts. In the four mammographically defined subtypes (Fig. 3a–d) that originate within the TDLUs, the use of the term “ductal” carcinoma (both in situ and invasive) leads to confusion.
Breast Cancers of Ductal Origin (DAB)
Breast cancer subtypes originating from the major ducts (DAB) have entirely different mammographic/3D histologic/conventional histologic presentations and outcomes compared to malignancies originating from the TDLUs (AAB) (Figs. 4 and 5). The diffusely infiltrating cancers of ductal origin, approximately 26% of all breast cancers, have a fatality rate 2.9 times higher than the unifocal AAB carcinomas originating from the TDLU.21 Breast cancers of ductal origin (DAB) and the diffusely infiltrating invasive lobular carcinoma can be grouped into eight subgroups having a similarly poor long-term outcome.
Subgroups DAB 1 and 2
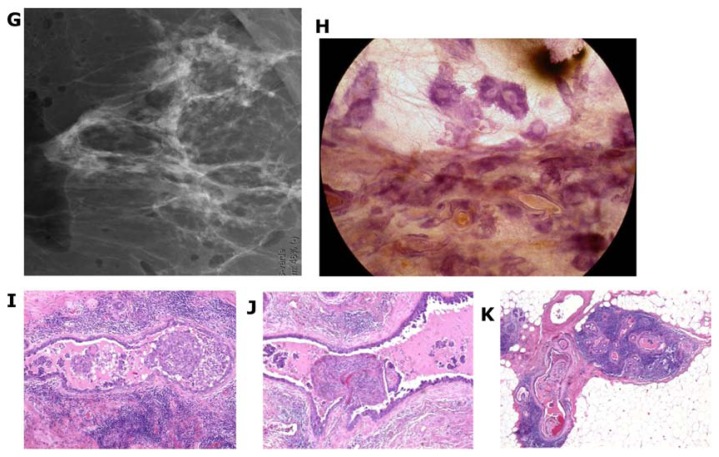
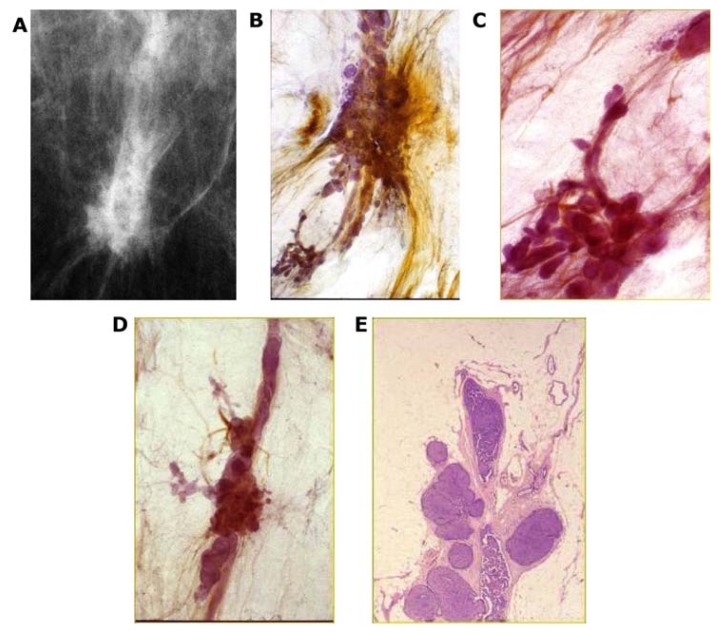
Two subgroups originate from the major ducts and present with central necrosis as the distinguishing feature. The carcinoma cells are predominantly of Grade 3 with mammographically demonstrable amorphous calcifications within the central necrosis. The mammographic presentation reflects the cell architecture: the fragmented casting-type calcifications are associated with predominantly solid cell proliferation (Fig. 15a–c) and the dotted casting-type calcifications are associated with micropapillary cell-architecture undergoing necrosis (Fig. 16a–c). The two subtypes are the archetypical examples of “neoductgenesis/duct forming poorly differentiated invasive breast carcinoma” with extensive necrosis (often erroneously called Grade 3 “DCIS”).5,6
Figure 15.
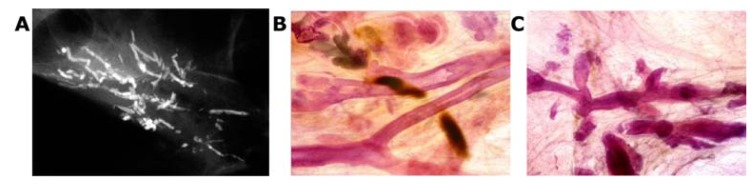
Casting-type calcifications on the mammogram (A). Large thick-section (3D) histology images (B,C) demonstrate solid cell proliferation, intraluminal necrosis, and amorphous calcifications. Histology: Grade 3 carcinoma in situ in the major ducts.
Figure 16.
Dotted casting-type calcifications on the mammogram (A) correspond to the calcifications of necrotic micropapillary cancer in situ developing within the large ducts. The micropapillary growths break off and calcify in the lumen, resulting in the individual dots of the dotted casting-type calcifications (B,C).
In these subtypes, the high-grade cancer cells originate from the major ducts,5 hence the name DAB. We have proposed that this high-grade malignancy, while resembling in situ cancer histologically, often acts as an advanced, poorly differentiated invasive carcinoma.5,6 Study of the large section, subgross (3D) histology specimens brings us closer to understanding why this disease subtype can be highly fatal. The large, thick-section histology image graphically demonstrates the unnaturally high density of tightly packed, tortuous, cancer-filled duct-like structures, which have no associated TDLUs, but are surrounded by a thick desmoplastic reaction and lymphocytic infiltration, indicating a strong immunologic reaction (Figs. 17d,f,g and 23k). The combination of these aberrant features strongly suggests that these duct-like structures are not the patient’s preexisting ducts; instead, they are newly formed, a phenomenon we have termed neoductgenesis.5,6 Our correlative studies suggest that the new duct formation (neoductgenesis) is produced by the ductal stem cells that have undergone malignant transformation, causing them to produce abnormal, tortuous duct-like structures populated by malignant cells and lacking TDLUs.
Figure 17.
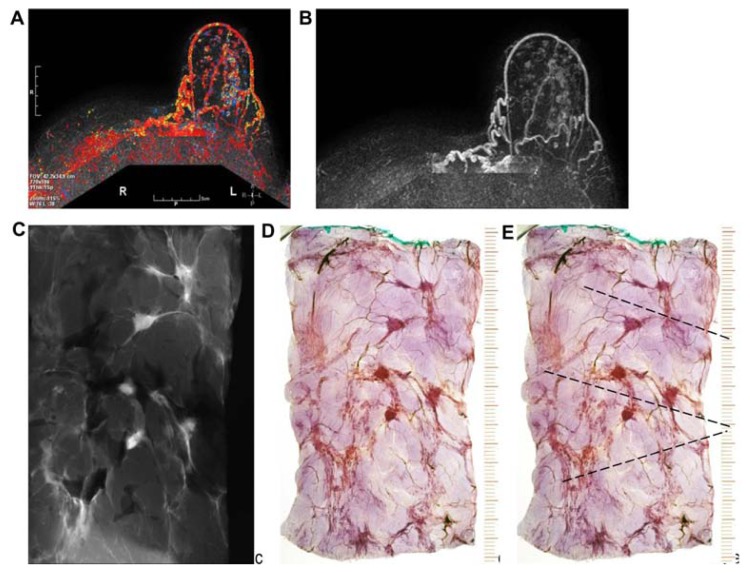
Details of right breast mammograms, craniocaudal (A) and mediolateral oblique (B) projections. A mixture of fragmented and dotted casting-type calcifications is seen throughout an entire lobe, starting in the nipple, outlining abnormally large number of ducts and branches toward the chest wall. The radiographs of the mastectomy specimen slices are compared with thick-section (3D) histology images (C–H). These demonstrate a large number of cancer-filled duct-like structures that have unnatural appearance, and are surrounded by thick desmoplastic reaction and lymphocytic infiltration.
Figure 23.
A–K Mammographically detected architectural distortion in the upper-outer quadrant of the right breast with no associated microcalcifications. There is also a pathologic lymph node in the right axilla (A,B). Microfocus magnification (C) and breast MRI (D) demonstrate the large extent of the disease. Specimen slice radiographs (E,G) compared with large thin-section (F) and large thick-section histology (H) images show the cancer-filled, tortuous, newly formed ducts tightly packed together. Low power histology images (I,J,K) demonstrate that the abnormal, cancer-filled, duct-like structures with micropapillary cell architecture are surrounded by a thick desmoplastic reaction and lymphocytic infiltration, which are the histologic signs of neoductgenesis.
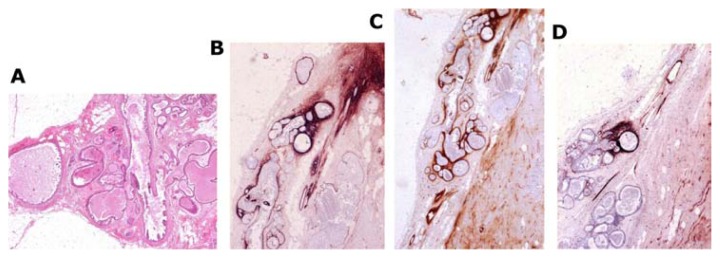
Tenascin staining showing overexpression (Fig. 18b–d) demonstrates that the epithelial cells actually penetrate into the surrounding tissue.5,6,26
Figure 18.
Large thin-section histology image (A) shows abnormal, contorted ducts with “micropapillary cancer in situ.” The accumulated fluid distends the ducts and branches. Tenascin staining (B–D) demonstrates overexpression, indicating that the torturous, duct-like structures correspond to neoductgenesis.
This breast cancer subtype should be regarded as a special form of invasive breast cancer: a poorly differentiated, duct-forming invasive carcinoma. The enormous tumor burden of poorly differentiated cancer cells and the frequent presence of lymph and blood vessel invasion can explain the high fatality rate of this subtype.
Subgroups DAB 3 and 4
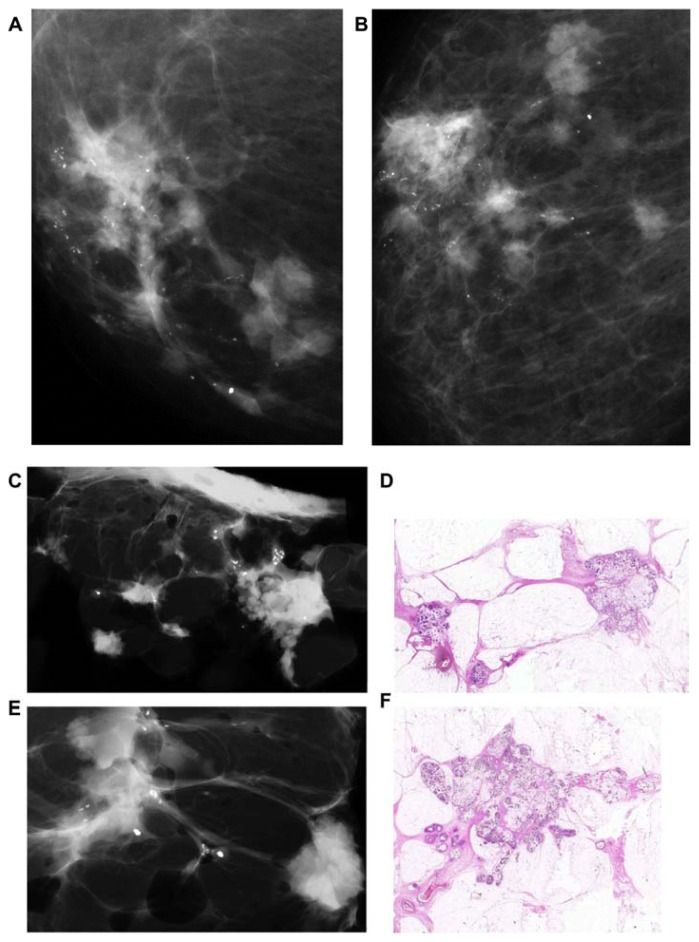
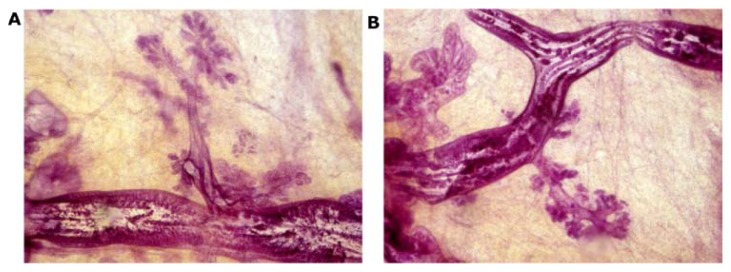
Two additional subgroups of in situ carcinoma can develop within the major ducts, in which fluid production dominates the picture, rather than necrosis. The fluid-producing Grades 1–3 carcinoma cells show micropapillary and/or cribriform architecture (Figs. 19a,b, 20a–f, and 21a–c). The clinical finding may be a palpable thickening corresponding to an increased number of cancerous, fluid-filled ducts. Nipple discharge may be present without demonstrable microcalcifications on the mammogram. In these cases, the galactogram and breast MRI can demonstrate the true extent of the disease. In cases with no nipple discharge, skipping stone-like calcifications can be shown on the mammograms (Fig. 20f).
Figure 19.
(A,B) The diffuse, fluid-producing micropapillary DCIS foci are seen on the pleats of the major ducts. The associated TDLUs are normal. The fluid distends the ducts and branches and either serous or bloody nipple discharge will be observed, or/and skipping stone-like calcifications will be formed in the gradually concentrating fluid.
Figure 20.
Thick-section (3D) histology images of micropapillary (A) and cribriform (B) in situ carcinoma in major ducts. The protein-rich fluid distends the ducts and branches (C,D). Over time, skipping stone-like oval-shaped, smoothly contoured calcifications will be formed in the viscous fluid (F). These will be seen diffusely scattered within a lobe (E).
Figure 21.
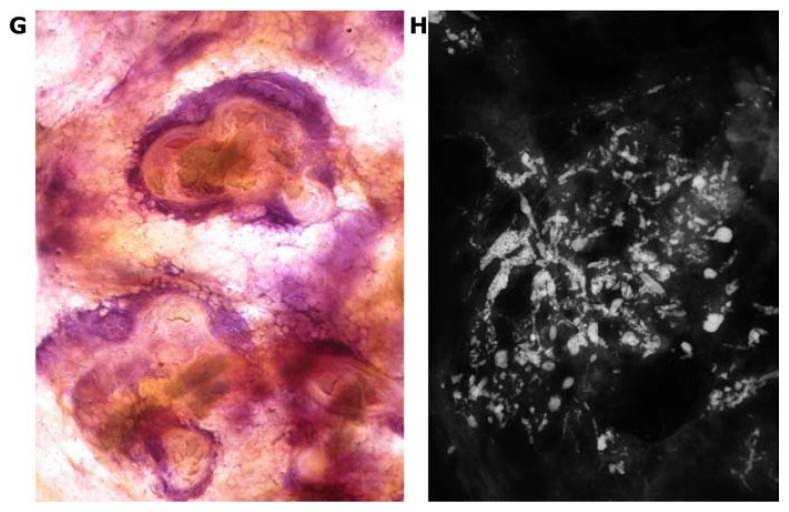
Large psammoma body-like calcifications outline the duct and its branches on the mammogram (A). 3D histology (B) and low power thin-section histology (C) images show this Grade 1 in situ carcinoma localized within the duct.
Subgroup DAB 4 is different from the characteristic histologic and imaging features of subgroups DAB 1 and 2. This subtype is also a fluid-producing in situ cancer that develops in the major ducts, but the histologic malignancy grade is Grade 1 or 2 and is associated with large psammoma body-like calcifications. These are seen on the mammograms as pearl necklace-like calcifications (Fig. 21a–c).
Subgroup DAB 5
In this subgroup of neoductgenesis, the mammograms show architectural distortion without demonstrable microcalcifications (Figs. 22a–e and 23a–k). These cases usually present as a palpable “thickening” corresponding to the region with the mammographic finding. Large section histology reveals a concentration of tortuous, cancer-filled abnormal duct-like structures, giving a striking appearance. Central necrosis is occasionally present with or without histologically demonstrable amorphous calcifications, whereas in other cases only fluid accumulation is seen within the cancerous ducts (Fig. 23h–k). The following two cases are representative of this subgroup.
Figure 22.
Palpable asymmetric density with architectural distortion on the mammogram (A). Large thick-section (3D) (B–D) and large thin-section (E) histology images demonstrate the corresponding duct-like structures distended by cancer cells with solid cell architecture. Histology: solid carcinoma in situ in major ducts.
The first case of extensive neoductgenesis without associated calcifications is demonstrated in Figure 22a–e.
The second of these two cases is a 74-year-old asymptomatic woman who was called back from mammography screening to assess the newly developed asymmetric density with architectural distortion in the upper-outer quadrant of her right breast.
Comment
These unnatural ducts have no associated TDLUs; the cancer cells fill newly formed ducts through the process of neoductgenesis. This is an example of “duct forming invasive carcinoma.” Because the tumor cells are not filling pre-existing ducts, this is not an “in situ cancer.”
Subgroup DAB 6
In addition to neoductgenesis, the diffuse breast cancer cases of ductal origin often contain an additional invasive breast cancer of the AAB type, coexisting within the same lobe. This common invasive carcinoma originating from the TDLU, stellate or circular with ill-defined borders, is in the majority of cases a moderately differentiated cancer with favorable biomarkers (ER positive, PR positive, HER2 negative, etc.), and is traditionally presumed to determine the patient’s prognosis. However, the associated DAB tumor is usually a large, poorly differentiated duct forming invasive carcinoma (most probably, a stem cell cancer), but is conventionally termed Grade 3 DCIS. The current TNM classification system focuses only on the invasive AAB component (T1a–c) and fails to recognize the potentially fatal nature of the associated poorly differentiated, duct-forming carcinoma presented on the mammogram with the features detailed above (Subgroups DAB 1–6). The poor survival statistics of these cases can in no way be attributed to an associated small AAB invasive tumor with favorable prognostic features. In these neoductgenesis cases, there is a striking discrepancy between the favorable TNM classification (T1a and T1b) and the unpredictable, often fatal long-term outcome. This inadequacy of the current classification system necessitates a simpler yet more predictive terminology emphasizing the site of origin (acinar vs. ductal) that determines the tumor appearance on imaging. This information should be added to the standard histopathologic description, including biomarkers. The long-term disease-specific survival curves based on the mammographic tumor features (supported by large thin- and thick-section histology) clearly show that the distinction between acinar and ductal origin of breast cancer has profound clinical relevance. In the classification of early breast cancers, the anatomic site of origin of the cancer cells has greater relevance to patient outcome (Fig. 2c) than do histologic prognostic factors.2,27–29
Comment
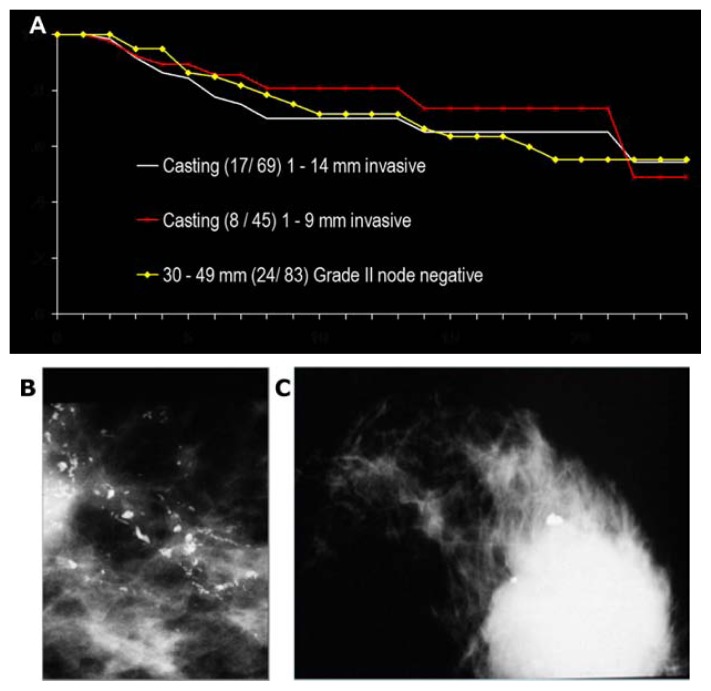
The breast cancers of ductal origin exhibiting casting-type calcifications on the mammogram (either fragmented or dotted) with associated 1–9 mm/10–14 mm invasive breast cancer have a remarkably poor outcome, similar to that of unifocal invasive AAB tumors measuring 30–50 mm (Fig. 24).
Figure 24.
Comparison of the 24-year survival curves (A) of women with 1–9 mm/1–14 mm invasive breast cancer according to the TNM classification, when the mammogram also shows extensive casting-type calcifications (B) corresponding to Grade 3 in situ carcinoma with women having moderately differentiated node-negative 30–49 mm AAB breast cancer (C). Data from Falun Central Hospital, Sweden.
Subgroup DAB 7
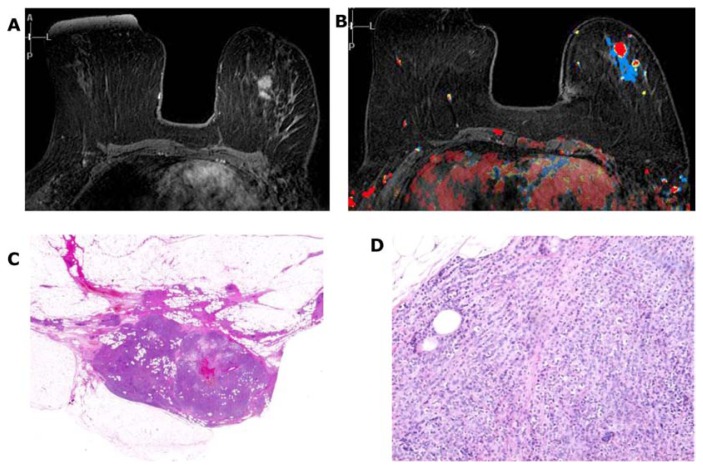
A special subgroup of intracystic papillary cancer also develops in the larger ducts (Fig. 25).
Figure 25.
Spot microfocus magnification mammograms of a right retroareolar low-density, oval-shaped lesion (A,B). Hand-held ultrasound examination (C–E) reveals a vascular intracystic growth. Histology images (F–H) show malignant intracystic tumor.
A special subgroup of diffuse invasive lobular carcinoma (subgroup DAB 8) with mammographic features and a poor outcome is reminiscent of subgroups DAB 1–6. An important subset of the diffuse invasive breast cancers is the large invasive lobular carcinoma, which presents on the mammogram with architectural distortion, reminiscent of a “spider’s web.” Figure 26 illustrates a characteristic example of this subgroup. A 78-year-old woman noticed a gradual deformation and shrinkage of her right breast. Clinical breast examination confirmed a large, hard tumor with skin changes and palpable axillary lymph nodes.
Figure 26.
Right (A) and left (B) mediolateral oblique mammograms. There is a large region with spider’s web-like architectural distortion with no central tumor mass throughout much of the right breast, best demonstrated on the mediolateral (C) and craniocaudal (D) microfocus magnification images. There are also bilateral calcified fibroadenomas. (E,F) Low and high power histology images of this “diffuse invasive lobular carcinoma.”
The poor long-term outcome of the diffuse subtype of invasive lobular carcinoma30 justifies differentiating it from three other invasive lobular carcinoma subtypes. Further justification for differentiating the diffuse form of invasive lobular carcinoma from the “solid,” “tubulolobular,” and “alveolar” type of invasive lobular carcinoma comes from its distinct mammographic presentation and the considerable poorer long-term outcome:
-
Mammographic appearance
The diffuse invasive lobular carcinoma has the mammographic presentation of extensive architectural distortion with no central tumor mass and usually occupies an entire lobe. This appearance is remarkably similar to the DAB subtypes described above (Figs. 26a–f and 27a–c).
The solid invasive lobular carcinoma has a circular/oval-shaped mammographic/ultrasound/MRI image (Fig. 28a–d).
The solid subtype of invasive lobular cancer is a spherical lesion, having a circular/oval shape on breast imaging. The breast MRI pictures (a, b) are compared in this case with the low and intermediate power large thin-section histology images (c, d).
The tubulolobular carcinoma is either a unifocal or a multifocal stellate lesion on the mammogram (Figs. 11a–l and 29a–c).
The alveolar type of invasive lobular carcinoma is most frequently occult at mammography, or can be seen as a subtle equivocal asymmetric density.
-
Site of origin
The solid, the tubulolobular, and the alveolar subtypes of invasive lobular carcinoma appear to originate from the TDLUs and all should be considered AAB.
The diffuse subtype of invasive lobular carcinoma, appearing on the mammogram as architectural distortion, is a diffusely infiltrating breast cancer from the outset, as are the breast cancers of ductal origin. Lacking calcifications and a central tumor mass, these malignancies are notoriously difficult to perceive on the mammogram even when they are large and palpable. However, the associated connective tissue response makes this subtype highly visible at breast ultrasound.
-
Long-term outcome
The long-term outcome of the solid, the tubulolobular, and the alveolar subtypes of invasive lobular carcinoma is good to excellent provided that they are detected and treated in the 1–14 mm size range.
The diffuse invasive lobular carcinoma has often invaded the underlying pectoral major muscle, skin, and nerves at the time of detection. This subtype has a poorer long-term outcome than the unifocal or even the multifocal invasive cancers originating from the TDLUs. Its long-term outcome is poor and is similar to that of the breast cancers of ductal origin presenting on the mammogram with casting-type calcifications, even with the use of modern therapeutic regimens (Fig. 30).
Figure 27.
The diffuse invasive lobular carcinoma lacks central tumor mass; it resembles a “spider’s web.” Its reflection on the mammogram (A) and on the specimen radiograph (B) is an architectural distortion, often difficult to detect. (C) The large section histologic image of a diffuse invasive lobular carcinoma.
Figure 28.
Breast MRI (A,B) and low and intermediate power histology images (C,D) of this solid invasive lobular carcinoma.
Figure 29.
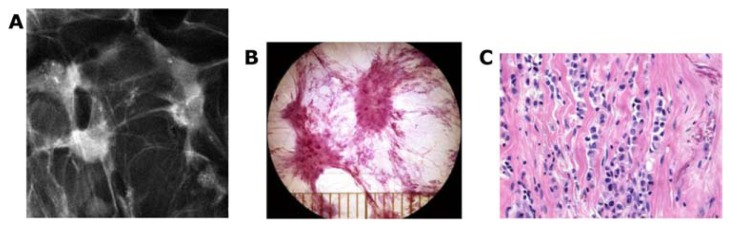
The stellate form of invasive lobular carcinoma (A) has its origin in the TDLU. Thick-section (3D) histology image (B) and higher power conventional histology image (C) of this stellate invasive lobular breast cancer.
Figure 30.
Long-term cumulative survival of women with breast cancers of “ductal origin” presenting as casting-type calcifications on the mammogram, compared with the long-term outcome of the diffuse subtype of invasive lobular carcinoma cases, diagnosed during 1977–2009 in Dalarna County, Sweden.
Comment
In most of the diffuse invasive lobular carcinoma cases, E-cadherin staining is negative, and the conventional terminology is therefore invasive “lobular” carcinoma, but when E-cadherin staining is positive, the term invasive “ductal” carcinoma is chosen by some pathologists, even if the mammographic and histologic appearances are identical in both instances. This inconsistency causes confusion among clinicians and imagers, because the conventional terminology relies on a staining method (E-cadherin) to indicate the presumed site of tumor origin (ductal vs. lobular). This is unfortunate, because a staining method such as E-cadherin is not an anatomic determinant of either ductal or lobular structure of the breast. Furthermore, the biomarkers in diffuse invasive lobular carcinoma do not accurately predict the poor long-term outcome, because these tumors are practically always ER and PR positives and HER2 negative, the proliferation index (Ki67) is low, and the histologic malignancy grade is generally moderately differentiated. Both the neoductgenesis cases and the diffuse invasive lobular carcinoma cases have a similarly poor prognosis, a diffusely infiltrating nature, and extensive lobar spread. All of these features apply to tumors with DAB etiology.
Summary
It is important to make the following distinction between the malignant breast processes at the initial evaluation of every newly diagnosed breast cancer case: the subtypes originating from the TDLUs (AAB cases with an excellent outcome in their early phase) and the diffusely infiltrating breast cancer subtypes having a considerably poor outcome (DAB cases as well as the diffuse form of invasive lobular carcinoma). This distinction should assist in planning more specifically targeted therapy, which should minimize overtreatment and undertreatment. In the new era, when the majority of breast cancers are detected mammographically, it is sensible to make full use of the mammographic tumor features when planning the management of breast cancer patients. The multimodality approach, including breast MRI, is now able to preoperatively demonstrate the unifocal, multifocal, and diffusely infiltrating nature of breast cancer. Inclusion of this information in future tumor classification systems would be beneficial for treatment planning and would also predict the long-term outcome more accurately. We suggest taking the terminology already in use to describe prostate cancer and adapting it to describe the breast cancer subtypes: DAB corresponding to DAP, and AAB corresponding to AAP. This simple, easily communicable terminology could lead to better communication between the diagnostic and therapeutic team members for the benefit of their patients.
Footnotes
COMPETING INTERESTS: Author(s) disclose no potential conflicts of interest.
Author Contributions
Conceived and designed the experiments: LT, MT. Analyzed the data: LT, AM-FY, MT, SY-HC, SL-SC, JC-YF, TH-HC. Wrote the first draft of the manuscript: LT, PBD. Contributed to the writing of the manuscript: LT, PBD, MT, TH-HC. Agree with manuscript results and conclusions: LT, PBD, AM-FY, MT, SY-HC, SL-SC, JC-YF, TH-HC. Jointly developed the structure and arguments for the paper: LT, PBD, AM-FY, MT, SY-HC, SL-SC, JC-YF, TH-HC. Made critical revisions and approved final version: LT, PBD, AM-FY, MT, SY-HC, SL-SC, JC-YF, TH-HC. All authors reviewed and approved of the final manuscript.
DISCLOSURES AND ETHICS
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
FUNDING: Author(s) disclose no funding sources.
REFERENCES
- 1.Tabár L, Vitak B, Chen TH, et al. Swedish two-county trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology. 2011;260(3):658–63. doi: 10.1148/radiol.11110469. [DOI] [PubMed] [Google Scholar]
- 2.Tabár L, Tot T, Dean PB. Breast cancer. The Art and Science of Early Detection with Mammography Perception, Interpretation, Histopathologic Correlation. Stuttgart/New York: Thieme; 2005. [Google Scholar]
- 3.Tarján M, Chen HH, Tot T, et al. Improved differentiation between ductal and acinar prostate cancer using three-dimensional histology and biomarkers. Scand J Urol Nephrol. 2012;46(4):258–66. doi: 10.3109/00365599.2012.675586. [DOI] [PubMed] [Google Scholar]
- 4.Mills SE, Carter D, Greenson JK, Ruter VE, Stoler MH, editors. Sternberg’s diagnostic surgical pathology. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2010. p. 1901. [Google Scholar]
- 5.Tabár L, Tot T, Dean PB. Breast Cancer: Early Detection with Mammography Casting Type Calcifications: Sign of a Subtype with Deceptive Features. Stuttgart/New York: Thieme; 2007. [Google Scholar]
- 6.Tabár L, Tony Chen HH, et al. Mammographic tumor features can predict long-term outcomes reliably in women with 1–14mm invasive breast carcinoma. Cancer. 2004;101(8):1745–59. doi: 10.1002/cncr.20582. [DOI] [PubMed] [Google Scholar]
- 7.Tabár L, Dean PB, Chen HHT, Duffy SW, Yen AM-F, Chiu SY-H. Early detection of breast cancer challenges current standards of care. In: Silberman H, Silberman AW, editors. Principles and Practice of Surgical Oncology. Philadelphia: Wolters Kluwer; 2010. pp. 276–91. [Google Scholar]
- 8.Tabár L, Tot T, Dean PB. Breast Cancer: Early Detection with Mammography Crushed Stone-like Calcifications: The Most Frequent Malignant Type. Stuttgart/New York: Thieme; 2008. [Google Scholar]
- 9.Tabár L, Dean PB, Chen TH-H, et al. The impact of mammography screening on the diagnosis and management of early phase breast cancer. In: Francescatti D, Silverstein M, editors. Selected Topics in Breast Surgery. Berlin: Springer; 2013. [Google Scholar]
- 10.Jensen HM, Rice JR, Wellings SR. Preneoplastic lesions in the human breast. Science. 1976;191:295–7. doi: 10.1126/science.1246614. [DOI] [PubMed] [Google Scholar]
- 11.Holland R, Veling SHJ, Mravunac M, Hendriks JHCL. Histologic multifocality of Tis, T1–2 breast carcinomas. Implications for clinical trials of breast conserving surgery. Cancer. 1985;56:979–90. doi: 10.1002/1097-0142(19850901)56:5<979::aid-cncr2820560502>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 12.Tot T. Clinical relevance of the distribution of the lesions in 500 consecutive breast cancer cases documented in large-format histologic sections. Cancer. 2007;110(11):2551–60. doi: 10.1002/cncr.23052. [DOI] [PubMed] [Google Scholar]
- 13.Tot T. The role of large-format histopathology in assessing subgross morphological prognostic parameters: a single institution report of 1000 consecutive breast cancer cases. Int J Breast Cancer. 2012;2012:395415. doi: 10.1155/2012/395415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tabár L, Lindhe L, Yen AM-F, et al. Radiotherapy after surgery for small breast cancers of stellate appearance. In: Tabár L, editor. Imaging of the Breast – Technical Aspects and Clinical Implication. InTech; http://www.intechopen.com/books/imaging-of-the-breast-technical-aspects-and-clinical-implication/radiotherapy-after-surgery-for-small-breast-cancers-of-stellate-appearance. [Google Scholar]
- 15.Chua B, Ung O, Taylor R, Boyages J. Frequency and predictors of axillary lymph node metastases in invasive breast cancer. ANZ J Surg. 2001;71:723–8. doi: 10.1046/j.1445-1433.2001.02266.x. [DOI] [PubMed] [Google Scholar]
- 16.Coombs NJ, Boyages J. Multifocal and multicentric breast cancer: does each focus matter? J Clin Oncol. 2005;23:7497–502. doi: 10.1200/JCO.2005.02.1147. [DOI] [PubMed] [Google Scholar]
- 17.Andea AA, Wallis T, Newman LA, Bouwman D, Dey J, Wisscher DW. Pathologic analysis of tumor size and lymph node status in multifocal/multicentric breast carcinoma. Cancer. 2002;94:1383–90. doi: 10.1002/cncr.10331. [DOI] [PubMed] [Google Scholar]
- 18.Tot T. The metastatic capacity of multifocal breast carcinomas: extensive tumors versus tumors of limited extent. Hum Pathol. 2009;40:199–205. doi: 10.1016/j.humpath.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Tot T, Tabár L. Mammographic-pathologic correlation of ductal carcinoma in situ of the breast using two- and three-dimensional large histologic sections. Semin Breast Dis. 2005;8:144–51. [Google Scholar]
- 20.Egan RL. Multicentric breast carcinomas: clinical-radiographic-pathologic whole organ studies and 10-year survival. Cancer. 1982;49:1123–30. doi: 10.1002/1097-0142(19820315)49:6<1123::aid-cncr2820490610>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 21.Tot T, Gere M, Pekár G, et al. Breast cancer multifocality, disease extent, and survival. Hum Pathol. 2011;42(11):1761–9. doi: 10.1016/j.humpath.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Weissenbacher TM, Zschage M, Janni W, et al. Multicentric and multifocal versus unifocal breast cancer: is the tumor-node-metastasis classification justified? Breast Cancer Res Treat. 2010;122(1):27–34. doi: 10.1007/s10549-010-0917-9. [DOI] [PubMed] [Google Scholar]
- 23.Tabár LK, Dean PB, Tot T, Lindhe N, Ingvarsson M, Yen AM-F. The implications of the imaging manifestations of multifocal and diffuse breast cancers. In: Tot T, editor. Breast Cancer: A Lobar Disease. Berlin: Springer; 2011. pp. 87–52. [Google Scholar]
- 24.Chung AP, Huynh K, Kidner T, Mirzadehgan P, Sim MS, Giuliano AE. Comparison of outcomes of breast conserving therapy in multifocal and unifocal invasive breast cancer. J Am Coll Surg. 2012;215(1):137–46. doi: 10.1016/j.jamcollsurg.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Tucker FL. New era pathologic techniques in the diagnosis and reporting of breast cancers. Semin Breast Dis. 2008;11:140–7. [Google Scholar]
- 26.Jahkola T, Toivonen T, Nordling S, von Smitten K, Virtanen I. Expression of tenascin-C in intraductal carcinoma of human breast: relationship to invasion. Eur J Cancer. 1998;34:1687–92. doi: 10.1016/s0959-8049(98)00215-9. [DOI] [PubMed] [Google Scholar]
- 27.Tot T, Kahán Z. New approach to early breast cancer. In: Kahán Z, Tot T, editors. Breast Cancer, A Heterogeneous Disease Entity. London: Springer; 2011. pp. 1–22. [Google Scholar]
- 28.Tabár L, Duffy SW, Vitak B, Chen Hsiu-Hsi, Prevost T. The natural history of breast cancer: what have we learned from screening? Cancer. 1999;86:449–62. [PubMed] [Google Scholar]
- 29.Tot T. The limited prognostic value of measuring and grading small invasive breast carcinomas: the whole sick lobe versus the details within it. Med Sci Monit. 2006;12(8):RA170–5. [PubMed] [Google Scholar]
- 30.Tot T. The diffuse type of invasive lobular carcinoma of the breast: morphology and prognosis. Virchows Arch. 2003;443(6):718–24. doi: 10.1007/s00428-003-0881-4. [DOI] [PubMed] [Google Scholar]