Abstract
Objective
To evaluate and compare the distribution and density of primordial follicles within a whole sheep ovary and to gain insight into how to overcome the impact of natural follicular heterogeneity on the experimental results.
Design
Histological study.
Setting
Academic research center.
Animals
Five- to nine-month-old ewes.
Interventions
Freshly sampled whole sheep ovaries were collected and prepared for histological analysis.
Main Outcome Measure(s)
The follicular densities and distributions were determined for hematoxylin and eosin sections. A mathematical model was derived based on the follicle counts and Monte-Carlo simulations.
Results
Heterogeneous distributions and densities of primordial follicles were identified 1) for distinct areas of the same ovarian cortex, 2) between the ovaries of the same animal and 3) across different ewes. A mathematical model based on the analysis of 37,153 primordial follicles from 8 different ovaries facilitated the estimation of the number of cortical biopsies and sections that had to be analyzed to overcome such heterogeneity.
Conclusion
The influence of physiological follicular heterogeneity on experimental and clinical results can be overcome when a definite number of cortical pieces and sections are taken into consideration.
Introduction
Ovarian cryopreservation is a promising technique for fertility preservation in young women with cancer prior to sterilization by chemotherapy or radiotherapy. This technique creates the hope of restoring gonadal function in iatrogenically sterilized patients upon autografting [1], [2]. Moreover, ovarian cryopreservation is the most appropriate strategy when embryonic cryopreservation is not feasible [3].
Fifteen years of clinical advances in ovarian tissue cryopreservation and cryobanking demonstrate that the procedure is safe, easy and promising. The pregnancy rate after the autografting of cryopreserved tissue to orthotopic sites is estimated to be approximately 30%, although the precise denominator is currently unknown [4]. To date, approximately 24 infants have been born worldwide using this procedure [5].
Even if human ovarian tissue has been occasionally used in studies of animal models [6], ethical barriers and the limited availability of human ovarian tissue preclude the widespread use of human ovaries for research. Consequently, there is an unmet need for a robust animal model comparable with humans. The “ideal” animal model should be similar to humans in terms of biochemical, physiological and anatomical characteristics. The greatest contribution of animal models, especially those of nonhuman primates, is that they offer the ability to conduct controlled experiments that would be logistically or ethically proscribed in women [7], [8]. In the case of cryopreservation studies, additional criteria for suitability as an animal model research tool would be similar ovarian architectures, a limited number of developed follicles and a single ovulation with primordial follicles distributed superficially in the cortex [9]. The sheep ovary is an adequate candidate for research according to these criteria. Its size is 80% of the human premenopausal ovary [9], [10], [11], and it has a comparable collagen-dense cortical layer containing the pool of primordial follicles. Indeed, the sheep ovary has been used for many studies [12], [13], [14], [15], [16].
The pool of human primordial follicles in cryopreserved ovarian cortical biopsies is of major importance for the subsequent restoration of fertility. The cryopreservation of live human ovarian tissue requires that cortical slices be no more than 1 mm thick. When the follicular reserve is low, this technical limitation decreases the likelihood of adequate follicle collection and increases the risk of subsequently transplanting empty biopsies. In women, follicle density and follicle distribution have been described as being highly variable according to the age and physiologic status [17], [18], [19], [20]. Different strategies have been pursued to facilitate the assessment of follicle density in situ as a research tool to ensure that only tissue pieces with abundant follicles are used [21], [22]. No precise data, methodology or guidelines for ovarian tissue analysis are available.
The purpose of the present study was to evaluate and compare the distribution and density of primordial follicles within a whole sheep ovary. Furthermore, we established a mathematical model to estimate the number of cortical pieces or sections that should be analyzed to limit the impact of follicular heterogeneity on experimental results.
Materials and Methods
1) Collection and preparation of ovarian tissue
The Animal Ethics Committee of the University of Namur approved the use of sheep ovarian tissue. Eight ovaries harvested from four ewes (5 ½, 7, 6 and 9 months old) were obtained from the Ovine Research Center (University of Namur). After euthanasia, the ovaries were immersed in Leibovitz L-15 medium (Lonza, Verviers, Belgium, BE12-700F) supplemented with 10% normal sheep serum and transported at 4°C to the laboratory within 1 h. For each ovary, the medulla was removed, and the cortex was cut into small pieces (2.5×2.5×1 mm). The cortical fragments were fixed in 4% paraformaldehyde, embedded in paraffin and fully cut into 5-µm-thick serial sections stained with hematoxylin and eosin (H&E) (Tribune Stainer HCS 33).
2) Virtual images acquisition
Virtual images were acquired with the fully automated digital microscopy system dotSlide (Olympus, BX51TF, Aartselaar, Belgium) coupled with a Peltier-cooled high-resolution digital color camera (1376×1032 pixels) (Olympus, XC10, Aartselaar, Belgium). Digital images of the whole tissue sections were digitized at high magnification (100×), producing virtual images with a pixel size of 1.510 µm.
3) Ovarian cortex analysis
The whole scanned H&E sections were analyzed using Image J software. The follicles were quantified manually, and to avoid double counting, only follicles with visible nuclei were taken into account. Follicles were then classified according to their maturity (primordial, primary or secondary follicles), as previously described [23]. The follicular densities (number/mm2) were calculated after manually outlining the cortical surface (Image J software).
4) Statistical analysis
To model the impact of the ewes, ovaries and ovarian fragments on the primordial follicle density, a linear mixed model was applied to the density of primordial follicles 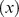 after subjecting the variable to the following base-10 logarithmic transformation:
after subjecting the variable to the following base-10 logarithmic transformation: 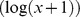 . All data analyses were performed using JMP v10.0 (SAS institute, Cary, USA).
. All data analyses were performed using JMP v10.0 (SAS institute, Cary, USA).
Results
1) Primordial follicle distributions and densities within whole sheep ovaries
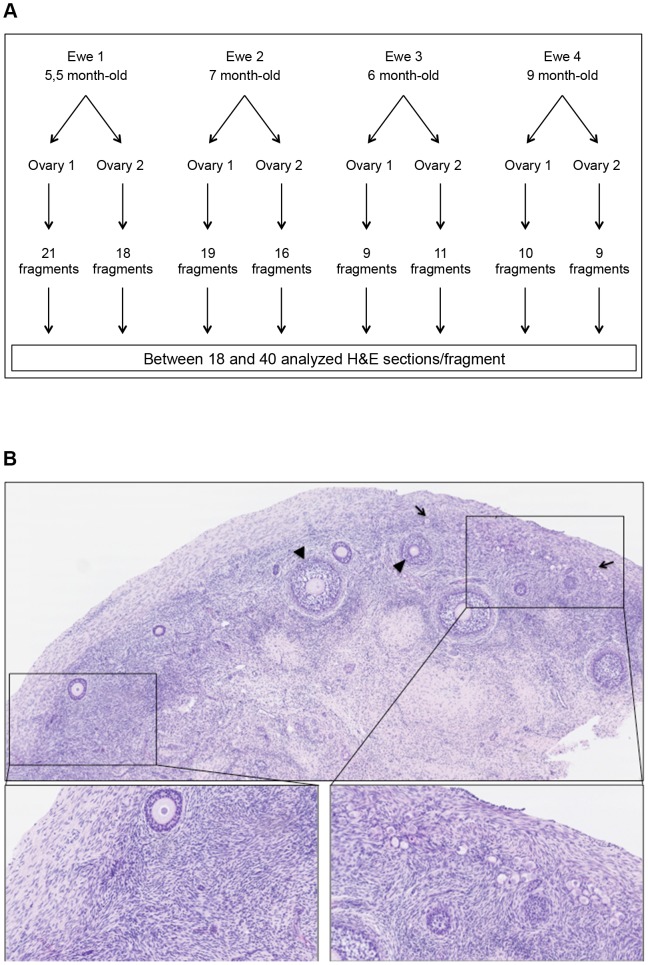
To assess the primordial follicle distributions in the sheep cortex, we analyzed the whole ovaries using the procedure depicted in the flow chart presented in Figure 1A. Both ovaries from 4 young ewes were collected. After removal of the medulla, the remaining cortex was cut into small fragments (9–21 fragments/ovary). Each fragment was completely serially sectioned (5 µm thick). Because sheep primordial follicle diameters range from 20 to 30 µm, we analyzed every sixth section of the serially sectioned ovarian cortex fragments. In total, 3,852 H&E sections were analyzed. Figure 1B illustrates one H&E section of a hemi-ovary where uneven primordial follicle distribution is obvious.
Figure 1. Flow chart of the experimental design (A) and representative histology of a sheep cortex section (B).
Ovaries were harvested from two ewes and fully cut into cortical fragments. Subsequently, each fragment was serially and completely sectioned, and approximately 40 H&E sections, each 30 µm apart from one another, were further used for the follicular quantification (A). The uneven repartition of follicles within the sheep ovarian cortex is obvious (B). The left part of the H&E section is completely devoid of primordial follicles, whereas the right part contains mostly primordial follicles. Primordial follicles (plain arrows) and secondary follicles (arrowhead).
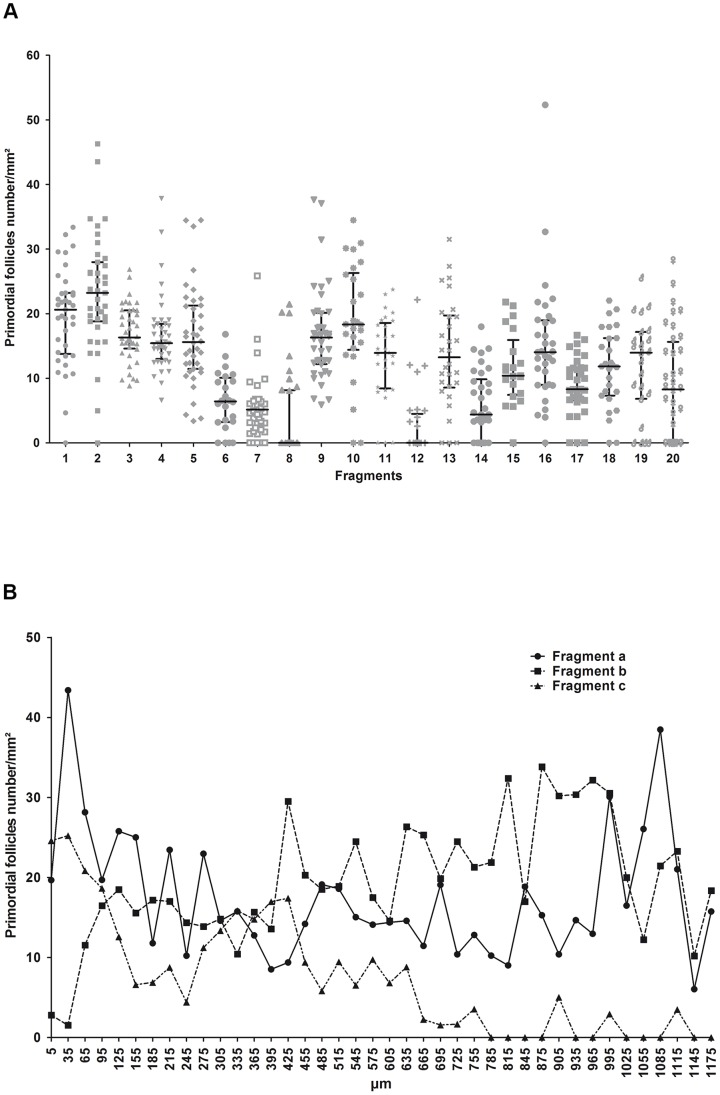
In this study, we only quantified primordial and primary follicles (Table 1). Secondary follicles representing less than 1% of the entire population were not taken into account. Figure 2 illustrates the primordial follicle density analysis for a single whole sheep ovary. The primordial follicle density is highly variable both between fragments belonging to the same ovary (Figure 2A) and among serial sections belonging to the same fragment (Figure 2B). In each ovary, there was a wide variation in the number of primordial follicles across the fragments. This analysis highlights the importance of adequately determining the number of fragments or sections necessary to overcome physiological follicular heterogeneity.
Table 1. Follicle quantification in the whole ovaries.
| H&E sections analyzed | Primordial follicle number | Primary follicle number | |
| Ewe 1, ovary 1 | 657 | 5624 | 325 |
| Ewe 1, ovary 2 | 669 | 8572 | 317 |
| Ewe 2, ovary 1 | 632 | 7746 | 235 |
| Ewe 2, ovary 2 | 567 | 6808 | 628 |
| Ewe 3, ovary 1 | 301 | 2409 | 619 |
| Ewe 3, ovary 2 | 360 | 2369 | 320 |
| Ewe 4, ovary 1 | 378 | 2345 | 267 |
| Ewe 4, ovary 2 | 288 | 1285 | 267 |
| Total | 3,852 | 37,153 | 2,978 |
Figure 2. Representative illustration of the primordial follicular quantification.
Mean primordial follicle densities (number/mm2) of 40 sections per fragment for 20 fragments from the same ovary (A) are shown. An example of the primordial follicle density within serial sections (at a 30-µm distance) of three fragments from the same ovary is also illustrated (a, b and c) (B).
2) Mathematical model
To create a linear mixed model of the follicle counts, the ewes and ovaries were treated as fixed factors. The ovaries were nested into the ewes, and the ovarian fragments were treated as random factors. After logarithmically transforming the primordial follicle densities, the weights of the fixed factors could be determined: the ewes had a significant effect on the primordial follicle density (p-value<0.0001). This finding indicated that the primordial follicle density was not identical between the two ewes. Moreover, the ovaries had a significant effect on the primordial follicle density (p-value<0.0001). Indeed, the follicular density was different between the two ovaries collected from the same animal. These findings indicated that in an experimental setup aiming to study the effects of specific treatments on follicular density, a single ovary should be used to limit the impact of the fixed factors (such as ewes and ovaries). Alternatively, if several ewes or several ovaries are required, then their effects should be included in the statistical model to extract their interferential influences over the treatment effects.
In a specific ovary, 9 to 21 pieces of cortex were analyzed, and the histological evaluation of the primordial follicle density revealed a wide variation among the sections of the same fragment (Figure 2). When ovarian fragments were treated as random factors with the residual error representing the number of sections per ovarian fragment, the statistical analysis clearly showed that the major source of variability was due to the sections per ovary fragment, accounting for 90% of the total variance (Table 2). The residual variability linked to the fragments accounted for only 10% of the total variance. This finding suggests that to increase the precision of the primordial follicular density measurement, increasing the number of sections per fragment rather than the number of analyzed fragments is more rewarding.
Table 2. Variance components of the random-effects fragments and sections and their respective standard errors.
| Random effects | Variance component | Standard error | Proportion of variance |
| Fragment | 0.116 | 0.037 | 10.46±3.00% |
| Section | 0.993 | 0.023 | 89.54±2.06% |
In the ovarian cortex, the primordial follicles represented the most important follicle population; therefore, we based the rest of our statistical analysis on their density. The primary follicles, however, displayed the same distribution profiles and density heterogeneities (data not shown).
3) Determination of the sample size
Based on the results obtained from the analysis of the follicular densities, Monte-Carlo simulations were performed to determine the best experimental design required to evaluate the effects of a specific ovarian treatment. The same linear mixed model as described for the analyses above was used. The logarithmically transformed primordial follicle densities were hence supposed to follow a normal distribution. Estimations of the effect of the ewes and ovaries obtained from the previous statistical analysis were used as fixed values in the model to define the mean of the distribution used for simulation. The estimated variance components of the ovarian fragments and sections were used to define the variance of the logarithmically transformed primordial follicle densities. An additional fixed factor was added to the mean of the model corresponding to a treatment effect that could have an impact on the follicle densities. The treatment fixed effects (i.e., the effects of a treatment on follicular density that should be detected) were arbitrarily set at 50, 25 and 10% modification of follicular densities with respect to a control treatment. For each of these three possibilities, 2000 simulations were performed for different combinations of numbers of ovarian fragments (20, 10 or 5) and numbers of sections per ovarian fragments (40, 20 or 10) defining the sample size. The probability of detecting the prespecified effect size on follicular density, i.e., the power of the test, was computed (Table 3). In the simulations, the treatment effect was defined as being significant for p-values<0.05. If a 50% improvement in the follicular preservation is required, all experimental setups that were tested allowed for detecting this improvement with 100% probability. To detect a more subtle effect, e.g., 25% follicular preservation with at least 95% probability, two experimental setups out of five allowed us to limit the total number of analyzed sections to 200 per group: either 10 fragments and 20 sections per fragment or 20 fragments and 10 sections per fragment. To power the system to detect an improvement in follicular preservation as small as 10%, at least 20 fragments with 40 sections per fragment had to be examined for the probability of detecting the effect to remain above 80%.
Table 3. Results of the Monte-Carlo simulations.
| Fixed effect (%) | Number of fragments | Number of sections | Probability of detecting the effect (%) |
| 50 | 20 or 10 or 5 | 40 or 20 or 10 | 100 |
| 25 | 20 | 40 | 100 |
| 25 | 20 | 20 | 100 |
| 25 | 20 | 10 | 99 |
| 25 | 10 | 40 | 100 |
| 25 | 10 | 20 | 98 |
| 25 | 10 | 10 | 93 |
| 25 | 5 | 40 | 94 |
| 25 | 5 | 20 | 87 |
| 25 | 5 | 10 | 74 |
| 10 | 20 | 40 | 84 |
| 10 | 20 | 20 | 71 |
| 10 | 20 | 10 | 54 |
| 10 | 10 | 40 | 70 |
| 10 | 10 | 20 | 53 |
| 10 | 10 | 10 | 39 |
| 10 | 5 | 40 | 56 |
| 10 | 5 | 20 | 44 |
| 10 | 5 | 10 | 29 |
The probability of detecting an effect on the primordial follicular density (i.e., power) was calculated with the fixed effects arbitrarily set at 50, 25 and 10% and a confidence level of alpha = 0.05 for various combinations of the numbers of ovary fragments and sections per fragment.
Discussion
We used a methodologically rigorous and laborious research technique to measure follicular distribution within the ovine ovarian cortex. Serial sections of different fragments covering several whole sheep ovaries displayed uneven primordial follicle densities, as has already been reported for the human cortex [17], [19], [20]. In our study, 3,852 sections belonging to 113 cortical fragments extracted from eight ovaries were analyzed and subsequently used to build a mathematical model useful for further application in the field of reproductive medicine. The primary focus of this study was not to perform comparisons among individuals; our specific aim was to provide guidelines regarding the number of samples and histological sections required to achieve specific objectives in the improvement of follicular cryopreservation.
Currently, the cryopreservation of cortical fragments followed by transplantation represents the only opportunity for fertility preservation available to young women with cancer who need immediate chemotherapy. However, this strategy is still considered an experimental technique that requires improvement. The initial follicle density within cryopreserved ovarian tissue has a profound impact on the transplantation success. The lifespan of the graft depends on the number of preserved viable primordial follicles [24], [25].
The study of follicle preservation is still hampered by the lack of a clearly defined experimental model that takes into account the physiological heterogeneity within an ovarian cortex. A diagnostic test that has the ability to confirm the presence of follicles and to indicate the survival of cryopreserved follicles would be a valuable tool in the field of reproductive medicine, particularly for ovarian tissue cryopreservation. The accuracy of estimating the follicle density in the human ovary based on a single biopsy or sample has been questioned [26], [27], [28], and studies have shown large variations in follicle density between and within ovaries when multiple samples are examined [19] or a lack of homogeneous follicle distribution even within a single sample [17], [29], [30], [31]. In situ follicle identification methods should be non-detrimental to the tissue and should allow long-term follicle survival and growth. Several methods of follicle detection using stereomicroscopic localization, fluorescent probes (Calcein AM or rhodamine 123) and the dye neutral red (NR) have been established [21], [22], [32]. All of these methods for follicle visualization have their own advantages and disadvantages (e.g., the expense and specificity of the required equipment, operator experience, toxicity toward oocytes and the thickness of cortical pieces [less than 500 µm]). Therefore, none of these methods is suitable for evaluating the follicular density in frozen-thawed fragments prior to transplantation or for analyzing transplants after a defined period of transplantation.
Our histological analysis followed by mathematical simulation provides useful information for establishing a follicular quantification method that considers the physiological follicular heterogeneity existing within the ovarian cortex and between ovaries or individuals (Tables 2 and 3). This method can be usefully applied in the field of ovarian tissue transplantation as an option for fertility preservation. For example, to perform in vivo or in vitro treatment comparisons, a sufficient number of cortical pieces from the same ovary of the same animal with a sufficient number of serial sections or fragments should be analyzed.
Additionally, our study clearly indicates that the number of ovarian fragments and/or the number of sections/fragments should be increased proportionately to the decreased expected effect (Table 3). This method is also useful for determining the number of host animals to be grafted for obtaining results that are independent of the follicular heterogeneity.
In conclusion, our study offers a valuable tool for evaluating the efficacy and safety of multiple treatments that may be beneficial for follicular preservation within xenograft models, using either human or sheep ovarian tissue.
Acknowledgments
The authors thank I. Dasoul, E. Feyereisen and P Gavitelli for their excellent technical assistance. M.F and L.H. are Televie grant-supported Ph.D. students (F.R.S.-FNRS, Belgium). C.M. is a Research Associate from the F.R.S.-FNRS (Belgium).
Funding Statement
This study was supported by grants from the “Fonds de la Recherche Scientifique” (F.R.S.-FNRS, Belgium), the Interuniversity Attraction Poles program of the Belgian Science Policy (Brussels, Belgium), the “Fonds Spéciaux de la Recherche” (University of Liège, Belgium) and the “Fonds Léon Fredericq” (University of Liège, Belgium). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Aubard Y, Poirot C, Piver P, Galinat S, Teissier MP (2001) Are there indications for ovarian tissue cryopreservation? Fertil Steril 76: 414–415. [DOI] [PubMed] [Google Scholar]
- 2. Donnez J, Jadoul P, Squifflet J, Van Langendonckt A, Donnez O, et al. (2010) Ovarian tissue cryopreservation and transplantation in cancer patients. Best Pract Res Clin Obstet Gynaecol 24: 87–100. [DOI] [PubMed] [Google Scholar]
- 3.Demeestere I, Moffa F, Peccatori F, Poirot C, Shalom-Paz E (2012) Multiple approaches for individualized fertility protective therapy in cancer patients. Obstet Gynecol Int: 961232. [DOI] [PMC free article] [PubMed]
- 4. Dolmans MM, Jadoul P, Gilliaux S, Amorim CA, Luyckx V, et al. (2013) A review of 15 years of ovarian tissue bank activities. J Assist Reprod Genet 30: 305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Sanchez Serrano M, et al. (2013) Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril 99: 1503–1513. [DOI] [PubMed] [Google Scholar]
- 6. Nisolle M, Casanas-Roux F, Qu J, Motta P, Donnez J (2000) Histologic and ultrastructural evaluation of fresh and frozen-thawed human ovarian xenografts in nude mice. FertilSteril 74: 122–129. [DOI] [PubMed] [Google Scholar]
- 7. VandeBerg JL (2004) Need for primate models in biomedical research. Gynecol Obstet Invest 57: 6–8. [PubMed] [Google Scholar]
- 8. Archer DF (2004) Role of the nonhuman primate for research related to women's health. ILAR J 45: 212–219. [DOI] [PubMed] [Google Scholar]
- 9. Gerritse R, Beerendonk CC, Tijink MS, Heetkamp A, Kremer JA, et al. (2008) Optimal perfusion of an intact ovary as a prerequisite for successful ovarian cryopreservation. Hum Reprod 23: 329–335. [DOI] [PubMed] [Google Scholar]
- 10. Munn CS, Kiser LC, Wetzner SM, Baer JE (1986) Ovary volume in young and premenopausal adults: US determination. Work in progress. Radiology 159: 731–732. [DOI] [PubMed] [Google Scholar]
- 11. Oktay K, Karlikaya GG, Aydin BA (2000) Ovarian cryopreservation and transplantation: basic aspects. MolCell Endocrinol 169: 105–108. [DOI] [PubMed] [Google Scholar]
- 12. Gosden RG, Baird DT, Wade JC, Webb R (1994) Restoration of fertility to oophorectomized sheep by ovarian autografts stored at -196 degrees C. Hum Reprod 9: 597–603. [DOI] [PubMed] [Google Scholar]
- 13. Lornage J, Courbiere B, Mazoyer C, Odagescu V, Baudot A, et al. (2006) [Ovarian tissue vitrification: Cortex and whole ovary in sheep]. Gynecol Obstet Fertil 34: 746–753. [DOI] [PubMed] [Google Scholar]
- 14. Courbiere B, Massardier J, Salle B, Mazoyer C, Guerin JF, et al. (2005) Follicular viability and histological assessment after cryopreservation of whole sheep ovaries with vascular pedicle by vitrification. Fertil Steril 84 Suppl 21065–1071. [DOI] [PubMed] [Google Scholar]
- 15. Sauvat F, Bouilly J, Capito C, Lefevre A, Blachere T, et al. (2013) Ovarian function is restored after grafting of cryopreserved immature ovary in ewes. FASEB J 27: 1511–1518. [DOI] [PubMed] [Google Scholar]
- 16. Baird DT (1999) Long-Term Ovarian Function in Sheep after Ovariectomy and Transplantation of Autografts Stored at -196 C. Endocrinology 140: 462–471. [DOI] [PubMed] [Google Scholar]
- 17. Qu J, Godin PA, Nisolle M, Donnez J (2000) Distribution and epidermal growth factor receptor expression of primordial follicles in human ovarian tissue before and after cryopreservation. HumReprod 15: 302–310. [DOI] [PubMed] [Google Scholar]
- 18. David A, Dolmans MM, Van Langendonckt A, Donnez J, Amorim CA (2011) Immunohistochemical localization of growth factors after cryopreservation and 3 weeks' xenotransplantation of human ovarian tissue. FertilSteril 95: 1241–1246. [DOI] [PubMed] [Google Scholar]
- 19. Schmidt KL, Byskov AG, Nyboe Andersen A, Muller J, Yding Andersen C (2003) Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Hum Reprod 18: 1158–1164. [DOI] [PubMed] [Google Scholar]
- 20. Campos JR, Rosa-e-Silva JC, Carvalho BR, Vireque AA, Silva-de-Sa MF, et al. (2011) Cryopreservation time does not decrease follicular viability in ovarian tissue frozen for fertility preservation. Clinics (Sao Paulo) 66: 2093–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Soleimani R, De Vos W, Van Oostveldt P, Lierman S, Van den BR, et al. (2006) Two novel techniques to detect follicles in human ovarian cortical tissue. HumReprod 21: 1720–1724. [DOI] [PubMed] [Google Scholar]
- 22. Chambers EL, Gosden RG, Yap C, Picton HM (2010) In situ identification of follicles in ovarian cortex as a tool for quantifying follicle density, viability and developmental potential in strategies to preserve female fertility. HumReprod 25: 2559–2568. [DOI] [PubMed] [Google Scholar]
- 23. Lundy T, Smith P, O'Connell A, Hudson NL, McNatty KP (1999) Populations of granulosa cells in small follicles of the sheep ovary. J Reprod Fertil 115: 251–262. [DOI] [PubMed] [Google Scholar]
- 24. Kim SS (2006) Fertility preservation in female cancer patients: current developments and future directions. Fertil Steril 85: 1–11. [DOI] [PubMed] [Google Scholar]
- 25. Bedaiwy MA, Shahin AY, Falcone T (2008) Reproductive organ transplantation: advances and controversies. Fertil Steril 90: 2031–2055. [DOI] [PubMed] [Google Scholar]
- 26. Lass A (2004) Assessment of ovarian reserve Is there still a role for ovarian biopsy in the light of new data? Hum Reprod 19: 467–469. [DOI] [PubMed] [Google Scholar]
- 27. Sharara FI, Scott RT (2004) Assessment of ovarian reserve. Is there still a role for ovarian biopsy? First do no harm! Hum Reprod 19: 470–471. [DOI] [PubMed] [Google Scholar]
- 28. Tilly JL (2003) Ovarian follicle counts–not as simple as 1, 2, 3. Reprod Biol Endocrinol 1: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lass A, Silye R, Abrams D-C, Krausz T, Hovatta O, et al. (1997) Follicular density in ovarian biopsy of infertile women: a novel method to assess ovarian reserve. Hum Reprod 12: 1028–1031. [DOI] [PubMed] [Google Scholar]
- 30. Poirot C, Vacher-Lavenu MC, Helardot P, Guibert J, Brugieres L, et al. (2002) Human ovarian tissue cryopreservation: indications and feasibility. Hum Reprod 17: 1447–1452. [DOI] [PubMed] [Google Scholar]
- 31. Lambalk CB, de Koning CH, Flett A, Van Kasteren Y, Gosden R, et al. (2004) Assessment of ovarian reserve. Ovarian biopsy is not a valid method for the prediction of ovarian reserve. Hum Reprod 19: 1055–1059. [DOI] [PubMed] [Google Scholar]
- 32.Cortvrindt RG, Smitz JEJ (2001) Fluorescent probes allow rapid and precise recording of follicle density and staging in human ovarian cortical biopsy samples. Fertil Steril 75. [DOI] [PubMed] [Google Scholar]