Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare hematological malignancy with a clinically aggressive phenotype arising from CD4/CD56-expressing plasmacytoid dendritic cell precursors [1]. BPDCN was categorized under "AML and related precursor neoplasms" by the 2008 WHO classification, with most cases having been previously classified as blastic natural killer (NK)-cell lymphoma/leukemia or agranular CD4+, CD56+ hematodermic neoplasm [2]. BPDCN cells generally express CD4, CD56, CD123, and TCL-1 but are negative for other T-, B-, NK-cell, or myeloid markers [3]. Clinically, patients with this disease typically present with a high incidence of cutaneous involvement described as generalized, localized, or solitary macules, plaques, and/or tumors, as the first manifestation, followed by involvement of bone marrow (BM), peripheral blood (PB), and lymph nodes (LNs). BM involvement usually occurs with leukemic progression of advanced or relapsed disease and is associated with a poor prognosis [4]. Diagnosis of leukemic BPDCN without cutaneous manifestation is rare. Here, we report a 41-yr-old man who was diagnosed with BPDCN in the absence of skin manifestation.
This patient was admitted for evaluation and management of palpable multiple neck lymphadenopathies that had developed a week previously. No improvement was noted despite antibiotic therapy, and the lymphadenopathies spread to the left axillary and inguinal LNs. The initial blood analysis revealed a white blood cell (WBC) count of 6.1×109/L (reference range, 4.1-10.2×109/L), an Hb level of 12.7 g/dL (reference range, 13.7-16.8 g/dL), and a platelet count of 329×109/L (reference range, 158-332×109/L). Approximately 25% of cells appeared atypical on PB smear. Other laboratory findings were not significant except for an increased lactate dehydrogenase (LDH) level of 719 IU/L (reference range, 252-458 IU/L).
Computed tomography (CT) imaging revealed multiple enlargements of the supraclavicular, right interlobar, inguinal, and iliac LNs, with no evidence of hepatosplenomegaly. Right supraclavicular LN biopsy and BM examination were also performed to characterize the atypical cells in the PB. Microscopic examination of the LN biopsies revealed diffuse infiltration by uniform, medium-sized cells together with effacement of the normal LN architecture. The tumor cells exhibited round or polygonal nuclei, coarse chromatin, and prominent nucleoli. Analysis of the BM aspirate revealed that blastoid cells accounted for up to 61% of all nucleated cells and had a similar morphology to those tumor cells that had infiltrated the LN in the BM study (Fig. 1).
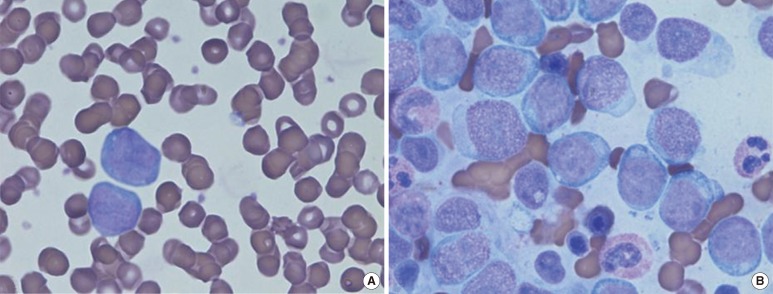
Fig. 1.
(A) Peripheral blood smear (Wright-Giemsa stain, ×1,000): large atypical cells with high nuclear cytoplasmic (N/C) ratios, basophilic cytoplasm, and prominent nucleoli accounted for up to 25% of the leukocyte population. (B) In the bone marrow aspirate (Wright-Giemsa stain, ×1,000), 61.4% of all nucleated cells were atypical in appearance.
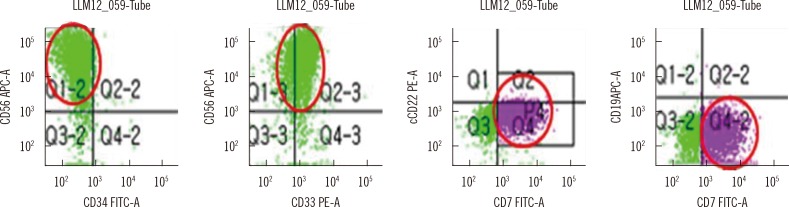
Flow cytometric analysis of the BM aspirate revealed 48% CD45dim positive cells within the blast gate, which were positive for CD33, CD7, CD56, CD117, HLA-DR, CD13 weak (w), CD11cw, and negative for myeloperoxidase (MPO), CD14, CD64, CD2, CD3, CD5, cytoplasmic CD3, CD10, CD19, CD20, CD22, CD79a, kappa and lambda light chain, terminal deoxynucleotidyl transferase (TdT), and CD34 (Fig. 2). Immunohistochemical staining of the BM section revealed it to be negative for MPO, non-specific esterase (NSE), and Periodic acid-Schiff (PAS) stain. The acute leukemic presentation and expression of myeloid lineage-associated markers with the exception of MPO was suggestive of AML with minimal differentiation.
Fig. 2.
Flow cytometric analysis of the bone marrow aspirate. CD45dim cells within the blast gated cells were negative for CD34 and positive for CD56, CD33, and CD7 (red circles).
The neoplastic BPDCN cells are characterized as medium-sized cells with round nuclei and finely dispersed chromatin with absent or indistinct nucleoli, which thus resemble blasts. In addition, the cytoplasm is scant and has no granules [1]. Likewise, in this case, it could be confused with acute leukemia and the BPDCN could consequently be missed because the neoplastic BPDCN cells are similar to blasts that lack lineage-specific markers. Further evaluation is necessary for an accurate diagnosis. Therefore, we performed immunohistochemical staining of the LN biopsy and found that the tumor cells were positive for leukocyte common antigen (LCA), CD4, CD56 and bcl-2 (Fig. 3); negative for MPO, CD3, CD5, CD8, CD10, CD20, CD21, CD15, CD30, CD1a, CD34, TdT, multiple myeloma oncogene 1 (MUM1), anaplastic lymphoma kinase (ALK), and bcl-6; and had a ki-67 labeling index of 60%. Based on these findings, the initial differential diagnosis included leukemic infiltration of acute leukemia, T cell lymphoma, myeloid leukemia/sarcoma, or acute leukemia presenting as BPDCN. We examined the whole body for skin involvement, but found no skin lesions in this patient. Nonetheless, the leukemic presentation with strong positivity for CD4 and CD56, combined with the absence of lineage-specific markers in blastoid cells were indicative of BPDCN. We additionally confirmed immunohistochemical positivity for CD4 and CD123 in BM and CD123 in the LN biopsy. Taken together, the diagnosis was most consistent with the current WHO category of BPDCN within the "AML and related precursor neoplasms" group of malignancies.
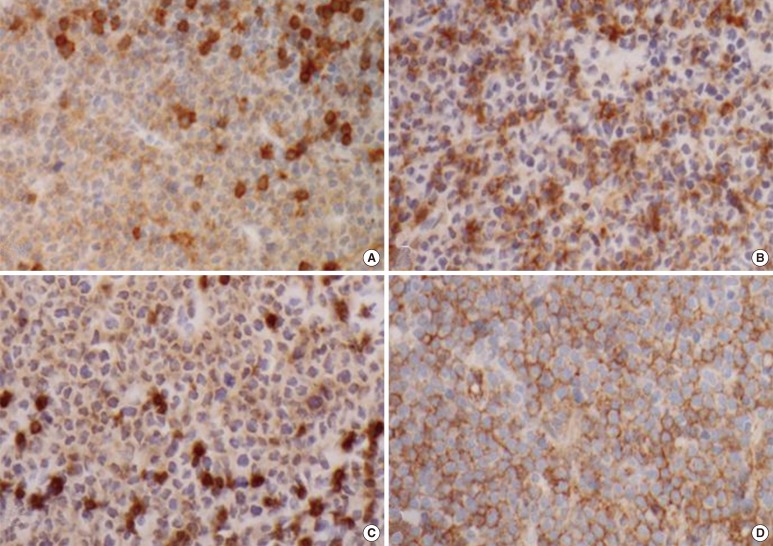
Fig. 3.
Immunohistochemical staining of the right supraclavicular lymph nodes biopsy. (A) CD4+, (B) CD56+, (C) leukocyte common antigen (LCA)+, and (D) CD123+.
In a cytogenetic study, complex structural abnormalities were observed and the karyotype was found to be 45,XY,der(3;7)(q10;q10),t(6;19)(p21.1;p13.3),t(8;18)(q24.1;q21.1) in 16 out of 20 analyzed metaphase cells. Leroux et al. [5] reported that chromosomal alterations in BPDCN seemed to occur most frequently at 6 chromosomal sites: 5q (72%), 12p and 13q (each 64%), 6q (50%), 15q (43%), and 9 (28%). However, the malignant cells in this case did not show aberrations at any of these chromosomal sites. BPDCN might be linked to genetic disorders that involve CDKN1B, CDKN2A, ARF, CDKN2, RB1, and MLL-ENL rearrangement, although the etiology and prognosis of BPDCN remains unclear [6-8].
BPDCN is initially highly sensitive to chemotherapy, with complete response rates ranging from 47% to 86%. However, the prognosis is poor even for patients who achieve a complete response, with a median survival period of 14 months (range, 10-18 months), and 2- and 5-yr overall survival rates of 33% and 6%, respectively [9, 10]. In leukemic BPDCN patients without cutaneous manifestations, the median survival was even lower at only 10 months. However, this survival data should be interpreted with caution because the therapeutic management of the patients was inhomogeneous and often not optimal [11]. It was recently suggested that patients with BPDCN should be treated with chemotherapy similar to that used for AML, followed by allogeneic stem cell transplantation (SCT) on complete response [12]. We performed further molecular studies of prognostic markers such as NPM1, cKIT, and FLT3-ITD, but detected no mutations. Induction chemotherapy with cytarabine and daunorubicin was administered, and a follow-up BM biopsy at day 14 after the start of chemotherapy revealed a marrow hypocellularity of 15% with no residual leukemic cells. The patient was transferred to another hospital for allogenic stem cell transplantation.
Although a few case reports and literature reviews of BPDCN without cutaneous lesion have been reported worldwide [9, 11], there has been no report in Korea. We describe, to our knowledge, the first Korean case of acute leukemic presentation of BPDCN without cutaneous lesion. Even in the absence of characteristic cutaneous manifestations of BPDCN, patients with leukemic presentation and an absence of lineage-specific markers should undergo immunophenotyping including CD4, CD56, and CD123 to exclude this malignancy. Cytogenetic molecular analysis is necessary to further investigate the pathogenesis and genetic background of BPDCN.
Footnotes
No potential conflicts of interest relevant to this article were reported.
References
- 1.Feuillard J, Jacob MC, Valensi F, Maynadie M, Gressin R, Chaperot L, et al. Clinical and biologic features of CD4(+)CD56(+) malignancies. Blood. 2002;99:1556–1563. doi: 10.1182/blood.v99.5.1556. [DOI] [PubMed] [Google Scholar]
- 2.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 3.Burg G, Kempf W, Cozzio A, Feit J, Willemze R, E SJ, et al. WHO/EORTC classification of cutaneous lymphomas 2005: histological and molecular aspects. J Cutan Pathol. 2005;32:647–674. doi: 10.1111/j.0303-6987.2005.00495.x. [DOI] [PubMed] [Google Scholar]
- 4.Cota C, Vale E, Viana I, Requena L, Ferrara G, Anemona L, et al. Cutaneous manifestations of blastic plasmacytoid dendritic cell neoplasm-morphologic and phenotypic variability in a series of 33 patients. Am J Surg Pathol. 2010;34:75–87. doi: 10.1097/PAS.0b013e3181c5e26b. [DOI] [PubMed] [Google Scholar]
- 5.Leroux D, Mugneret F, Callanan M, Radford-Weiss I, Dastugue N, Feuillard J, et al. CD4(+), CD56(+) DC2 acute leukemia is characterized by recurrent clonal chromosomal changes affecting 6 major targets: a study of 21 cases by the Groupe Francais de Cytogenetique Hematologique. Blood. 2002;99:4154–4159. doi: 10.1182/blood.v99.11.4154. [DOI] [PubMed] [Google Scholar]
- 6.Lucioni M, Novara F, Fiandrino G, Riboni R, Fanoni D, Arra M, et al. Twenty-one cases of blastic plasmacytoid dendritic cell neoplasm: focus on biallelic locus 9p21.3 deletion. Blood. 2011;118:4591–4594. doi: 10.1182/blood-2011-03-337501. [DOI] [PubMed] [Google Scholar]
- 7.Wiesner T, Obenauf AC, Cota C, Fried I, Speicher MR, Cerroni L. Alterations of the cell-cycle inhibitors p27(KIP1) and p16(INK4a) are frequent in blastic plasmacytoid dendritic cell neoplasms. J Invest Dermatol. 2010;130:1152–1157. doi: 10.1038/jid.2009.369. [DOI] [PubMed] [Google Scholar]
- 8.Toya T, Nishimoto N, Koya J, Nakagawa M, Nakamura F, Kandabashi K, et al. The first case of blastic plasmacytoid dendritic cell neoplasm with MLL-ENL rearrangement. Leuk Res. 2012;36:117–118. doi: 10.1016/j.leukres.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Cao J, Hong X. Blastic plasmacytoid dendritic cell neoplasm without cutaneous lesion at presentation: case report and literature review. Acta Haematol. 2012;127:124–127. doi: 10.1159/000334703. [DOI] [PubMed] [Google Scholar]
- 10.Bekkenk MW, Jansen PM, Meijer CJ, Willemze R. CD56+ hematological neoplasms presenting in the skin: a retrospective analysis of 23 new cases and 130 cases from the literature. Ann Oncol. 2004;15:1097–1108. doi: 10.1093/annonc/mdh268. [DOI] [PubMed] [Google Scholar]
- 11.Rauh MJ, Rahman F, Good D, Silverman J, Brennan MK, Dimov N, et al. Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation, lacking cutaneous involvement: Case series and literature review. Leuk Res. 2012;36:81–86. doi: 10.1016/j.leukres.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 12.Ham JC, Janssen JJ, Boers JE, Kluin PM, Verdonck LF. Allogeneic stem-cell transplantation for blastic plasmacytoid dendritic cell neoplasm. J Clin Oncol. 2012;30:e102–e103. doi: 10.1200/JCO.2011.35.8143. [DOI] [PubMed] [Google Scholar]