Abstract
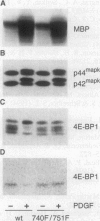
It has previously been argued that the repressor of protein synthesis initiation factor 4E, 4E-BP1, is a direct in vivo target of p42mapk. However, the immunosuppressant rapamycin blocks serum-induced 4E-BP1 phosphorylation and, in parallel, p70s6k activation, with no apparent effect on p42mapk activation. Consistent with this finding, the kinetics of serum-induced 4E-BP1 phosphorylation closely follow those of p70s6k activation rather than those of p42mapk. More striking, insulin, which does not induce p42mapk activation in human 293 cells or Swiss mouse 3T3 cells, induces 4E-BP1 phosphorylation and p70s6k activation in both cell types. Anisomycin, which, like insulin, does not activate p42mapk, promotes a small parallel increase in 4E-BP1 phosphorylation and p70s6k activation. The insulin effect on 4E-BP1 phosphorylation and p70s6k activation in both cell types is blocked by SQ20006, wortmannin, and rapamycin. These three inhibitors have no effect on p42mapk activation induced by phorbol 12-tetradecanoate 13-acetate, though wortmannin partially suppresses both the p70s6k response and the 4E-BP1 response. Finally, in porcine aortic endothelial cells stably transfected with either the wild-type platelet-derived growth factor receptor or a mutant receptor bearing the double point mutation 740F/751F, p42mapk activation in response to platelet-derived growth factor is unimpaired, but increased 4E-BP1 phosphorylation is ablated, as previously reported for p70s6k. The data presented here demonstrate that 4E-BP1 phosphorylation is mediated by the FRAP-p70s6k pathway and is independent of mitogen-activated protein kinase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballou L. M., Luther H., Thomas G. MAP2 kinase and 70K S6 kinase lie on distinct signalling pathways. Nature. 1991 Jan 24;349(6307):348–350. doi: 10.1038/349348a0. [DOI] [PubMed] [Google Scholar]
- Barbet N. C., Schneider U., Helliwell S. B., Stansfield I., Tuite M. F., Hall M. N. TOR controls translation initiation and early G1 progression in yeast. Mol Biol Cell. 1996 Jan;7(1):25–42. doi: 10.1091/mbc.7.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beretta L., Gingras A. C., Svitkin Y. V., Hall M. N., Sonenberg N. Rapamycin blocks the phosphorylation of 4E-BP1 and inhibits cap-dependent initiation of translation. EMBO J. 1996 Feb 1;15(3):658–664. [PMC free article] [PubMed] [Google Scholar]
- Brown E. J., Albers M. W., Shin T. B., Ichikawa K., Keith C. T., Lane W. S., Schreiber S. L. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994 Jun 30;369(6483):756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- Brown E. J., Beal P. A., Keith C. T., Chen J., Shin T. B., Schreiber S. L. Control of p70 s6 kinase by kinase activity of FRAP in vivo. Nature. 1995 Oct 5;377(6548):441–446. doi: 10.1038/377441a0. [DOI] [PubMed] [Google Scholar]
- Chung J., Grammer T. C., Lemon K. P., Kazlauskas A., Blenis J. PDGF- and insulin-dependent pp70S6k activation mediated by phosphatidylinositol-3-OH kinase. Nature. 1994 Jul 7;370(6484):71–75. doi: 10.1038/370071a0. [DOI] [PubMed] [Google Scholar]
- Chung J., Kuo C. J., Crabtree G. R., Blenis J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell. 1992 Jun 26;69(7):1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- Flotow H., Thomas G. Substrate recognition determinants of the mitogen-activated 70K S6 kinase from rat liver. J Biol Chem. 1992 Feb 15;267(5):3074–3078. [PubMed] [Google Scholar]
- Graves L. M., Bornfeldt K. E., Argast G. M., Krebs E. G., Kong X., Lin T. A., Lawrence J. C., Jr cAMP- and rapamycin-sensitive regulation of the association of eukaryotic initiation factor 4E and the translational regulator PHAS-I in aortic smooth muscle cells. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7222–7226. doi: 10.1073/pnas.92.16.7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighat A., Mader S., Pause A., Sonenberg N. Repression of cap-dependent translation by 4E-binding protein 1: competition with p220 for binding to eukaryotic initiation factor-4E. EMBO J. 1995 Nov 15;14(22):5701–5709. doi: 10.1002/j.1460-2075.1995.tb00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J. W., Pearson R. B., Dennis P. B., Thomas G. Rapamycin, wortmannin, and the methylxanthine SQ20006 inactivate p70s6k by inducing dephosphorylation of the same subset of sites. J Biol Chem. 1995 Sep 8;270(36):21396–21403. doi: 10.1074/jbc.270.36.21396. [DOI] [PubMed] [Google Scholar]
- Haystead T. A., Haystead C. M., Hu C., Lin T. A., Lawrence J. C., Jr Phosphorylation of PHAS-I by mitogen-activated protein (MAP) kinase. Identification of a site phosphorylated by MAP kinase in vitro and in response to insulin in rat adipocytes. J Biol Chem. 1994 Sep 16;269(37):23185–23191. [PubMed] [Google Scholar]
- Jefferies H. B., Reinhard C., Kozma S. C., Thomas G. Rapamycin selectively represses translation of the "polypyrimidine tract" mRNA family. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4441–4445. doi: 10.1073/pnas.91.10.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardalinou E., Zhelev N., Hazzalin C. A., Mahadevan L. C. Anisomycin and rapamycin define an area upstream of p70/85S6k containing a bifurcation to histone H3-HMG-like protein phosphorylation and c-fos-c-jun induction. Mol Cell Biol. 1994 Feb;14(2):1066–1074. doi: 10.1128/mcb.14.2.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane H. A., Fernandez A., Lamb N. J., Thomas G. p70s6k function is essential for G1 progression. Nature. 1993 May 13;363(6425):170–172. doi: 10.1038/363170a0. [DOI] [PubMed] [Google Scholar]
- Lane H. A., Morley S. J., Dorée M., Kozma S. C., Thomas G. Identification and early activation of a Xenopus laevis p70s6k following progesterone-induced meiotic maturation. EMBO J. 1992 May;11(5):1743–1749. doi: 10.1002/j.1460-2075.1992.tb05226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaris-Karatzas A., Montine K. S., Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5' cap. Nature. 1990 Jun 7;345(6275):544–547. doi: 10.1038/345544a0. [DOI] [PubMed] [Google Scholar]
- Lin T. A., Kong X., Haystead T. A., Pause A., Belsham G., Sonenberg N., Lawrence J. C., Jr PHAS-I as a link between mitogen-activated protein kinase and translation initiation. Science. 1994 Oct 28;266(5185):653–656. doi: 10.1126/science.7939721. [DOI] [PubMed] [Google Scholar]
- Lin T. A., Kong X., Saltiel A. R., Blackshear P. J., Lawrence J. C., Jr Control of PHAS-I by insulin in 3T3-L1 adipocytes. Synthesis, degradation, and phosphorylation by a rapamycin-sensitive and mitogen-activated protein kinase-independent pathway. J Biol Chem. 1995 Aug 4;270(31):18531–18538. doi: 10.1074/jbc.270.31.18531. [DOI] [PubMed] [Google Scholar]
- Mader S., Lee H., Pause A., Sonenberg N. The translation initiation factor eIF-4E binds to a common motif shared by the translation factor eIF-4 gamma and the translational repressors 4E-binding proteins. Mol Cell Biol. 1995 Sep;15(9):4990–4997. doi: 10.1128/mcb.15.9.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan L. C., Edwards D. R. Signalling and superinduction. Nature. 1991 Feb 28;349(6312):747–748. doi: 10.1038/349747c0. [DOI] [PubMed] [Google Scholar]
- Marais R., Wynne J., Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993 Apr 23;73(2):381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- Ming X. F., Burgering B. M., Wennström S., Claesson-Welsh L., Heldin C. H., Bos J. L., Kozma S. C., Thomas G. Activation of p70/p85 S6 kinase by a pathway independent of p21ras. Nature. 1994 Sep 29;371(6496):426–429. doi: 10.1038/371426a0. [DOI] [PubMed] [Google Scholar]
- Olivier A. R., Ballou L. M., Thomas G. Differential regulation of S6 phosphorylation by insulin and epidermal growth factor in Swiss mouse 3T3 cells: insulin activation of type 1 phosphatase. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4720–4724. doi: 10.1073/pnas.85.13.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pause A., Belsham G. J., Gingras A. C., Donzé O., Lin T. A., Lawrence J. C., Jr, Sonenberg N. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5'-cap function. Nature. 1994 Oct 27;371(6500):762–767. doi: 10.1038/371762a0. [DOI] [PubMed] [Google Scholar]
- Pearson R. B., Dennis P. B., Han J. W., Williamson N. A., Kozma S. C., Wettenhall R. E., Thomas G. The principal target of rapamycin-induced p70s6k inactivation is a novel phosphorylation site within a conserved hydrophobic domain. EMBO J. 1995 Nov 1;14(21):5279–5287. doi: 10.1002/j.1460-2075.1995.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. M., Erdjument-Bromage H., Lui M., Tempst P., Snyder S. H. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994 Jul 15;78(1):35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- Sabers C. J., Martin M. M., Brunn G. J., Williams J. M., Dumont F. J., Wiederrecht G., Abraham R. T. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J Biol Chem. 1995 Jan 13;270(2):815–822. doi: 10.1074/jbc.270.2.815. [DOI] [PubMed] [Google Scholar]
- Shama S., Avni D., Frederickson R. M., Sonenberg N., Meyuhas O. Overexpression of initiation factor eIF-4E does not relieve the translational repression of ribosomal protein mRNAs in quiescent cells. Gene Expr. 1995;4(4-5):241–252. [PMC free article] [PubMed] [Google Scholar]
- Thomas G., Martin-Pérez J., Siegmann M., Otto A. M. The effect of serum, EGF, PGF2 alpha and insulin on S6 phosphorylation and the initiation of protein and DNA synthesis. Cell. 1982 Aug;30(1):235–242. doi: 10.1016/0092-8674(82)90029-0. [DOI] [PubMed] [Google Scholar]
- Wennström S., Siegbahn A., Yokote K., Arvidsson A. K., Heldin C. H., Mori S., Claesson-Welsh L. Membrane ruffling and chemotaxis transduced by the PDGF beta-receptor require the binding site for phosphatidylinositol 3' kinase. Oncogene. 1994 Feb;9(2):651–660. [PubMed] [Google Scholar]
- Westermark B., Siegbahn A., Heldin C. H., Claesson-Welsh L. B-type receptor for platelet-derived growth factor mediates a chemotactic response by means of ligand-induced activation of the receptor protein-tyrosine kinase. Proc Natl Acad Sci U S A. 1990 Jan;87(1):128–132. doi: 10.1073/pnas.87.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]