Abstract
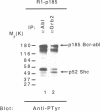
The cytosolic 185 and 210 kDa Bcr-Abl protein tyrosine kinases play important roles in the development of Philadelphia chromosome positive (Ph+) chronic myelogenous leukemia (CML) and acute lymphoblastic leukemia (Ph+ ALL). p185 and p210 Bcr-Abl contain identical abl-encoded sequences juxtaposed to a variable number of bcr-derived amino acids. As the mitogenic and transforming activities of tyrosine kinases involve stimulation of the Ras pathway, we analyzed Bcr-Abl oncoproteins for interactions with cytoplasmic proteins that mediate Ras activation. Such polypeptides include Grb2, which comprises a single Src homology 2 (SH2) domain flanked by two SH3 domains, and the 66, 52 and 46 kDa Shc proteins which possess an SH2 domain in their carboxy-terminus. Grb2 associates with tyrosine phosphorylated proteins through its SH2 domain, and with the Ras guanine nucleotide releasing protein mSos1 through its SH3 domains. mSos1 stimulates conversion of the inactive GDP-bound form of Ras to the active GTP-bound state. In bcr-abl-transformed cells, Grb2 and mSos1 formed a physical complex with Bcr-Abl. In vitro, the Grb2 SH2 domain bound Bcr-Abl through recognition of a tyrosine phosphorylation site within the amino-terminal bcr-encoded sequence (p.Tyr177-Val-Asn-Val), that is common to both Bcr-Abl proteins. These results suggest that autophosphorylation within the Bcr element of Bcr-Abl creates a direct physical link to Grb2-mSos1, and potentially to the Ras pathway, and thereby modifies the target specificity of the Abl tyrosine kinase.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowtell D., Fu P., Simon M., Senior P. Identification of murine homologues of the Drosophila son of sevenless gene: potential activators of ras. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6511–6515. doi: 10.1073/pnas.89.14.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buday L., Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993 May 7;73(3):611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- Burkhardt A. L., Brunswick M., Bolen J. B., Mond J. J. Anti-immunoglobulin stimulation of B lymphocytes activates src-related protein-tyrosine kinases. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7410–7414. doi: 10.1073/pnas.88.16.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. L., Li W., Arlinghaus R. B. P210 BCR-ABL is complexed to P160 BCR and ph-P53 proteins in K562 cells. Oncogene. 1990 May;5(5):773–776. [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Chardin P., Camonis J. H., Gale N. W., van Aelst L., Schlessinger J., Wigler M. H., Bar-Sagi D. Human Sos1: a guanine nucleotide exchange factor for Ras that binds to GRB2. Science. 1993 May 28;260(5112):1338–1343. doi: 10.1126/science.8493579. [DOI] [PubMed] [Google Scholar]
- Clark S. S., McLaughlin J., Crist W. M., Champlin R., Witte O. N. Unique forms of the abl tyrosine kinase distinguish Ph1-positive CML from Ph1-positive ALL. Science. 1987 Jan 2;235(4784):85–88. doi: 10.1126/science.3541203. [DOI] [PubMed] [Google Scholar]
- Daley G. Q., McLaughlin J., Witte O. N., Baltimore D. The CML-specific P210 bcr/abl protein, unlike v-abl, does not transform NIH/3T3 fibroblasts. Science. 1987 Jul 31;237(4814):532–535. doi: 10.1126/science.2440107. [DOI] [PubMed] [Google Scholar]
- Dhut S., Chaplin T., Young B. D. Normal c-abl gene protein--a nuclear component. Oncogene. 1991 Aug;6(8):1459–1464. [PubMed] [Google Scholar]
- Eck M. J., Shoelson S. E., Harrison S. C. Recognition of a high-affinity phosphotyrosyl peptide by the Src homology-2 domain of p56lck. Nature. 1993 Mar 4;362(6415):87–91. doi: 10.1038/362087a0. [DOI] [PubMed] [Google Scholar]
- Egan S. E., Giddings B. W., Brooks M. W., Buday L., Sizeland A. M., Weinberg R. A. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature. 1993 May 6;363(6424):45–51. doi: 10.1038/363045a0. [DOI] [PubMed] [Google Scholar]
- Elefanty A. G., Hariharan I. K., Cory S. bcr-abl, the hallmark of chronic myeloid leukaemia in man, induces multiple haemopoietic neoplasms in mice. EMBO J. 1990 Apr;9(4):1069–1078. doi: 10.1002/j.1460-2075.1990.tb08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobedo J. A., Kaplan D. R., Kavanaugh W. M., Turck C. W., Williams L. T. A phosphatidylinositol-3 kinase binds to platelet-derived growth factor receptors through a specific receptor sequence containing phosphotyrosine. Mol Cell Biol. 1991 Feb;11(2):1125–1132. doi: 10.1128/mcb.11.2.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig L. A., Cooper G. M. Inhibition of NIH 3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Mol Cell Biol. 1988 Aug;8(8):3235–3243. doi: 10.1128/mcb.8.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder S., Zhou M., Hu P., Ureña J., Ullrich A., Chaudhuri M., White M., Shoelson S. E., Schlessinger J. SH2 domains exhibit high-affinity binding to tyrosine-phosphorylated peptides yet also exhibit rapid dissociation and exchange. Mol Cell Biol. 1993 Mar;13(3):1449–1455. doi: 10.1128/mcb.13.3.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale N. W., Kaplan S., Lowenstein E. J., Schlessinger J., Bar-Sagi D. Grb2 mediates the EGF-dependent activation of guanine nucleotide exchange on Ras. Nature. 1993 May 6;363(6424):88–92. doi: 10.1038/363088a0. [DOI] [PubMed] [Google Scholar]
- Gishizky M. L., Johnson-White J., Witte O. N. Efficient transplantation of BCR-ABL-induced chronic myelogenous leukemia-like syndrome in mice. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3755–3759. doi: 10.1073/pnas.90.8.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goga A., McLaughlin J., Pendergast A. M., Parmar K., Muller A., Rosenberg N., Witte O. N. Oncogenic activation of c-ABL by mutation within its last exon. Mol Cell Biol. 1993 Aug;13(8):4967–4975. doi: 10.1128/mcb.13.8.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Furuya W. Chemoattractant-induced tyrosine phosphorylation and activation of microtubule-associated protein kinase in human neutrophils. J Biol Chem. 1992 Sep 5;267(25):18122–18125. [PubMed] [Google Scholar]
- Hanazono Y., Chiba S., Sasaki K., Mano H., Miyajima A., Arai K., Yazaki Y., Hirai H. c-fps/fes protein-tyrosine kinase is implicated in a signaling pathway triggered by granulocyte-macrophage colony-stimulating factor and interleukin-3. EMBO J. 1993 Apr;12(4):1641–1646. doi: 10.1002/j.1460-2075.1993.tb05809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisterkamp N., Jenster G., ten Hoeve J., Zovich D., Pattengale P. K., Groffen J. Acute leukaemia in bcr/abl transgenic mice. Nature. 1990 Mar 15;344(6263):251–253. doi: 10.1038/344251a0. [DOI] [PubMed] [Google Scholar]
- Hermans A., Heisterkamp N., von Linden M., van Baal S., Meijer D., van der Plas D., Wiedemann L. M., Groffen J., Bootsma D., Grosveld G. Unique fusion of bcr and c-abl genes in Philadelphia chromosome positive acute lymphoblastic leukemia. Cell. 1987 Oct 9;51(1):33–40. doi: 10.1016/0092-8674(87)90007-9. [DOI] [PubMed] [Google Scholar]
- Kashishian A., Kazlauskas A., Cooper J. A. Phosphorylation sites in the PDGF receptor with different specificities for binding GAP and PI3 kinase in vivo. EMBO J. 1992 Apr;11(4):1373–1382. doi: 10.1002/j.1460-2075.1992.tb05182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher M. A., McLaughlin J., Witte O. N., Rosenberg N. Induction of a chronic myelogenous leukemia-like syndrome in mice with v-abl and BCR/ABL. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6649–6653. doi: 10.1073/pnas.87.17.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Kolanus W., Romeo C., Seed B. T cell activation by clustered tyrosine kinases. Cell. 1993 Jul 16;74(1):171–183. doi: 10.1016/0092-8674(93)90304-9. [DOI] [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Witte O. N. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984 Jul;37(3):1035–1042. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Letwin K., Yee S. P., Pawson T. Novel protein-tyrosine kinase cDNAs related to fps/fes and eph cloned using anti-phosphotyrosine antibody. Oncogene. 1988 Dec;3(6):621–627. [PubMed] [Google Scholar]
- Li N., Batzer A., Daly R., Yajnik V., Skolnik E., Chardin P., Bar-Sagi D., Margolis B., Schlessinger J. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling. Nature. 1993 May 6;363(6424):85–88. doi: 10.1038/363085a0. [DOI] [PubMed] [Google Scholar]
- Li W. J., Kloetzer W. S., Arlinghaus R. B. A novel 53 kDa protein complexed with P210bcr-abl in human chronic myelogenous leukemia cells. Oncogene. 1988 Jun;2(6):559–566. [PubMed] [Google Scholar]
- Liu J., Campbell M., Guo J. Q., Lu D., Xian Y. M., Andersson B. S., Arlinghaus R. B. BCR-ABL tyrosine kinase is autophosphorylated or transphosphorylates P160 BCR on tyrosine predominantly within the first BCR exon. Oncogene. 1993 Jan;8(1):101–109. [PubMed] [Google Scholar]
- Lowenstein E. J., Daly R. J., Batzer A. G., Li W., Margolis B., Lammers R., Ullrich A., Skolnik E. Y., Bar-Sagi D., Schlessinger J. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992 Aug 7;70(3):431–442. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Lu D., Liu J., Campbell M., Guo J. Q., Heisterkamp N., Groffen J., Canaani E., Arlinghaus R. Tyrosine phosphorylation of P160 BCR by P210 BCR-ABL. Blood. 1993 Aug 15;82(4):1257–1263. [PubMed] [Google Scholar]
- Mandanas R. A., Leibowitz D. S., Gharehbaghi K., Tauchi T., Burgess G. S., Miyazawa K., Jayaram H. N., Boswell H. S. Role of p21 RAS in p210 bcr-abl transformation of murine myeloid cells. Blood. 1993 Sep 15;82(6):1838–1847. [PubMed] [Google Scholar]
- Maxwell S. A., Kurzrock R., Parsons S. J., Talpaz M., Gallick G. E., Kloetzer W. S., Arlinghaus R. B., Kouttab N. M., Keating M. J., Gutterman J. U. Analysis of P210bcr-abl tyrosine protein kinase activity in various subtypes of Philadelphia chromosome-positive cells from chronic myelogenous leukemia patients. Cancer Res. 1987 Mar 15;47(6):1731–1739. [PubMed] [Google Scholar]
- Mayer B. J., Jackson P. K., Baltimore D. The noncatalytic src homology region 2 segment of abl tyrosine kinase binds to tyrosine-phosphorylated cellular proteins with high affinity. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):627–631. doi: 10.1073/pnas.88.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlade J., Cheng A., Pelicci G., Pelicci P. G., Pawson T. Shc proteins are phosphorylated and regulated by the v-Src and v-Fps protein-tyrosine kinases. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8869–8873. doi: 10.1073/pnas.89.19.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J., Chianese E., Witte O. N. Alternative forms of the BCR-ABL oncogene have quantitatively different potencies for stimulation of immature lymphoid cells. Mol Cell Biol. 1989 May;9(5):1866–1874. doi: 10.1128/mcb.9.5.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhirter J. R., Wang J. Y. Activation of tyrosinase kinase and microfilament-binding functions of c-abl by bcr sequences in bcr/abl fusion proteins. Mol Cell Biol. 1991 Mar;11(3):1553–1565. doi: 10.1128/mcb.11.3.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhirter J. R., Wang J. Y. An actin-binding function contributes to transformation by the Bcr-Abl oncoprotein of Philadelphia chromosome-positive human leukemias. EMBO J. 1993 Apr;12(4):1533–1546. doi: 10.1002/j.1460-2075.1993.tb05797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mes-Masson A. M., McLaughlin J., Daley G. Q., Paskind M., Witte O. N. Overlapping cDNA clones define the complete coding region for the P210c-abl gene product associated with chronic myelogenous leukemia cells containing the Philadelphia chromosome. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9768–9772. doi: 10.1073/pnas.83.24.9768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura O., D'Andrea A., Kabat D., Ihle J. N. Induction of tyrosine phosphorylation by the erythropoietin receptor correlates with mitogenesis. Mol Cell Biol. 1991 Oct;11(10):4895–4902. doi: 10.1128/mcb.11.10.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran M. F., Polakis P., McCormick F., Pawson T., Ellis C. Protein-tyrosine kinases regulate the phosphorylation, protein interactions, subcellular distribution, and activity of p21ras GTPase-activating protein. Mol Cell Biol. 1991 Apr;11(4):1804–1812. doi: 10.1128/mcb.11.4.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy L. S., Smith M. R., Stacey D. W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature. 1985 Jan 17;313(5999):241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- Muller A. J., Young J. C., Pendergast A. M., Pondel M., Landau N. R., Littman D. R., Witte O. N. BCR first exon sequences specifically activate the BCR/ABL tyrosine kinase oncogene of Philadelphia chromosome-positive human leukemias. Mol Cell Biol. 1991 Apr;11(4):1785–1792. doi: 10.1128/mcb.11.4.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A., Gibson T., Lehto V. P., Saraste M. SH3--an abundant protein domain in search of a function. FEBS Lett. 1992 Jul 27;307(1):55–61. doi: 10.1016/0014-5793(92)80901-r. [DOI] [PubMed] [Google Scholar]
- Naumovski L., Morgan R., Hecht F., Link M. P., Glader B. E., Smith S. D. Philadelphia chromosome-positive acute lymphoblastic leukemia cell lines without classical breakpoint cluster region rearrangement. Cancer Res. 1988 May 15;48(10):2876–2879. [PubMed] [Google Scholar]
- Olivier J. P., Raabe T., Henkemeyer M., Dickson B., Mbamalu G., Margolis B., Schlessinger J., Hafen E., Pawson T. A Drosophila SH2-SH3 adaptor protein implicated in coupling the sevenless tyrosine kinase to an activator of Ras guanine nucleotide exchange, Sos. Cell. 1993 Apr 9;73(1):179–191. doi: 10.1016/0092-8674(93)90170-u. [DOI] [PubMed] [Google Scholar]
- Pawson T., Gish G. D. SH2 and SH3 domains: from structure to function. Cell. 1992 Oct 30;71(3):359–362. doi: 10.1016/0092-8674(92)90504-6. [DOI] [PubMed] [Google Scholar]
- Payne G., Shoelson S. E., Gish G. D., Pawson T., Walsh C. T. Kinetics of p56lck and p60src Src homology 2 domain binding to tyrosine-phosphorylated peptides determined by a competition assay or surface plasmon resonance. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):4902–4906. doi: 10.1073/pnas.90.11.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicci G., Lanfrancone L., Grignani F., McGlade J., Cavallo F., Forni G., Nicoletti I., Grignani F., Pawson T., Pelicci P. G. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992 Jul 10;70(1):93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- Pendergast A. M., Muller A. J., Havlik M. H., Maru Y., Witte O. N. BCR sequences essential for transformation by the BCR-ABL oncogene bind to the ABL SH2 regulatory domain in a non-phosphotyrosine-dependent manner. Cell. 1991 Jul 12;66(1):161–171. doi: 10.1016/0092-8674(91)90148-r. [DOI] [PubMed] [Google Scholar]
- Piccione E., Case R. D., Domchek S. M., Hu P., Chaudhuri M., Backer J. M., Schlessinger J., Shoelson S. E. Phosphatidylinositol 3-kinase p85 SH2 domain specificity defined by direct phosphopeptide/SH2 domain binding. Biochemistry. 1993 Apr 6;32(13):3197–3202. doi: 10.1021/bi00064a001. [DOI] [PubMed] [Google Scholar]
- Ponzetto C., Bardelli A., Maina F., Longati P., Panayotou G., Dhand R., Waterfield M. D., Comoglio P. M. A novel recognition motif for phosphatidylinositol 3-kinase binding mediates its association with the hepatocyte growth factor/scatter factor receptor. Mol Cell Biol. 1993 Aug;13(8):4600–4608. doi: 10.1128/mcb.13.8.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronk G. J., McGlade J., Pelicci G., Pawson T., Bos J. L. Insulin-induced phosphorylation of the 46- and 52-kDa Shc proteins. J Biol Chem. 1993 Mar 15;268(8):5748–5753. [PubMed] [Google Scholar]
- Ren R., Mayer B. J., Cicchetti P., Baltimore D. Identification of a ten-amino acid proline-rich SH3 binding site. Science. 1993 Feb 19;259(5098):1157–1161. doi: 10.1126/science.8438166. [DOI] [PubMed] [Google Scholar]
- Rottapel R., Reedijk M., Williams D. E., Lyman S. D., Anderson D. M., Pawson T., Bernstein A. The Steel/W transduction pathway: kit autophosphorylation and its association with a unique subset of cytoplasmic signaling proteins is induced by the Steel factor. Mol Cell Biol. 1991 Jun;11(6):3043–3051. doi: 10.1128/mcb.11.6.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozakis-Adcock M., Fernley R., Wade J., Pawson T., Bowtell D. The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1. Nature. 1993 May 6;363(6424):83–85. doi: 10.1038/363083a0. [DOI] [PubMed] [Google Scholar]
- Rozakis-Adcock M., McGlade J., Mbamalu G., Pelicci G., Daly R., Li W., Batzer A., Thomas S., Brugge J., Pelicci P. G. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature. 1992 Dec 17;360(6405):689–692. doi: 10.1038/360689a0. [DOI] [PubMed] [Google Scholar]
- Schiff-Maker L., Burns M. C., Konopka J. B., Clark S., Witte O. N., Rosenberg N. Monoclonal antibodies specific for v-abl- and c-abl-encoded molecules. J Virol. 1986 Mar;57(3):1182–1186. doi: 10.1128/jvi.57.3.1182-1186.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J. Mitogenic response to colony-stimulating factor 1. Trends Genet. 1991 Nov-Dec;7(11-12):398–402. doi: 10.1016/0168-9525(91)90263-p. [DOI] [PubMed] [Google Scholar]
- Shtivelman E., Lifshitz B., Gale R. P., Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985 Jun 13;315(6020):550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- Simon M. A., Bowtell D. D., Dodson G. S., Laverty T. R., Rubin G. M. Ras1 and a putative guanine nucleotide exchange factor perform crucial steps in signaling by the sevenless protein tyrosine kinase. Cell. 1991 Nov 15;67(4):701–716. doi: 10.1016/0092-8674(91)90065-7. [DOI] [PubMed] [Google Scholar]
- Simon M. A., Dodson G. S., Rubin G. M. An SH3-SH2-SH3 protein is required for p21Ras1 activation and binds to sevenless and Sos proteins in vitro. Cell. 1993 Apr 9;73(1):169–177. doi: 10.1016/0092-8674(93)90169-q. [DOI] [PubMed] [Google Scholar]
- Skolnik E. Y., Lee C. H., Batzer A., Vicentini L. M., Zhou M., Daly R., Myers M. J., Jr, Backer J. M., Ullrich A., White M. F. The SH2/SH3 domain-containing protein GRB2 interacts with tyrosine-phosphorylated IRS1 and Shc: implications for insulin control of ras signalling. EMBO J. 1993 May;12(5):1929–1936. doi: 10.1002/j.1460-2075.1993.tb05842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Smith M. R., DeGudicibus S. J., Stacey D. W. Requirement for c-ras proteins during viral oncogene transformation. Nature. 1986 Apr 10;320(6062):540–543. doi: 10.1038/320540a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z., Shoelson S. E., Chaudhuri M., Gish G., Pawson T., Haser W. G., King F., Roberts T., Ratnofsky S., Lechleider R. J. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993 Mar 12;72(5):767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- Suen K. L., Bustelo X. R., Pawson T., Barbacid M. Molecular cloning of the mouse grb2 gene: differential interaction of the Grb2 adaptor protein with epidermal growth factor and nerve growth factor receptors. Mol Cell Biol. 1993 Sep;13(9):5500–5512. doi: 10.1128/mcb.13.9.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S., Wong T. W. A manual sequencing method for identification of phosphorylated amino acids in phosphopeptides. Anal Biochem. 1991 Aug 15;197(1):65–68. doi: 10.1016/0003-2697(91)90356-x. [DOI] [PubMed] [Google Scholar]
- Torigoe T., O'Connor R., Santoli D., Reed J. C. Interleukin-3 regulates the activity of the LYN protein-tyrosine kinase in myeloid-committed leukemic cell lines. Blood. 1992 Aug 1;80(3):617–624. [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Bolen J. B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988 Oct 21;55(2):301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Waksman G., Shoelson S. E., Pant N., Cowburn D., Kuriyan J. Binding of a high affinity phosphotyrosyl peptide to the Src SH2 domain: crystal structures of the complexed and peptide-free forms. Cell. 1993 Mar 12;72(5):779–790. doi: 10.1016/0092-8674(93)90405-f. [DOI] [PubMed] [Google Scholar]
- Wang L. M., Keegan A. D., Paul W. E., Heidaran M. A., Gutkind J. S., Pierce J. H. IL-4 activates a distinct signal transduction cascade from IL-3 in factor-dependent myeloid cells. EMBO J. 1992 Dec;11(13):4899–4908. doi: 10.1002/j.1460-2075.1992.tb05596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]