Abstract
Mass and body composition are indices of overall animal health and energetic balance and are often used as indicators of resource availability in the environment. This study used morphometric models and isotopic dilution techniques, two commonly used methods in the marine mammal field, to assess body composition of Weddell seals (Leptonychotes weddellii, N = 111). Findings indicated that traditional morphometric models that use a series of circular, truncated cones to calculate marine mammal blubber volume and mass overestimated the animal’s measured body mass by 26.9±1.5% SE. However, we developed a new morphometric model that uses elliptical truncated cones, and estimates mass with only −2.8±1.7% error (N = 10). Because this elliptical truncated cone model can estimate body mass without the need for additional correction factors, it has the potential to be a broadly applicable method in marine mammal species. While using elliptical truncated cones yielded significantly smaller blubber mass estimates than circular cones (10.2±0.8% difference; or 3.5±0.3% total body mass), both truncated cone models significantly underestimated total body lipid content as compared to isotopic dilution results, suggesting that animals have substantial internal lipid stores (N = 76). Multiple linear regressions were used to determine the minimum number of morphometric measurements needed to reliably estimate animal mass and body composition so that future animal handling times could be reduced. Reduced models estimated body mass and lipid mass with reasonable accuracy using fewer than five morphometric measurements (root-mean-square-error: 4.91% for body mass, 10.90% for lipid mass, and 10.43% for % lipid). This indicates that when test datasets are available to create calibration coefficients, regression models also offer a way to improve body mass and condition estimates in situations where animal handling times must be short and efficient.
Introduction
Establishing links among variations in environmental conditions, prey availability, foraging success, and population status has become increasingly important as ecosystems face climate and anthropogenic threats. While monitoring ecosystem processes can be difficult, changes in the mass and body condition of apex predators can be used as indices of ecosystem health [1]–[5]. Accurate estimates of body mass and condition are also essential for a wide range of ecological and physiological studies, as they represent animals’ net energetic costs or gains [6]–[8]. In addition to being a proxy for overall animal health and fitness, in marine mammals, body composition also influences animal streamlining, buoyancy, metabolic demand, and thermoregulatory costs [9]–[14].
In fieldwork situations, mass and body composition (e.g., lipid stores) can be most directly measured by weighing animals and using hydrogen-isotope dilution techniques, respectively. Isotopic dilution methods measure the animal’s total body water (TBW) volume by allowing a bolus of labeled water to dilute within the body water pool. Measured TBW volume, coupled with estimates of the hydration state of lean tissue (73% water) and adipose (10% water), allows for relatively accurate (mean error: 3.7%) estimates of body composition in mammals [7], [15], [16]. Errors arise from the generation of metabolic water, exchange of isotope with non-aqueous hydrogen ions, dilution in stomach water, and evaporative water loss [6], [16], [17]. Method accuracy is also influenced by errors in the assumed hydration state of body tissue, as water content in the blubber and lean tissue may differ by species, season, and age [18]–[20]. Additionally, validations of isotopic dilution to true TBW and lipid stores by desiccation and dissection comparisons have only been performed in a select number of studies because these destructive methods are so labor intensive [21]–[25]. Still, isotopic dilution methods have been used in a wide range of species, including pinnipeds, and are generally assumed to be the “golden standard.” Despite the potential sources of error, isotopic dilution has the advantage of accounting for both the subcutaneous and internal lipid stores, and in most study systems, body composition determined by these methods is considered to be the most reliable field measure of total body lipid content [6], [20].
However, isotopic dilution protocols can be logistically difficult, costly, and time consuming. As a result, a wide variety of proxy variables have been identified to serve as indicators of marine mammal body condition. Because marine mammals store large amounts of energy in their large subcutaneous blubber layer, these simpler methods have placed a large emphasis on blubber volume. Proxies range from models using a single length and girth measurement to estimate body mass, to a single blubber depth or bioelectrical impedance analysis to indicate animal body condition [25]–[28]. As these overly simplistic models are often poor predictors of body composition, Gales & Burton [29] outlined a technique for determining blubber volume in pinnipeds. Using morphometric (lengths and girths) and ultrasound blubber depth measurements, the animal’s body shape is reconstructed as a series of circular truncated cones. Blubber and core tissue density estimates are then used to convert the calculated volumes of the cones to blubber mass. This truncated cone method has been used to determine blubber mass in multiple pinniped [8], [9], [11], [30], [31] and cetacean species [32], [33]. However, soon after this method was developed, Slip et al. [34] described the phenomenon of “fat slumping” wherein gravity causes blubber along the lateral sides of the animal to “slump” and, therefore, causes the animal’s true shape to deviate from circular towards elliptical.
Since Gales & Burton [29], many studies have used modeled estimates of blubber volume to calculate body composition, despite the fact that blubber mass measurements are not equivalent to lipid mass. Adult marine mammal blubber contains structural proteins and is not composed entirely of lipid. Blubber lipid content ranges from ∼30−95%, depending on species, reproductive status, overall health, and season [19], [35]–[38]. Still, very few studies incorporate actual blubber lipid content into calculations [31], [39]. In addition, pinniped studies that solely use the truncated cones method as a measure of condition cannot account for internal lipid stores. Thus, potential sources of error should be acknowledged when evaluating the success with which a morphometric technique can determine body mass and condition.
However, morphometric models are so attractive because studies at the population level require large sample sizes to detect potentially small but significant changes in mass and condition. For large animals such as marine mammals, determining body mass via direct weighing is difficult, and isotopic dilution requires long sedation and equilibration times. Morphometric models thus offer a good alternative, yet very few studies have attempted to construct predictive models that employ only a few non-invasive measurements to estimate body mass and condition [34], [40].
This study compared methods of estimating Weddell seal (Leptonychotes weddellii) body composition, including morphometric models and isotopic dilution techniques. In addition, we developed a modified truncated cone method that accounts for blubber and core body slumping by modeling animal cross-sectional shape as ellipses instead of circles, and compared accuracy of body mass and condition estimates. Then, we developed models to estimate body mass and composition from a few non-invasive morphometric measurements. Our findings provide a quantitative basis for choosing efficient and logistically feasible methods of assessing marine mammal body mass and condition under constrained field conditions.
Materials and Methods
Ethics Statement
Animal handling protocols were approved by the University of Alaska Anchorage and University of California Santa Cruz’s Institutional Animal Care and Use Committees. Research and sample import to the United States was authorized under the Marine Mammal permit No. 87-1851-04 issued by the Office of Protected Resources, National Marine Fisheries Service. Research activities were approved through Antarctic Conservation Act permits while at McMurdo Station.
Animal Capture
Adult Weddell seals (N = 111; Table 1) were captured on fast-ice in Erebus Bay (∼77°S, 165°E) and the Victorialand coastline (∼76°S, 162°E), Antarctica from 2010−2012. Animals were handled in Jan/Feb (Austral fall) after the molt period when seals are typically in their poorest condition (lowest lipid stores) and in Oct/Nov (Austral spring; pre-breeding period) after the animals have been actively foraging for ∼8 months [41].
Table 1. Sample sizes and means ± SE for body mass and composition.
| Season | Reproductive Status | Total Body Mass (MT; kg) | TBW (%MT) | Lipid by HTO (%MT) | Blubber Biopsy Lipid Content (%) |
| Jan/Feb | Skip-Breeding Female | 320.7±10.3 (52) | 50.7±0.5 (32) | 30.5±0.7 (32) | 79.3±1.3 (49) |
| Male | 231.8±12.5 (10) | 50.2±0.7 (7) | 31.4±1.1 (7) | 86.2±2.2 (5) | |
| Oct/Nov | Non-Reproductive Female | 335.8±14.2 (28) | 45.9±0.6 (22) | 37.6±0.8 (22) | 83.5±1.4 (24) |
| Reproductive Female | 413.7±13.3 (16) | 46.4±0.6 (13) | 36.8±0.9 (13) | 84.1±1.9 (17) | |
| Male | 294.6±11.7 (5) | 47.5±1.2 (2) | 35.4±1.9 (2) | --- | |
| Overall | All | 328.8±7.6 (111) | 48.5±0.4 (76) | 33.9±0.6 (76) | 81.6±0.9 (95) |
Mean ± SE total body mass (MT), total body water (TBW) and lipid stores as determined by isotopic dilution (as %MT), and lipid content of blubber biopsies (% wet mass) for animals handled throughout this study. Animals are classed by season and reproductive status, and sample sizes are in parentheses.
Animals were sedated with an initial intramuscular dose of 1.0 mg·kg−1 tiletamine/zolazepam HCl, followed by intravenous injections of ketamine and diazepam (1∶1 ratio; 100 mg·mL−1 and 5 mg·mL−1) as necessary.
Direct Measures of Total Body Mass and Lipid Stores
Total body mass (MT) was determined by direct weighing (MSI-7200-1T Dyna-Link digital dynamometer, capacity 1,000±1.0 kg).
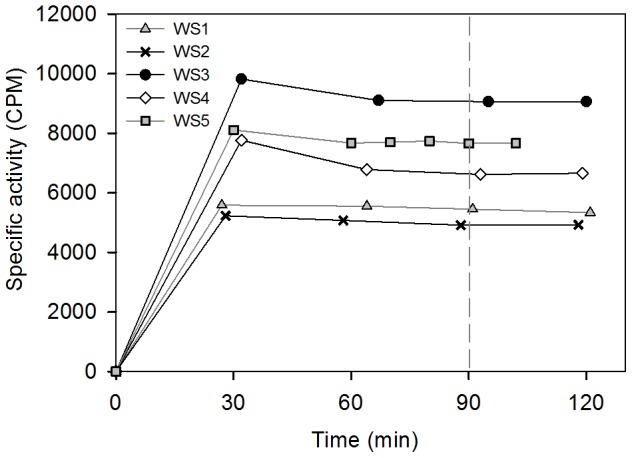
The body composition of 76 animals (Table 1) was determined by isotopic dilution. Following collection of an initial blood sample, 1−1.5 mCi tritiated water (HTO) was injected into the extradural vein. Each syringe was gravimetrically calibrated prior to use, and syringes were flushed with blood after injection to ensure complete administration of the dose. Blood was collected from the extradural vein in serum separator vacutainers at 15−30 min intervals for 90−120 min post-injection. Serum was separated from whole blood samples via centrifugation and stored at −80°C until analysis. Water was extracted from samples in triplicate using the freeze-capture technique as described in Ortiz et al. [15]. HTO specific activity (counts per minute; CPM) was determined using a Packard Tri-Carb 2900TR liquid scintillation counter (Packard Bioscience Co., Meriden, CT) by adding 100 µL distillate into 10 mL ScintiSafe scintillation cocktail (Fisher Scientific, Inc.). Each of the triplicate distillate samples was counted twice for 20 min (∼10,000−20,000 total counts). Triplicates were only accepted if CV’s were <2%. Pre-injection activity determined for each animal was subtracted from all post-injection activities. Injectate standards were distilled in six replicates before and after analyzing animal serum samples to ensure minimal intra-assay variation. Dilution curves plateaued by 90 min (Fig. 1), and total body water (TBW) was calculated as:
| (1) |
Figure 1. HTO equilibration curve for five Weddell seals showing plateau by 90 min.
TBW values were reduced by 3.3% to account for post-injection isotope losses due to exchanging hydrogen ions and ventilation [42]. Total body lipid mass (TBLHTO) was calculated from TBW following Reilly & Fedak [24]:
| (2) |
where MT is total body mass, and both TBW and MT are in kg. Isotopic dilution techniques yielded total lipid mass, regardless of its location subcutaneously or internally, and lean mass was considered to be fat free tissue.
Truncated Cone Estimates of Body Mass and Composition
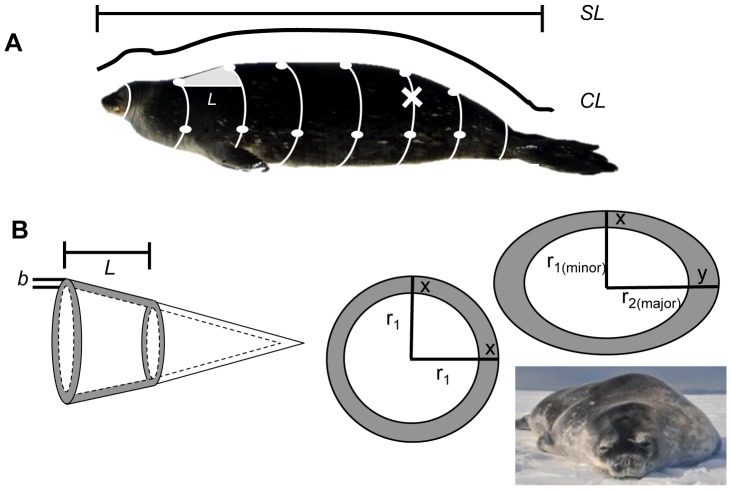
After animals were captured and weighed, a series of morphometric measurements were taken in order to model mass and body composition: girths, straight lengths (sLengths), and curvilinear lengths (cLengths) measured from the animal’s nose to eight consecutive sites along the body (Fig. 2A). Subcutaneous blubber thickness was measured at six dorsal and lateral sites (Fig. 2A) using a SonoSite Vet180Plus portable ultrasound and 3.5 MHz convex transducer (SonoSite Inc., Bothell, Washington, USA) while the animal was in sternal recumbency. Blubber depth measurements were used to calculate blubber volume and mass using the traditional truncated cones method as described by Gales & Burton [29]. The animal’s body shape was reconstructed as a series of circular truncated cones, and blubber volume was calculated as the volume of the outer cone (total body volume) minus the volume of the inner cone (core body volume; Fig. 2B). The volume of the head and tail were estimated using full cones composed entirely of lean mass, while flippers were not included in truncated cone models (Table 2) because there is very little blubber around the head, tail, fore-, and hind-flippers [43].
Figure 2. Morphometric measurements taken for each study animal.
(A) SL = Standard length, CL = Curve length, Girths = white lines, Blubber depths = white dots, Cone section length calculations = grey triangle and “L”. Site of blubber biopsy is marked with “X”. (B) Reconstruction of truncated cones with segment length “L” and blubber depth “b” (At left). Circular and elliptical cross-sections shown (At right). Because an ellipse has a major and minor radius “r,” the model can account for different dorsal and lateral blubber depths (x and y) and more accurately reflect true animal shape.
Table 2. Calculation of subcutaneous fat for seal WS12-22 using traditional truncated cones with circular cross-sections.
| Outer Cone Measurements | Inner Cone Measurements | Body Volume | Mass Conversions | |||||||||||
| Cone Position | Girth (cm) | Radius (Outer; cm) | Radius Difference (cm) | Curvilinear Length (cm) | Straight Length (cm) | Average Blubber Depth (cm) | Radius (Inner; cm) | Outer Cone (L) | Inner Cone (L) | Blubber (L) | Core (kg) | Blubber (kg) | Total Body (kg) | |
| 1 | nose | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | nose → ears | 75 | 11.9 | 11.9 | 20 | 16.1 | 0 | 11.9 | 2.4 | 2.4 | 0 | 2.6 | 0 | 2.6 |
| 3 | ears → neck | 137 | 21.8 | 9.9 | 27 | 25.1 | 5.99 | 15.8 | 23.1 | 15.3 | 7.8 | 16.8 | 7.3 | 24.2 |
| 4 | neck → axillary | 202 | 32.2 | 10.4 | 37 | 35.5 | 6.13 | 26.0 | 82.2 | 49.8 | 32.4 | 54.8 | 30.5 | 85.3 |
| 5 | axillary → sternum | 121 | 33.7 | 1.6 | 31 | 31.0 | 5.64 | 28.1 | 105.6 | 71.3 | 34.3 | 78.4 | 32.3 | 110.7 |
| 6 | sternum → middle | 206 | 32.8 | 1.0 | 38 | 38.0 | 5.32 | 27.5 | 132.1 | 92.1 | 39.9 | 101.3 | 37.5 | 138.9 |
| 7 | middle → umbilicus | 181 | 28.8 | 4.0 | 37 | 36.8 | 5.48 | 23.3 | 109.8 | 74.7 | 35.1 | 82.2 | 33.0 | 115.1 |
| 8 | umbilicus → pelvis | 127 | 20.2 | 8.6 | 42 | 41.1 | 4.55 | 15.7 | 78.4 | 49.7 | 28.7 | 54.7 | 26.9 | 81.6 |
| 9 | pelvis → ankles | 76 | 12.1 | 8.1 | 23 | 21.5 | 0 | 12.1 | 18.0 | 13.1 | 4.9 | 14.4 | 4.6 | 19.0 |
| 10 | ankles → tail | 0 | 0 | 12.1 | 21 | 17.2 | 0 | 0 | 2.6 | 2.6 | 0 | 2.9 | 0 | 2.9 |
| Total: | 554.2 | 371.0 | 183.1 | 408.2 | 172.1 | 580.3 | ||||||||
| Measured Mass (kg): | 451.0 | |||||||||||||
| Error from Measured Mass: | 28.7% | |||||||||||||
Radius outer cone = Girth/2π;
Radius difference = base − roof of cone;
Straight Length = sqrt(Curvilinear Length2 − Radius Difference2);
Radius inner cone = Radius outer cone – (2 × Blubber Depth);
Outer/Inner Cone Volume = (1/3)π × [(Radius Outer/Inner1)2 + (Radius Outer/Inner1 × Radius Outer/Inner2) + (Radius Outer/Inner2)2];
Blubber Volume = Outer Cone Volume − Inner Cone Volume;
Mass Conversions: Core Mass = Core Volume × 1.1; Blubber Mass = Blubber Volume × 0.94; Total Body Mass = Core Mass + Blubber Mass.
Because seals did not appear circular in cross-section when lying on the ice, the procedure was adjusted to include measurements of animal height and width at each site along the body for both outer and inner body cones (N = 11). The circular truncated cones calculations [29] were then modified for elliptical body cross-sections in animals for which all measurements were taken (N = 10; Fig. 2B; Table 3). In the modified elliptical cone method, the straight length for each truncated cone segment was calculated by using right triangles along the animal (Fig. 2A), with the measured curvilinear length as the hypotenuse and half the height difference between cone segments as the adjacent side of each triangle:
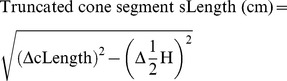 |
(3) |
Table 3. Calculation of subcutaneous fat for seal WS12-22 using truncated cones with modified, elliptical cross-sections.
| Outer Cone Measurements | Inner Cone Measurements | Body Volume | Mass Conversions | ||||||||||||||
| Cone Position | Body Height (cm) | Body Width (cm) | Minor Radius (Outer Cone; cm) | Minor Radius Difference (cm) | Curvilinear Length (cm) | Straight Length (cm) | Dorsal Blubber Depth (cm) | Lateral Blubber Depth (cm) | Diameter Minor Axis (cm) | Diameter Major Axis (cm) | Outer Cone (L) | Inner Cone (L) | Blubber (L) | Core (kg) | Blubber (kg) | Total Body (kg) | |
| 1 | nose | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | nose → ears | 20 | 33 | 10.0 | 10.0 | 20 | 17.3 | 0 | 0 | 20.0 | 33.0 | 3.0 | 3.0 | 0 | 3.3 | 0 | 3.3 |
| 3 | ears → neck | 27 | 57 | 13.5 | 3.5 | 27 | 26.8 | 5.25 | 6.72 | 16.5 | 43.6 | 22.5 | 14.5 | 8.0 | 15.9 | 7.5 | 23.4 |
| 4 | neck → axillary | 42 | 73 | 21.0 | 7.5 | 37 | 36.2 | 5.10 | 7.15 | 31.8 | 58.7 | 64.3 | 35.5 | 28.8 | 39.1 | 27.0 | 66.1 |
| 5 | axillary → sternum | 44 | 81 | 22.0 | 1.0 | 31 | 31.0 | 5.57 | 5.71 | 32.9 | 69.6 | 80.6 | 50.4 | 30.1 | 55.5 | 28.3 | 83.8 |
| 6 | sternum → middle | 41 | 85 | 20.5 | 1.5 | 38 | 38.0 | 5.82 | 4.82 | 29.4 | 75.4 | 105.1 | 67.1 | 38.0 | 73.8 | 35.7 | 109.5 |
| 7 | middle → umbilicus | 34 | 68 | 17.0 | 3.5 | 37 | 36.8 | 5.68 | 5.28 | 22.6 | 57.4 | 83.3 | 50.2 | 33.0 | 55.3 | 31.1 | 86.3 |
| 8 | umbilicus → pelvis | 30 | 45 | 15.0 | 2.0 | 42 | 42.0 | 4.67 | 4.42 | 20.7 | 36.2 | 59.6 | 33.3 | 26.3 | 36.6 | 24.7 | 61.4 |
| 9 | pelvis → ankles | 12 | 35 | 6.0 | 9.0 | 23 | 21.2 | 0 | 0 | 12.0 | 35.0 | 14.0 | 9.6 | 4.4 | 10.5 | 4.1 | 14.7 |
| 10 | ankles → tail | 0 | 0 | 0 | 6.0 | 21 | 20.1 | 0 | 0 | 0 | 0 | 2.2 | 2.2 | 0 | 2.4 | 0 | 2.4 |
| Total: | 434.6 | 265.8 | 168.6 | 292.4 | 158.6 | 451.0 | |||||||||||
| Measured Mass (kg): | 451.0 | ||||||||||||||||
| Error from Measured Mass: | 0.0% | ||||||||||||||||
Minor radius outer cone = Body Height/2;
Minor radius difference = base − roof of cone minor radius;
Straight Length = sqrt(Curvilinear Length2 – Minor Radius Difference2);
Diameter inner cone major/minor axis = Body Height/Width – (2 × Blubber Depth);
Outer/Inner Cone Volume = [(Straight length × π)/12] × [D1D2 + D3D4 + sqrt(D1D2D3D4)];
Blubber Volume = Outer Cone Volume − Inner Cone Volume;
Mass Conversions: Core Mass = Core Volume × 1.1; Blubber Mass = Blubber Volume × 0.94; Total Body Mass = Core Mass + Blubber Mass.
where ΔcLength is the curvilinear length of the elliptical truncated cone segment and Δ½H is the height difference from the center of the frustum (half the animal height) for that segment of the animal. The volume of the total body outer elliptical truncated cone was calculated as:
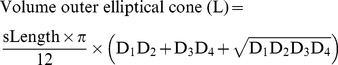 |
(4) |
where D1 and D2 are the major (measured animal width) and minor (height) diameters of the anterior end of the cone segment, and D3 and D4 are the major and minor diameters of the posterior end of the cone segment, respectively. The summation of these elliptical truncated cone segments yielded total body volume. To determine the volume of the animal’s inner core, blubber depths were subtracted from the body diameter:
| (5) |
| (6) |
The same equations were used to find D3, inner and D4, inner at the posterior end of the cone segment. Once blubber depths were subtracted, Eqn (4) was used to calculate the volume of the inner core for each truncated cone segment using these modified diameters. Summation of the core truncated cones yielded core body volume, and blubber volume was calculated as the difference between the outer and inner core volumes:
| (7) |
In both circular and elliptical truncated cone models, blubber and core volume estimates were converted to body mass by assuming that the lean body core and blubber layer had densities of 1.1 g·mL−1 and 0.94 g·mL−1, respectively [29], [30], [44]. Blubber and total body mass were estimated by summing the mass of each truncated cone segment, and the head and tail cones.
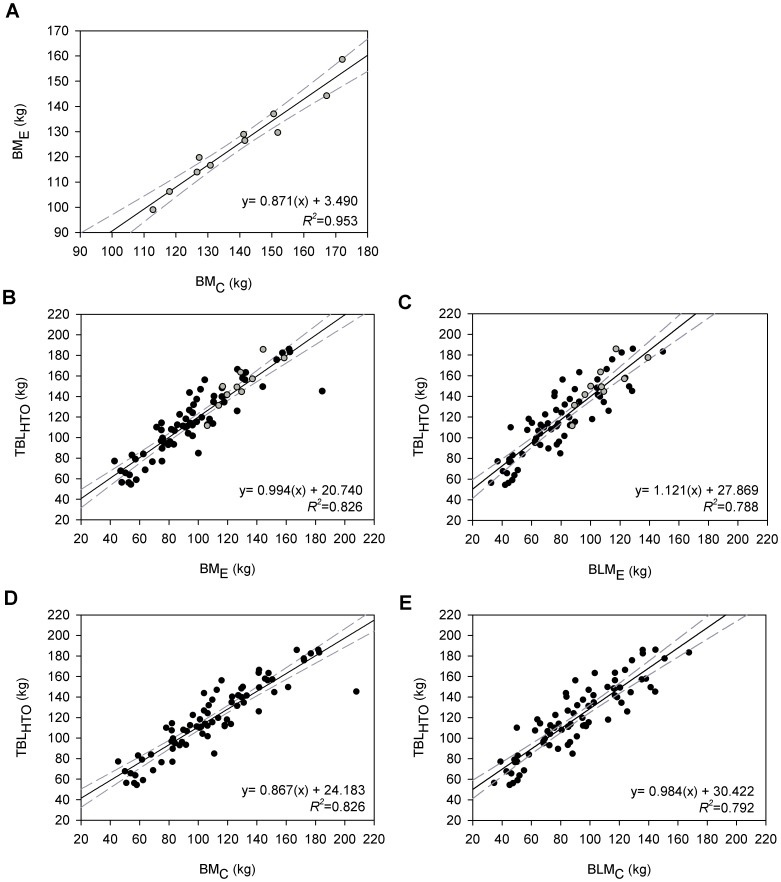
Blubber mass (BM) calculated using elliptical truncated cones (BME) was regressed against blubber mass estimated from traditional circular cones (BMC). This relationship was highly significant, and the regression equation was used to estimate BME for animals where it could not be directly calculated.
Blubber Lipid Content
A blubber biopsy was taken from each animal after the site was prepped with Betadine and Lidocaine, using a sterile 6-mm biopsy punch just below the midline at the umbilicus (Fig. 2A), flash frozen and stored at −80°C. To compare lipid content from the single biopsy site to average values across the body, blubber was collected opportunistically from a female that died of natural causes in McMurdo Sound, <24 hrs post-mortem, from all 12 sites where blubber depth was measured for truncated cone models.
Full thickness blubber biopsies were weighed to the nearest 0.001 g and lipid content of the samples was determined gravimetrically after extracting lipids using a 2∶1 chloroform-methanol rinse in a Soxhlet apparatus [45], [46]. In the event that blubber lipid content was not available for a particular animal, the average lipid content for that season and reproductive class was used to convert BM to lipid mass (Table 1). Lipid mass within the blubber layer (BLM) was determined for elliptical (BLME) and circular truncated cones (BLMC) using BM determined by morphometric models, as described above, and the lipid content per unit mass in the blubber biopsy:
| (8) |
Statistical Analyses
Prior to statistical analyses, data were assessed for outliers and normality. Body composition data were normally distributed and between 20−80%, and thus were not arcsine transformed. Results are reported as mean ± SE. To determine whether animals were indeed elliptical in cross-section, width:height ratios were compared to that of a circle (width:height = 1) using one-sample t-tests, while a two-way ANOVA was used to assess differences in the width:height ratio across the body, and between the inner and outer cones (N = 11). Paired t-tests were used to determine whether total body mass estimates derived from the circular truncated cone method differed from actual body mass (MT). Repeated-measures ANOVAs with Bonferroni post-hoc tests were used to determine whether mass and body composition estimates from both elliptical (N = 10) and circular (N = 76) truncated cone methods differed from the direct measurement of MT or TBLHTO. Regression analyses were used to determine significant relationships between calculated BM or BLM, and TBLHTO (N = 76).
To create models that maximized the R 2 and minimized root-mean-square-error (RMSE; equivalent to standard deviation), forward stepwise multiple regression models were used to estimate body mass and condition. MT was estimated from straight length (sLength) and the square of axillary girth (LG2) measurements to compare models to the simplest published methods [26], [27], and also estimated using the suite of lengths and girths measured during this study (N = 111). Animals for which all lengths, girths, and blubber depths could be measured in addition to TBLHTO (N = 76) were used to create regression models relating morphometric measures to body composition. TBLHTO was estimated with and without true MT included in models.
Second-order Akaike information criterion (AICc) tests were implemented using the R “MuMIn” package to select the best models, incorporating the fewest number of parameters, as would be useful if animal handling times in the field are constrained. Variables were only added to models when the ΔAICc of the added parameter was ≥ 2. Season, sex, and reproductive status were added as categorical “dummy” variables. If a categorical variable was an important parameter in the model, it was added to all regressions as this would be a known parameter in a fieldwork situation. When incorporating parameters into predictive models, multicollinearity was assessed by variance inflation factors (VIF); all were less than 7. While lower than a VIF of 10, which is typically considered to be a concern [47], [48], to further ensure that added parameters were not a spurious result of multicollinearity, RMSE of models was determined using k-fold cross-validations (with 10 folds) using the R “DAAG” package. Parameters were only added to the model when RMSE decreased. All analyses were conducted in R (v 2.15.2) and significance was assessed at the 95% level (P<0.05).
Results
Morphometric Estimates of Body Mass versus Weighing
Animals in this study varied widely in MT (181−502 kg), TBW (97.6−253.0 kg; 40.5−56.5% MT), and TBLHTO (54.3−186.0 kg; 22.0−45.5% MT) measurements (Table 1).
Animals were elliptical-shaped in cross-section as indicated by width:height ratios >1 at all eight sites along the body (Fig. 3; One-sample t-tests- ears: t 10 = 4.114, P = 0.002; neck to ankles: all t 10 > 10, all P<0.001). Width:height ratios differed by site along the body (Fig. 3; Two-Way ANOVA- F 7, 160 = 20.010, P<0.001) and, once blubber depths were subtracted, the inner core cone had even greater width:height ratios as compared to the outer cones (Two-Way ANOVA- F 1, 160 = 20.436, P<0.001). Larger animals and those in better condition were not more ellipsoid-shaped as compared to smaller seals (Multiple Regression- MT: F 8, 10 = 18.022, P = 0.054, TBLHTO: F 8, 9 = 5.268, P = 0.325).
Figure 3. Weddell seal body cross-sections are elliptical.
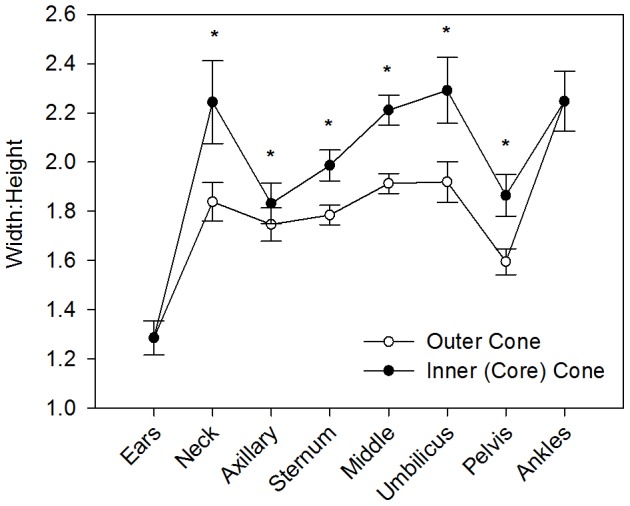
Mean ± SE width-to-height ratios along the body of adult female Weddell seals (N = 11), with a circle having a ratio = 1. Asterisk indicates that the width-to-height ratio of the inner core cone is significantly greater than the outer, total body cone.
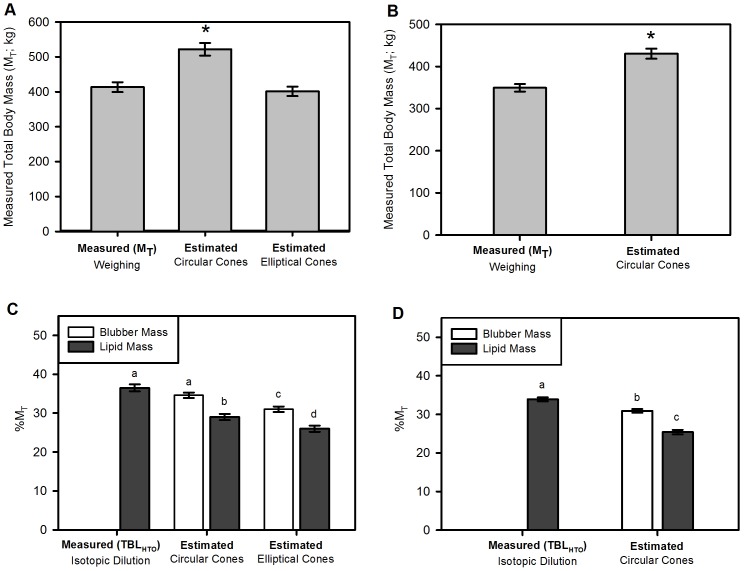
Using standard published values for blubber and body core density, estimated MT using truncated cone calculations with elliptical cross-sections were not significantly different from measured MT (Fig. 4A; Subset Study: Repeated measures ANOVA- F 2,18 = 167.442; Elliptical mean error from MT: −11.9±6.8 kg; −2.8±1.7% (range: −8.9 to +7.1%), Bonferroni post hoc- P = 0.340). Conversely, estimates using traditional circular cones were significantly higher than MT directly determined by weighing animals (Fig. 4A; Subset Study: Circular mean error from MT: 108.4±6.8 kg; 26.3±1.4% (range: +20.6 to +35.3%), Bonferroni post hoc- P<0.001; Fig. 4B; Full Study: Circular error from MT: 81.0±3.5 kg; 22.8±0.6% (range: +11.3 to +42.2%), Paired t-test, t 75 = −23.339, P<0.001).
Figure 4. Estimated mass and body composition using truncated cones methods relative to measured values.
Mean ± SE estimated total body mass (MT) from the “subset study” (A; N = 10) using both circular and elliptical truncated cones, and (B) from the “full study” (N = 76) using circular truncated cones. Body composition estimated (C) from circular and elliptical cones in the subset study and (D) circular cones from the full study are also shown. Blubber with or without corrections for lipid content were compared to total body lipid determined via isotopic dilution (TBLHTO). * = significant difference between estimated and measured MT. Different letters = significant difference between body composition estimates relative to measured lipid stores.
As the use of ellipses yielded smaller MT estimates, it also resulted in smaller blubber and core mass estimates. Core body mass estimated using elliptical truncated cones was significantly smaller than estimates using the traditional circular cones (66.2±1.5 vs. 91.7±1.2%MT; Paired t-test, t 9 = 19.981, P<0.001). The difference in core body mass between the two models was substantially greater than the difference in BM.
Morphometric versus Isotopic Dilution Estimates of Body Composition
Blubber lipid content determined via biopsy sample (Table 1; range: 61.1−97.4%) was used to convert BM to subcutaneous lipid mass (BLM) for each seal separately. Blubber lipid content at the dorsal umbilicus site was similar to the average blubber lipid content across the body (−2.37% error) in the necropsied seal. All BME, BLMC, and BLME models yielded significantly smaller blubber/lipid masses as compared to isotopic dilution (Fig. 4C; Subset Study: Repeated measures ANOVA- F 1.8,16 = 112.845, Bonferroni post-hoc- all P<0.001). The difference between TBLHTO and BLME was 10.5±0.8% body mass. BMC did not differ from TBLHTO in the study subset, but BMC and BLMC were both significantly lower than TBLHTO in the full study (Fig. 4D; Full Study: Repeated measures ANOVA- F 1.5,110.6 = 190.941, Bonferroni post-hoc- all P<0.001). BMC was significantly positively correlated with BME (Fig. 5A; Subset Study: Regression- F 1,9 = 182.2, P<0.001), and this relationship was used to predict BME for animals in which all measurements could not be taken.
Figure 5. Relationships between morphometric and isotopic dilution body composition results.
Linear regression between blubber mass determined using (A) elliptical and circular truncated cones (N = 11). Once this relationship (grey) was used to correct values to elliptical models for additional animals (black), regressions were made between lipid mass determined by HTO measurements and elliptical cones with (B) and without (C) corrections for blubber lipid content. Similar relationships exist when using traditional, circular truncated cones with (D) and without (E) corrections for blubber lipid content (N = 76).
Truncated cones calculations underestimated TBLHTO; however, regression models allowed BM to estimate TBLHTO. All regressions between morphometric cone models and TBLHTO were highly significant and produced low error (RMSE). Regression errors were similar between elliptical and circular truncated cone models (Fig. 5B-D; Regression- BME: F 1,74 = 351.8, P<0.001; RMSE = 14.76 kg, 12.38% TBLHTO; BLME: F 1,74 = 274.4, P<0.001, RMSE = 16.16 kg, 13.54% TBLHTO; BMC: F 1,74 = 350.5, P<0.001, RMSE = 14.80 kg, 12.41% TBLHTO; BLMC: F 1,74 = 281.1, P<0.001, RMSE = 16.03 kg, 13.44% TBLHTO). However, the slope correcting BMC to TBLHTO was significantly < 1 (95% CI: 0.774−0.959) indicating that traditional circular truncated cones underestimated TBLHTO to a greater extent in larger animals. All slopes relating BME, BLMC, or BLME to TBLHTO were not significantly different from 1. Adding season as a variable in regression models allowed for more accurate estimates of TBLHTO from BM (BME: tseason = 2.422, P = 0.018, R 2 = 0.839, RMSE = 14.25 kg, 11.94% TBLHTO; BMC: tseason = 2.444, P = 0.017, R 2 = 0.839, RMSE = 14.28 kg, 11.97% TBLHTO), but adding season did not improve fit between TBLHTO and BLM (e.g. BLME or BLMC).
Estimating Mass and Body Composition from Regression Models
In the absence of direct MT measurements, the best single morphometric measurements to take in order to estimate MT were sternum (F 1,109 = 794.458, P<0.001, R 2 = 0.879) and middle girths (F 1,109 = 790.550, P<0.001, R 2 = 0.879). Using either of these two girth measurements accounted for 8.8% more variance than using the axillary girth measurement alone to estimate MT (F 1,109 = 412.882, P<0.001, R 2 = 0.791). Adding length measurements to the multiple regression improved model fit and decreased the RMSE. The best model included sternum girth, cLength, middle girth, and sLength, and this estimated MT with RMSE of 16.16 kg or 4.91% MT (Table 4).
Table 4. Morphometric measurements to estimate body mass and composition.
| Estimated Parameter | Equation | AICc | ΔAICc | R 2 | RMSE; kg (%MT) |
| Mass (kg) | 4.676×10−5 (sLength × Girth:axillary2 ) − 11.399 | 1043.26 | 11.98 | 0.896 | 26.57 (8.08) |
| Traditional LG2 only | 4.553×10−5 (sLength × Girth:axillary2 ) + 18.442(Season) − 10.642 | 1031.28 | 0 | 0.908 | 25.14 (7.65) |
| Equation | AICc | ΔAICc | R 2 | RMSE; kg (%MT) | |
| Mass (kg) | 4.398(Girth:sternum) − 468.287 | 1059.47 | 125.10 | 0.879 | 28.34 (8.62) |
| All morphs | 3.003(Girth:sternum) + 1.589(cLength) − 613.603 | 988.98 | 54.65 | 0.937 | 20.62 (6.27) |
| 1.443(Girth:sternum) + 1.420(cLength) + 1.565(Girth:middle) − 565.268 * | 939.14 | 4.81 | 0.961 | 16.55 (5.03) | |
| 1.509(Girth:sternum) + 0.985(cLength) + 1.497(Girth:middle) + 0.534(sLength) − 580.934 * | 934.33 | 0 | 0.963 | 16.16 (4.91) | |
| Equation | AICc | ΔAICc | R 2 | RMSE; kg (%TBLHTO) | |
| TBLHTO (kg) | 0.388(MT) − 16.258 | 638.65 | 44.71 | 0.793 | 15.81 (13.25) |
| MT included | 0.349(MT) + 20.798(Season) − 12.840 | 600.6 | 6.66 | 0.878 | 12.29 (10.30) |
| 0.300(MT) + 16.327(Season) + 6.485(Blubb:middle dorsal) − 21.621 | 593.94 | 0 | 0.892 | 11.87 (9.95) | |
| Equation | AICc | ΔAICc | R 2 | RMSE; kg (%TBLHTO) | |
| TBLHTO (kg) | 1.799(Girth:sternum) − 213.980 | 646.33 | 38.28 | 0.771 | 16.70 (14.00) |
| MT not included | 1.609(Girth:sternum) + 18.227(Season) − 187.788 | 624.13 | 16.08 | 0.834 | 14.32 (12.00) |
| 1.305(Girth:sternum) + 8.406(Season) + 8.770(Blubb:sternum lateral) − 165.120 | 615.25 | 7.20 | 0.857 | 13.82 (11.58) | |
| 0.955(Girth:sternum) + 11.712(Season) + 8.334(Blubb:sternum lateral) + 0.373(cLength) − 195.099 | 608.05 | 0 | 0.874 | 13.00 (10.90) | |
| Equation | AICc | ΔAICc | R 2 | RMSE; %MT (%TBLHTO) | |
| TBLHTO (%MT) | 5.152(Season) + 1.287(Blubb:middle dorsal) + 25.749 | 408.53 | 0 | 0.508 | 3.54 (10.43) |
Stepwise forward multiple regressions using morphometric measurements to estimate total body mass (MT) and lipid mass (TBLHTO; absolute kg and as %MT). Factors that were included in each model are shown under the estimated parameter. Each step is shown to elucidate which measurements should be taken preferentially, if animal handling time is limited (all P<0.001). * = Note that the additional parameter in this model had slightly increased the variance inflation factor, and the variance in the coefficients. All lengths, girths, and blubber depths were measured in cm, and when season is a significant parameter, the coefficient should be multiplied by “0” for January and “1” for October study animals. Root-square-mean-error (RMSE) of models is presented as absolute (kg) and as a percentage of the study’s mean MT or TBLHTO.
The best model for estimating absolute lipid mass (TBLHTO) included MT, season, and blubber depth at the middle dorsal site (Table 4; RMSE: 11.87 kg; 9.95% TBLHTO). If MT could not be determined in the field, the best model to estimate TBLHTO included sternum girth, season, sternum lateral blubber depth, and cLength measurements (Table 4; RMSE: 13.00 kg; 10.90% TBLHTO). The best predictor of TBLHTO (as %MT) was season and blubber depth measured at the middle dorsal site (Table 4, RMSE: 3.54%MT; 10.43% for %TBLHTO).
Discussion
This study demonstrated the efficacy of the modified elliptical truncated cone model to estimate MT and TBLHTO, and showed that a reduced set of non-invasive measurements can be used to estimate these parameters with high accuracy. The traditional truncated cones model using circular animal cross-sections significantly overestimated MT and BM (absolute and as %MT), relative to elliptical cones. Still, both the circular and elliptical truncated cone models underestimated lipid stores measured by using isotopic dilution techniques.
That body lipid stores determined by isotopic dilution techniques (TBLHTO) are consistently higher than blubber lipid stores, both using elliptical and circular body cross-sections (BLME and BLMC), suggests Weddell seals have significant internal lipid deposits that would be overlooked by solely using morphometric measures of condition. Previous work has demonstrated the presence of intramuscular lipid reserves and lipid sheaths around internal organs and abdominal mesentery [18], [20], . These internal stores may be mobilized first during times of reduced foraging [52], and would also impact the animal’s net buoyancy and cost of locomotion [14], [53]. Therefore, ignoring internal lipid reserves could introduce biases when comparing body composition among species, populations, and seasons.
The errors in Weddell seal MT estimates using traditional circular truncated cones were not substantially improved when blubber and core tissue densities were slightly altered. Only if the total body average density of Weddell seals was assumed to be 0.83±0.01 g·mL−1 were estimates of MT equal to measured values. This density is well outside of the physiologically-relevant range, as measured blubber densities are 0.92−0.95 g·mL−1 [29], [54], and the average densities of lean mass components for mammals are approximately 1.1 g·mL−1 [29], [30], [44], [55]. In contrast, elliptical truncated cones provided estimates of MT that were not significantly different from measured values when using published blubber and core density estimates to convert body volume to mass. Further, elliptical models did not require additional empirically-determined correction factors to accurately estimate MT.
The fact that elliptical, but not circular, truncated cones closely approximated actual MT indicates that a major source of error in the traditional truncated cones method is the assumption that animals are circular in cross-section. Elliptical cross-sections much more accurately reflect the animals’ true body shape while hauled-out and lying flat against the ice. This deformation, or “slumping,” was first described by Slip et al. [34], and was supported by the fact that field measurements of sculp mass were smaller than estimates using the traditional circular truncated cones calculations [56]. However, this study is the first, to our knowledge, to demonstrate that the compression of the core body mass into non-circular form introduces error. Using circular truncated cones to estimate core body mass produced values that were much too high, at 91.7% MT. These errors arise because circles have the largest area per unit arc length, and therefore, the greater the degree of asymmetry of the animal (i.e. greater width:height ratio), the larger the overestimate in cross-sectional area.
The errors accompanying MT and BM estimates from circular cones are likely to be important when calculating animal drag forces, buoyancy, density, and metabolic costs; all of which are influenced by surface area and volume calculations (Table 5). Conversely, variations between circular and elliptical models likely would not impact the relative differences and trends when simply comparing body condition within a population. Thus, either method could be used as an ecosystem metric or index, provided it is understood that circular truncated cones are not yielding accurate estimates of mass or body composition unless additional correction factors are included in the model.
Table 5. Differences in additional physiological parameters determined by circular vs. elliptical truncated cones.
| Physiological Estimate | Circular Cones | Elliptical Cones |
| Total Volume (L)* | 490.3±17.3 | 379.8±13.4 |
| Surface Area (SA; cm2) | 3773.4±93.2 | 3773.4±93.2 |
| SA:V* | 7.73±0.10 | 9.98±0.15 |
| Net Buoyancy (N)* | −57.3±5.0 | −76.2±5.7 |
| Calculated Density (g·mL −1 )* | 0.83±0.01 | 1.07±0.02 |
Mean ± SE total body volume, surface area, surface area-to-volume ratios, buoyant force, and calculated density in Weddell seals (N = 11). Surface area remains the same between circular and elliptical cones. Buoyancy was calculated following Webb et al. [9], and density was calculated using measured MT. Asterisk indicates a significant difference in estimated parameter using circular versus elliptical models (paired t-tests; all P<0.001).
This study indicates that animal MT and BM can be accurately estimated with volumetric and morphometric models, but that accurate estimates of lipid stores require isotopic dilution techniques or additional calibration factors. In combination, direct weighing and isotopic dilution techniques were found here to be the most appropriate tools when precise measures of animal size, body composition, or energetic costs are required. As this is not always possible in a field setting, there is a need for predictive models that relate proxy variables, such as morphometric measurements, to mass or body composition [34], [40]. Such models do not make assumptions about animal shape, but instead rely on a “calibration dataset” for coefficient development. This study has shown that, once developed, these simplified models can be used to estimate mass and body composition quickly for a much larger sample size. Moreover, they can be quite accurate (RMSE: 4.91% for MT; 10.90% for TBLHTO; 10.43% for %TBLHTO), and require much less time and effort as compared to direct measures or the more complex truncated cones models. Indeed, the multiple linear regression approach predicted body composition more accurately than the truncated cone models, while using far fewer morphometric measurements. Surprisingly, the models that produced the most accurate estimate of MT included sternum rather than the axillary girth measure, which has traditionally been incorporated into the straight length × axillary girth2 (LG2) proxy [26], [27]. Similarly, the model that produced the most accurate absolute and percent TBLHTO used middle dorsal or sternum lateral blubber depth measures, respectively, instead of the traditional single axillary blubber measurement [28].
Identifying suitable proxy variables using hierarchical regressions can lead to reduced handling times and simpler procedures; however, predictive power will depend on model development using test datasets. This is because the relationship between morphometric measures with animal MT and TBLHTO are likely to be species-specific and vary seasonally. In contrast, the elliptical truncated cones method does not require such calibration coefficients and is; therefore, more broadly applicable and useful in new species and field situations. In addition to utilizing morphometric measurements to estimate MT, there have been some recent successes in photogrammetric methods. While these techniques to estimate MT have been validated within <2−10% accuracy in pinnipeds and allow researchers to avoid animal handling [57]–[59], photogrammetry can’t quantify total body lipid stores. Alternatively, dive loggers have been used to estimate net animal buoyancy by measuring changes in animal drift rates through the water column. Since buoyancy is influenced by total body lipid content (both in the blubber and internal stores), drift rates are used as a proxy of changes in body composition [53], [60], [61]; however, this method doesn’t provide estimates of MT. The optimal technique for determining animal mass and body composition clearly depends on multiple factors such as handling and analytical constraints, whether precise or index values are needed to accomplish study goals, and the availability of test datasets for development of appropriate correction factors.
Acknowledgments
We thank field team members: Kimberly Goetz, Dr. Patrick Robinson, Dr. Luis Hückstädt, and Michelle LaRue for sample collection, group B-009-M led by Dr. Robert Garrott, Dr. Jay Rotella, and Dr. Thierry Chambert, and also group B-470-M led by Drs. Jo-Ann Mellish, Markus Horning, and Allyson Hindle for their help locating study animals. We also thank an anonymous reviewer whose suggestions improved this manuscript. Logistical support was provided by the National Science Foundation (NSF) U.S. Antarctic Program, Raytheon Polar Services, and Lockheed Martin ASC; we thank all the support staff in Christchurch, NZ and McMurdo Station.
Funding Statement
This research was conducted with support from NSF ANT-0838892 to D.P.C. and ANT-0838937 to J.M.B. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship under Grant No. DGE-1242789. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. M.R.S. and this research were supported in part by a UAF Center for Global Change Student Research Grant with funds from the Cooperative Institute for Alaska Research and the Alaska Climate Science Center. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Costa DP, Croxall JP, Duck CD (1989) Foraging energetics of Antarctic fur seals in relation to changes in prey availability. Ecol 70: 596–606. [Google Scholar]
- 2. Boyd IL, Murray AWA (2001) Monitoring a marine ecosystem using responses of upper trophic level predators. J Anim Ecol 70: 747–760. [Google Scholar]
- 3. Derocher AE, Lunn NJ, Stirling I (2004) Polar bears in a warming climate. Integr Comp Biol 44: 163–174. [DOI] [PubMed] [Google Scholar]
- 4. Burek KA, Gulland FMD, O'Hara TM (2008) Effects of climate change on arctic marine mammal health. Ecol Applic 18: S126–S134. [DOI] [PubMed] [Google Scholar]
- 5. Reid K, Croxall JP (2001) Environmental response of upper trophic-level predators reveals a system change in an Antarctic marine ecosystem. Proc R Soc Lond B 268: 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Speakman JR (2001) Body composition analysis of animals: A handbook of non-destructive methods. Cambridge, UK: Cambridge University Press.
- 7. Costa DP, Le Boeuf BJ, Ortiz CL, Huntley AC (1986) The energetics of lactation in the northern elephant seal, Mirounga angustirostris . J Zool Lond 209: 21–33. [Google Scholar]
- 8. Crocker DE, Williams JD, Costa DP, Le Boeuf BJ (2001) Maternal traits and reproductive effort in northern elephant seals. Ecol 82: 3541–3555. [Google Scholar]
- 9. Webb PM, Crocker DE, Blackwell SB, Costa DP, Le Boeuf BJ (1998) Effects of buoyancy on the diving behavior of northern elephant seals. J Exp Biol 201: 2349–2358. [DOI] [PubMed] [Google Scholar]
- 10. Beck CA, Bowen WD, Iverson SJ (2000) Seasonal changes in buoyancy and diving behaviour of adult grey seals. J Exp Biol 203: 2323–2330. [DOI] [PubMed] [Google Scholar]
- 11. Noren SR, Pearson LP, Davis J, Trumble SJ, Kanatous SB (2008) Different thermoregulatory strategies in nearly weaned pup, yearling, and adult Weddell seals (Leptonychotes weddellii). Phys Bioch Zool 81: 868–879. [DOI] [PubMed] [Google Scholar]
- 12. Liwanag HE, Berta A, Costa DP, Budge SM, Williams TM (2013) Morphological and thermal properties of mammalian insulation: the evolutionary transition to blubber in pinnipeds. Biol J Linnean Soc 107: 774–787. [Google Scholar]
- 13. Crocker DE, Houser DS, Webb PM (2012) Impact of body reserves on energy expenditure, water flux, and mating success in breeding male northern elephant seals. Phys Bioch Zool 85: 11–20. [DOI] [PubMed] [Google Scholar]
- 14. Miller PJO, Biuw M, Watanuki YY, Thompson D, Fedak MA (2012) Sink fast and swim harder! Round-trip cost-of-transport for buoyant divers. J Exp Biol 215: 3622–3630. [DOI] [PubMed] [Google Scholar]
- 15. Ortiz CL, Costa DP, Le Boeuf BJ (1978) Water and energy flux in elephant seal pups fasting under natural conditions. Physiol Zool 51: 166–178. [Google Scholar]
- 16. Nagy KA, Costa DP (1980) Water flux in animals: analysis of potential errors in the tritiated water method. Am J Physiol 238: R454–R465. [DOI] [PubMed] [Google Scholar]
- 17.Costa DP (1987) Isotopic methods for quantifying material and energy intake of free-ranging marine mammals. In: Huntley AC, Costa DP, Worthy GAJ, Castellini MA, editors. Approaches to Marine Mammal Energetics. Lawrence, KA: Allen Press. pp. 43−61.
- 18. Beck GG, Smith TG, Hammill MO (1993) Evaluation of body condition in the Northwest Atlantic Harp seal (Phoca groenlandica). Can J Fish Aquat Sci 50: 1372–1381. [Google Scholar]
- 19. Dunkin RC, McLellan WA, Blum JE, Pabst DA (2005) The ontogenetic changes in the thermal properties of blubber from Atlantic bottlenose dolphin Tursiops truncatus . J Exp Biol 208: 1469–1480. [DOI] [PubMed] [Google Scholar]
- 20. Sheng H-P, Huggins RA (1979) A review of body composition studies with emphasis on total body water and fat. Am J Clin Nutr 32: 630–647. [DOI] [PubMed] [Google Scholar]
- 21. Lydersen C, Hammill MO, Ryg MS (1992) Water flux and mass gain during lactation in free-living ringed seal (Phoca hispida) pups. J Zool Lond 228: 361–369. [Google Scholar]
- 22. Oftedal OT, Bowen WD, Boness DJ (1993) Energy transfer by lactating hooded seals and nutrient deposition in their pups during the four days from birth to weaning. Physiol Zool 66: 412–436. [Google Scholar]
- 23. Oftedal OT, Bowen WD, Boness DJ (1996) Lactation performance and nutrient deposition in pups of the harp seal, Phoca groenlandica, on ice floes off southeast labrador. Physiol Zool 69: 635–657. [Google Scholar]
- 24. Reilly JJ, Fedak MA (1990) Measurement of body composition of living grey seals by hydrogen isotope dilution. J Appl Physiol 69: 885–891. [DOI] [PubMed] [Google Scholar]
- 25. Arnould JPY, Boyd IL, Speakman JR (1996) Measuring the body composition of Antarctic fur seals (arctocephalus gazella): Validation of hydrogen isotope dilution. Physiol Zool 69: 93–116. [Google Scholar]
- 26. Castellini MA, Kooyman GL (1990) Length, girth and mass relationships in Weddell seals (Leptonychotes weddellii). Mar Mamm Sci 6: 75–77. [Google Scholar]
- 27.Hofman RJ (1975) Distribution patterns and population structure of Antarctic seals [dissertation]. University of Minnesota, Minneapolis, MN.
- 28. Committee on Marine Mammals (1967) Standard measurements of seals. J Mammal 48: 459–462. [Google Scholar]
- 29. Gales NJ, Burton HR (1987) Ultrasonic measurement of blubber thichkness of the southern elephant seal, Mirounga leonina (Linn.). Aust J Zool 35: 207–217. [Google Scholar]
- 30. Worthy GAJ, Morris PA, Costa DP, Le Boeuf BJ (1992) Moult energetics of the northern elephant seal (Mirounga angustirostris). J Zool Lond 227: 257–265. [Google Scholar]
- 31. McDonald BI, Crocker DE, Burns JM, Costa DP (2008) Body condition as an index of winter foraging success in crabeater seals (Lobodon carcinophaga). Deep Sea Res II 55: 515–522. [Google Scholar]
- 32. Koopman HN, Pabst DA, McLellan WA, Dillaman RM, Read AJ (2002) Changes in blubber distribution and morphology associated with starvation in the harbor porpoise (Phocoena phocoena): Evidence for regional differences in blubber structure and function. Phys Bioch Zool 75: 498–512. [DOI] [PubMed] [Google Scholar]
- 33. Noren SR, Wells RS (2009) Blubber deposition during ontogeny in free-ranging bottlenose dolphins: balancing disparate roles of insulation and locomotion. J Mammal 90: 629–637. [Google Scholar]
- 34. Slip DJ, Burton HR, Gales NJ (1992) Determining blubber mass in the southern elephant seal, Mirounga leonina, by ultrasonic and isotopic techniques. Aust J Zool 40: 143–152. [Google Scholar]
- 35. Lockyer CH, McConnell LC, Waters TD (1984) The biochemical composition of fin whale blubber. Can J Zool 62: 2553–2562. [Google Scholar]
- 36. Struntz DJ, McLellan WA, Dillaman RM, Blum JE, Kucklick JR, et al. (2004) Blubber development in bottlenose dolphins (Tursiops truncatus). J Morph 259: 7–20. [DOI] [PubMed] [Google Scholar]
- 37. Koopman HN (2007) Phylogenetic, ecological, and ontogenetic factors influencing the biochemical structure of the blubber of odontocetes. Mar Biol 151: 277–291. [Google Scholar]
- 38. Aguilar A, Borrell A (1990) Patterns of lipid content and stratification in the blubber of fin whales (Balaenoptera physalus). J Mammal 71: 544–554. [Google Scholar]
- 39. Burns JM, Lestyk K, Folkow LP, Hammill MO, Blix AS (2007) Size and distribution of oxygen stores in harp and hooded seals from birth to maturity. J Comp Physiol B 177: 687–700. [DOI] [PubMed] [Google Scholar]
- 40. Tierney M, Hindell MA, Lea MA, Tollit DJ (2001) A comparison of techniques used to estimate body condition of southern elephant seals (Mirounga leonina). Wildl Res 28: 581–588. [Google Scholar]
- 41. Stirling I (1969) Ecology of the Weddell seal in McMurdo Sound, Antarctica. Ecol 50: 573–586. [Google Scholar]
- 42. Bowen WD, Iverson SJ (1998) Estimation of total body water in pinnipeds using hydrogen-isotope dilution. Physiol Zool 71: 329–332. [DOI] [PubMed] [Google Scholar]
- 43.Bryden MM (1967) The biology of the southern elephant seal, Mirounga leonina (Linn.): development and growth [dissertation]. University of Sydney.
- 44. Nordøy ES, Blix AS (1985) Energy sources in fasting grey seal pups evaluated with computed tomography. Am J Physiol 249: R471–R476. [DOI] [PubMed] [Google Scholar]
- 45. Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226: 497–509. [PubMed] [Google Scholar]
- 46. Budge SM, Iverson SJ, Koopman HN (2006) Studying the trophic ecology in marine ecosystems using fatty acids: a primer on analysis and interpretation. Mar Mamm Sci 22: 759–801. [Google Scholar]
- 47.Meyers LS, Gamst G, Guarino AJ (2006) Applied Multivariate Research: Design and Interpretation. Thousand Oaks, California: Sage Publications, Inc.
- 48. O'Brien RM (2007) A caution regarding rules of thumb for variance inflation factors. Quality & Quantity 41: 673–690. [Google Scholar]
- 49. Worthy GAJ, Lavigne DM (1983) Changes in energy stores during postnatal development of the harp seal, Phoca groenlandica . J Mammal 64: 89–96. [Google Scholar]
- 50. Gales R, Renouf D, Noseworthy E (1994) Body composition of harp seals. Can J Zool 72: 545–551. [Google Scholar]
- 51. Pond CM (1978) Morphological aspects and the ecological and mechanical consequences of fat deposition in wild vertebrates. Ann Rev Ecol Syst 9: 519–570. [Google Scholar]
- 52.Rice DW, Wolman AA (1971) The life history and ecology of the gray whale (Eschrichtius robustus).
- 53. Aoki K, Watanuki YY, Crocker DE, Robinson PW, Biuw M, et al. (2011) Northern elephant seals adjust gliding and stroking patterns with changes in buoyancy: validation of at-sea metrics of body density. J Exp Biol 214: 2973–2987. [DOI] [PubMed] [Google Scholar]
- 54. Beck GG, Smith TG (1995) Distribution of blubber in the northwest Atlantic harp seal, Phoca groenlandica . Can J Zool 73: 1991–1998. [Google Scholar]
- 55.Moore FD, Olsen KH, McMurray JD, Parker HV, Ball MR, et al.. (1963) The Body Cell Mass and its Supporting Environment: Body Composition in Health and Disease. Philadelphia.
- 56. Gales R, Renouf D (1994) Assessment of body condition of harp seals. Polar Biol 14: 381–387. [Google Scholar]
- 57. Bell CM, Hindell MA, Burton HR (1997) Estimation of body mass in the southern elephant seal, Mirounga leonina, by photogrammetry and morphometrics. Mar Mamm Sci 13: 669–682. [Google Scholar]
- 58. Waite JN, Schrader WJ, Mellish JE, Horning M (2007) Three-dimensional photogrammetry as a tool for estimating morphometrics and body mass of Steller sea lions (Eumetopias jubatus). Can J Fish Aquat Sci 64: 296–303. [Google Scholar]
- 59. de Bruyn PJN, Bester MN, Carlini AR, Oosthuizen WC (2009) How to weigh an elephant seal with one finger: a simple three-dimensional photogrammetric application. Aquatic Biology 5: 31–39. [Google Scholar]
- 60. Robinson PW, Simmons SE, Crocker DE, Costa DP (2010) Measurements of foraging success in a highly pelagic marine predator, the northern elephant seal. J Anim Ecol 79: 1146–1156. [DOI] [PubMed] [Google Scholar]
- 61.Schick RS, New LF, Thomas L, Costa DP, Hindell MA, et al.. (2013) Estimating resource acquisition and at-sea body conditon of a marine predator. J Anim Ecol. [DOI] [PMC free article] [PubMed]