Abstract
Neurons release neuropeptides via the regulated exocytosis of dense core vesicles (DCVs) to evoke or modulate behaviors. We found that Caenorhabditis elegans motor neurons send most of their DCVs to axons, leaving very few in the cell somas. How neurons maintain this skewed distribution and the extent to which it can be altered to control DCV numbers in axons or to drive release from somas for different behavioral impacts is unknown. Using a forward genetic screen, we identified loss-of-function mutations in UNC-43 (CaM kinase II) that reduce axonal DCV levels by ∼90% and cell soma/dendrite DCV levels by ∼80%, leaving small synaptic vesicles largely unaffected. Blocking regulated secretion in unc-43 mutants restored near wild-type axonal levels of DCVs. Time-lapse video microscopy showed no role for CaM kinase II in the transport of DCVs from cell somas to axons. In vivo secretion assays revealed that much of the missing neuropeptide in unc-43 mutants is secreted via a regulated secretory pathway requiring UNC-31 (CAPS) and UNC-18 (nSec1). DCV cargo levels in unc-43 mutants are similarly low in cell somas and the axon initial segment, indicating that the secretion occurs prior to axonal transport. Genetic pathway analysis suggests that abnormal neuropeptide function contributes to the sluggish basal locomotion rate of unc-43 mutants. These results reveal a novel pathway controlling the location of DCV exocytosis and describe a major new function for CaM kinase II.
Keywords: placeholder, C. elegans, CaM Kinase II, Dense core vesicle, Neuropeptide release, Regulated secretion
BOTH neurons and neuroendocrine cells rely on the controlled release of neuropeptides via dense core vesicle (DCV) exocytosis to evoke or modulate behaviors (Scheller and Axel 1984; Kupfermann 1991; T. Liu et al. 2007; Li and Kim 2008). The DCVs in neuroendocrine and PC12 cells are much more abundant and accessible to biochemical and physiological experiments than those in neurons. For example, in chromaffin and pancreatic β-cells (both neuroendocrine cells), DCVs can number in the tens of thousands per cell and can occupy 31 and 12% of the cell volume, respectively (Dean 1973; Plattner et al. 1997). Exploiting these advantages, studies in PC12 and neuroendocrine cells have revealed that DCVs arise from a regulated secretory pathway. The pathway begins in the trans Golgi, where various sorting mechanisms cause regulated secretory proteins, such as neuropeptides and their processing enzymes, to coalesce into vesicles that bud from the trans Golgi to form immature DCVs. Additional sorting of non-DCV cargos away from DCV cargos occurs as DCVs mature through this pathway (Arvan and Castle 1998; Tooze et al. 2001; Borgonovo et al. 2006).
DCVs must selectively retain and protect several distinct cargos that have different physical states as they mature. These include the neuropeptide core, which is thought to be in an aggregated state, the neuropeptide-processing enzymes PC-2 convertase and carboxypeptidase E, possibly soluble cargos, and transmembrane cargos.
While neurons and neuroendocrine cells share this core pathway for DCV production, neurons have evolved additional membrane-trafficking requirements that may necessitate modifications to this core pathway or additional levels of regulation. For example, neurons send most of their DCVs to the axon as opposed to amassing them in the cell soma as neuroendocrine cells do. As neuronal DCVs complete their maturation in the cell soma, but before they are transported to the axon, neurons may need a mechanism to prevent the loss of those newly formed DCVs due to exocytosis from the cell soma in response to electrical depolarization or chemical signals impinging on the soma.
The model organism Caenorhabditis elegans has several strengths for investigating neuronal DCV trafficking, including the ability to track and quantitatively image DCV cargos in live animals using fluorescently tagged cargos expressed from integrated transgenes, and the ability to perform large forward genetic screens to uncover the molecular requirements for DCV trafficking. Past studies used these strengths to show that null mutations in UNC-108 (Rab2) cause altered interactions between immature DCVs and early endosomes, resulting in the loss of soluble and transmembrane cargos without affecting the aggregated neuropeptide core (Edwards et al. 2009; Sumakovic et al. 2009).
In the current study, we performed a forward genetic screen aimed at finding other mutations that alter the distribution of DCVs and DCV cargos between cell somas and axons. From the screen we recovered loss-of-function (nonsense) mutations in UNC-43 (CaM kinase II) that reduce the axonal levels of DCVs and all DCV cargos examined by ∼90% while cell soma/dendrite levels of all nontransmembrane cargos were reduced by ∼60–80%. In contrast, small synaptic vesicles were largely unaffected. Our analysis of DCV distribution, movements, and exocytosis in unc-43 mutants revealed a surprising new function for CaM kinase II in blocking the regulated exocytosis of DCVs before they are transported into axons.
Materials and Methods
Worm culture and strains
Worm culture and manipulation essentially followed previously described methods (Brenner 1974; Stiernagle 2006). Briefly, culture media was modified NGM (referred to as NGM-LOB), containing no added calcium or magnesium, and consisted of the following (per liter): 2 g NaCl, 3.1 g peptone, 3.0 g KH2PO4, 0.5 g K2HPO4, and 20 g Sigma A-7002 agar. After autoclaving and cooling to 55°, the following was added (per liter): 1.6 ml of 5 mg/ml cholesterol in ethanol, 1.0 ml of 100 mg/ml streptomycin in ddH2O, 1.25 ml of 10 mg/ml mycostatin suspension in ethanol. Prior studies define the culture plate types as “spread plates,” “streak plates,” and “locomotion plates” (Miller et al. 1999; Edwards et al. 2008). Wild-type worms were the N2 strain and the mapping strain CB4856. Supporting Information lists the complete genotypes of all strains used in this study as well the molecular lesion of each mutation used in this study.
IDA-1-GFP forward genetic screen
For each screening cycle, we mutagenized 3000 L4s of the strain KG2445 ceIs76 (unc-17::IDA-1-GFP; four times outcrossed after integration) with 27.6 mM EMS in M9 supplemented with OP50 bacteria for 4 hr at 20°. After growing this P0 generation for 72 hr at 14° on 2 spread plates, we rinsed all adults and larvae from the plates and allowed the F1 generation eggs to hatch overnight at 20°. F1 hatchlings were collected and counted, and 3000 were plated on each of eight spread plates and grown for 3 days at 20°. All animals were scraped from these plates and treated with alkaline hypochlorite solution to release eggs. The egg suspension was plated on unseeded NGM-LOB culture plates and incubated overnight at 20° to allow the F2 generation eggs to hatch. F2 hatchlings were collected and counted, and 5000 were plated on each of 18 spread plates. Half of these plates were grown for 32 hr at 20° + 16 hr at 14°, and half were grown for 8 hr at 20° + 64 hr at 14° so that screening could be done on 2 successive days. These growth times produced mid-L4 stage synchronous F2 grandprogeny. For each half day of screening, we harvested animals from three of these plates using 5 ml of sterile PBS per plate and adding the suspension to 8 ml of sterile PBS, stirring slowly in a 50-ml beaker (stir bar trauma can harm animals). We counted 12, 50-μl aliquots of this suspension, obtained an average, and adjusted the suspension volume to 24 worms per 50 μl. At 20-min intervals, we pipetted 50 μl (∼24 worms) from the stirring suspension into each of the 12 center wells of a 96-well Mat-Tek glass-bottom plate (MatTek, Ashland, MA; P96G-1.5-F-F) that had been preloaded with 50 μl of 300 μM levamisole per well. We began screening 10 min after loading the first well and used ∼20 min to screen the animals in the 12 wells using a 0.75 numerical aperture ×20 dry objective on a Nikon TE-2000E inverted microscope with a Universal stage containing an inset sized for 96-well plates. In each half day of screening, over 2 successive days per week, we screened 8 such rows of 12 wells. We screened animals in each well for decreased fluorescence in the dorsal cord and/or increased fluorescence in cell somas. At the end of each 20-min screening session, after noting wells containing such mutants, we pipetted the contents of the well onto a predried streak plate using a Pasteur pipette, rinsed the well with 100 μl of M9, and then, immediately after the liquid dried, clonally distributed up to 24 animals to a 24-well solid-media culture plate. Depending on the relative size of the animal, we often plated fewer animals. In later experiments, we plated the animals on 96-well solid media culture plates containing a thick layer of OP50 bacteria. After 3 days at 20° + 1 day at 14°, we used a sterile toothpick to pick about six L4s from each well onto Mat-Tek plate wells containing 150 μM levamisole. After screening on the TE-2000E, wells with 100% mutant phenotype were noted, and we used the corresponding well on the 24- or 96-well plate to score behavioral and other phenotypes and to set up stocks. We screened 22,300 F2 animals for a calculated sevenfold genomic coverage.
Mapping, identification, and outcrossing of new mutations
We crossed the ceIs76 transgene into the CB4856 mapping strain 12 times and then used ceIs76 (CB4856) males to cross the CB4856 polymorphisms with the ce685; ceIs76 mutant, re-isolating 23 homozgyous mutant mapping lines by the fluorescence phenotype in the F2 generation. We then mapped the ce685 mutation relative to single nucleotide polymorphisms (SNPs) to the center region of chromosome IV using a previously described method (Schade et al. 2005). For high-resolution SNP mapping to a subregion of chromosome IV, we crossed the ce685 mutation away from the ceIs76 insertion to make a four-times outcrossed strain, which we then crossed to CB4856 to produce 95 mutant mapping lines isolated by their behavioral phenotype. The physical interval to which the mutation mapped was 8,502,939–10,603,456 for a size of 2,101,417. A complementation test in which we crossed ce725/+;ceIs76 males to ce685;ceIs76 revealed noncomplementation among ∼50% of the male cross-progeny, scoring by the fluorescence phenotype as well as by uncoordinated phenotype. A complementation test in which we crossed ce685/+ males to unc-43(n498n1186) revealed noncomplementation among ∼50% of the male cross-progeny, scoring by the uncoordinated phenotype. PCR amplification and sequencing of the unc-43 gene-coding region in ce685 and ce725 mutant genomic DNAs revealed a Q67Stop mutation (amino acid position relative to the K11E8.1g.2 isoform) in ce685. This mutation is identical to unc-43(n1186). Analysis of ce725 mutant genomic DNA revealed a W168Stop mutation relative to the K11E8.1g.2 isoform. We outcrossed ce685 and ce725 four and two times, respectively, to N2 before using them for the experiments in this article.
Plasmids
Supporting Information lists all of the DNA constructs used in this study along with sources and construction details. In all constructs involving the cloning of PCR fragments, we sequenced the inserts and used clones containing no mutations in the fragment of interest to establish the final plasmid stock.
Transgene production
We prepared plasmids for microinjection using the Qiagen Tip-20 system according to the manufacturer’s instructions, except that we added a 0.1-M potassium acetate/2-volume ethanol precipitation step after resuspending the isopropanol-precipitated pellet. We produced transgenic strains bearing extrachromosomal arrays by the method of Mello et al. (1991). For the unc-43 rescue experiments, the host was KG2550 unc-43(ce685); ceIs76. For all other injection experiments, N2 was the host. We used pBluescript carrier DNA to bring the final concentration of DNA in each injection mixture to 175 ng/μl and integrated the arrays into the genome as described (Reynolds et al. 2005), using 9100 Rad of gamma rays. Supporting Information lists all of the transgenic arrays in this study, their DNA contents, and the injection concentration of each DNA. We mapped the integration site of each new insertion by crossing the integrant through CB4856, re-isolating and cloning homozygous transgenic animals in the F2 generation, and using the resulting mapping lines to map the transgenes relative to SNPs as described (Schade et al. 2005).
C. elegans strain constructions
To cross an integrated transgene into a mutant background, we typically crossed a male integrant strain, made by the heat-shock method (Sulston and Hodgkin 1988), to the mutant, although in some cases we used integrant/+ heterozygous males. After incubating 3 days at 20°, we cloned five L4-stage cross-progeny carrying the fluorescently marked transgene. After 4 days at room temperature, we cloned 12 bright-fluorescent (putatively homozygous for the transgene) mutant adult hermaphrodites and grew them for 4 days at room temperature before choosing one homozygous mutant line that was also homozygous for the transgene to establish the stock. To construct strains carrying two mutations plus a transgene, we typically first crossed the insertion into each single mutant and then used homo- or hemizygous integrant males for the first cross to mutant A, allowing the entire construction to be done in a background that is homozygous for the insertion. We constructed these strains, as well as the unc-43; egl-3 double mutant, using the standard method of crossing heterozygous males of mutant A with homozygous hermaphrodites of mutant B and cloning virgin F1 cross-progeny. From plates segregating mutant A in their F2 progeny, we cloned mutant A and/or mutant B animals and looked for segregation of the double mutant in the next generation using behavioral phenotypes. After making a strain composed of two or more mutations, we verified the homozygosity of the mutations by PCR (for deletions) and/or by sequencing PCR products (for point mutants) of both loci from genomic DNA of the final strain.
Quantitative fluorescence imaging and image analysis of DCV cargos in live animals
Supporting Information provides complete details of the procedures used for quantitative fluorescence imaging and image analysis, including growth and mounting of strains, image acquisition, processing and quantifying images, and producing representative images.
Time-lapse video microscopy of DCV active transport in live animals
Supporting Information provides complete details of the procedures used for time-lapse imaging, including growth and mounting of strains, image acquisition, processing time-lapse images, and quantifying DCV movements from kymographs.
Antibody production
Supporting Information describes the EGL-21 antibody production.
Immunostaining
Supporting Information describes the immunostaining methods.
Electron microscopy
Supporting Information describes the electron microscopy (EM) methods used in this study.
Behavioral assays
Previous studies described the basal locomotion rate assays (Miller et al. 1999; Reynolds et al. 2005).
Statistical analysis
For analyses involving only two experimental groups, we used the unpaired t-test, Welch corrected. For all multiple comparison data (more than two groups), we performed ANOVAs followed by post-tests for statistical significance (Tukey’s test for any multiple comparison data in which at least some noncontrol samples were compared to each other and Dunnett’s test for any multiple comparison graphs that compared samples to only a single control population). The figure legends note which post test was used. We performed all statistical comparisons using Graphpad Instat 3 (Graphpad Software, Inc.).
Results
DCV cargos are largely absent from axons in mutants lacking UNC-43 (CaM kinase II)
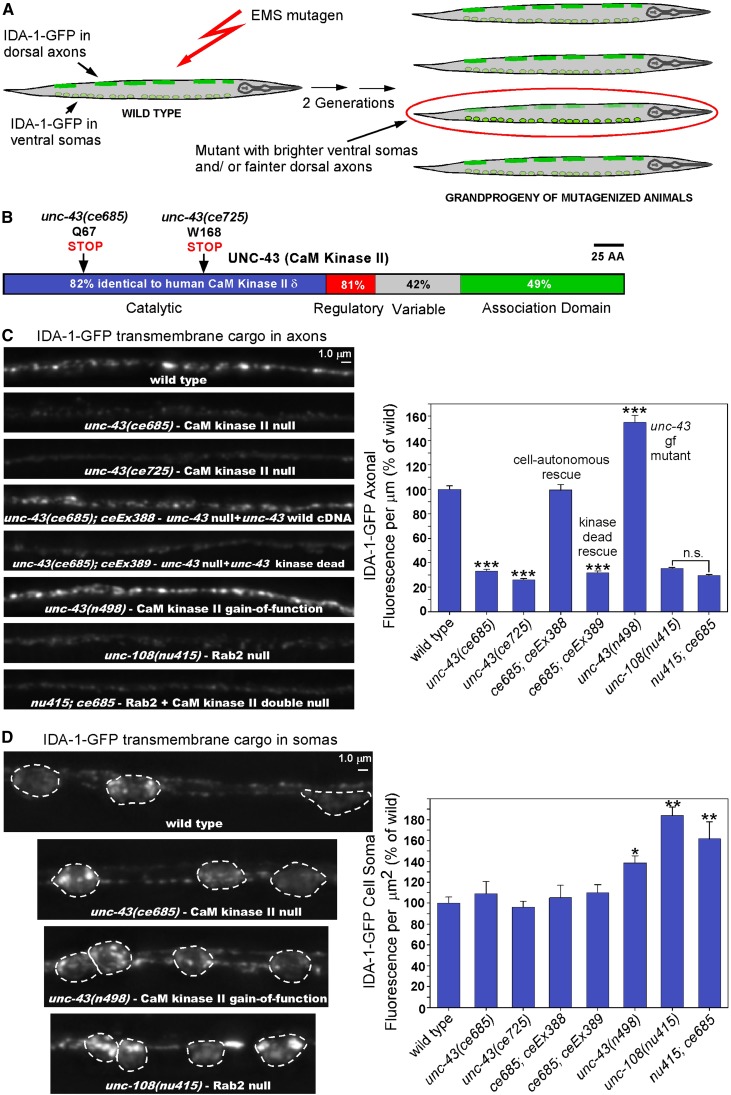
unc-108 (Rab2) null mutants have DCV cargo-trafficking defects that cause the transmembrane DCV cargo IDA-1-GFP to build up in neuronal cell somas and reduce its axonal levels by ∼70% (Edwards et al. 2009). IDA-1 is the C. elegans ortholog of insulinoma-associated protein 2, a protein that is specific to DCVs in vertebrate neurons (Solimena et al. 1996) and is a marker for DCVs in C. elegans (Cai et al. 2004; Zahn et al. 2004). To identify other proteins that control how DCVs and/or DCV cargos distribute between cell somas and axons, we mutagenized animals containing an integrated transgene that expresses IDA-1-GFP in cholinergic motor neurons and screened their grandprogeny for mutants with higher levels of IDA-1-GFP in their cell somas and/or lower levels of IDA-1-GFP in their axons (Figure 1A). Forward genetic screening of 22,300 F2 animals (∼sevenfold genomic coverage) yielded two nonsense (early stop codon) mutations that mapped to the unc-43 gene, which encodes the sole C. elegans ortholog of Ca++-calmodulin-dependent protein kinase II (Reiner et al. 1999) and is >80% identical to its human ortholog over much of its length. Both nonsense mutations occur in the highly conserved catalytic domain (Figure 1B) and are thus likely to strongly reduce or eliminate the catalytic activity of CaM kinase II. Hereafter we refer to the nonsense mutants as unc-43 lf (loss-of-function) mutants. Note that we also analyzed the unc-43(tm1605) deletion allele and found it to be homozygous viable with growth, behavioral, and soluble cargo-trafficking phenotypes similar to the nonsense mutants reported here (data not shown). tm1605 is a 651-bp in-frame deletion that removes H113 through R218 (amino acid numbering refers to the K11e8.1f.2 isoform), which is ∼39% of the catalytic domain.
Figure 1.
unc-43 mutant motor neuron axons are missing most of a DCV transmembrane cargo due to loss of UNC-43 function in the same neurons. (A) Schematic illustrating the forward genetic screen by which this study identified unc-43 mutants. We screened F2 grandprogeny of the mutagenized animals on 96-well glass-bottom Mat-Tek plates using an inverted microscope and selected animals with brighter cell somas and/or fainter dorsal axons. The cholinergic motor neuron cell somas are found only on the ventral side of the animal’s body. Narrow axonal commissures (not shown) connect many of the cell somas to the main axon tracts on the dorsal side of the animal. EMS, ethylmethanesulfonate. (B) Scale drawing depicting the UNC-43G isoform with mutation locations and percentage identities to its human ortholog CaM kinase II δ (accession no. NP_742113). Domain boundaries are from Wang (2008) (catalytic and regulatory domains) and from Rosenberg et al. (2006) (association domain). Percentage identity of the variable domain does not include a 39-aa insertion that is present in the UNC-43G splice isoform. (C) Representative, identically scaled images and quantification of IDA-1-GFP transmembrane cargo, expressed from the integrated transgene ceIs76, in cholinergic motor neuron axons in animals with the indicated genotypes. ceEx388 and ceEx389 are stable extrachromosomal transgenes that express the unc-43g (+) and unc-43g (K41R; kinase dead) cDNAs, respectively, from the same promoter used in the ceIs76 transgene. Graph data are means and standard errors of the background-adjusted total dorsal cord fluorescence per μm of cord length from 13 animals each. ***P ≤ 0.001 by Tukey’s Test when comparing the indicated genotypes to wild type or to each other; n.s., P > 0.05 (not significant). (D) Representative, identically scaled images and quantification of IDA-GFP transmembrane cargo expressed from the integrated transgene ceIs76 in ventral cord motor neuron somas in animals with the indicated genotypes. Dashed lines outline the cell soma boundaries. Graph data are means and standard errors of the background-adjusted total cell soma fluorescence per μm2 from 12 animals each. **P < 0.05 and *P = 0.01 by Dunnett’s test, when comparing the indicated genotypes to wild type. Bars without asterisks are not significantly different from wild type (P > 0.05).
The levels of IDA-1-GFP in unc-43 lf mutant axons are ∼30% of wild-type axons (Figure 1C). This defect results from the loss of UNC-43’s kinase activity in the same neurons because expressing wild-type UNC-43 in the same neurons completely rescued this defect, but a kinase-dead version of UNC-43 did not rescue (Figure 1C). A previously isolated unc-43 gain-of-function mutant showed the opposite phenotype. It accumulated IDA-1-GFP in its axons at levels that were ∼155% of wild type (Figure 1C).
To determine if UNC-43 (CaM kinase II) and UNC-108 (Rab2) act in the same pathway, we analyzed double mutants. An unc-43; unc-108 double mutant was only slightly worse than either single mutant with respect to the amount of IDA-1-GFP reaching axons and was not statistically significant, possibly suggesting a common pathway (Figure 1C). However, an analysis of cell somas revealed that unc-43 lf mutants, unlike unc-108 null mutants, do not accumulate IDA-1-GFP in their cell somas (Figure 1D), suggesting that the two mutations may disrupt different pathways.
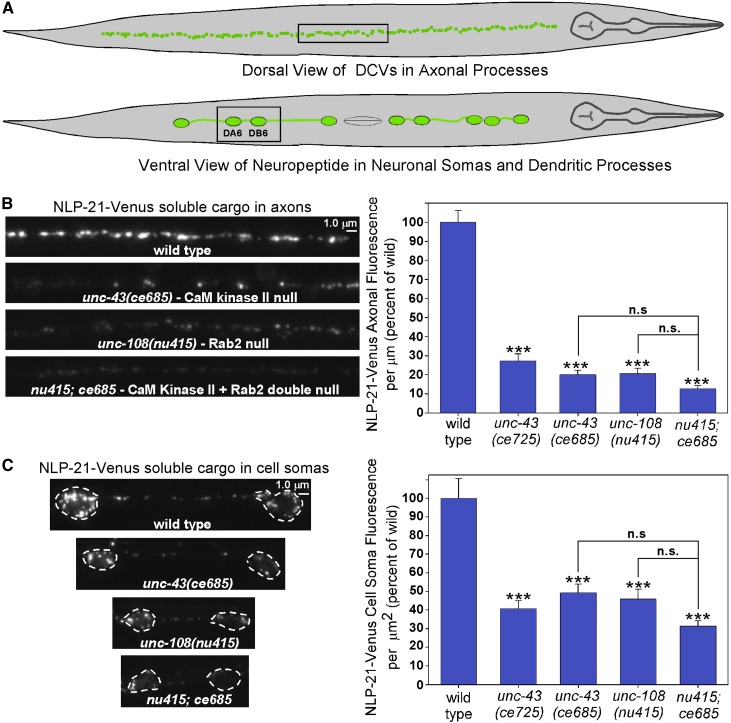
To determine if unc-43 lf mutations also disrupt the trafficking of soluble DCV cargo, we used the NLP-21 pro-neuropeptide tagged at its C terminus with the fluorescent protein Venus (Sieburth et al. 2007). This genomically integrated transgene expresses the tagged pro-neuropeptide in a set of nine DA and DB-type ventral cord motor neurons, which allows quantitative imaging of defined axonal, dendritic, and cell soma regions (Figure 2A). The Venus tag on NLP-21 behaves as a soluble cargo after EGL-3 (PC2 convertase) cleaves it from the neuropeptides inside DCVs (Figure S1) (Edwards et al. 2009). We found that both unc-43 and unc-108 null mutant axons are missing ∼80% of this soluble DCV cargo, while their cell somas were missing 60 and 50%, respectively (Figure 2B). As an important control, we found that expression of soluble GFP alone using the same transgenic promoter is not affected by an unc-43 lf mutation (Figure S2).
Figure 2.
Strongly decreased levels of a DCV soluble cargo in unc-43 mutant axons and cell somas. (A) Schematic illustrating the subset of DA/DB motors neurons in which the unc-129 promoter drives expression. This promoter was used in most transgenes in this study. Boxed regions indicate regions selected for imaging. The spacing between the DA6 and DB6 cell somas is variable and contains the dendrites from each cell. (B and C) Representative, identically scaled images and quantification of Venus cargo, expressed from the integrated transgene ceIs56 in DA/DB motor neuron axons (B) and DA6/DB6 cell somas (C) in animals with the indicated genotypes. Dashed lines outline the cell soma boundaries in C. The fluorescent signal represents soluble intravesicular Venus that has been cleaved from the NLP-21-Venus pro-neuropeptide by EGL-3 (PC2 convertase) (Figure S1) (Edwards et al. 2009). Graph data are means and standard errors of the background-adjusted total dorsal cord fluorescence per μm of cord length (B) or background-adjusted cell soma fluorescence per μm2 from 13 (B) or 12 (C) animals each. ***P ≤ 0.001 by Tukey’s test when comparing the indicated genotypes to wild type. n.s., not significant (P > 0.05).
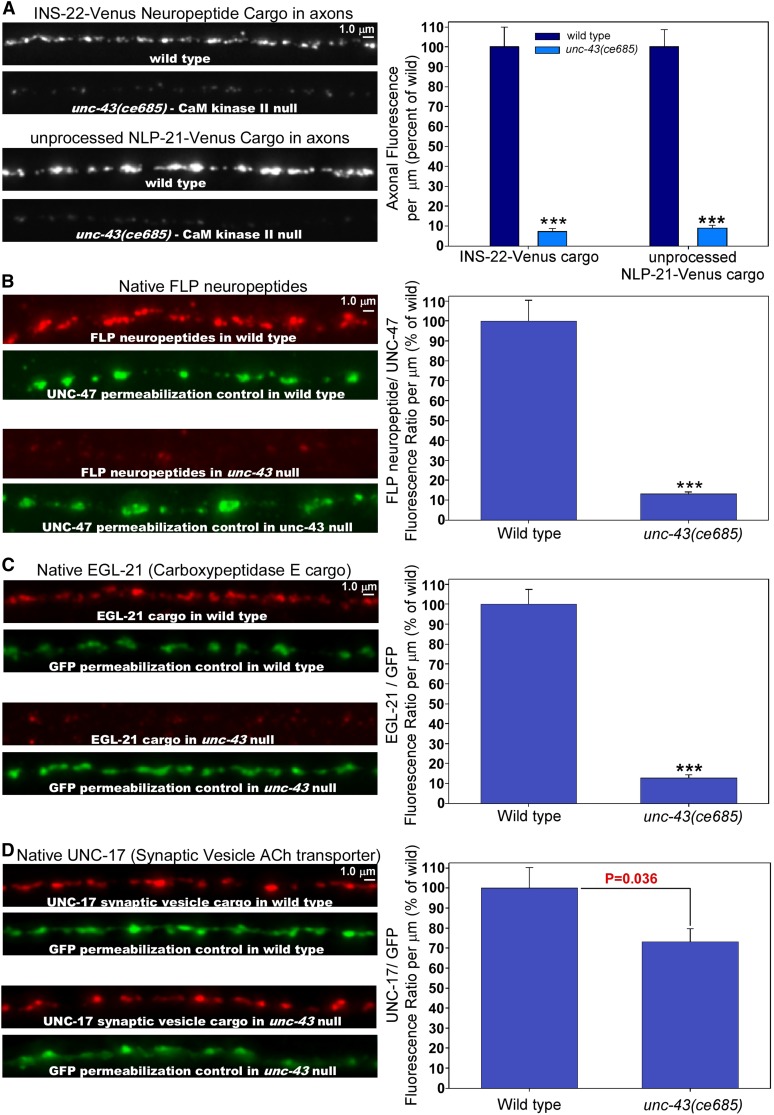
To determine whether unc-43 lf mutations affect the trafficking of actual neuropeptide cargos, we tracked this cargo class using an INS-22-Venus transgene. INS-22 is one of a group of insulin-like peptides in C. elegans, and it lacks EGL-3 (PC2 convertase) cleavage sites. Thus, the Venus tag should stay with the aggregated neuropeptide cargo rather than being separated from the neuropeptides during the neuropeptide processing that occurs in DCVs (Figure S1). unc-108 null mutations have little or no effect on INS-22-Venus cargos (Edwards et al. 2009). In strong contrast, unc-43 lf mutant axons are missing >90% of their INS-22-Venus cargo (Figure 3A). We saw similar results when we tracked aggregated neuropeptide cargo by viewing the NLP-21-Venus transgene in an egl-3 (PC2 convertase) null mutant background, which should also keep the Venus tag with the aggregated neuropeptide since the neuropeptides cannot be cleaved away from the tag in this background (Figure 3A and Figure S1). Again, this contrasts strongly with unc-108 mutants, which can traffic unprocessed NLP-21-Venus to axons similar to wild type (Edwards et al. 2009).
Figure 3.
unc-43 mutant axons are missing ∼90% of their neuropeptide and processing enzyme DCV cargos, but a synaptic vesicle cargo is only mildly affected. (A) Representative, identically scaled images and quantification of INS-22-Venus cargo expressed from the integrated transgene nuIs195 (first pair of bars) or unprocessed NLP-21-Venus cargo expressed from the ceIs56 transgene in an egl-3 null mutant background (second pair of bars) in DA/DB motor neuron axons in animals with the indicated genotypes. The fluorescent signals in this experiment represent aggregated neuropeptide cargos (Figure S1) (Edwards et al. 2009). Graph data are means and standard errors of the total background-adjusted dorsal cord fluorescence per μm of cord length from 13 animals each. ***P ≤ 0.001 by the unpaired t-test with Welch correction when comparing the indicated genotypes to wild type. (B–D) Representative images and quantification from immunostaining of native FLP neuropeptides (B; red signal), native EGL-21 (C; red signal) and native UNC-17 (D; red signal) in the dorsal cord axons of animals with the indicated genotypes. In B, we costained with an antibody that recognizes UNC-47 (the SV GABA transporter; bottom green image in each pair in B) as a permeabilization control and then plotted the ratio of the two signals. In C and D, we immunostained in a ceIs123 genetic background, which expresses soluble GFP in the dorsal cord axons. We co-immuostained the strains in C and D with an anti-GFP antibody as a permeabilization control (bottom green image in each pair in C and D). We visualized the anti-GFP antibody using a far-red Dylight 650 secondary antibody and then plotted the ratio of the two signals. Graphs show means and standard errors of the ratios of the red/green signals from 18 animals each. ***P ≤ 0.001 by the unpaired t-test with Welch correction when comparing the indicated genotypes to wild type.
To determine whether unc-43 lf mutations affect the trafficking of endogenous DCV cargos, we performed quantitative immunostaining experiments using antibodies that recognize endogenous neuropeptides as well as endogenous EGL-21(carboxypeptidase E), which is a neuropeptide processing enzyme known to be copackaged into DCVs with neuropeptides (Fricker 1988; Cool et al. 1997; Rindler 1998). To detect endogenous neuropeptides, we used an antibody that recognizes processed FMRFamide-related neuropeptides in C. elegans (Schinkmann and Li 1992) and observed an 86.9% reduction in the endogenous neuropeptide signal in unc-43 mutant axons compared to wild type (Figure 3B). To detect endogenous EGL-21, we produced an affinity-purified polyclonal antibody against EGL-21 from purified, recombinant EGL-21. The antibody specifically recognizes EGL-21 because the signal is nearly absent in an egl-21 mutant that contains a large in-frame deletion (Figure S2). We observed an 87.2% reduction in the endogenous EGL-21 signal in unc-43 mutant axons compared to wild type (Figure 3C). Thus, in agreement with the transgenic cargo data, endogenous DCV cargos in unc-43 mutant axons are present at only ∼10% of wild-type levels.
In addition to DCVs, neurons also contain synaptic vesicles (SVs) that secrete small-molecule neurotransmitters via a separate regulated secretory pathway. To determine the extent to which unc-43 lf mutations disrupt the trafficking of an endogenous SV cargo, we immunostained animals with an antibody that recognizes UNC-17 (VAChT, the SV ACh transporter) (Duerr et al. 2001) and observed a small but significant decrease in UNC-17 in unc-43 mutant axons compared to wild type (Figure 3D). Thus, UNC-43’s role in the trafficking of SV cargos is minimal compared to its role in the trafficking of DCV cargos.
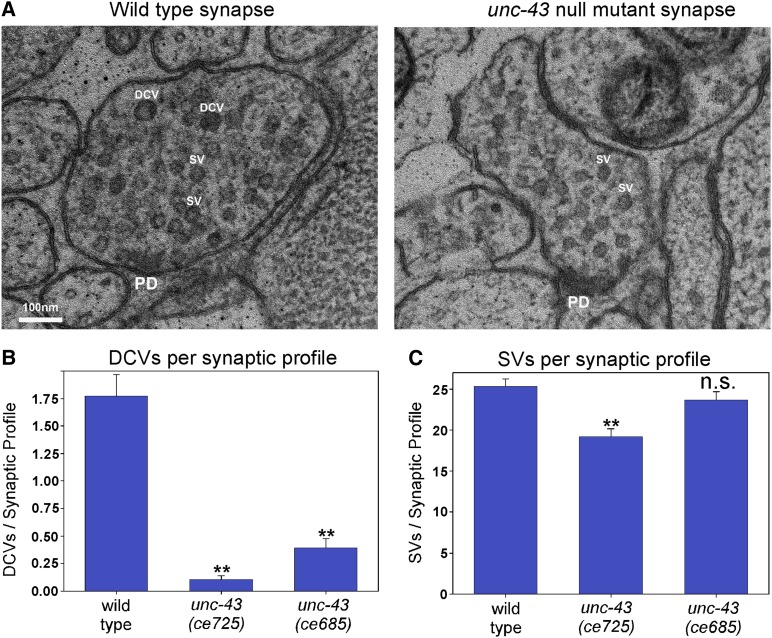
Electron microscopy analysis: unc-43 lf mutant axons are missing up to 90% of their DCVs, but synaptic vesicles are only mildly affected
To determine whether the DCV cargo losses in unc-43 mutant axons correspond to similar reductions in actual DCV numbers, we used high-pressure freezing EM to count DCVs in unc-43 mutant synapses. The DCVs in EM micrographs of unc-43 mutant axons appeared indistinguishable from DCVs in wild-type axons, showing that unc-43 lf mutants are capable of forming normal-looking DCVs. However, the two unc-43 nonsense mutants ce685 and ce725 had 78 and 94% fewer DCVs, respectively, than wild-type animals at their synapses (Figure 4, A and B). In contrast, synaptic vesicle numbers were reduced by only 7% (not significant) and 24% (significant) in the two mutants. Thus, the EM data corroborate the quantitative fluorescence data and demonstrate that the cargos that are missing in axons correspond to similar reductions in actual DCVs, with only mild effects on SV numbers. This contrasts with unc-108 mutants, which are missing about two-thirds of their soluble and transmembrane DCV cargos, but have normal levels of actual DCVs and SVs in their axons (Edwards et al. 2009; Sumakovic et al. 2009).
Figure 4.
Electron microscopy analysis: unc-43 mutant axons are missing up to 90% of their dense core vesicles, but synaptic vesicles are only mildly affected. (A) Representative electron micrographs of ACh motor neuron synaptic profiles from the dorsal nerve cord axons of wild type and the unc-43(ce725) nonsense mutant as indicated. PD, presynaptic density; SV, synaptic vesicle; DCV, dense core vesicle. (B and C) Quantification of the number of DCVs (B) or SVs (C) per synaptic profile in animals with the indicated genotypes. Data are means and standard errors from 57 wild type, 77 unc-43(ce725), or 66 unc-43(ce685) synaptic profiles from 8, 14, or 12 ACh synapses, respectively, collected from a total of two animals for each genotype. **P ≤ 0.01 by Dunnett’s test when comparing the indicated genotypes to wild type. n.s., not significant (P > 0.05).
UNC-68 (ryanodine receptor) contributes to UNC-43-mediated DCV cargo trafficking
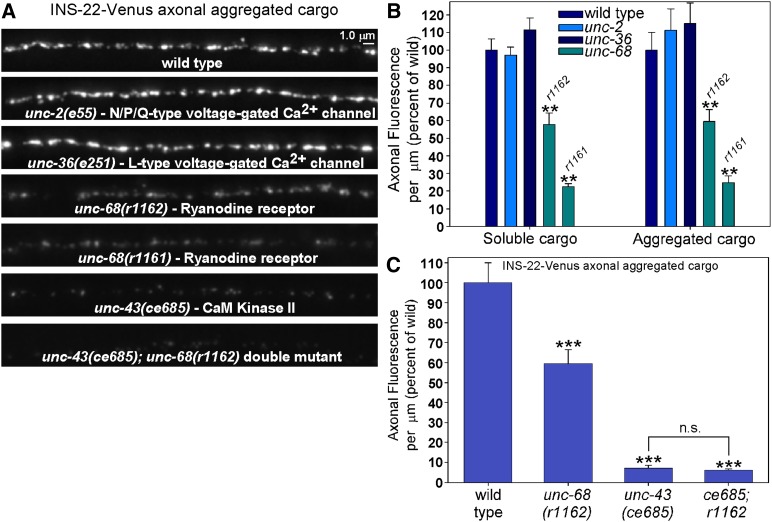
Because CaM kinase II is known to be activated by calcium and calmodulin, we tested lf mutants of known calcium channels for similar DCV cargo phenotypes. C. elegans has three predicted voltage-gated calcium channel α1 subunits: egl-19 (CaV1; L type), unc-2 (CaV2; N/P/Q type), and cca-1 (CaV3). Nonsense mutations in unc-2 and unc-36 [an α2δ subunit that contributes to the trafficking and function of both CaV1 and CaV2 voltage-gated Ca2+ channels (Bauer Huang et al. 2007; Saheki and Bargmann 2009)] had axonal levels of both soluble and aggregated neuropeptide cargos that were not significantly different from wild type (Figure 5, A and B). We note that a previous study, using the same mutant and same aggregated neuropeptide cargo transgene, did show a slight (10%) significant increase in axonal levels of the cargo (Ch’ng et al. 2008). Although egl-19 (CaV1) null mutants are embryonic lethal (Lee et al. 1997), we found that the egl-19(ad1006) reduction-of-function mutant (Lee et al. 1997) also showed wild-type axonal levels of aggregated neuropeptide cargo using the nuIs195 transgene (96 ± 10% of wild type; n = 13 dorsal cords). We have not yet tested cca-1 (CaV3) mutants. However, two deletion alleles of unc-68 (the sole worm ortholog of the ryanodine receptor, which releases Ca2+ from internal stores in the endoplasmic reticulum (ER), showed significantly reduced levels of soluble and aggregated neuropeptide cargos (Figure 5, A and B). Eliminating unc-68 did not further enhance the cargo loss in an unc-43 lf mutant (Figure 5C), which suggests that the ryanodine receptor and CaM kinase II function in the same pathway to regulate DCV trafficking. None of the calcium channel mutations affected expression from the transgenic promoter used for this experiment (Figure S3).
Figure 5.
UNC-68 (ryanodine receptor) contributes to UNC-43-mediated DCV cargo trafficking. (A) Representative, identically scaled images of axonal INS-22-Venus neuropeptide cargo expressed from the nuIs195 integrated transgene in animals with the indicated genotypes. (B) Quantification of axonal fluorescence from soluble DCV cargo from NLP-21-Venus expressed from the ceIs56 transgene (first set of bars), or aggregated neuropeptide cargo from INS-22-Venus expressed from the nuIs195 transgene (second set of bars). The last two bars in each set are two different unc-68 mutants. The data are means and standard errors of the total background-adjusted dorsal cord fluorescence per μm of cord length from 13 animals. **P ≤ 0.01 by Dunnett’s test when comparing the indicated genotypes to wild type. Bars lacking asterisks are not significantly different from wild type (P > 0.05). (C) Quantification of axonal fluorescence from aggregated neuropeptide cargo from INS-22-Venus expressed from the nuIs195 transgene in unc-68 and unc-43 single and double mutants, showing that impairing UNC-68 does not further worsen the unc-43 INS-22-Venus trafficking phenotype. The data are means and standard errors of the total background-adjusted dorsal cord fluorescence per μm of cord length from 13 animals. ***P < 0.001 by Tukey’s test when comparing the indicated genotypes to wild type. n.s., not significant (P > 0.05).
DCVs largely fail to enter axons in unc-43 lf mutants
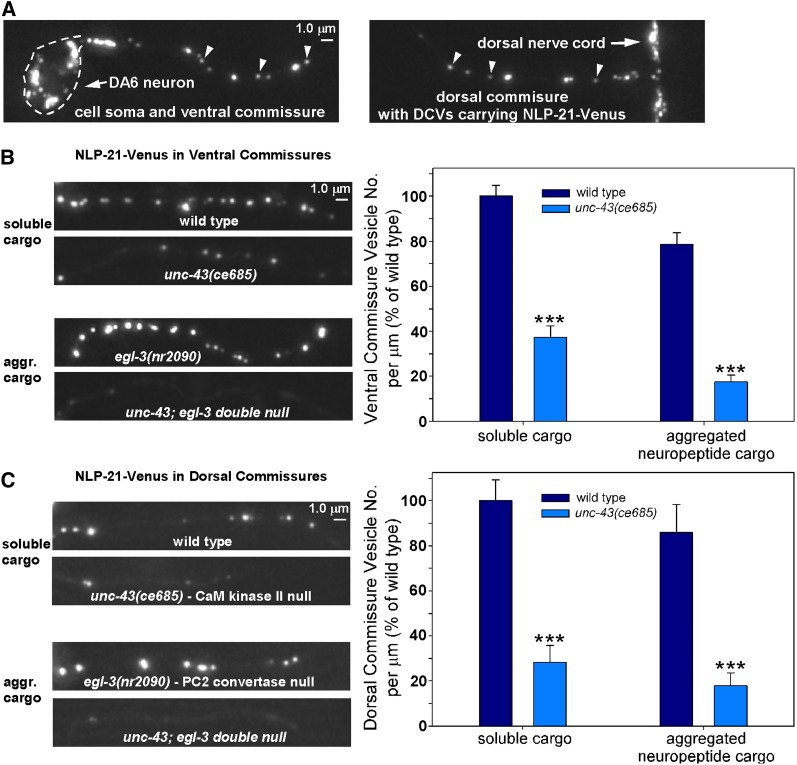
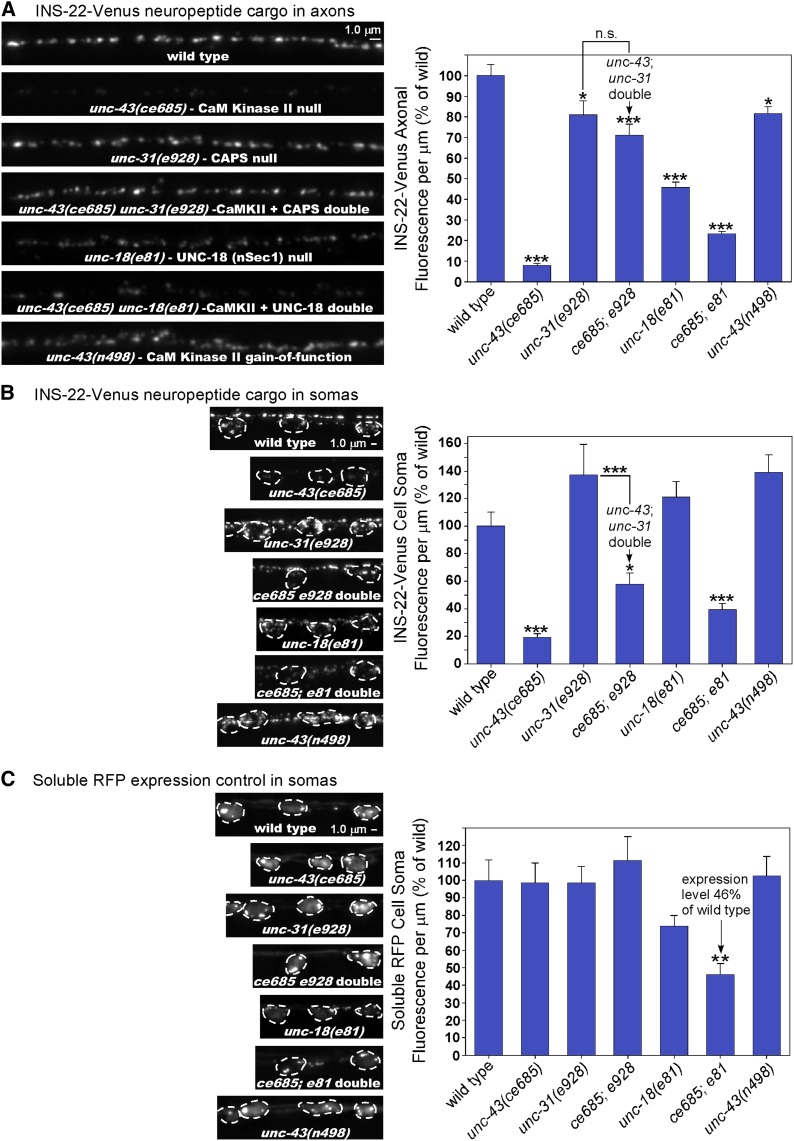
The near absence of DCVs in unc-43 mutant axons could result either from hyperactive exocytosis of DCVs upon reaching the synaptic region of axons or from a failure of DCVs to exit the cell soma and enter axons, the latter of which could have a number of different causes. To distinguish between these two possibilities, we analyzed single-vesicle DCV cargos in axonal commissures. A commissure is the initial segment of the axon that does not contain synapses. It contains only those DCVs that the neuron is transporting between the cell soma and the distal synaptic region of the axon (in both directions). In C. elegans DA and DB motor neurons, the commissures extend from the cell somas on the ventral side of the animal across the body to the synaptic region of the axon in the dorsal nerve cord (Figure 6A). The concentration of DCVs in a wild-type commissure is much lower than in the synaptic region, and time-lapse imaging studies suggest that many fluorescently tagged neuropeptide puncta within commissures represent individual vesicles or packets of cotransported vesicles (Goodwin et al. 2012; also supported by Figure 10 in this study).
Figure 6.
DCV neuropeptide cargos largely fail to enter axons in unc-43 mutants. (A) Images of the DA6 motor neuron soma (outlined in dashes) with its axonal commissure exiting the cell soma on the ventral side of the animal (left) and the same commissure entering the nerve cord on the dorsal side of the animal (right). The fluorescent signal comes from NLP-21-Venus neuropeptide in dense core vesicles expressed from the integrated transgene ceIs56. Time-lapse imaging demonstrates that most of these puncta represent individual vesicles (Goodwin et al. 2012). (B and C) Representative, identically scaled images and quantification of soluble NLP-21-Venus cargo, expressed from the integrated transgene ceIs56 (first pair of bars), or unprocessed NLP-21-Venus cargo (aggregated neuropeptide cargo; Figure S1) (Edwards et al. 2009), expressed from the ceIs56 transgene in an egl-3 null mutant background (second pair of bars) in DA/DB motor neuron axons in animals with the indicated genotypes. Graph for (B) are the data means and standard errors from the following numbers of puncta: 391 (wild type; from 24 commissures), 138 (unc-43 mutant; from 23 commissures), 358 (egl-3 mutant; from 26 commissures), or 36 (unc-43; egl-3 double mutant; 19 commissures). Graph data for (C) are means and standard errors from the following numbers of puncta: 153 (wild type; from 13 commissures), 39 (unc-43 mutant; from 14 commissures), 112 (egl-3 mutant; from 13 commissures), or 19 (unc-43; egl-3 double mutant; from 12 commissures). ***P ≤ 0.001 by the unpaired t-test with Welch correction when comparing the indicated genotypes to wild type (or to the egl-3 null mutant for the aggregated cargo experiments).
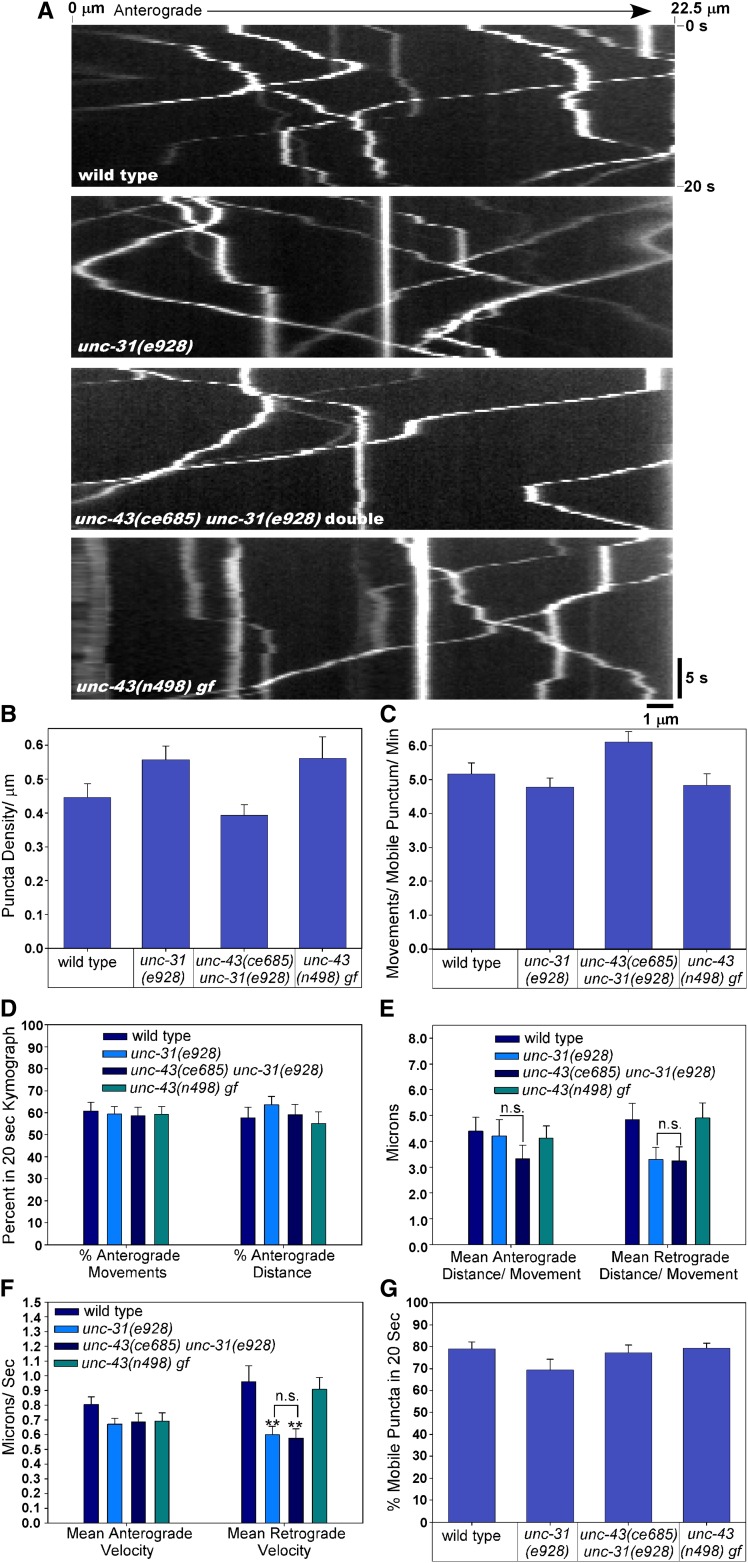
Figure 10.
CaM kinase II is not required for active transport of DCVs between cell somas and the synaptic region of axons. (A) Representative kymographs of DCV movements in motor neuron commissures of the indicated genotypes. The left part of each image is closest to the cell soma, while the right part extends to near the start of the synaptic region in the dorsal axon. Each movie represents 20 sec of DCV movements. Anterograde movements angle toward the lower right, while retrograde movements angle toward the lower left. Stationary puncta are vertical lines. (B–G) Graphs plotting various parameters extracted from the kymographs as indicated. For all graphs, the indicated parameter was calculated as an average or a percentage based on all movements in each kymograph, and then the average of all of the kymographs was plotted (n = 23, 22, 25, and 20 wild type, unc-31, unc-43 unc-31 double mutant, and unc-43 gf, respectively). Error bars represent standard errors. **P ≤ 0.01 by Dunnett’s test when comparing to wild type. Bars without asterisks are not significantly different from wild type when compared by Dunnett’s test. n.s., not significant when non-wild-type strains are compared using Tukey’s test.
We compared the number of detectable fluorescent puncta containing soluble and aggregated neuropeptide cargos in wild-type and unc-43 lf mutant commissures. In the ventral commissure region, which is the closest region to the cell soma, the number of detectable puncta containing soluble cargo and aggregated neuropeptide cargo in an unc-43 lf mutant was reduced by 63 and 82%, respectively (Figure 6B). We saw similar results for the dorsal commissure region (72 and 79%, respectively; Figure 6C). The results thus suggest that DCV neuropeptide cargos do not properly exit the cell somas of unc-43 mutants or that they exit the cell soma but are secreted from the axon initial segment before being transported to the synaptic region.
unc-43 lf mutants lose neuropeptide cargos via secretion
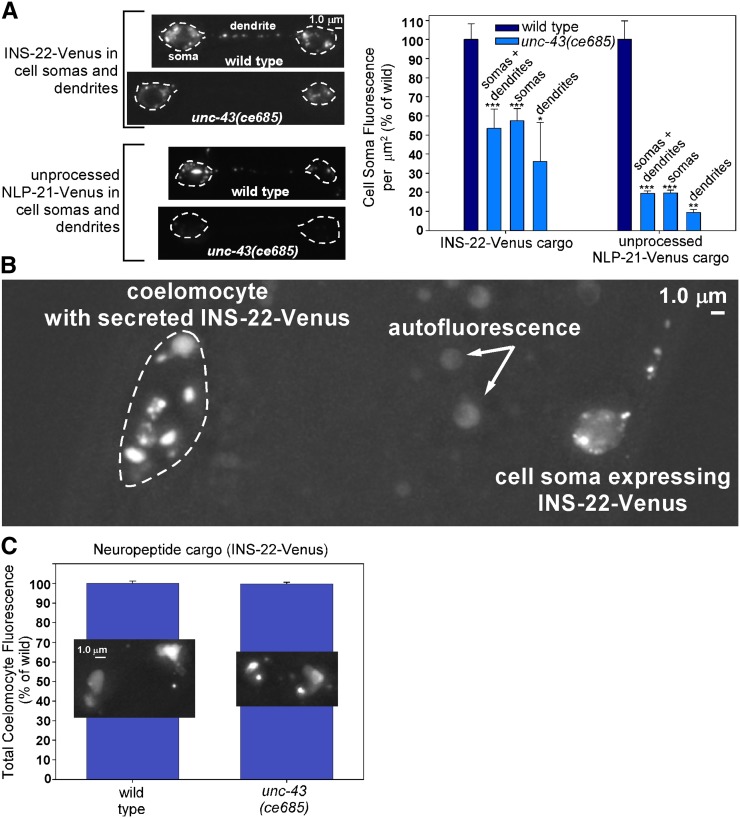
C. elegans motor neurons use the UNC-104 (KIF1A) motor to transport both synaptic vesicles and dense core vesicles to axons (Hall and Hedgecock 1991; Jacob and Kaplan 2003). If the drastic reduction in axonal neuropeptide cargos in unc-43 lf mutants results from an active transport defect that causes DCVs to simply remain in cell somas rather than being transported to axons, then DCV cargos should accumulate in cell somas, as they do in unc-104 mutants (Jacob and Kaplan 2003). However, that is not the case. The soluble DCV cargo NLP-21-Venus is reduced by ∼60% in unc-43 lf mutant somas (Figure 2C), and the aggregated neuropeptide cargos INS-22-Venus and unprocessed NLP-21-Venus are reduced by ∼45 and 80%, respectively (Figure 7A). Prior studies have found that mutants that affect the polarity of DCV trafficking, such as cdk-5 (cyclin-dependent kinase-5) and syd-2 (Liprin-α) mutants, have lower levels of DCV cargos in their DB motor neuron axons, but the missing cargos can be found in dendrites (Goodwin et al. 2012; Goodwin and Juo 2013). However, that is not the case here, since unc-43 mutant dendrites also have lower levels of the DCV cargos (Figure 7A). This suggests that the cargos are degraded in, and/or secreted from, the cell somas and/or dendrites.
Figure 7.
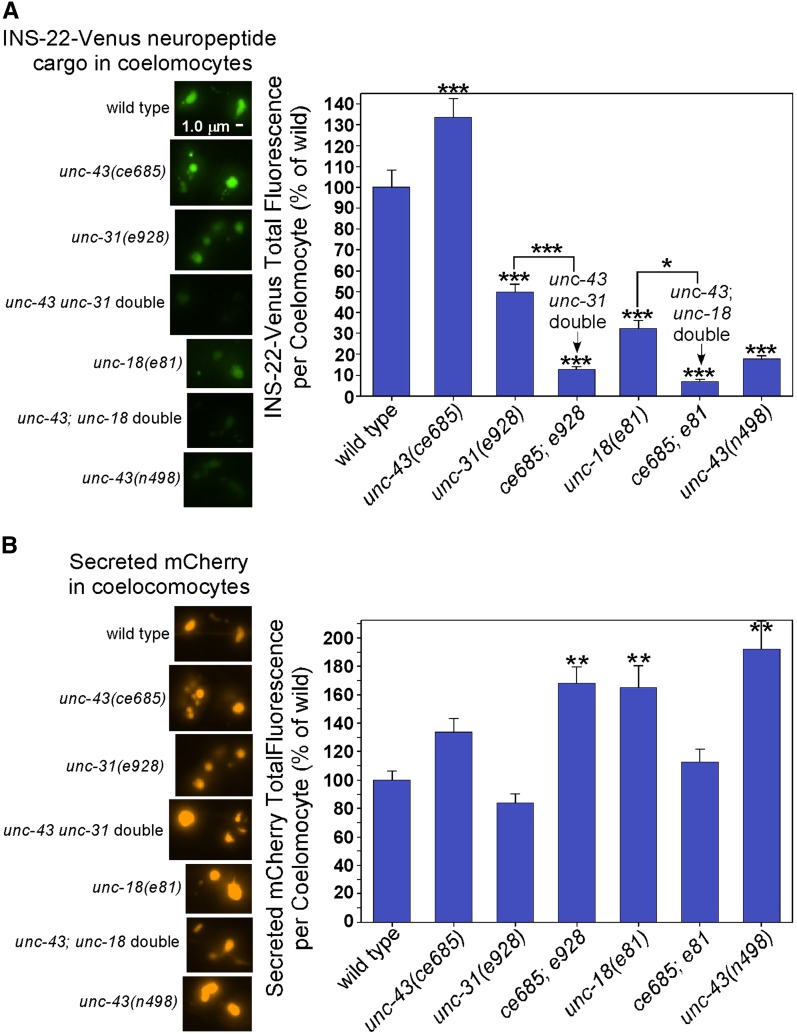
unc-43 mutants lose neuropeptide cargoes via secretion. (A) Representative, identically scaled images and quantification of INS-22-Venus cargo, expressed from the integrated transgene nuIs195 (first pair of bars), or unprocessed NLP-21-Venus cargo, expressed from the ceIs56 transgene in an egl-3 null mutant background (second pair of bars), in DA6/DB6 cell somas and dendrites in animals with the indicated genotypes. The fluorescent signals in this experiment represent aggregated neuropeptide cargos. Graph data are means and standard errors from 12 animals each. ***P ≤ 0.001, **P ≤ 0.0012, and *P ≤ 0.02 by the unpaired t-test with Welch correction when comparing the indicated genotypes to wild type (or the egl-3 mutant for the unprocessed NLP-21-Venus cargo data). Error bars for control strains are for soma + dendrite combined data. (B) Representative image of a coelomocyte containing secreted INS-22-Venus. The bright patches in the coelomocyte are endosomes that have endocytosed the secreted neuropeptide from the animal’s pseudocoelom. All of the INS-22-Venus comes from expression of the integrated transgene nuIs195 in a set of nine motor neuron cell somas, one of which is indicated. Also indicated are autofluorescent patches from intestinal granules. (C) Graph and representative, identically scaled images of secreted INS-22-Venus levels in coelomocytes of wild-type and unc-43 loss-of-function mutants. Graph data are means and standard errors of the background-adjusted total coelomocyte fluorescence from 27 wild-type and 32 unc-43 mutant animals.
To determine the extent to which the missing cargos are secreted, we looked for their presence in coelomocytes, which are scavenger cells in the animal’s body that endocytose fluid from the pseudocoelom and concentrate secreted proteins in their endosomes (Figure 7B). A previous study developed this method specifically for quantifying INS-22-Venus neuropeptide secretion (Ch’ng et al. 2008). We observed neuropeptide cargos at wild-type levels in the endosomes of unc-43 mutant coelomocytes (Figure 7C). The only possible sources of the neuropeptide cargo in the coelomocyte endosomes are the axons, cell somas, and dendrites of the nine neurons where the INS-22-Venus transgene is expressed. The wild-type levels of neuropeptide cargos in unc-43 mutant coelomocytes, along with the near absence of the cargos in axons, suggest that a substantial amount of the neuropeptide cargo that fails to enter unc-43 mutant axons is secreted prior to axonal transport. According to this hypothesis, the neuropeptide cargo in wild-type coelomocytes originates mostly from axonal release, whereas the neuropeptide cargo in unc-43 mutant coelomoctyes originates mostly from cell soma and/or dendrite release, resulting in the observed wild-type neuropeptide levels in coelomocytes and in low neuropeptide levels in unc-43 mutant somas and dendrites.
Loss of neuropeptide from unc-43 mutant neurons requires regulated secretion machinery
To further test whether the neuropeptide secretion in unc-43 mutants is regulated or constitutive, we used the coelomocyte uptake assay to test whether the neuropeptide secretion in unc-43 lf mutants requires UNC-31 (CAPS), a protein that docks/primes dense core vesicles for regulated exocytosis (Grishanin et al. 2004; Gracheva et al. 2007; Hammarlund et al. 2008). For this experiment we used a new integrated transgene that co-expresses the neuropeptide marker INS-22-Venus along with a constitutively secreted protein (secreted mCherry, or S-mCherry) in cholinergic motor neurons. The S-mCherry marker controls for defects in coelomocyte function and provides an easy way to locate coelomocytes for quantitative imaging in strains with strongly impaired regulated secretion.
Using this system of markers, unc-43 lf mutants showed significantly higher-than-wild-type levels of secreted neuropeptide in their coelomocytes (133% of wild type), while constitutive secretion was not significantly different (Figure 8, A and B). unc-31 null single mutants accumulated neuropeptide in their coelomocytes at ∼50% of wild type, consistent with the partial block in neuropeptide secretion observed in prior studies (Sieburth et al. 2007; Speese et al. 2007). However, in unc-43unc-31 double mutants, secreted neuropeptide in coelomocytes was reduced by ∼96% relative to unc-43 single mutants (Figure 8A). This suggests that most of the neuropeptide that is secreted from unc-43 mutant neurons exits via an unc-31-dependent regulated secretory pathway rather than via constitutive secretion. The neuropeptide secretion in unc-43 lf mutants was also dependent on the conventional synaptic t-SNARE syntaxin because an unc-18 (nSec1) null mutation also reduced secreted neuropeptide levels in coelomocytes by ∼90% (Figure 8A). UNC-18 interacts closely with syntaxin to regulate conventional neurotransmitter release from SVs in C. elegans (Weimer et al. 2003; McEwen and Kaplan 2008; Johnson et al. 2009), and syntaxin is required for DCV docking in C. elegans (Hammarlund et al. 2008), so this result again supports the conclusion that the neuropeptide secretion in unc-43 lf mutants occurs via a regulated secretory pathway.
Figure 8.
The loss of neuropeptide from unc-43 mutant neurons requires regulated secretion machinery. (A) Representative, identically scaled images and quantification of coelomocytes containing secreted INS-22-Venus. The bright patches in the coelomocyte are endosomes that have endocytosed the secreted neuropeptide from the animal’s pseudocoelom. All of the INS-22-Venus comes from expression of the integrated transgene ceIs201 in cholinergic motor neurons. Graph data are means and standard errors of the background-adjusted total coelomocyte fluorescence from 28 to 32 coelomocytes (from 28 to 32 different animals). ***P < 0.001 and *P < 0.05 by Tukey’s test when comparing the indicated genotypes to wild type or to each other, as indicated. (B) Representative, identically scaled images and quantification of coelomocytes containing constitutively secreted mCherry. These are the same coelomocytes shown in A because the ceIs201 transgene also co-expresses mCherry with a signal sequence as an internal control for constitutive secretion and coelomocyte function. Graph data are means and standard errors of the background-adjusted total coelomocyte fluorescence from 28 to 32 coelomocytes (from 28–32 different animals). **P < 0.01 by Dunnett’s test when comparing the indicated genotypes to wild type.
None of the four strains with decreased neuropeptide secretion (unc-31 null, unc-18 null, and the doubles with unc-43) were associated with corresponding decreases in constitutive S-mCherry in coelomocytes, although two of these strains showed significant increases in S-mCherry in coelomocytes (Figure 8B). As expected, an unc-43 gain-of-function mutant had the opposite phenotype: strongly decreased levels of secreted neuropeptide in coelomocytes (∼17% of wild type) although it also had significantly high levels of S-mCherry in coelomocytes (Figure 8, A and B). As judged by quantification of nonsecreted mCherry in cell somas, all of the strains that we tested showed wild-type expression levels with the exception of the unc-43; unc-18 double, whose slow growth may have impaired expression of the transgenes (46% of wild type; Figure 9B). However, even after normalizing for the decreased expression level of this strain, we can conclude that the unc-18 null mutation reduced secreted neuropeptide in unc-43 mutant coelomocytes by ∼90%.
Figure 9.
Blocking regulated secretion machinery allows neuropeptide cargoes to accumulate in unc-43 mutant axons. (A and B) Representative, identically scaled images and quantification of INS-22-Venus cargo in dorsal axons (A) and cell somas (B) in animals with the indicated genotypes. INS-22-Venus is expressed from the integrated transgene ceIs201 in cholinergic motor neurons. The fluorescent signals represent aggregated neuropeptide cargos. Dashed lines outline the cell soma boundaries in B as determined by tracing the cell soma soluble mCherry signal from the identical cells in C. Graph data are means and standard errors from 12 to 13 animals each. ***P < 0.001 and *P <0.05 by Tukey’s test when comparing the indicated genotypes to wild type or each other as indicated. n.s., not significant (P ≥ 0.05). (C) Representative, identically scaled images and quantification of mCherry in cell somas as a control for transgene expression in animals with the indicated genotypes. mCherry is co-expressed with INS-22-Venus from the integrated transgene ceIs201, and the representative images are of the same cell somas shown in B. Dashed lines outline the cell somas. **P ≤ 0.01 by Dunnett’s test when comparing to wild type.
If neuropeptide is being secreted by an UNC-31 (CAPS)-dependent regulated secretory pathway from unc-43 mutant somas, it is possible that the aberrant distribution and exocytosis of DCVs results from a mislocalization of UNC-31 itself. For example, if eliminating UNC-43 impaired the trafficking of UNC-31 to axons, unc-43 mutant somas might be more likely to exocytose DCVs prior to axonal transport. However, using immunostaining we found normal levels of UNC-31 in unc-43 mutant dorsal axons (Figure S4), suggesting that mislocalization of UNC-31 is not the cause of the aberrant neuropeptide secretion in unc-43 mutants.
Blocking the function of regulated secretory proteins allows neuropeptide cargos to accumulate in unc-43 lf mutant axons
Taken together, the above data suggest that, in the absence of UNC-43 (CaM kinase II), DCVs are secreted prior to axonal transport via an UNC-31 (CAPS)-dependent regulated secretory pathway. If DCVs are indeed properly formed in unc-43 lf mutants, then blocking their secretion using unc-43unc-31 double mutants should restore wild or near-wild-type levels of DCVs to axons by allowing them to escape the cell soma instead of being exocytosed. To test this, we quantified neuropeptide cargo in the axons and cell somas of the same strains used for the above in vivo secretion experiment. The results showed that unc-43unc-31 double mutants transport neuropeptide-carrying vesicles into axons where they accumulate at levels that are not significantly different from unc-31 single mutants (Figure 9A). This suggests that DCVs in unc-43 mutants are stored in the absence of the DCV regulated secretory pathway and thus are not constitutively secreted. We saw similar results using an unc-18 null mutation to block regulated secretion in unc-43 lf mutants, although the levels of neuropeptide cargo in unc-43; unc-18 double-mutant axons were much lower than those in unc-43unc-31 double-null mutant axons (Figure 9A). A significant part of this difference is due to a 54% decrease in expression of the transgene caused by combining the unc-43 and unc-18 null mutations (Figure 9C), which severely impaired the growth of the animals.
In cell somas, blocking regulated secretion with the unc-31 null mutation restored neuropeptide levels in unc-43 mutants to 58% of wild type despite having no significant effect on expression of the transgene. The fact that neuropeptide levels are still lower than wild type in unc-43unc-31 double-mutant somas could indicate that degradation of neuropeptide cargos, either before or after DCVs are formed, also contributes to the loss of neuropeptides in unc-43 mutants. Alternatively, or in addition, the secretion that still occurs in unc-43unc-31 double mutants (Figure 8A; ∼10% of wild type) could contribute to the reduced cell soma neuropeptide levels.
CaM kinase II is not required for the active transport of DCVs between cell somas and the synaptic region of axons
One model suggested by our data is that DCVs in unc-43 lf mutants fail to enter the axon because they are exocytosed from cell somas and/or dendrites by a regulated secretory pathway before they have a chance to be transported to axons. However, this model does not preclude an additional defect in the active transport of DCVs from cell somas to axons in unc-43 lf mutants, since this phenotype would be masked if DCVs are lost by secretion prior to transport. Our initial analysis of unc-43unc-31 double mutants argues against a DCV transport defect because blocking regulated secretion restored near-wild-type levels of DCVs in axons that are >100 μm away from the cell soma (Figure 9A). That said, a rigorous test of DCV transport is warranted because there is precedent for CaM kinase II affecting the movement of DCVs. Two prior studies used pharmacological approaches to acutely inhibit CaM kinase II in dissected Drosophila larvae and found that this abolished the DCV movements between synaptic boutons that normally occur after high-frequency tetanic stimulation (Shakiryanova et al. 2007) as well as the subsequent capture of DCVs as they transit through synaptic boutons after stimulation (Wong et al. 2009).
To determine whether genetic ablation of CaM kinase II affects the axonal transport of DCVs in intact animals, we used time-lapse microscopy to analyze DCV movements in the axonal commisssures of unc-43 lf mutants (i.e., between cell somas and the synaptic region). Since most DCVs fail to enter the axonal commissure in unc-43 lf mutants, we analyzed DCV movements in unc-43unc-31 double mutants and compared them to unc-31 single mutants and wild type as well as an unc-43 gain-of-function mutant. The density of puncta in the commissures of the three mutant strains that we analyzed was not significantly different from wild type (Figure 10B). Our analysis of kymographs produced from time-lapse movies of these strains (Figure 10A) showed that active transport in unc-31 null mutants is not significantly different from unc-31unc-43 double mutants with respect to the percentage of puncta that were mobile, the number of movements per mobile punctum per micron, the percentage of movements that were in the anterograde direction, the percentage of total distance that was in the anterograde direction, the mean anterograde and retrograde distance per movement, or the mean anterograde and retrograde velocities (Figure 10, C–G). Both strains containing the unc-31 null mutation (the unc-31 single mutant and the unc-31unc-43 double mutant) showed a significantly reduced retrograde velocity; however, they were not significantly different from each other, indicating that the unc-43 lf mutation did not contribute to this phenotype (Figure 10F). Furthermore, the unc-43 gain-of-function mutant was not significantly different from wild type for any of the parameters that we measured (Figure 10, B–G). These data suggest that an axonal transport defect does not contribute to the failure of DCVs to populate unc-43 mutant axons.
Wild-type DCV densities in cell somas are ∼2% of DCV densities in axons
Taken together, the above data suggest that wild-type neurons have a mechanism to inhibit or otherwise tightly regulate how much DCV secretion occurs from cell somas. Inhibiting release from somas would allow the UNC-104 motor (KIF1A) to transport DCVs out of cell somas to the synaptic region of axons where they could be stored until appropriate signals stimulate their release. If this is true, wild-type animals should have a relatively low concentration of DCVs in their cell somas and a much higher concentration in axons. To test this prediction, we reconstructed two wild-type motor neuron cell somas and a dorsal axon synaptic region spanning several synapses at high resolution using serial electron micrographs and then counted the DCVs in each region. The motor neuron somas contained an average of 40 DCVs each at an average concentration of 4.4 DCVs/μm3, whereas the much smaller axonal region contained an average of 25 DCVs per synapse at an average concentration of 187 DCV/μm3, or ∼50-fold higher than the concentration in somas (Figure 11, A and B). Thus, although there are no data to rule out that secretion of DCVs normally occurs from wild-type motor neuron somas, the reconstruction data suggest that motor neurons do not use somas to store a large reservoir of DCVs for secretion. This contrasts with neuroendocrine cells, such as chromaffin cells and pancreatic β-cells, in which DCVs number in the tens of thousands per cell and can occupy 31 and 12% of the cell soma volume, respectively (Dean 1973; Plattner et al. 1997).
Figure 11.
DCV densities in wild-type cell somas are ∼2% of DCV densities at synapses. (A and B) Three-dimensional reconstructions of motor neuron cell somas (A) and axonal synapses (B) from electron micrographs of serial thin sections cut from high-pressure-frozen wild-type animals. Light blue spheres, synaptic vesicles; black spheres, dense core vesicles; red, active zone dense projection; green, mitochondria; brown, nucleus; blue, Golgi and Golgi-adjacent vesicles; dark gray, endoplasmic reticulum; light gray, multivesicular bodies.
Abnormal neuropeptide function contributes to the sluggish locomotion of unc-43 lf mutants
If unc-43 mutants secrete native neuropeptides abnormally from their motor neuron somas instead of their axons, this could affect the animal’s locomotion behavior. Similarly, the ∼90% reduction in axonal DCVs in motor neurons could also affect the animal’s locomotion behavior. To test this in living animals, we compared the basal locomotion rates of unc-43 mutants in the presence and absence of native neuropeptides by making unc-43; egl-3 double null mutants. Eliminating EGL-3 (PC2 convertase) or EGL-21 (carboxypeptidase E) causes a similar sluggish locomotion rate that is not further worsened in an egl-3; egl-21 double null mutant (Edwards et al. 2009). Since these processing enzymes act sequentially, this demonstrates that knocking out either processing enzyme effectively eliminates most or all of the neuropeptide function that regulates basal locomotion rate.
Although both unc-43 nulls and egl-3 nulls have basal locomotion rates that are significantly slower than wild type, we found that the locomotion rate of the egl-3 null was not further reduced by additionally removing UNC-43 function (Figure 12). This suggests that the sluggish basal locomotion of unc-43 null mutants largely results from abnormal neuropeptide function because if it had a different cause, the double with the egl-3 null would be worse. The sluggish locomotion could result from ectopic somatic release of neuropeptides and/or from a reduction in the axonal supply of DCVs. These data contrast strongly with the unc-108 (Rab2) null, which has a sluggish locomotion rate not significantly different from the unc-43 null, but which strongly reduces the locomotion of the egl-3 null (Figure 12), consistent with Rab2 having a function that does not overlap with neuropeptides (Edwards et al. 2009).
Figure 12.
Abnormal neuropeptide function contributes to the sluggish basal locomotion of unc-43 lf mutants. The graph compares the basal (unstimulated) locomotion rates of wild type with the indicated mutants. All alleles are null deletion or early nonsense loss-of-function mutants. Error bars are SEMs of 10 animals each. P-values are from the unpaired t-test with Welch correction.
Discussion
A novel function for CaM kinase II
In this study we used the strengths of C. elegans to discover a previously unknown membrane-trafficking control pathway that, when disrupted, has profound effects on the distribution of DCVs within neurons as well as on the sites at which neuropeptides are released. No less surprising and unexpected was our finding that CaM kinase II plays a major role in this novel pathway. Discovered 35 years ago (Schulman and Greengard 1978a,b), CaM kinase II is a ubiquitous enzyme, but it is especially abundant in the brain, where it makes up 1–2% of total protein depending on the brain region (Erondu and Kennedy 1985). Most prior studies of the role of CaM kinase II in neurons have focused on two major areas: (1) presynaptic effects on synaptic strength and plasticity and (2) postsynaptic effects on glutamate receptors, especially with respect to long-term potentiation (LTP) in hippocampal dendritic spines.
Presynaptic studies in vertebrates have revealed both positive and negative roles for CaM kinase II as a regulator of synaptic transmission (Wang 2008). Similarly, studies of CaM kinase II in the C. elegans nervous system also showed that both loss- and gain-of-function unc-43 (CaM kinase II) mutations reduce spontaneous and evoked ACh and GABA synaptic transmission (Q. Liu et al. 2007; Vashlishan et al. 2008). The gain-of-function effect on synaptic transmission requires the BK channel SLO-1 (a Ca++-activated K+ channel) (Q. Liu et al. 2007).
The postsynaptic LTP studies show that CaM kinase II is one of the major signaling proteins that is activated by the increase in Ca++ entry that occurs through glutamate-gated ion channels in dendrites during the initiation phase of LTP (Lisman et al. 2012). Activated CaM kinase II then contributes to the long-term strengthening of hippocampal synapses by phosphorylating AMPA-type glutamate receptors in the dendrites, resulting in even more Ca++ entry upon presynaptic stimulation (Lisman et al. 2012).
The techniques used in both the pre- and the postsynaptic studies could detect only the release of classical neurotransmitters from small synaptic vesicles and could not directly detect effects on DCVs or effects mediated by DCVs. In the current study, we used an unbiased forward genetic strategy to investigate the requirements for DCV trafficking between cell somas and axons. Our discovery that CaM kinase II has a crucial role in a membrane-trafficking pathway is not unprecedented. Two prior studies showed that CaM kinase II regulates the membrane transport of AMPA-type glutamate receptors into postsynaptic regions in C. elegans and in vertebrate hippocampal slices (Rongo and Kaplan 1999; Hayashi et al. 2000; Rongo 2002). However, no prior study has implicated CaM kinase II in the major DCV trafficking pathway reported here.
Normal axonal transport of DCVs in CaM kinase II mutants
Two prior studies used pharmacological approaches to acutely inhibit CaM kinase II in dissected Drosophila larvae and found that this abolished the DCV movements within and between synaptic boutons that occur after high-frequency tetanic stimulation (Shakiryanova et al. 2007) as well as the subsequent capture of DCVs as they transit through synaptic boutons after stimulation (Wong et al. 2009). Although we found no defect in DCV motility between cell somas and the synaptic region of axons in CaM kinase II lf mutants, our data do not rule out a defect in DCV motility in the synaptic region itself after high-frequency tetanic stimulation. Neither Drosophila study investigated the role of CaM kinase II in regulating the exocytosis of DCVs prior to axonal transport or the effects of CaM kinase II inhibition on DCV levels in axons and cell somas. Because it is unclear how a defect in the intersynaptic motility/synaptic capture of DCVs could be related to the massive loss of DCV cargos via regulated exocytosis that we see prior to axonal transport, attempts to explain these disparate phenotypes in terms of a common underlying mechanism should be made with caution.
Model for CaM kinase II’s DCV function
Our data show that eliminating CaM kinase II reduces axonal and cell soma levels of DCVs by 90 and 80%, respectively. Mutationally blocking regulated secretion in unc-43 lf mutants restored near wild-type axonal levels of DCVs, and time-lapse microscopy of such double mutants showed no defect in active transport. In vivo secretion assays revealed that much of the missing neuropeptide in unc-43 mutants is secreted by a regulated secretory mechanism requiring UNC-31 (CAPS) and UNC-18 (nSec1). Analysis of DCVs at cell soma/axon boundaries suggested that most DCVs never escape unc-43 mutant somas. Taken together, these data suggest that CaM kinase II inhibits the regulated exocytosis of DCVs from neuronal somas and/or dendrites.
By this model, most of the “missing” neuropeptide cargo in unc-43 lf mutants is secreted, although we cannot rule out that some degradation of neuropeptide cargos prior to secretion (either before or after DCV biogenesis) also contributes to the loss. Although we did not directly observe neuropeptide release from cell somas, our finding that IDA-1-GFP levels are not reduced in unc-43 mutant somas provides indirect evidence of DCV exocytosis from unc-43 mutant somas when coupled with our observation of reduced neuropeptide levels in the somas. IDA-1 is a transmembrane DCV protein, so it should not disappear from somas upon DCV exocytosis, unlike the neuropeptide cargo. It is a little surprising that we do not see even higher levels of IDA-1-GFP in the somas because we might expect it to accumulate in the plasma membrane due to increased neuropeptide secretion from the soma. This could indicate that postfusion endocytosis and degradation of IDA-1 is very efficient or that some IDA-1 is diverted to a degradative pathway prior to DCV biogenesis in unc-43 mutants.
The proposed model prompts an interesting question about UNC-31’s role in cell soma neuropeptide release. Specifically, what is the pathway that allows an unc-31 null mutant to secrete at ∼50% of wild-type levels, but that is blocked by >90% in the unc-43unc-31 double mutant? One possibility is that there are two cell soma neuropeptide release pathways: one UNC-31-dependent and one UNC-31-independent but CaM kinase II-dependent. By this model, eliminating CaM kinase II blocks the UNC-31-independent secretion and at the same time removes an inhibition from the UNC-31-dependent pathway, causing all neuropeptides to be released from the soma by the UNC-31-dependent pathway. This is consistent with our observation that virtually all (96%) of the secretion in an unc-43 lf mutant is UNC-31-dependent.
Source of calcium for activating the CaM kinase II’s DCV function
The search for the source(s) of Ca++ that activates CaM kinase II’s DCV function is complicated by the fact that Ca++ has multiple roles that could affect DCV levels in opposite ways. For example, Ca++ is known to activate DCV exocytosis in endocrine cells (Martin 2003). Thus, reducing Ca++ influx through a channel that regulates exocytosis could cause a build-up of DCVs. In contrast, reducing Ca++ influx through a channel that regulates CaM kinase II’s DCV function would cause reduced levels of DCVs in neurons. Furthermore, it is possible that one or more of the same channel types could regulate both functions and that blocking both functions could result in intermediate, possibly even wild-type, levels of DCVs. In two other functions of UNC-43 in C. elegans, glutamate receptor trafficking and olfactory neuronal fate specification, the source of Ca++ for activating UNC-43 (CaM kinase II) is external Ca++ entering through voltage-gated calcium channels (Rongo and Kaplan 1999; Troemel et al. 1999). We found that the same voltage-gated calcium channel mutations used in those studies had no effect on DCV levels in axons. Although we cannot rule out that the wild-type DCV levels result from the “cancelling out” of different Ca++ functions, our study suggests that at least some of the calcium for activating UNC-43’s DCV function comes through the ryanodine receptor, which releases calcium from internal stores in the ER. Several studies have found that disrupting either CaM kinase II or ryanodine receptors causes similar phenotypes and, in one of those studies, the data suggested that CaM kinase II functioned downstream of the Ca++ released by the ryanodine receptor (He et al. 2000; Jin and Hawkins 2003; Shakiryanova et al. 2007). However, in the vertebrate heart, CaM kinase II functions upstream of the ryanodine receptor and modulates its activity via phosphorylation (Currie 2009). Because an unc-68 (ryanodine receptor) null mutant does not disrupt DCV trafficking to the same extent as a CaM kinase II lf mutant, it is possible that eliminating the ryanodine receptor also reduces DCV exocytosis, thus causing a build-up of DCVs that would counteract some of the losses incurred by disrupting the CaM kinase II pathway. Alternatively, there could be another source of Ca++ that contributes to activating CaM kinase II’s DCV function such as CCA-1 (CaV3) or Ca++ exiting the ER through the inositol trisphosphate (IP3) receptor. Indeed, IP3 receptors appear to be present in the C. elegans nervous system (Baylis et al. 1999), and a prior study found evidence that both IP3 receptors and ryanodine receptors contribute to O2-induced tonic signaling, which involves the release of neuropeptides from O2-sensing neurons in C. elegans (Busch et al. 2012).
Why do neurons need the CaM kinase II DCV pathway?
The involvement of CaM kinase II and proteins known to function in regulated exocytosis suggests that this DCV pathway is part of a regulated signal transduction cascade. There are at least three DCV trafficking-related needs that might have driven neurons to evolve this pathway: (1) preventing DCV exocytosis from cell somas before axonal transport of DCVs to axons (i.e., protecting DCVs before they are transported); (2) regulating DCV numbers in neurons; and (3) regulating DCV release from cell somas vs. axons in response to cell-specific needs or specific cues.
First, CaM kinase II may function in a pathway that physically changes or sequesters DCVs so they are fusion-incompetent within the cell soma, thus allowing them to be transported to axons where they can serve their intended function. For example, DCVs that have been modified by the CaM kinase II pathway may no longer be responsive to electrical depolarization or other signals that they might encounter in the cell soma, but they may now be responsive to different signals, such as those generated by the network of Gα pathways that controls synaptic activity in axons (Perez-Mansilla and Nurrish 2009). Indeed, a past study found that CaM kinase II and Gαo act in the same genetic pathway to regulate signaling during the locomotion behavior (Robatzek and Thomas 2000).
Second, CaM kinase II may be part of a regulatory system that maintains DCV numbers within an optimal range. According to this hypothesis, DCV production in cell somas is relatively constant, but rates of exocytosis can vary greatly. When rates of DCV exocytosis decrease, the excess DCVs would accumulate in the absence of a CaM kinase II-based mechanism to eliminate them (i.e., turning off the CaM kinase II pathway). One problem with this hypothesis is that turning off the CaM kinase II pathway would cause nondirected release of potentially behavior-altering neuropeptides and thus could have deleterious effects.
Third, neurons may use the CaM kinase II DCV pathway to regulate neuropeptide release from cell somas vs. axons in response to neuron-specific requirements for establishing or altering a behavior or in response to environmental cues. For example, neurons may turn off the CaM kinase II pathway to drive cell soma release to distribute neuropeptides globally. This would be useful to initiate a complex, coordinated set of behaviors that is not dependent on directed release, such as egg laying in Aplysia, in which a complex mixture of neuropeptides is released from the bag cell neurons to initiate a coordinated set of behaviors (Scheller and Axel 1984). In contrast, turning on the CaM kinase II pathway would drive release from axons, which would be expected to produce more local effects such as altering the strength of synaptic connections. Different neurons may use the CaM kinase II pathway in opposite ways to affect cell-specific functions, with some neurons requiring cell soma neuropeptide release to mediate their functions (CaM kinase II pathway off) and others requiring axonal release of neuropeptides (CaM kinase II pathway on). Alternatively, neurons may change how they release neuropeptides, either allowing or inhibiting cell soma secretion in response to environmental cues that regulate the CaM kinase II pathway.
Concluding remarks
Using the strengths of the genetic model C. elegans, we have uncovered a previously unrecognized DCV trafficking control pathway within neurons and an unprecedented function for the heavily investigated signaling protein CaM kinase II. The key factors contributing to this discovery include lack of CaM kinase II genetic redundancy in C. elegans (mice and humans have four isoforms), a strategic unbiased forward genetic screen, and the ability to perform high-resolution, quantitative imaging of DCV cargos in different physical states in defined regions of identified neurons in live animals. In future studies, new forward genetic screens can identify positive and negative regulators of this unusual new pathway, which may reveal connections to other signal transduction and membrane-trafficking pathways within neurons.
Supplementary Material
Acknowledgments
We thank Joshua Kaplan, Derek Sieburth, Jim Rand, Andrew Fire, Oliver Hobert, and John Tesmer for generously providing some of the plasmids and strains that we used in this study (specified in Supporting Information) and Mike Nonet for providing the unc-68 alleles. Some strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health Office of Research Infrastructure Programs (P40 OD010440). We thank Chris Li for generously providing the FaRP neuropeptide antiserum and Janet Duerr and Jim Rand for their gift of anti-UNC-17 monoclonal ascites. This work was supported by grants from the National Institute of General Medical Sciences and the National Institute of Mental Health of the National Institutes of Health (R01GM080765 to K.G.M.; R01MH073156 to J.E.R.) and by a grant from the Oklahoma Center for the Advancement of Science (HR09-070 to K.G.M.). Stefan Eimer was supported by the European Neuroscience Institute at Goettingen, which is jointly funded by the Max-Planck Society and the University Medical Center Göttingen. M.K. was supported by the Education Abroad Program Göttingen, the German–American Fulbright Program, and the Göttingen Graduate School for Neurosciences and Molecular Biosciences. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the granting agencies.
Footnotes
Communicating editor: M. Sundaram
Literature Cited
- Arvan P., Castle D., 1998. Sorting and storage during secretory granule biogenesis: looking backward and looking forward. Biochem. J. 332(Pt 3): 593–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer Huang S. L., Saheki Y., VanHoven M. K., Torayama I., Ishihara T., et al. , 2007. Left-right olfactory asymmetry results from antagonistic functions of voltage-activated calcium channels and the Raw repeat protein OLRN-1 in C. elegans. Neural Dev. 2: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis H. A., Furuichi T., Yoshikawa F., Mikoshiba K., Sattelle D. B., 1999. Inositol 1,4,5-trisphosphate receptors are strongly expressed in the nervous system, pharynx, intestine, gonad and excretory cell of Caenorhabditis elegans and are encoded by a single gene (itr-1). J. Mol. Biol. 294: 467–476. [DOI] [PubMed] [Google Scholar]
- Borgonovo B., Ouwendijk J., Solimena M., 2006. Biogenesis of secretory granules. Curr. Opin. Cell Biol. 18: 365–370. [DOI] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of C. elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch K. E., Laurent P., Soltesz Z., Murphy R. J., Faivre O., et al. , 2012. Tonic signaling from O(2) sensors sets neural circuit activity and behavioral state. Nat. Neurosci. 15: 581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai T., Fukushige T., Notkins A. L., Krause M., 2004. Insulinoma-associated protein IA-2, a vesicle transmembrane protein, genetically interacts with UNC-31/CAPS and affects neurosecretion in Caenorhabditis elegans. J. Neurosci. 24: 3115–3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch’ng Q., Sieburth D., Kaplan J. M., 2008. Profiling synaptic proteins identifies regulators of insulin secretion and lifespan. PLoS Genet. 4: e1000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool D. R., Normant E., Shen F., Chen H. C., Pannell L., et al. , 1997. Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpe(fat) mice. Cell 88: 73–83. [DOI] [PubMed] [Google Scholar]
- Currie S., 2009. Cardiac ryanodine receptor phosphorylation by CaM Kinase II: keeping the balance right. Front. Biosci. 14: 5134–5156. [DOI] [PubMed] [Google Scholar]
- Dean P. M., 1973. Ultrastructural morphometry of the pancreatic β-cell. Diabetologia 9: 115–119. [DOI] [PubMed] [Google Scholar]
- Duerr J. S., Gaskin J., Rand J. B., 2001. Identified neurons in C. elegans coexpress vesicular transporters for acetylcholine and monoamines. Am. J. Physiol. Cell Physiol. 280: C1616–C1622. [DOI] [PubMed] [Google Scholar]
- Edwards S. L., Charlie N. K., Milfort M. C., Brown B. S., Gravlin C. N., et al. , 2008. A novel molecular solution for ultraviolet light detection in Caenorhabditis elegans. PLoS Biol. 6: e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. L., Charlie N. K., Richmond J. E., Hegermann J., Eimer S., et al. , 2009. Impaired dense core vesicle maturation in Caenorhabditis elegans mutants lacking Rab2. J. Cell Biol. 186: 881–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erondu N. E., Kennedy M. B., 1985. Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J. Neurosci. 5: 3270–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker L. D., 1988. Carboxypeptidase E. Annu. Rev. Physiol. 50: 309–321. [DOI] [PubMed] [Google Scholar]
- Goodwin P. R., Juo P., 2013. The scaffolding protein SYD-2/liprin-alpha regulates the mobility and polarized distribution of dense-core vesicles in C. elegans motor neurons. PLoS ONE 8: e54763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin P. R., Sasaki J. M., Juo P., 2012. Cyclin-dependent kinase 5 regulates the polarized trafficking of neuropeptide-containing dense-core vesicles in Caenorhabditis elegans motor neurons. J. Neurosci. 32: 8158–8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracheva E. O., Burdina A. O., Touroutine D., Berthelot-Grosjean M., Parekh H., et al. , 2007. Tomosyn negatively regulates CAPS-dependent peptide release at Caenorhabditis elegans synapses. J. Neurosci. 27: 10176–10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishanin R. N., Kowalchyk J. A., Klenchin V. A., Ann K., Earles C. A., et al. , 2004. CAPS acts at a prefusion step in dense-core vesicle exocytosis as a PIP2 binding protein. Neuron 43: 551–562. [DOI] [PubMed] [Google Scholar]
- Hall D. H., Hedgecock E. M., 1991. Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell 65: 837–847. [DOI] [PubMed] [Google Scholar]
- Hammarlund M., Watanabe S., Schuske K., Jorgensen E. M., 2008. CAPS and syntaxin dock dense core vesicles to the plasma membrane in neurons. J. Cell Biol. 180: 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Shi S. H., Esteban J. A., Piccini A., Poncer J. C., et al. , 2000. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287: 2262–2267. [DOI] [PubMed] [Google Scholar]
- He X., Yang F., Xie Z., Lu B., 2000. Intracellular Ca(2+) and Ca(2+)/calmodulin-dependent kinase II mediate acute potentiation of neurotransmitter release by neurotrophin-3. J. Cell Biol. 149: 783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob T. C., Kaplan J. M., 2003. The EGL-21 carboxypeptidase E facilitates acetylcholine release at Caenorhabditis elegans neuromuscular junctions. J. Neurosci. 23: 2122–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin I., Hawkins R. D., 2003. Presynaptic and postsynaptic mechanisms of a novel form of homosynaptic potentiation at aplysia sensory-motor neuron synapses. J. Neurosci. 23: 7288–7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. R., Ferdek P., Lian L. Y., Barclay J. W., Burgoyne R. D., et al. , 2009. Binding of UNC-18 to the N-terminus of syntaxin is essential for neurotransmission in Caenorhabditis elegans. Biochem. J. 418: 73–80. [DOI] [PubMed] [Google Scholar]
- Kupfermann I., 1991. Functional studies of cotransmission. Physiol. Rev. 71: 683–732. [DOI] [PubMed] [Google Scholar]
- Lee R. Y., Lobel L., Hengartner M., Horvitz H. R., Avery L., 1997. Mutations in the alpha1 subunit of an L-type voltage-activated Ca2+ channel cause myotonia in Caenorhabditis elegans. EMBO J. 16: 6066–6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C., and Kim, K., 2008 Neuropeptides, WormBook, ed. The C. elegans Research Community, WormBook, /10.1895/wormbook.1.142.1, http://www.wormbook.org.
- Lisman J., Yasuda R., Raghavachari S., 2012. Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 13: 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Chen B., Ge Q., Wang Z. W., 2007. Presynaptic Ca2+/calmodulin-dependent protein kinase II modulates neurotransmitter release by activating BK channels at Caenorhabditis elegans neuromuscular junction. J. Neurosci. 27: 10404–10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Kim K., Li C., Barr M. M., 2007. FMRFamide-like neuropeptides and mechanosensory touch receptor neurons regulate male sexual turning behavior in Caenorhabditis elegans. J. Neurosci. 27: 7174–7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. F., 2003. Tuning exocytosis for speed: fast and slow modes. Biochim. Biophys. Acta 1641: 157–165. [DOI] [PubMed] [Google Scholar]
- McEwen J. M., Kaplan J. M., 2008. UNC-18 promotes both the anterograde trafficking and synaptic function of syntaxin. Mol. Biol. Cell 19: 3836–3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D., Ambros V., 1991. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10(12): 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. G., Emerson M. D., Rand J. B., 1999. Goα and diacylglycerol kinase negatively regulate the Gqα pathway in C. elegans. Neuron 24: 323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Mansilla B., Nurrish S., 2009. A network of G-protein signaling pathways control neuronal activity in C. elegans. Adv. Genet. 65: 145–192. [DOI] [PubMed] [Google Scholar]
- Plattner H., Artalejo A. R., Neher E., 1997. Ultrastructural organization of bovine chromaffin cell cortex-analysis by cryofixation and morphometry of aspects pertinent to exocytosis. J. Cell Biol. 139: 1709–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner D. J., Newton E. M., Tian H., Thomas J. H., 1999. Diverse behavioural defects caused by mutations in Caenorhabditis elegans unc-43 CaM kinase II. Nature 402: 199–203. [DOI] [PubMed] [Google Scholar]
- Reynolds N. K., Schade M. A., Miller K. G., 2005. Convergent, RIC-8 dependent Gα signaling pathways in the Caenorhabditis elegans synaptic signaling network. Genetics 169: 650–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rindler M. J., 1998. Carboxypeptidase E, a peripheral membrane protein implicated in the targeting of hormones to secretory granules, co-aggregates with granule content proteins at acidic pH. J. Biol. Chem. 273: 31180–31185. [DOI] [PubMed] [Google Scholar]
- Robatzek M., Thomas J. H., 2000. Calcium/calmodulin-dependent protein kinase II regulates Caenorhabditis elegans locomotion in concert with a Go/Gq signaling network. Genetics 156: 1069–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongo C., 2002. A fresh look at the role of CaMKII in hippocampal synaptic plasticity and memory. Bioessays 24: 223–233. [DOI] [PubMed] [Google Scholar]
- Rongo C., Kaplan J. M., 1999. CaMKII regulates the density of central glutamatergic synapses in vivo. Nature 402: 195–199. [DOI] [PubMed] [Google Scholar]
- Rosenberg O. S., Deindl S., Comolli L. R., Hoelz A., Downing K. H., et al. , 2006. Oligomerization states of the association domain and the holoenyzme of Ca2+/CaM kinase II. FEBS J. 273: 682–694. [DOI] [PubMed] [Google Scholar]
- Saheki Y., Bargmann C. I., 2009. Presynaptic CaV2 calcium channel traffic requires CALF-1 and the alpha(2)delta subunit UNC-36. Nat. Neurosci. 12: 1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schade M. A., Reynolds N. K., Dollins C. M., Miller K. G., 2005. Mutations that rescue the paralysis of Caenorhabditis elegans ric-8 (synembryn) mutants activate the Gαs pathway and define a third major branch of the synaptic signaling network. Genetics 169: 631–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller R. H., Axel R., 1984. How genes control an innate behavior. Sci. Am. 250: 54–62. [DOI] [PubMed] [Google Scholar]
- Schinkmann K., Li C., 1992. Localization of FMRFamide-like peptides in Caenorhabditis elegans. J. Comp. Neurol. 316: 251–260. [DOI] [PubMed] [Google Scholar]
- Schulman H., Greengard P., 1978a Ca2+-dependent protein phosphorylation system in membranes from various tissues, and its activation by “calcium-dependent regulator.” Proc. Natl. Acad. Sci. USA 75: 5432–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman H., Greengard P., 1978b Stimulation of brain membrane protein phosphorylation by calcium and an endogenous heat-stable protein. Nature 271: 478–479. [DOI] [PubMed] [Google Scholar]
- Shakiryanova D., Klose M. K., Zhou Y., Gu T., Deitcher D. L., et al. , 2007. Presynaptic ryanodine receptor-activated calmodulin kinase II increases vesicle mobility and potentiates neuropeptide release. J. Neurosci. 27: 7799–7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburth D., Madison J. M., Kaplan J. M., 2007. PKC-1 regulates secretion of neuropeptides. Nat. Neurosci. 10: 49–57. [DOI] [PubMed] [Google Scholar]
- Solimena M., Dirkx R., Jr, Hermel J. M., Pleasic-Williams S., Shapiro J. A., et al. , 1996. ICA 512, an autoantigen of type I diabetes, is an intrinsic membrane protein of neurosecretory granules. EMBO J. 15: 2102–2114. [PMC free article] [PubMed] [Google Scholar]
- Speese S., Petrie M., Schuske K., Ailion M., Ann K., et al. , 2007. UNC-31 (CAPS) is required for dense-core vesicle but not synaptic vesicle exocytosis in Caenorhabditis elegans. J. Neurosci. 27: 6150–6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiernagle, T., 2006 Maintenance of C. elegans, WormBook, ed. The C. elegans Research Community, WormBook, /10.1895/wormbook.1.101.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston, J., and J. Hodgkin, 1988 Methods, pp. 596–597 in The Nematode Caenorhabditis elegans, edited by W. B. Wood. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Sumakovic M., Hegermann J., Luo L., Husson S. J., Schwarze K., et al. , 2009. UNC-108/RAB-2 and its effector RIC-19 are involved in dense core vesicle maturation in Caenorhabditis elegans. J. Cell Biol. 186: 897–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze S. A., Martens G. J., Huttner W. B., 2001. Secretory granule biogenesis: rafting to the SNARE. Trends Cell Biol. 11: 116–122. [DOI] [PubMed] [Google Scholar]
- Troemel E. R., Sagasti A., Bargmann C. I., 1999. Lateral signaling mediated by axon contact and calcium entry regulates asymmetric odorant receptor expression in C. elegans. Cell 99: 387–398. [DOI] [PubMed] [Google Scholar]
- Vashlishan A. B., Madison J. M., Dybbs M., Bai J., Sieburth D., et al. , 2008. An RNAi screen identifies genes that regulate GABA synapses. Neuron 58: 346–361. [DOI] [PubMed] [Google Scholar]
- Wang Z. W., 2008. Regulation of synaptic transmission by presynaptic CaMKII and BK channels. Mol. Neurobiol. 38: 153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer R. M., Richmond J. E., Davis W. S., Hadwiger G., Nonet M. L., et al. , 2003. Defects in synaptic vesicle docking in unc-18 mutants. Nat. Neurosci. 6: 1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. Y., Shakiryanova D., Levitan E. S., 2009. Presynaptic ryanodine receptor-CamKII signaling is required for activity-dependent capture of transiting vesicles. J. Mol. Neurosci. 37: 146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn T. R., Angleson J. K., MacMorris M. A., Domke E., Hutton J. F., et al. , 2004. Dense core vesicle dynamics in Caenorhabditis elegans neurons and the role of kinesin UNC-104. Traffic 5: 544–559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.