Eggerthella lenta, previously Eubacterium lentum, is a non-motile, non-spore-forming anaerobic gram-positive bacillus. In 1999, the designation Eggerthella gen. nov. (named in honor of Arnold Eggerth, who published the first description) was proposed as a substitute for Eubacterium lentum on the basis of 16S rRNA gene analysis and G+C content comparisons, and Eggerthella lenta was only species in that genus [1, 2]. To date, 2 species, Eggerthella lenta and Eggerthella sinensis, have been assigned to the genus Eggerthella. Eggerthella hongkongensis, which was previously included in the genus Eggerthella, has been classified under a new genus and renamed Paraeggerthella hongkongensis since 2009, based on chemotaxonomic data [3]. E. lenta is a causative pathogen in appendicitis, bacteremia, cutaneous abscess, genitourinary tract infection, liver abscess, peritonitis, spondylodiscitis, and wound infection, but reports of E. lenta bacteremia are rare [4-13]. Here we report a case of E. lenta bacteremia in a rectal cancer patient. To the best of our knowledge, this is the first report of E. lenta bacteremia in Korea.
A 53-yr-old man was admitted to our hospital with fever and chills following the replacement of a double-J stent in the outpatient clinic on August 16, 2013. The patient was previously diagnosed with rectal cancer and underwent laparoscopic ultralow anterior resection with ileostomy in 2011. He underwent Hartmann's operation for recurrence in 2012 and received chemotherapy from August 2012 to April 2013. Following chemotherapy, left kidney hydronephrosis, recurrent mass in the left pelvic wall, and peritoneal seeding was observed in the follow-up abdominal computed tomography, and he underwent left double-J stent insertion for obstructive acute kidney injury in April 2013.
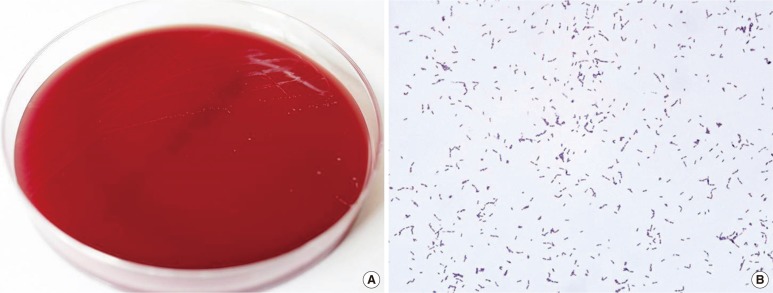
On admission, the patient had a temperature of 39.5℃, blood pressure of 157/93 mmHg, pulse of 98/min, and respiratory rate of 20 breaths/min. Laboratory investigation showed a Hb of 11.4 g/dL, leukocyte count of 2.2×109/L, platelet count of 255×109/L, C-reactive protein (CRP) level of 9.55 mg/dL, blood urea nitrogen (BUN)/creatinine of 20/1.3 mg/dL, and total protein/albumin level of 7.1/3.5 g/dL. A urine sample and two sets of blood samples were collected, and the patient received empirical antibiotic therapy. After three days of incubation, gram-positive bacilli grew in an anaerobic culture bottle, but no microorganisms were detected in the urine culture. The positive culture broth was inoculated onto a Brucella agar plate and anaerobically cultured for 48 hr. Small and translucent colonies grew on the Brucella agar plate, and gram-positive coccobacilli were observed by using Gram staining (Fig. 1). The organism was identified as E. lenta by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) (Bruker Daltonik GmbH, Bremen, Germany), with a score of 2.223. To confirm the identity of the isolate, 16S rRNA gene sequencing was conducted using the MicroSeq 500 system (Applied Biosystems, Foster City, CA, USA). PCR and sequencing kits were designed with universal primers to cover all bacteria. Sequences were analyzed by using an ABI PRISM 3730 Series DNA Analyzer (Applied Biosystems). In the first 500 bp of the 16S rRNA gene sequence, the isolate showed a 100% identity match with GenBank sequence NR_074377.1 (Eggerthella lenta) and 96.8% identity match with GenBank sequence AY321958.1 (Eggerthella sinensis). The patient was diagnosed as having E. lenta bacteremia.
Fig. 1.
(A) Small and translucent colonies of Eggerthella lenta on a Brucella agar plate. (B) Gram-positive coccobacilli from smear preparations from the colonies on Brucella agar plate (×1,000).
Empirical treatment with intravenous cefotaxime and amikacin was started before the culture results were obtained. During the four day treatment course, the patient's fever subsided and he was discharged with a prescription for oral antibiotics. There were no microorganisms in the follow-up urine sample or the two sets of blood cultures.
E. lenta is a non-motile, non-spore-forming anaerobic gram-positive bacillus. It does not produce acids from glucose, does not produce indole or liquefy gelatin, and produces little or no gas [14]. E. lenta colonies on blood agar are 0.25 to 0.75 mm in diameter, slightly raised, smooth, and gray [14]. E. lenta is a common gastrointestinal commensal [2, 14], but has been identified as the causative pathogen in various conditions, including appendicitis, skin abscesses, and spondylodiscitis [4-6]. There have been infrequent reports of E. lenta bacteremia, which is characterized by a high proportion of polymicrobial infection (about 40%) and a high mortality rate (20-30%) [7-13]. In 1994, Jang et al. [15] reported E. lenta (E. lentum) isolate from expressed prostatic secretion sample from a chronic prostatitis patient, which was the only case reported in Korea. Patients with gastrointestinal tract disease, malignancy, hepatobiliary disease, bed sores, diabetes mellitus, and stroke are prone to E. lenta bacteremia [7-13].
Biochemical identification of E. lenta by using the API system (bioMeriéux, Marcy l'Etoile, France) and the VITEK 2 system (bioMeriéux) was reported to be reliable, but many reported cases were confirmed by 16S rRNA sequence analysis [9, 11, 16]. MALDI-TOF MS, recently introduced in the clinical microbiologic laboratory, has been used successfully for the identification of E. lenta, with 100% success at the genus level and about 80% success at the species level [17-19]. MALDI-TOF MS is fast and useful in the identification of clinically important anaerobic bacteria such as E. lenta, although it is limited by the number of databases available for anaerobic bacteria [17-19].
E. lenta is susceptible to ampicillin-sulbactam, metronidazole, carbapenems, tigecycline, and daptomycin, but the two strains depending on colony morphology exhibit variable susceptibility to cephalosporins. Translucent-colony coccobacilli are susceptible to cephalosporins, while speckled-colony pleomorphic bacilli are resistant to them [12, 20]. Antibiotics, such as ampicillin-sulbactam, metronidazole, and carbapenems, to which E. lenta is known to be susceptible, have been conventionally used for the treatment of E. lenta bacteremia and are appropriate choices for patient management. However, bacteremia induced by translucent-colony-forming E. lenta as observed in the current case, could be treated effectively by using empirical antibiotics containing third-generation cephalosporins.
In the present case, we confirmed the identity of the blood isolate as E. lenta by MALDI-TOF MS and 16S rRNA sequence analysis. Although E. lenta bacteremia is associated with significant mortality and morbidity [7-13], this patient made a quick recovery, possibly because of prompt treatment at an early stage. The isolated E. lenta grew only in an anaerobic blood culture bottle, and the patient had high temperature, elevated heart rate, elevated CRP levels, and decreased leukocyte count and gastrointestinal tract malignancy as a predisposing factor for E. lenta bacteremia. On the basis of these observations and laboratory results, we concluded that the patient had E. lenta bacteremia. The occurrence of gram-positive bacillus in blood should not be ignored and warrants further laboratory investigation to avoid complications.
To the best of our knowledge, this is the first report of E. lenta bacteremia in Korea. MALDI-TOF MS is a fast, efficient method for identification of clinically anaerobic bacteria, such as E. lenta.
Footnotes
No potential conflicts of interest relevant to this article were reported.
References
- 1.Kageyama A, Benno Y, Nakase T. Phylogenetic evidence for the transfer of Eubacterium lentum to the genus Eggerthella as Eggerthella lenta gen. nov., comb. nov. Int J Syst Bacteriol. 1999;49:1725–1732. doi: 10.1099/00207713-49-4-1725. [DOI] [PubMed] [Google Scholar]
- 2.Eggerth AH. The gram-positive non-spore-bearing anaerobic bacilli of human feces. J Bacteriol. 1935;30:277–299. doi: 10.1128/jb.30.3.277-299.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Würdemann D, Tindall BJ, Pukall R, Lünsdorf H, Strömpl C, Namuth T. Gordonibacterpamelaeae gen. nov., sp. nov., a new member of the Coriobacteriaceae isolated from a patient with Crohn's disease, and reclassification of Eggerthella hongkongensis Lau et al. 2006 as Paraeggerthella hongkongensis gen. nov., comb. nov. Int J Syst Evol Microbiol. 2009;59:1405–1415. doi: 10.1099/ijs.0.005900-0. [DOI] [PubMed] [Google Scholar]
- 4.Bok CW, Ng YS. Eggerthella lenta as a cause of anaerobic spondylodiscitis. Singapore Med J. 2009;50:e393–e396. [PubMed] [Google Scholar]
- 5.Lattuada E, Zorzi A, Lanzafame M, Antolini D, Fontana R, Vento S, et al. Cutaneous abscess due to Eubacterium lentum in injection drug user: a case report and review of the literature. J Infect. 2005;51:E71–E72. doi: 10.1016/j.jinf.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 6.Rautio M, Saxén H, Siitonen A, Nikku R, Jousimies-Somer H. Bacteriology of histopathologically defined appendicitis in children. Pediatr Infect Dis J. 2000;19:1078–1083. doi: 10.1097/00006454-200011000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Brook I, Frazier EH. Significant recovery of nonsporulating anaerobic rods from clinical specimens. Clin Infect Dis. 1993;16:476–480. doi: 10.1093/clind/16.4.476. [DOI] [PubMed] [Google Scholar]
- 8.Chan RC, Mercer J. First Australian description of Eggerthellalenta bacteraemia identified by 16S rRNA gene sequencing. Pathology. 2008;40:409–410. doi: 10.1080/00313020802036772. [DOI] [PubMed] [Google Scholar]
- 9.Landais C, Doudier B, Imbert G, Fenollar F, Brouqui P. Application of rrs gene sequencing to elucidate the clinical significance of Eggerthelalenta infection. J Clin Microbiol. 2007;45:1063–1065. doi: 10.1128/JCM.01805-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau SK, Woo PC, Woo GK, Fung AM, Wong MK, Chan KM. Eggerthella hongkongensis sp. nov. and eggerthella sinensis sp. nov., two novel Eggerthella species, account for half of the cases of Eggerthella bacteremia. Diagn Microbiol Infect Dis. 2004;49:255–263. doi: 10.1016/j.diagmicrobio.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Liderot K, Larsson M, Boräng S, Ozenci V. Polymicrobial bloodstream infection with Eggerthella lenta and Desulfovibrio desulfuricans. J Clin Microbiol. 2010;48:3810–3812. doi: 10.1128/JCM.02481-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee MR, Huang YT, Liao CH, Chuang TY, Wang WJ, Lee SW, et al. Clinical and microbiological characteristics of bacteremia caused by Eggerthella, Paraeggerthella, and Eubacterium species at a university hospital in Taiwan from 2001 to 2010. J Clin Microbiol. 2012;50:2053–2055. doi: 10.1128/JCM.00548-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venugopal AA, Szpunar S, Johnson LB. Risk and prognostic factors among patients with bacteremia due to Eggerthella lenta. Anaerobe. 2012;18:475–478. doi: 10.1016/j.anaerobe.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Moore WE, Cato EP, Holdeman LV. Eubacteriumlentum (Eggerth) Prévot 1938: emendation of description and designation of the neotype strain. Int J Syst Bacteriol. 1971;21:299–303. [Google Scholar]
- 15.Jang JH, Kim SJ. Anaerobic bacterial isolation in patients with chronic prostatitis syndrome. Korean J Urol. 1994;35:640–645. [Google Scholar]
- 16.Thota VR, Dacha S, Natarajan A, Nerad J. Eggerthella lenta bacteremia in a Crohn's disease patient after ileocecal resection. Future Microbiol. 2011;6:595–597. doi: 10.2217/fmb.11.31. [DOI] [PubMed] [Google Scholar]
- 17.Justesen US, Holm A, Knudsen E, Andersen LB, Jensen TG, Kemp M, et al. Species identification of clinical isolates of anaerobic bacteria: a comparison of two matrix-assisted laser desorption ionization-time of flight mass spectrometry systems. J Clin Microbiol. 2011;49:4314–4318. doi: 10.1128/JCM.05788-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coltella L, Mancinelli L, Onori M, Lucignano B, Menichella D, Sorge R, et al. Advancement in the routine identification of anaerobic bacteria by MALDI-TOF mass spectrometry. Eur J Clin Microbiol Infect Dis. 2013;32:1183–1192. doi: 10.1007/s10096-013-1865-1. [DOI] [PubMed] [Google Scholar]
- 19.Barreau M, Pagnier I, La Scola B. Improving the identification of anaerobes in the clinical microbiology laboratory through MALDI-TOF mass spectrometry. Anaerobe. 2013;22:123–125. doi: 10.1016/j.anaerobe.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Mosca A, Summanen P, Finegold SM, De Michele G, Miragliotta G. Cellular fatty acid composition, soluble-protein profile, and antimicrobial resistance pattern of Eubacterium lentum. J Clin Microbiol. 1998;36:752–755. doi: 10.1128/jcm.36.3.752-755.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]