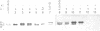Abstract
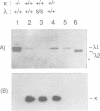
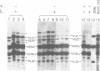
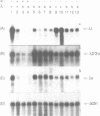
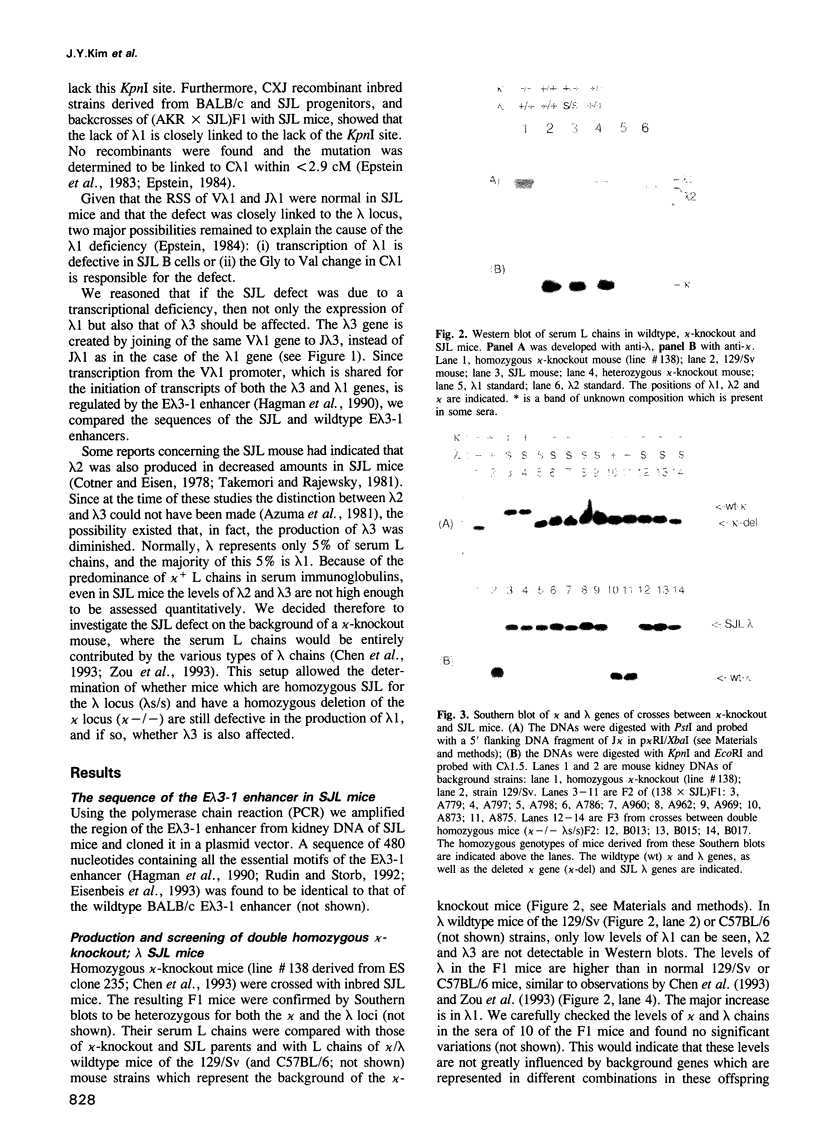
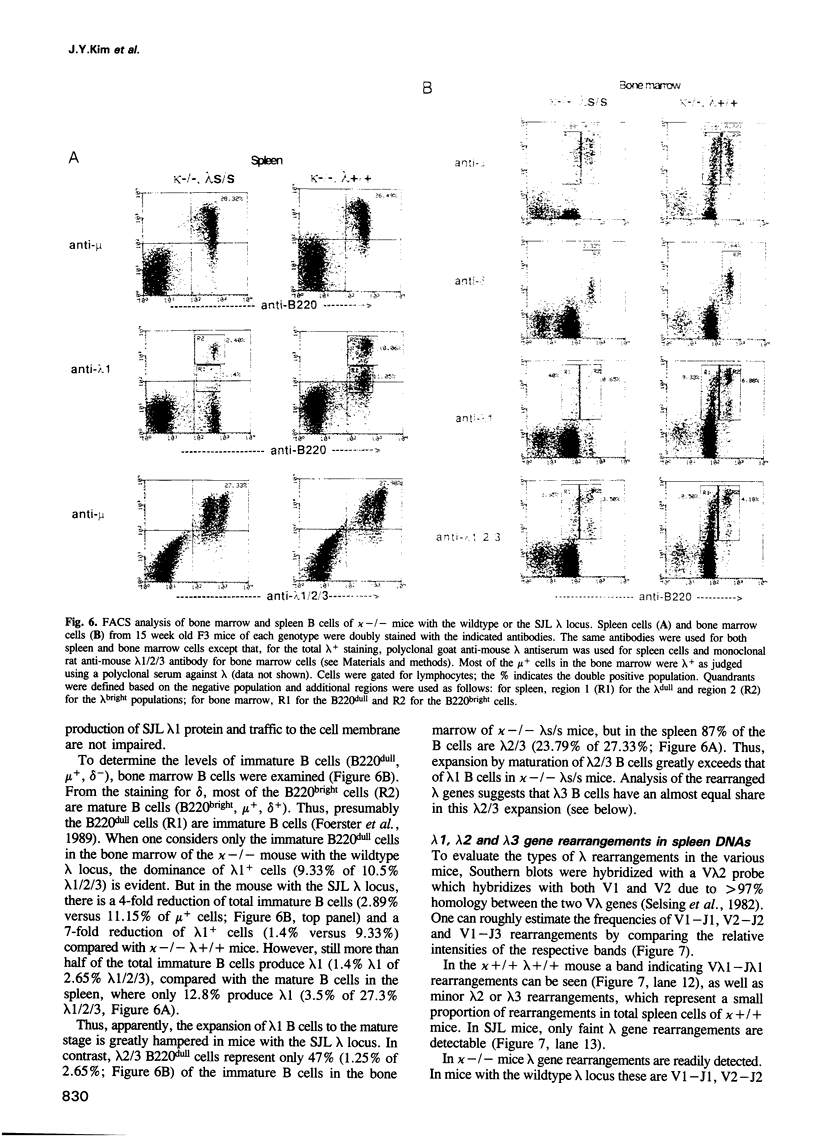
Mice of the SJL strain produce approximately 50 times less serum lambda 1 immunoglobulin light chains than other mouse strains. The defect is genetically linked to the lambda locus, but it is unknown whether it is due to regulatory alterations or known structural changes. We find no mutation in the SJL lambda 3-1 enhancer which regulates both lambda 1 and lambda 3. To investigate the defect further, the production of lambda light chains was amplified by crossing SJL with kappa-knockout mice. In kappa-knockout mice with the wildtype lambda locus (kappa -/- lambda +/+), the majority of serum light chains are lambda 1. In contrast, kappa-knockout mice with the SJL lambda locus (kappa -/- lambda s/s) show a pronounced expression of lambda 2 and lambda 3, with only some expression of lambda 1. The results show that the SJL defect is lambda 1 specific, since the linked lambda 3 expression is normal. As the transcription and rearrangement of lambda 1 appear normal in SJL, the defective lambda 1 synthesis is most likely due to a point mutation in the lambda 1 constant region resulting in a glycine to valine substitution. At the cellular level, in kappa-knockout mice with the SJL lambda locus there are fewer immature, and especially mature, lambda 1 B cells and the production of lambda 1 plasma cells is strongly inhibited. The lambda 1 specificity of the defect suggests that the point mutation in SJL C lambda 1 creates an immunoglobulin receptor complex which is dysfunctional in B cell differentiation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arp B., McMullen M. D., Storb U. Sequences of immunoglobulin lambda 1 genes in a lambda 1 defective mouse strain. Nature. 1982 Jul 8;298(5870):184–187. doi: 10.1038/298184a0. [DOI] [PubMed] [Google Scholar]
- Azuma T., Steiner L. A., Eisen H. N. Identification of a third type of lambda light chain in mouse immunoglobulins. Proc Natl Acad Sci U S A. 1981 Jan;78(1):569–573. doi: 10.1073/pnas.78.1.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg B., Tonegawa S. DNA sequences of the joining regions of mouse lambda light chain immunoglobulin genes. Proc Natl Acad Sci U S A. 1982 Jan;79(2):530–533. doi: 10.1073/pnas.79.2.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg B., Traunecker A., Eisen H., Tonegawa S. Organization of four mouse lambda light chain immunoglobulin genes. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3765–3769. doi: 10.1073/pnas.78.6.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Schwartz R. C., Sonenshein G. E., Gefter M. L., Baltimore D. Dual expression of lambda genes in the MOPC-315 plasmacytoma. Nature. 1981 Mar 5;290(5801):65–67. doi: 10.1038/290065a0. [DOI] [PubMed] [Google Scholar]
- Carson S., Wu G. E. A linkage map of the mouse immunoglobulin lambda light chain locus. Immunogenetics. 1989;29(3):173–179. doi: 10.1007/BF00373642. [DOI] [PubMed] [Google Scholar]
- Chen J., Trounstine M., Kurahara C., Young F., Kuo C. C., Xu Y., Loring J. F., Alt F. W., Huszar D. B cell development in mice that lack one or both immunoglobulin kappa light chain genes. EMBO J. 1993 Mar;12(3):821–830. doi: 10.1002/j.1460-2075.1993.tb05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cotner T., Eisen H. N. The natural abundance of lambda2-light chains in inbred mice. J Exp Med. 1978 Nov 1;148(5):1388–1399. doi: 10.1084/jem.148.5.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dildrop R., Gause A., Müller W., Rajewsky K. A new V gene expressed in lambda-2 light chains of the mouse. Eur J Immunol. 1987 May;17(5):731–734. doi: 10.1002/eji.1830170525. [DOI] [PubMed] [Google Scholar]
- Eisen H. N., Reilly E. B. Lambda chains and genes in inbred mice. Annu Rev Immunol. 1985;3:337–365. doi: 10.1146/annurev.iy.03.040185.002005. [DOI] [PubMed] [Google Scholar]
- Eisenbeis C. F., Singh H., Storb U. PU.1 is a component of a multiprotein complex which binds an essential site in the murine immunoglobulin lambda 2-4 enhancer. Mol Cell Biol. 1993 Oct;13(10):6452–6461. doi: 10.1128/mcb.13.10.6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott B. W., Jr, Eisen H. N., Steiner L. A. Unusual association of V, J and C regions in a mouse immunoglobulin lambda chain. Nature. 1982 Oct 7;299(5883):559–561. doi: 10.1038/299559a0. [DOI] [PubMed] [Google Scholar]
- Epstein R., Lehmann K., Cohn M. Induction of lambda 1-immunoglobulin is determined by a regulatory gene (r lambda 1) linked (or identical) to the structural (c lambda 1) gene. J Exp Med. 1983 May 1;157(5):1681–1686. doi: 10.1084/jem.157.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster I., Vieira P., Rajewsky K. Flow cytometric analysis of cell proliferation dynamics in the B cell compartment of the mouse. Int Immunol. 1989;1(4):321–331. doi: 10.1093/intimm/1.4.321. [DOI] [PubMed] [Google Scholar]
- Geckeler W., Faversham J., Cohn M. On a regulatory gene controlling the expression of the murine lambda1 light chain. J Exp Med. 1978 Nov 1;148(5):1122–1136. doi: 10.1084/jem.148.5.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagman J., Lo D., Doglio L. T., Hackett J., Jr, Rudin C. M., Haasch D., Brinster R., Storb U. Inhibition of immunoglobulin gene rearrangement by the expression of a lambda 2 transgene. J Exp Med. 1989 Jun 1;169(6):1911–1929. doi: 10.1084/jem.169.6.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagman J., Rudin C. M., Haasch D., Chaplin D., Storb U. A novel enhancer in the immunoglobulin lambda locus is duplicated and functionally independent of NF kappa B. Genes Dev. 1990 Jun;4(6):978–992. doi: 10.1101/gad.4.6.978. [DOI] [PubMed] [Google Scholar]
- Kitamura D., Kudo A., Schaal S., Müller W., Melchers F., Rajewsky K. A critical role of lambda 5 protein in B cell development. Cell. 1992 May 29;69(5):823–831. doi: 10.1016/0092-8674(92)90293-l. [DOI] [PubMed] [Google Scholar]
- Kitamura D., Roes J., Kühn R., Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991 Apr 4;350(6317):423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Miller J., Bothwell A., Storb U. Physical linkage of the constant region genes for immunoglobulins lambda I and lambda III. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3829–3833. doi: 10.1073/pnas.78.6.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., Ogden S., McMullen M., Andres H., Storb U. The order and orientation of mouse lambda-genes explain lambda-rearrangement patterns. J Immunol. 1988 Oct 1;141(7):2497–2502. [PubMed] [Google Scholar]
- Onodera Y., Reilly E. B., Eisen H. N. Synthesis of lambda light chain subtypes by stimulated and unstimulated mouse B cells. Eur J Immunol. 1983 Sep;13(9):739–746. doi: 10.1002/eji.1830130909. [DOI] [PubMed] [Google Scholar]
- Persiani D. M., Durdik J., Selsing E. Active lambda and kappa antibody gene rearrangement in Abelson murine leukemia virus-transformed pre-B cell lines. J Exp Med. 1987 Jun 1;165(6):1655–1674. doi: 10.1084/jem.165.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard D., Schaffner W. A lymphocyte-specific enhancer in the mouse immunoglobulin kappa gene. Nature. 1984 Jan 5;307(5946):80–82. doi: 10.1038/307080a0. [DOI] [PubMed] [Google Scholar]
- Poljak R. J., Amzel L. M., Avey H. P., Chen B. L., Phizackerley R. P., Saul F. Three-dimensional structure of the Fab' fragment of a human immunoglobulin at 2,8-A resolution. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3305–3310. doi: 10.1073/pnas.70.12.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly E. B., Blomberg B., Imanishi-Kari T., Tonegawa S., Eisen H. N. Restricted association of V and J-C gene segments for mouse lambda chains. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2484–2488. doi: 10.1073/pnas.81.8.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly E. B., Frackelton A. R., Jr, Eisen H. N. Synthesis of lambda 1, lambda 2, and lambda 3 light chains by mouse spleen B cells. Eur J Immunol. 1982 Jul;12(7):552–557. doi: 10.1002/eji.1830120705. [DOI] [PubMed] [Google Scholar]
- Reth M. Antigen receptors on B lymphocytes. Annu Rev Immunol. 1992;10:97–121. doi: 10.1146/annurev.iy.10.040192.000525. [DOI] [PubMed] [Google Scholar]
- Rudin C. M., Storb U. Two conserved essential motifs of the murine immunoglobulin lambda enhancers bind B-cell-specific factors. Mol Cell Biol. 1992 Jan;12(1):309–320. doi: 10.1128/mcb.12.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez P., Cazenave P. A. A new variable region in mouse immunoglobulin lambda light chains. J Exp Med. 1987 Jul 1;166(1):265–270. doi: 10.1084/jem.166.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlissel M. S., Baltimore D. Activation of immunoglobulin kappa gene rearrangement correlates with induction of germline kappa gene transcription. Cell. 1989 Sep 8;58(5):1001–1007. doi: 10.1016/0092-8674(89)90951-3. [DOI] [PubMed] [Google Scholar]
- Selsing E., Miller J., Wilson R., Storb U. Evolution of mouse immunoglobulin lambda genes. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4681–4685. doi: 10.1073/pnas.79.15.4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storb U., Haasch D., Arp B., Sanchez P., Cazenave P. A., Miller J. Physical linkage of mouse lambda genes by pulsed-field gel electrophoresis suggests that the rearrangement process favors proximate target sequences. Mol Cell Biol. 1989 Feb;9(2):711–718. doi: 10.1128/mcb.9.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemori T., Rajewsky K. Lambda chain expression at different stages of ontogeny in C57BL/6, BALB/c and SJL mice. Eur J Immunol. 1981 Aug;11(8):618–625. doi: 10.1002/eji.1830110806. [DOI] [PubMed] [Google Scholar]
- Weiss S., Lehmann K., Cohn M. A monoclonal lambda 1-bearing anti-dextran antibody from a lambda-defective mouse strain. Eur J Immunol. 1985 Aug;15(8):768–772. doi: 10.1002/eji.1830150806. [DOI] [PubMed] [Google Scholar]
- Weiss S., Lehmann K., Raschke W. C., Cohn M. Mice completely suppressed for the expression of immunoglobulin kappa light chain. Proc Natl Acad Sci U S A. 1984 Jan;81(1):211–215. doi: 10.1073/pnas.81.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. E., Govindji N., Hozumi N., Murialdo H. Nucleotide sequence of a chromosomal rearranged lambda 2 immunoglobulin gene of mouse. Nucleic Acids Res. 1982 Jul 10;10(13):3831–3843. doi: 10.1093/nar/10.13.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y. R., Takeda S., Rajewsky K. Gene targeting in the Ig kappa locus: efficient generation of lambda chain-expressing B cells, independent of gene rearrangements in Ig kappa. EMBO J. 1993 Mar;12(3):811–820. doi: 10.1002/j.1460-2075.1993.tb05721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]