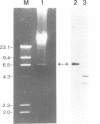
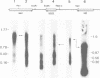
Abstract
Theileria parva, an intralymphocytic protozoan parasite of cattle, contains a linear 7.1 kb DNA element with terminal inverted repeat sequences. The molecule is transcribed into low molecular weight RNA, and both DNA strands encode short stretches of unique sequences, usually < 100 nucleotides, which are similar to large (LSU) or small (SSU) ribosomal subunit RNA. Phylogenetically conserved conformational rRNA domains were assembled from the discontinuous rDNA sequences using comparative secondary structure modelling. For example, a minimum of four predicted sequences, two derived from each DNA strand, is required to assemble domain V of LSU rRNA which participates in peptidyl transferase activity. The discontinuities in the identified rRNA domains fall within regions of no known functional significance. Hence, it is likely that the element encodes fragmented rDNA genes and the mature rRNA is unconventional, consisting of several fragments of RNA, primarily held together by intermolecular and intramolecular base pairing. The element also has ORFs for components of the last two mitochondrial electron transport enzyme complexes. The structure of the parasite DNA element, its protein coding capacity and scrambled rDNA gene sequences, are reminiscent of the mitochondrial genome of Chlamydomonas reinhardtii. We propose that the 7.1 kb element is equivalent to the mitochondrial DNA of T. parva, although a number of its features are unusual for this family of extrachromosomal DNA molecules.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Boer P. H., Gray M. W. Scrambled ribosomal RNA gene pieces in Chlamydomonas reinhardtii mitochondrial DNA. Cell. 1988 Nov 4;55(3):399–411. doi: 10.1016/0092-8674(88)90026-8. [DOI] [PubMed] [Google Scholar]
- Daldal F., Tokito M. K., Davidson E., Faham M. Mutations conferring resistance to quinol oxidation (Qz) inhibitors of the cyt bc1 complex of Rhodobacter capsulatus. EMBO J. 1989 Dec 20;8(13):3951–3961. doi: 10.1002/j.1460-2075.1989.tb08578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feagin J. E., Werner E., Gardner M. J., Williamson D. H., Wilson R. J. Homologies between the contiguous and fragmented rRNAs of the two Plasmodium falciparum extrachromosomal DNAs are limited to core sequences. Nucleic Acids Res. 1992 Feb 25;20(4):879–887. doi: 10.1093/nar/20.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. W. Organelle origins and ribosomal RNA. Biochem Cell Biol. 1988 May;66(5):325–348. doi: 10.1139/o88-042. [DOI] [PubMed] [Google Scholar]
- Gray M. W. Origin and evolution of mitochondrial DNA. Annu Rev Cell Biol. 1989;5:25–50. doi: 10.1146/annurev.cb.05.110189.000325. [DOI] [PubMed] [Google Scholar]
- Gutell R. R., Schnare M. N., Gray M. W. A compilation of large subunit (23S-like) ribosomal RNA sequences presented in a secondary structure format. Nucleic Acids Res. 1990 Apr 25;18 (Suppl):2319–2330. doi: 10.1093/nar/18.suppl.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge W. E., Dave D., Richards W. H. Conversion of dihydroorotate to orotate in parasitic protozoa. Biochim Biophys Acta. 1979 Feb 1;582(3):390–401. doi: 10.1016/0304-4165(79)90131-4. [DOI] [PubMed] [Google Scholar]
- Hall R., Coggins L., McKellar S., Shiels B., Tait A. Characterisation of an extrachromosomal DNA element from Theileria annulata. Mol Biochem Parasitol. 1990 Jan 15;38(2):253–260. doi: 10.1016/0166-6851(90)90028-k. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988 Dec 15;73(1):237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- Howell N., Gilbert K. Mutational analysis of the mouse mitochondrial cytochrome b gene. J Mol Biol. 1988 Oct 5;203(3):607–618. doi: 10.1016/0022-2836(88)90195-7. [DOI] [PubMed] [Google Scholar]
- Hudson A. T., Randall A. W., Fry M., Ginger C. D., Hill B., Latter V. S., McHardy N., Williams R. B. Novel anti-malarial hydroxynaphthoquinones with potent broad spectrum anti-protozoal activity. Parasitology. 1985 Feb;90(Pt 1):45–55. doi: 10.1017/s0031182000049003. [DOI] [PubMed] [Google Scholar]
- Jasmer D. P., Reduker D. W., Goff W. L., Stiller D., McGuire T. C. DNA probes distinguish geographical isolates and identify a novel DNA molecule of Babesia bovis. J Parasitol. 1990 Dec;76(6):834–841. [PubMed] [Google Scholar]
- Johnston R. C., Farias N. A., Gonzales J. C., Dewes H., Masuda A., Termignoni C., Amako K., Ozaki L. S. A putative RNA virus in Babesia bovis. Mol Biochem Parasitol. 1991 Mar;45(1):155–158. doi: 10.1016/0166-6851(91)90037-7. [DOI] [PubMed] [Google Scholar]
- Joseph J. T., Aldritt S. M., Unnasch T., Puijalon O., Wirth D. F. Characterization of a conserved extrachromosomal element isolated from the avian malarial parasite Plasmodium gallinaceum. Mol Cell Biol. 1989 Sep;9(9):3621–3629. doi: 10.1128/mcb.9.9.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine N. D., Corliss J. O., Cox F. E., Deroux G., Grain J., Honigberg B. M., Leedale G. F., Loeblich A. R., 3rd, Lom J., Lynn D. A newly revised classification of the protozoa. J Protozool. 1980 Feb;27(1):37–58. doi: 10.1111/j.1550-7408.1980.tb04228.x. [DOI] [PubMed] [Google Scholar]
- Megson A., Inman G. J., Hunt P. D., Baylis H. A., Hall R. The gene for apocytochrome B of Theileria annulata resides on a small linear extrachromosomal element. Mol Biochem Parasitol. 1991 Sep;48(1):113–115. doi: 10.1016/0166-6851(91)90171-2. [DOI] [PubMed] [Google Scholar]
- Michaelis G., Vahrenholz C., Pratje E. Mitochondrial DNA of Chlamydomonas reinhardtii: the gene for apocytochrome b and the complete functional map of the 15.8 kb DNA. Mol Gen Genet. 1990 Sep;223(2):211–216. doi: 10.1007/BF00265056. [DOI] [PubMed] [Google Scholar]
- Morzaria S. P., Spooner P. R., Bishop R. P., Musoke A. J., Young J. R. SfiI and NotI polymorphisms in Theileria stocks detected by pulsed field gel electrophoresis. Mol Biochem Parasitol. 1990 May;40(2):203–211. doi: 10.1016/0166-6851(90)90042-k. [DOI] [PubMed] [Google Scholar]
- Ossorio P. N., Sibley L. D., Boothroyd J. C. Mitochondrial-like DNA sequences flanked by direct and inverted repeats in the nuclear genome of Toxoplasma gondii. J Mol Biol. 1991 Dec 5;222(3):525–536. doi: 10.1016/0022-2836(91)90494-q. [DOI] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Robertson D. E., Daldal F., Dutton P. L. Mutants of ubiquinol-cytochrome c2 oxidoreductase resistant to Qo site inhibitors: consequences for ubiquinone and ubiquinol affinity and catalysis. Biochemistry. 1990 Dec 25;29(51):11249–11260. doi: 10.1021/bi00503a014. [DOI] [PubMed] [Google Scholar]
- Saraste M. Location of haem-binding sites in the mitochondrial cytochrome b. FEBS Lett. 1984 Jan 30;166(2):367–372. doi: 10.1016/0014-5793(84)80114-3. [DOI] [PubMed] [Google Scholar]
- Schnare M. N., Gray M. W. Sixteen discrete RNA components in the cytoplasmic ribosome of Euglena gracilis. J Mol Biol. 1990 Sep 5;215(1):73–83. doi: 10.1016/S0022-2836(05)80096-8. [DOI] [PubMed] [Google Scholar]
- Spencer D. F., Collings J. C., Schnare M. N., Gray M. W. Multiple spacer sequences in the nuclear large subunit ribosomal RNA gene of Crithidia fasciculata. EMBO J. 1987 Apr;6(4):1063–1071. doi: 10.1002/j.1460-2075.1987.tb04859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suplick K., Morrisey J., Vaidya A. B. Complex transcription from the extrachromosomal DNA encoding mitochondrial functions of Plasmodium yoelii. Mol Cell Biol. 1990 Dec;10(12):6381–6388. doi: 10.1128/mcb.10.12.6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya A. B., Akella R., Suplick K. Sequences similar to genes for two mitochondrial proteins and portions of ribosomal RNA in tandemly arrayed 6-kilobase-pair DNA of a malarial parasite. Mol Biochem Parasitol. 1989 Jun 15;35(2):97–107. doi: 10.1016/0166-6851(89)90112-6. [DOI] [PubMed] [Google Scholar]
- Vaidya A. B., Arasu P. Tandemly arranged gene clusters of malarial parasites that are highly conserved and transcribed. Mol Biochem Parasitol. 1987 Jan 15;22(2-3):249–257. doi: 10.1016/0166-6851(87)90056-9. [DOI] [PubMed] [Google Scholar]
- Wang A. L., Wang C. C. Discovery of a specific double-stranded RNA virus in Giardia lamblia. Mol Biochem Parasitol. 1986 Dec;21(3):269–276. doi: 10.1016/0166-6851(86)90132-5. [DOI] [PubMed] [Google Scholar]
- Widger W. R., Cramer W. A., Herrmann R. G., Trebst A. Sequence homology and structural similarity between cytochrome b of mitochondrial complex III and the chloroplast b6-f complex: position of the cytochrome b hemes in the membrane. Proc Natl Acad Sci U S A. 1984 Feb;81(3):674–678. doi: 10.1073/pnas.81.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Rago J. P., Colson A. M. Molecular basis for resistance to antimycin and diuron, Q-cycle inhibitors acting at the Qi site in the mitochondrial ubiquinol-cytochrome c reductase in Saccharomyces cerevisiae. J Biol Chem. 1988 Sep 5;263(25):12564–12570. [PubMed] [Google Scholar]
- ole-MoiYoi O. K., Sugimoto C., Conrad P. A., Macklin M. D. Cloning and characterization of the casein kinase II alpha subunit gene from the lymphocyte-transforming intracellular protozoan parasite Theileria parva. Biochemistry. 1992 Jul 14;31(27):6193–6202. doi: 10.1021/bi00142a004. [DOI] [PubMed] [Google Scholar]
- von Jagow G., Link T. A. Use of specific inhibitors on the mitochondrial bc1 complex. Methods Enzymol. 1986;126:253–271. doi: 10.1016/s0076-6879(86)26026-7. [DOI] [PubMed] [Google Scholar]