Abstract
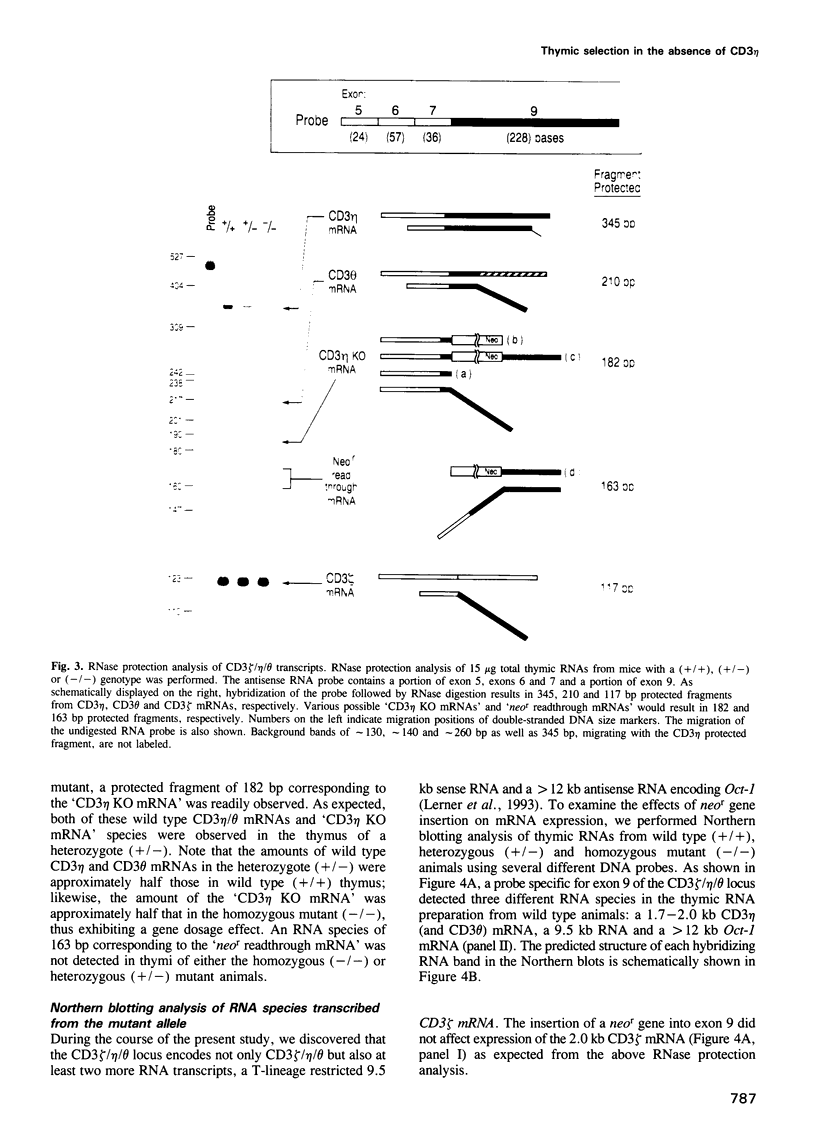
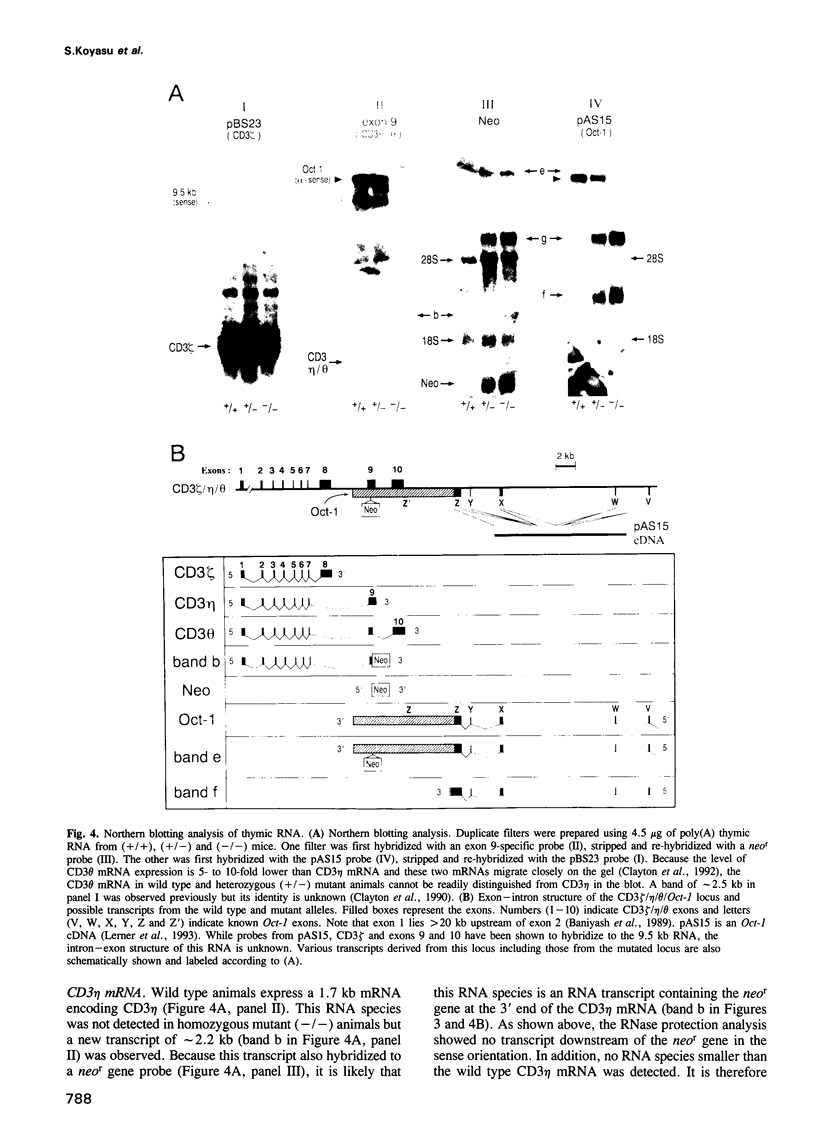
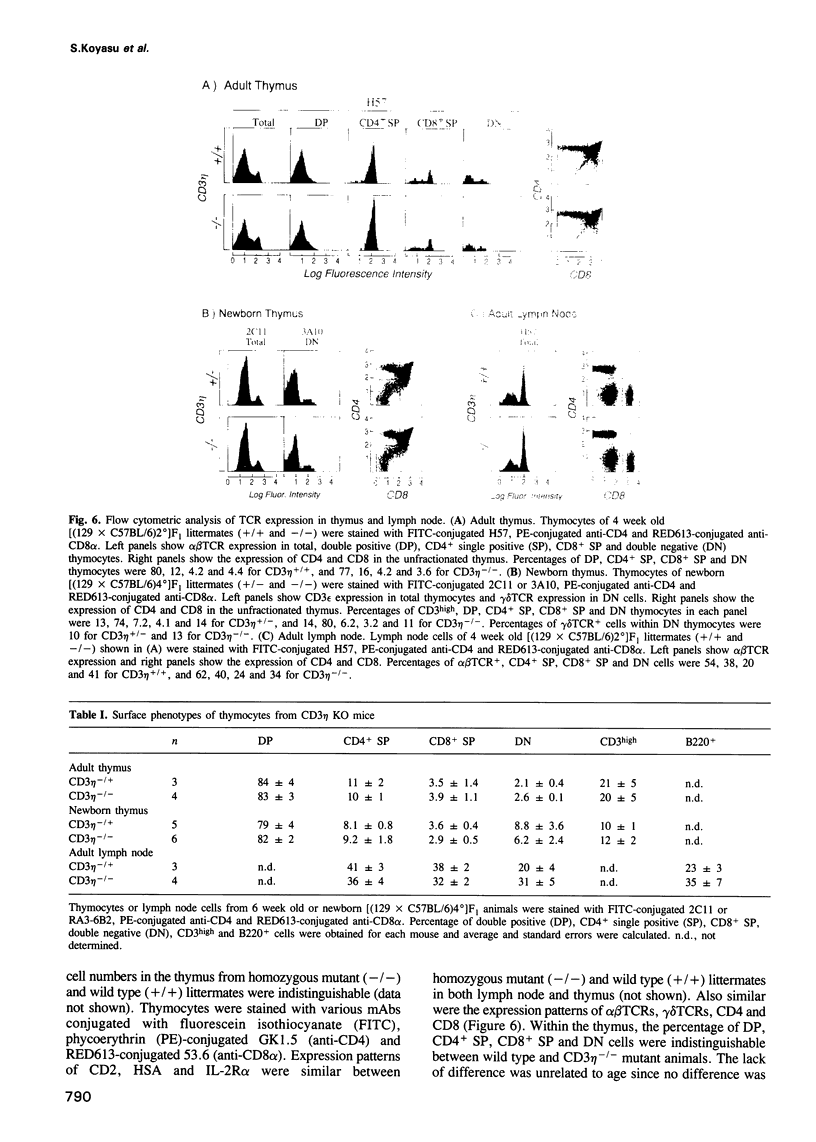
To elucidate the role of the CD3 eta subunit of the T cell receptor (TCR) in thymic development, a CD3 eta -/- mouse was generated by gene targeting. Insertion of a neomycin resistance gene into exon 9 of the CD3 zeta/eta/phi locus disrupted expression of CD3 eta and CD3 phi without affecting the expression of CD3 zeta. Little difference was observed between wild type and CD3 eta -/- mice with regard to cellularity or subset composition in thymus and peripheral lymphoid organs. Furthermore, neither alloproliferative responses nor cytotoxic T lymphocyte generation and effector function was affected by the mutation. The effect of the CD3 eta -/- mutation on thymic selection was examined by crossing the CD3 eta knockout animals with anti-HY TCR transgenic animals: the absence of the CD3 eta subunit altered neither positive nor negative selection. Thus, CD3 eta is not required for thymic selection. Of note, the birth rate of the CD3 eta -/- animals was significantly lower than that of wild type or heterozygous animals (P = 0.041-0.002). This unexpected result is probably the consequence of an alteration in mRNA expression of the Oct-1 nuclear transcription factor in CD3 eta -/- animals. The CD3 zeta/eta/phi locus partially overlaps the gene encoding Oct-1 whose transcription is dysregulated by the CD3 eta -/- mutation. Our results clearly underscore the value of characterizing all products of a genetic locus disrupted by gene targeting.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baniyash M., Garcia-Morales P., Bonifacino J. S., Samelson L. E., Klausner R. D. Disulfide linkage of the zeta and eta chains of the T cell receptor. Possible identification of two structural classes of receptors. J Biol Chem. 1988 Jul 15;263(20):9874–9878. [PubMed] [Google Scholar]
- Baniyash M., Hsu V. W., Seldin M. F., Klausner R. D. The isolation and characterization of the murine T cell antigen receptor zeta chain gene. J Biol Chem. 1989 Aug 5;264(22):13252–13257. [PubMed] [Google Scholar]
- Bauer A., McConkey D. J., Howard F. D., Clayton L. K., Novick D., Koyasu S., Reinherz E. L. Differential signal transduction via T-cell receptor CD3 zeta 2, CD3 zeta-eta, and CD3 eta 2 isoforms. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3842–3846. doi: 10.1073/pnas.88.9.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg R. S., Ley S., Sancho J., Lonberg N., Lacy E., McDermott F., Schad V., Greenstein J. L., Terhorst C. Structure of the T-cell antigen receptor: evidence for two CD3 epsilon subunits in the T-cell receptor-CD3 complex. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7220–7224. doi: 10.1073/pnas.87.18.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M. B., Strominger J. L., Krangel M. S. The gamma delta T cell receptor. Adv Immunol. 1988;43:133–192. [PubMed] [Google Scholar]
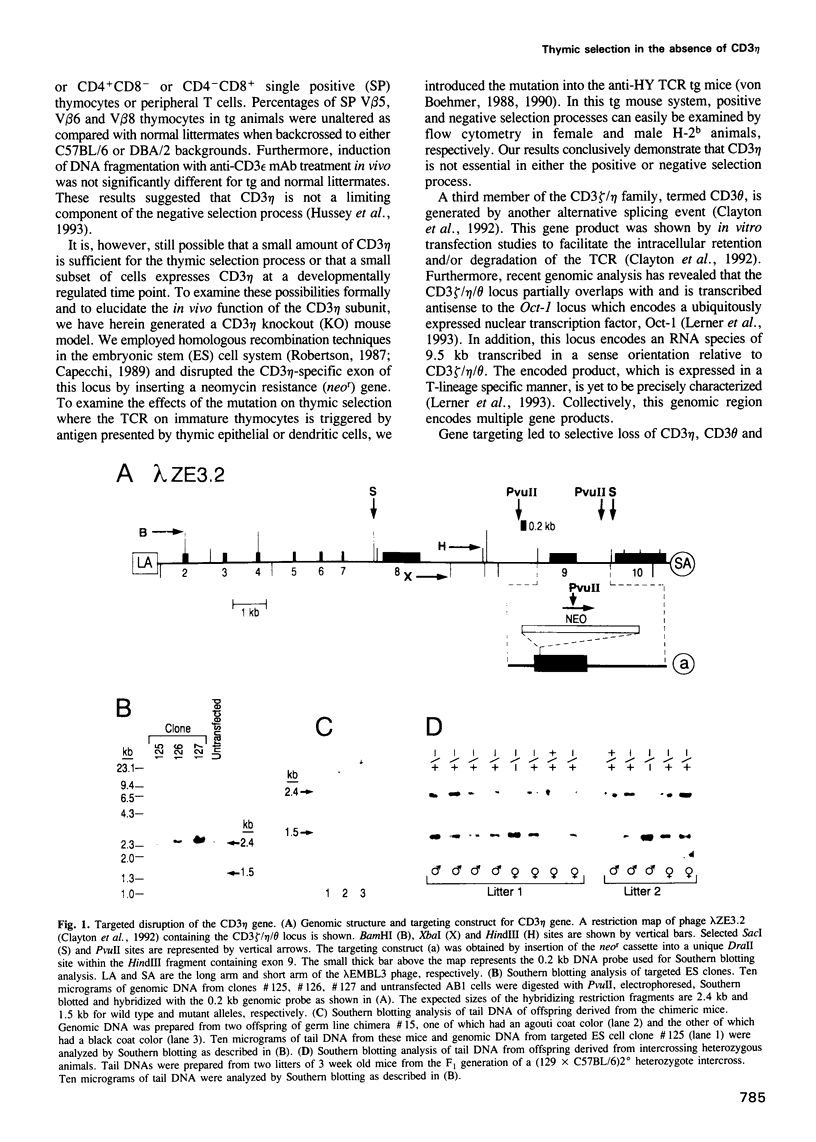
- Capecchi M. R. Altering the genome by homologous recombination. Science. 1989 Jun 16;244(4910):1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Clayton L. K., Bauer A., Jin Y. J., D'Adamio L., Koyasu S., Reinherz E. L. Characterization of thymus-derived lymphocytes expressing Ti alpha-beta CD3 gamma delta epsilon zeta-zeta, Ti alpha-beta CD3 gamma delta epsilon eta-eta or Ti alpha-beta CD3 gamma delta epsilon zeta-zeta/zeta-eta antigen receptor isoforms: analysis by gene transfection. J Exp Med. 1990 Oct 1;172(4):1243–1253. doi: 10.1084/jem.172.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton L. K., D'Adamio L., Howard F. D., Sieh M., Hussey R. E., Koyasu S., Reinherz E. L. CD3 eta and CD3 zeta are alternatively spliced products of a common genetic locus and are transcriptionally and/or post-transcriptionally regulated during T-cell development. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5202–5206. doi: 10.1073/pnas.88.12.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton L. K., Diener A. C., Lerner A., Tse A. G., Koyasu S., Reinherz E. L. Differential regulation of T-cell receptor processing and surface expression affected by CD3 theta, an alternatively spliced product of the CD3 zeta/eta gene locus. J Biol Chem. 1992 Dec 25;267(36):26023–26030. [PubMed] [Google Scholar]
- Clevers H., Alarcon B., Wileman T., Terhorst C. The T cell receptor/CD3 complex: a dynamic protein ensemble. Annu Rev Immunol. 1988;6:629–662. doi: 10.1146/annurev.iy.06.040188.003213. [DOI] [PubMed] [Google Scholar]
- Coffman R. L. Surface antigen expression and immunoglobulin gene rearrangement during mouse pre-B cell development. Immunol Rev. 1982;69:5–23. doi: 10.1111/j.1600-065x.1983.tb00446.x. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Hussey R. E., Clayton L. K., Diener A., McConkey D. J., Howard F. D., Rodewald H. R., D'Adamio L., Dallenbach F., Stein H., Schmidt E. V. Overexpression of CD3 eta during thymic development does not alter the negative selection process. J Immunol. 1993 Feb 15;150(4):1183–1194. [PubMed] [Google Scholar]
- Itohara S., Nakanishi N., Kanagawa O., Kubo R., Tonegawa S. Monoclonal antibodies specific to native murine T-cell receptor gamma delta: analysis of gamma delta T cells during thymic ontogeny and in peripheral lymphoid organs. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5094–5098. doi: 10.1073/pnas.86.13.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J. P., Cenciarelli C., Hou D., Rellahan B. L., Dean M., Weissman A. M. T cell antigen receptor-eta subunit. Low levels of expression and limited cross-species conservation. J Immunol. 1993 Jan 1;150(1):122–130. [PubMed] [Google Scholar]
- Jensen J. P., Hou D., Ramsburg M., Taylor A., Dean M., Weissman A. M. Organization of the human T cell receptor zeta/eta gene and its genetic linkage to the Fc gamma RII-Fc gamma RIII gene cluster. J Immunol. 1992 Apr 15;148(8):2563–2571. [PubMed] [Google Scholar]
- Jin Y. J., Clayton L. K., Howard F. D., Koyasu S., Sieh M., Steinbrich R., Tarr G. E., Reinherz E. L. Molecular cloning of the CD3 eta subunit identifies a CD3 zeta-related product in thymus-derived cells. Proc Natl Acad Sci U S A. 1990 May;87(9):3319–3323. doi: 10.1073/pnas.87.9.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y. J., Koyasu S., Moingeon P., Steinbrich R., Tarr G. E., Reinherz E. L. A fraction of CD3 epsilon subunits exists as disulfide-linked dimers in both human and murine T lymphocytes. J Biol Chem. 1990 Sep 15;265(26):15850–15853. [PubMed] [Google Scholar]
- Kisielow P., Blüthmann H., Staerz U. D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988 Jun 23;333(6175):742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Koning F., Maloy W. L., Coligan J. E. The implications of subunit interactions for the structure of the T cell receptor-CD3 complex. Eur J Immunol. 1990 Feb;20(2):299–305. doi: 10.1002/eji.1830200211. [DOI] [PubMed] [Google Scholar]
- Koyasu S., D'Adamio L., Arulanandam A. R., Abraham S., Clayton L. K., Reinherz E. L. T cell receptor complexes containing Fc epsilon RI gamma homodimers in lieu of CD3 zeta and CD3 eta components: a novel isoform expressed on large granular lymphocytes. J Exp Med. 1992 Jan 1;175(1):203–209. doi: 10.1084/jem.175.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo R. T., Born W., Kappler J. W., Marrack P., Pigeon M. Characterization of a monoclonal antibody which detects all murine alpha beta T cell receptors. J Immunol. 1989 Apr 15;142(8):2736–2742. [PubMed] [Google Scholar]
- Küster H., Thompson H., Kinet J. P. Characterization and expression of the gene for the human Fc receptor gamma subunit. Definition of a new gene family. J Biol Chem. 1990 Apr 15;265(11):6448–6452. [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
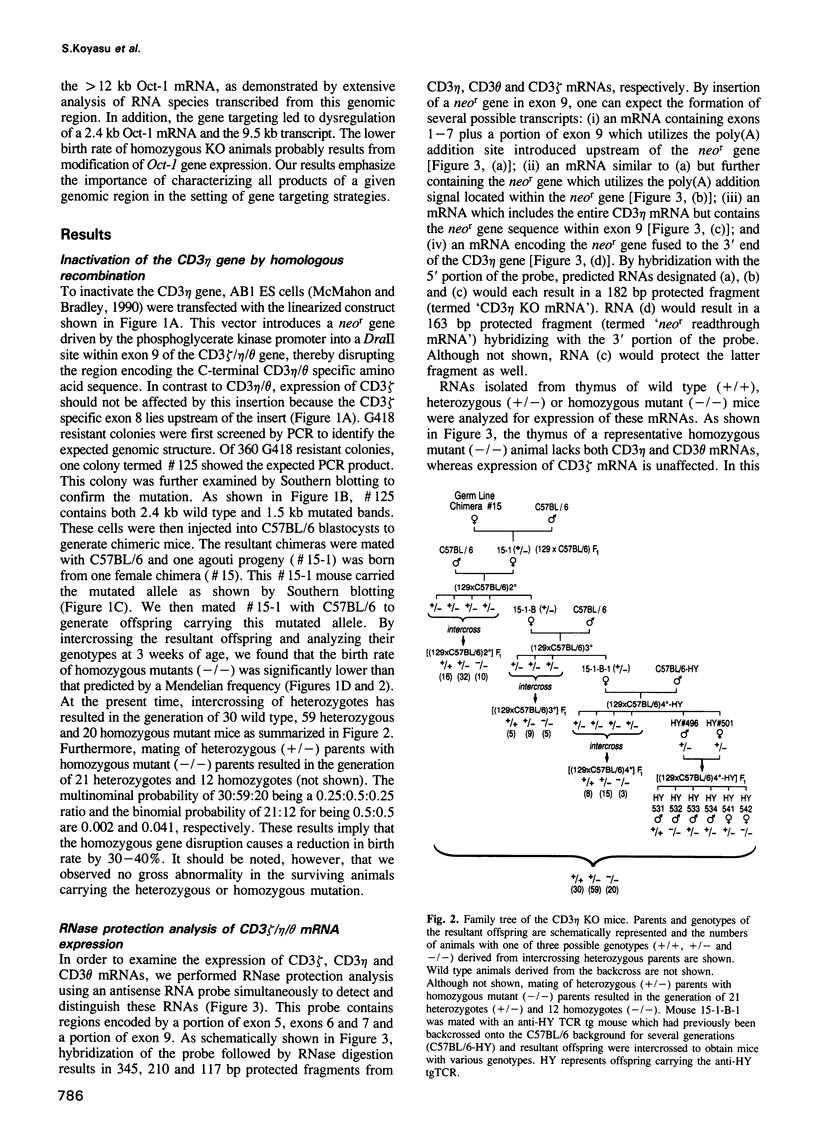
- Lerner A., D'Adamio L., Diener A. C., Clayton L. K., Reinherz E. L. CD3 zeta/eta/theta locus is colinear with and transcribed antisense to the gene encoding the transcription factor Oct-1. J Immunol. 1993 Sep 15;151(6):3152–3162. [PubMed] [Google Scholar]
- Lerner A., Diener A. C., Reinherz E. L., Clayton L. K. Human genomic sequences corresponding to murine CD3 eta-related transcripts: lack of conservation or expression of homologous human products. Eur J Immunol. 1992 Aug;22(8):2135–2140. doi: 10.1002/eji.1830220826. [DOI] [PubMed] [Google Scholar]
- Love P. E., Shores E. W., Johnson M. D., Tremblay M. L., Lee E. J., Grinberg A., Huang S. P., Singer A., Westphal H. T cell development in mice that lack the zeta chain of the T cell antigen receptor complex. Science. 1993 Aug 13;261(5123):918–921. doi: 10.1126/science.7688481. [DOI] [PubMed] [Google Scholar]
- Lowenthal J. W., Corthésy P., Tougne C., Lees R., MacDonald H. R., Nabholz M. High and low affinity IL 2 receptors: analysis by IL 2 dissociation rate and reactivity with monoclonal anti-receptor antibody PC61. J Immunol. 1985 Dec;135(6):3988–3994. [PubMed] [Google Scholar]
- Malissen M., Gillet A., Rocha B., Trucy J., Vivier E., Boyer C., Köntgen F., Brun N., Mazza G., Spanopoulou E. T cell development in mice lacking the CD3-zeta/eta gene. EMBO J. 1993 Nov;12(11):4347–4355. doi: 10.1002/j.1460-2075.1993.tb06119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolios N., Letourneur F., Bonifacino J. S., Klausner R. D. Pairwise, cooperative and inhibitory interactions describe the assembly and probable structure of the T-cell antigen receptor. EMBO J. 1991 Jul;10(7):1643–1651. doi: 10.1002/j.1460-2075.1991.tb07687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The antigen-specific, major histocompatibility complex-restricted receptor on T cells. Adv Immunol. 1986;38:1–30. doi: 10.1016/s0065-2776(08)60005-x. [DOI] [PubMed] [Google Scholar]
- McMahon A. P., Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990 Sep 21;62(6):1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merćep M., Weissman A. M., Frank S. J., Klausner R. D., Ashwell J. D. Activation-driven programmed cell death and T cell receptor zeta eta expression. Science. 1989 Dec 1;246(4934):1162–1165. doi: 10.1126/science.2531464. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Acuto O., Hercend T., Schlossman S. F., Reinherz E. L. The human T-cell receptor. Annu Rev Immunol. 1984;2:23–50. doi: 10.1146/annurev.iy.02.040184.000323. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H., O'Shea J. J., Longo D. L., Loeffler C. M., McVicar D. W., Ochoa A. C. Alterations in signal transduction molecules in T lymphocytes from tumor-bearing mice. Science. 1992 Dec 11;258(5089):1795–1798. doi: 10.1126/science.1465616. [DOI] [PubMed] [Google Scholar]
- Ohno H., Aoe T., Taki S., Kitamura D., Ishida Y., Rajewsky K., Saito T. Developmental and functional impairment of T cells in mice lacking CD3 zeta chains. EMBO J. 1993 Nov;12(11):4357–4366. doi: 10.1002/j.1460-2075.1993.tb06120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H., Saito T. CD3 zeta and eta chains are produced by alternative splicing from a common gene. Int Immunol. 1990;2(11):1117–1119. doi: 10.1093/intimm/2.11.1117. [DOI] [PubMed] [Google Scholar]
- Orloff D. G., Frank S. J., Robey F. A., Weissman A. M., Klausner R. D. Biochemical characterization of the eta chain of the T-cell receptor. A unique subunit related to zeta. J Biol Chem. 1989 Sep 5;264(25):14812–14817. [PubMed] [Google Scholar]
- Orloff D. G., Ra C. S., Frank S. J., Klausner R. D., Kinet J. P. Family of disulphide-linked dimers containing the zeta and eta chains of the T-cell receptor and the gamma chain of Fc receptors. Nature. 1990 Sep 13;347(6289):189–191. doi: 10.1038/347189a0. [DOI] [PubMed] [Google Scholar]
- Raulet D. H. The structure, function, and molecular genetics of the gamma/delta T cell receptor. Annu Rev Immunol. 1989;7:175–207. doi: 10.1146/annurev.iy.07.040189.001135. [DOI] [PubMed] [Google Scholar]
- Rodewald H. R., Arulanandam A. R., Koyasu S., Reinherz E. L. The high affinity Fc epsilon receptor gamma subunit (Fc epsilon RI gamma) facilitates T cell receptor expression and antigen/major histocompatibility complex-driven signaling in the absence of CD3 zeta and CD3 eta. J Biol Chem. 1991 Aug 25;266(24):15974–15978. [PubMed] [Google Scholar]
- Samelson L. E., Harford J. B., Klausner R. D. Identification of the components of the murine T cell antigen receptor complex. Cell. 1985 Nov;43(1):223–231. doi: 10.1016/0092-8674(85)90027-3. [DOI] [PubMed] [Google Scholar]
- Soriano P., Montgomery C., Geske R., Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991 Feb 22;64(4):693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Springer T., Galfrè G., Secher D. S., Milstein C. Monoclonal xenogeneic antibodies to murine cell surface antigens: identification of novel leukocyte differentiation antigens. Eur J Immunol. 1978 Aug;8(8):539–551. doi: 10.1002/eji.1830080802. [DOI] [PubMed] [Google Scholar]
- Staerz U. D., Rammensee H. G., Benedetto J. D., Bevan M. J. Characterization of a murine monoclonal antibody specific for an allotypic determinant on T cell antigen receptor. J Immunol. 1985 Jun;134(6):3994–4000. [PubMed] [Google Scholar]
- Sussman J. J., Bonifacino J. S., Lippincott-Schwartz J., Weissman A. M., Saito T., Klausner R. D., Ashwell J. D. Failure to synthesize the T cell CD3-zeta chain: structure and function of a partial T cell receptor complex. Cell. 1988 Jan 15;52(1):85–95. doi: 10.1016/0092-8674(88)90533-8. [DOI] [PubMed] [Google Scholar]
- Teh H. S., Kisielow P., Scott B., Kishi H., Uematsu Y., Blüthmann H., von Boehmer H. Thymic major histocompatibility complex antigens and the alpha beta T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature. 1988 Sep 15;335(6187):229–233. doi: 10.1038/335229a0. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979 Sep 19;150(3):580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman A. M., Baniyash M., Hou D., Samelson L. E., Burgess W. H., Klausner R. D. Molecular cloning of the zeta chain of the T cell antigen receptor. Science. 1988 Feb 26;239(4843):1018–1021. doi: 10.1126/science.3278377. [DOI] [PubMed] [Google Scholar]
- Yagita H., Nakamura T., Karasuyama H., Okumura K. Monoclonal antibodies specific for murine CD2 reveal its presence on B as well as T cells. Proc Natl Acad Sci U S A. 1989 Jan;86(2):645–649. doi: 10.1073/pnas.86.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Hera A., Müller U., Olsson C., Isaaz S., Tunnacliffe A. Structure of the T cell antigen receptor (TCR): two CD3 epsilon subunits in a functional TCR/CD3 complex. J Exp Med. 1991 Jan 1;173(1):7–17. doi: 10.1084/jem.173.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H. Developmental biology of T cells in T cell-receptor transgenic mice. Annu Rev Immunol. 1990;8:531–556. doi: 10.1146/annurev.iy.08.040190.002531. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. The developmental biology of T lymphocytes. Annu Rev Immunol. 1988;6:309–326. doi: 10.1146/annurev.iy.06.040188.001521. [DOI] [PubMed] [Google Scholar]