Abstract
Quantification of muscle protein synthesis (MPS) remains a cornerstone for understanding the control of muscle mass. Traditional [13C]amino acid tracer methodologies necessitate sustained bed rest and intravenous cannulation(s), restricting studies to ∼12 h, and thus cannot holistically inform on diurnal MPS. This limits insight into the regulation of habitual muscle metabolism in health, aging, and disease while querying the utility of tracer techniques to predict the long-term efficacy of anabolic/anticatabolic interventions. We tested the efficacy of the D2O tracer for quantifying MPS over a period not feasible with 13C tracers and too short to quantify changes in mass. Eight men (22 ± 3.5 yr) undertook one-legged resistance exercise over an 8-day period (4 × 8–10 repetitions, 80% 1RM every 2nd day, to yield “nonexercised” vs. “exercise” leg comparisons), with vastus lateralis biopsies taken bilaterally at 0, 2, 4, and 8 days. After day 0 biopsies, participants consumed a D2O bolus (150 ml, 70 atom%); saliva was collected daily. Fractional synthetic rates (FSRs) of myofibrillar (MyoPS), sarcoplasmic (SPS), and collagen (CPS) protein fractions were measured by GC-pyrolysis-IRMS and TC/EA-IRMS. Body water initially enriched at 0.16–0.24 APE decayed at ∼0.009%/day. In the nonexercised leg, MyoPS was 1.45 ± 0.10, 1.47 ± 0.06, and 1.35 ± 0.07%/day at 0–2, 0–4, and 0–8 days, respectively (∼0.05–0.06%/h). MyoPS was greater in the exercised leg (0–2 days: 1.97 ± 0.13%/day; 0–4 days: 1.96 ± 0.15%/day, P < 0.01; 0–8 days: 1.79 ± 0.12%/day, P < 0.05). CPS was slower than MyoPS but followed a similar pattern, with the exercised leg tending to yield greater FSRs (0–2 days: 1.14 ± 0.13 vs. 1.45 ± 0.15%/day; 0–4 days: 1.13 ± 0.07%/day vs. 1.47 ± 0.18%/day; 0–8 days: 1.03 ± 0.09%/day vs. 1.40 ± 0.11%/day). SPS remained unchanged. Therefore, D2O has unrivaled utility to quantify day-to-day MPS in humans and inform on short-term changes in anabolism and presumably catabolism alike.
Keywords: deuterium oxide, protein synthesis, skeletal muscle
skeletal muscle is the body's largest organ and represents an important tissue for maintenance of metabolic health. Muscle not only provides locomotory function but also acts as a major glucose disposal sink and a fuel reservoir for other organs in times of fasting and stress (60). Thus, unsurprisingly, declines in muscle mass with aging (sarcopenia) or during acute or chronic illness [i.e., sepsis, chronic obstructive pulmonary disease, cancer cachexia, and organ failure (31, 52, 56, 59)] lead to frailty and loss of mobility and independence in addition to increased risk of cardiovascular and metabolic disease [e.g., type 2 diabetes; (54)] or, in severe cases, even death (2).
Over recent years, considerable effort has been focused on research into techniques and interventions for understanding the regulation of skeletal muscle mass and minimizing loss of muscle mass with aging and disease (26, 41). Despite this, effective treatments to mitigate muscle loss remain limited. Indeed, despite extensive investment in pharmaceutical interventions (6, 39), the current most effective and safe means by which to maintain or increase muscle mass remains resistance exercise training (RET) with accompanying protein nutrition (11, 19, 25, 29, 40). The hypertrophic response to RET is regulated via cumulative postexercise increases in muscle protein synthesis (MPS) (22, 30, 42), which after several weeks/months leads to muscle hypertrophy that is quantifiable using imaging techniques such as dual-energy X-ray absorptiometry (DEXA), ultrasound, MRI, or CT.
Traditionally, MPS has been measured in vivo using stable isotope tracers of amino acids (AA) with either heavy carbon (13C), deuterium (2H), or nitrogen (15N) motifs. The incorporation of these labeled AAs into muscle protein allows for the calculation of a fractional synthetic rate (FSR) of MPS (45, 61). Although they provide great utility, these methods are not without their inherent limitations. For instance, 13C-AA tracers permit measurements to be performed only over short durations (typically <8–12 h) and require preparation of sometimes costly sterile infusions, venous/arterial cannulation, and multiple biopsy collection, all within a controlled laboratory environment. Therefore, there is a need to develop less restrictive and longer-term means to determine MPS in “free-living” humans. It would clearly be advantageous were this method to be sensitive enough to detect changes in MPS and anabolic and/or catabolic scenarios over periods in which it is not plausible to measure changes in mass via imaging methods.
Recent advances in mass spectrometry, in particular the development of pyrolysis-isotope ratio mass spectrometry (IRMS) systems for high-precision measurement of hydrogen and oxygen stable isotopes (21, 47), have led to the recent reintroduction of the first stable isotope tracer used in metabolic research, deuterium oxide (D2O, or “heavy water”) (48, 51). Upon oral ingestion, D2O equilibrates within the body water pool [∼20 min in rats (7) and 2 h in humans (24)], and through exchange with hydrogen the deuterium can be incorporated into multiple metabolic pools and tissues. This negates the need for intravenous administration and allows subjects to administer tracer while performing normal activities. D2O has been successfully integrated into the measurement of protein synthesis in both animal models (7, 9, 12a, 14, 18, 27, 37, 62) and humans (9, 16, 33, 43, 50). Nonetheless, to date few studies have concentrated on its application and validity in the arena of human muscle.
Robinson et al. (50) reported that it was possible to measure MPS over extended periods (i.e., 6 wk) in groups of free-living adults, highlighting its unique suitability for determining longer-term MPS. Furthermore, Gasier et al. (16) showed stimulation of myofibrillar protein synthesis (MyoPS) but not mixed-muscle protein synthesis 24 h following a single exercise bout highlighting the importance of delineating fraction-specific differences. Nonetheless, the authors of that study did not measure responses over longer periods in an attempt to resolve the temporal and cumulative relationship between successive bouts of resistance exercise and MPS, the latter being the key underlying feature regulating muscle hypertrophy. Finally, both the Gasier et al. (16) and Robinson et al. (50) studies used relatively large doses of D2O to be able to chart MPS due to sensitivity issues involved with gas chromatography-mass spectrometry (GC-MS) as an analytical tool. In comparison, IRMS adds ≥100-fold increases in sensitivity for measuring tissue isotopic enrichment compared with GC-MS, as demonstrated by MacDonald et al., (33), who showed that MyoPS could be measured over a period of 4–14 days following a small single bolus of 100 ml of 70 atom% D2O in healthy adults under rested conditions.
Our goal was to assess the utility of D2O for quantifying temporal and cumulative MPS under habitual and stimulated conditions (to assess its potential sensitivity to inform on anabolic/anticatabolic interventions). We employed unilateral exercise (28), where one leg is exercised and the contralateral serves as internal control, to determine the efficacy of D2O for quantifying cumulative MPS at rest and in response to exercise. We hypothesized that D2O alongside IRMS techniques could be used to distinguish the synthesis profiles of muscle protein fractions (myofibrillar, collagen, sarcoplasmic) over a period of short-term RET (4 bouts over 8 days). Our rationale for this was that 1) it is unfeasible to use AA tracers over this time, 2) it would inform about diurnal MPS in real-life settings, and 3) it is a period too short to quantify increases in muscle mass as a consequence of RET in healthy, young volunteers.
MATERIALS AND METHODS
Subject characteristics and ethics.
Eight young healthy males (22 ± 3.5 yr, body mass index 23.5 ± 0.8 kg/m−2) were recruited. All volunteers were screened by means of a medical questionnaire, physical examination, and resting electrocadiogram, with exclusions for metabolic, respiratory, and cardiovascular disorders or other symptoms of ill health. Subjects had normal blood chemistry, were normotensive (blood pressure <140/90), and were not prescribed any medications; all subjects performed activities of daily living and recreation but did not participate routinely in any formal exercise. All subjects gave their written, informed consent to participate after all procedures and risks were explained. This study was approved by The University of Nottingham Ethics Committee and complied with the Declaration of Helsinki.
Study procedures.
Volunteers were asked to refrain from heavy exercise for the 72 h before the start of the study and performed no exercise over the course of the study other than that prescribed as part of the protocol. One week before the first visit, strength was assessed for the training leg using an isokinetic dynamometer (Isocom; Isokinetic Technologies, Eurokinetics, UK), with a one-repetition maximum (1RM) also assessed (Dominant leg; Technogym, Gambettola, Italy). In addition, muscle architecture was assessed by ultrasound (Mylab 70; Esaote Biomedica). The same measurements were performed 8 days later following the final training and biopsy session. Participants then completed a unilateral resistance exercise training program over a period of 8 days; training consisted of single-leg knee extension exercise (4 × 8 reps at 80% 1RM) performed on days 0, 2, 4, 6, and 8. Twenty grams of whey protein isolate (Pro-Isolate Tech; Muscletech) was provided postexercise to ensure sufficient substrate to support the increased demands of exercise on MPS processes. Bilateral biopsies (nonexercised and exercised) of m. vastus lateralis were taken under sterile conditions, using the conchotome biopsy technique (13) with 1% lidocaine (B. Braun Melsungen) as local anaesthetic, on days 0 (basal), 2, 4, and 8. A rested biopsy was taken prior to the exercise bout to avoid acute stimulation from the exercise and whey protein feed. Muscle was rapidly dissected free of fat and connective tissue, washed in ice-cold saline, and then frozen in liquid N2 and stored at −80°C until further analysis. Single venous blood samples were collected on days 0 and 8 into lithium heparin-coated tubes; these were immediately cold centrifuged at 3,200 rpm, and the plasma fraction was then aliquoted and frozen at −80°C until analysis.
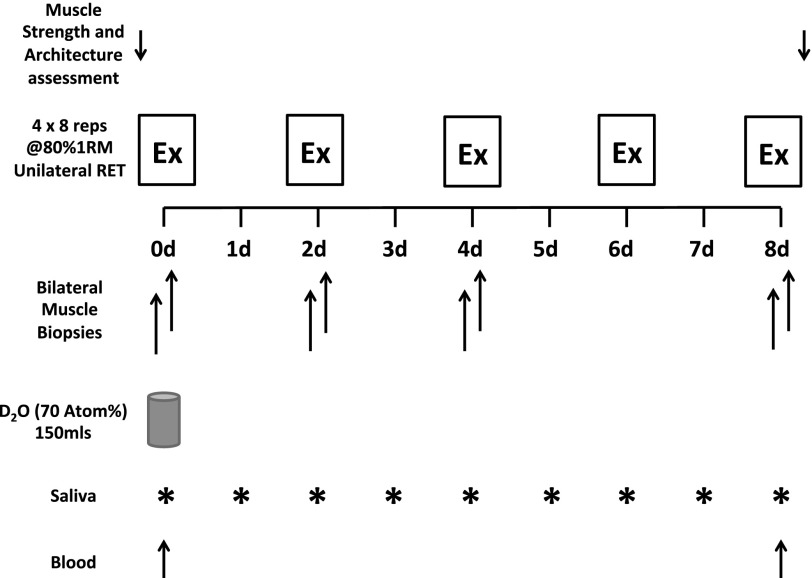
Immediately post-biopsy on day 0, participants provided a saliva sample (collected in sterile plastic tubes) and were asked to consume a single 150-ml oral bolus of D2O (70 atom%; Sigma-Aldrich, Poole, UK), and this was performed with the aim to label the body water pool to ∼0.2%. To monitor the body water enrichment throughout the study, each participant was asked to provide a single daily saliva sample collected at midday ≥30 min after their last meal or drink. These were collected in sterile plastic tubes and kept refrigerated, and participants were asked to bring these to each training session. Upon receipt of saliva samples, they were immediately cold centrifuged at 16,000 g to remove any debris that might have been present; they were then aliquoted into 2-ml glass vials and frozen at −20°C until analysis. A detailed schematic of the study protocol is provided in Fig. 1.
Fig. 1.
Schematic of resistance exercise (Ex) study protocol. RET, resistance exercise training; 1RM, 1-repetition maximum.
Body water enrichment.
Body water enrichment was determined through direct liquid injection of saliva samples (0.1-μl volume) into a high-temperature conversion elemental analyzer (TC/EA; Thermo Finnigan, Thermo Scientific, Hemel Hempstead, UK) connected to an isotope ratio mass spectrometer (IRMS; Delta V Advantage, Thermo). To minimize the effect of carryover between samples, each was injected a minimum of four times. To validate the accuracy of the TC/EA for measuring body water enrichment from saliva, the analysis was repeated with two participants' saliva sets using GC-Pyrolysis-IRMS (Trace GC isolink Delta V Advantage; Thermo Scientific) and a modification of the protocol by Mahsut et al. (34). Briefly, 100 μl of each saliva sample was incubated with 2 μl of 10 M NaOH and 1 μl of acetone for 24 h at room temperature; this high pH incubation leads to the exchange of deuterium from water with the hydrogen positions on the acetone. Following incubation the acetone was extracted into 200 μl of n-heptane, and 0.5 μl of the heptane phase was injected into the GC for analysis. A standard curve of known D2O enrichment was run alongside the saliva samples for calculation of enrichment. Further validation was also provided via analysis of water extracted from day 0 and day 8 plasma. In addition, 50- to 100-μl aliquots of plasma were placed in the cap of inverted autosampler vials; these were then placed on a heating block set at 90°C for 2 h. Water distillate was then collected by rapidly cooling the vials on ice for 10 min and transferred to fresh vials for direct liquid injection into the TC/EA, same as for the saliva.
Isolation and derivatization of myofibrillar, sarcoplasmic, and collagen protein fractions.
For isolation of myofibrillar, sarcoplasmic, and collagen fractions, ∼30–50 mg of muscle was used. The muscle was homogenized in ice-cold homogenization buffer [50 mM Tris·HCl (pH 7.4), 50 mM NaF, 10 mM β-glycerophosphate disodium salt, 1 mM EDTA, 1 mM EGTA, and 1 mM activated Na3VO4 (all from Sigma-Aldrich)] and a complete protease inhibitor cocktail tablet (Roche, West Sussex, UK) at 10 μl/μg tissue. Homogenates were rotated for 10 min, and the supernatant was collected by centrifugation at 13,000 g for 5 min at 4°C. The myofibrillar pellet was solubilized in 0.3 M NaOH and separated from the insoluble collagen by centrifugation, and the myofibrillar protein was precipitated with 1 M perchloric acid. The sarcoplasmic proteins were precipitated from the initial homogenate supernatant fraction with 1 M perchloric acid and separated by centrifugation. The insoluble collagen fraction was washed sequentially with 0.3 M NaOH, 70% ethanol, 0.5 M acetic acid, and 0.5 M acetic acid and isolated by centrifugation. Protein-bound AAs from myofibrillar, sarcoplasmic, and collagen were released using acid hydrolysis by incubating in 0.1 M HCl in Dowex H+ resin slurry overnight before being eluted from the resin with 2 M NH4OH and evaporated to dryness. The AAs were then derivatized as their n-methoxycarbonyl methyl esters (MCME) according to the protocol of Husek and Liebich (23), with slight modification. The dried samples were resuspended in 60 μl of distilled water and 32 μl of methanol, and following a brief vortex, 10 μl of pyridine and 8 μl of methylchloroformate were added. Samples were vortexed for 30 s and left to react at room temperature for 5 min. The newly formed MCME AAs were then extracted into 100 μl of chloroform; any remaining water was removed from the sample with the addition of a molecular sieve. Incorporation of deuterium into protein bound alanine was determined by gas chromatography-pyrolysis-isotope ratio mass spectrometry (Delta V Advantage; Thermo Scientific) alongside a standard curve of known l-Alanine-2,3,3,3-d4 enrichment.
Plasma alanine enrichment.
Plasma (200 μl) proteins were precipitated with 100% ethanol, the supernatant evaporated to dryness and was reconstituted in 0.5 M HCl, and the lipid fraction was removed using ethyl acetate extraction; alanine was then converted to its MCME derivative, as described above. All samples were run alongside an l-alanine-2,3,3,3-d4 standard curve. Enrichment (mole %excess) of alanine was then determined using gas chromatography-mass spectrometry (GC-MS; MD800, Fison, UK) and single ion monitoring of m/z 102, 103, 104, 105, and 106.
GC-pyrolysis IRMS deuterium analyses.
Prior to each analysis, the IRMS system was calibrated and tested for measurement accuracy and was not used unless it fell within the manufacturer's technical specifications. For GC-pyrolysis-IRMS analysis of acetone and MCME alanine, samples were separated on a DB-wax column (30 m × 0.32 mm × 0.25 μm; Agilent J & W) following splitless injection. The oven temperature program for acetone was start at 50°C and hold for 2 min and then ramp at 30°C/min to 240°C and hold for 2 min, and for alanine it was start at 70°C and hold for 3 mins and then ramp at 10°C/min to 240°C and hold for 15 min. The separated samples were then passed through a high temperature (1,420°C) conversion reactor, where the analytes were converted to H2 gas before being directed to the IRMS, where the 2H/1H ratio was determined. For TC/EA analysis of body water enrichment, following direct liquid injection into the TC/EA, where samples were immediately converted to H2 gas, sample gases were directed to the IRMS, where the 2H/1H ratio was determined. The deuterium isotopic enrichment provided as δ2H was converted to atom% using the following equation:
where AR represents the absolute ratio constant for deuterium based on the VSMOW standard and equates to 0.00015595. This was then converted to atom percent excess (APE) by correcting for baseline sample, i.e., background enrichment. If any sample fell outside the dynamic range of the instrument, these were re-injected or re-prepared.
Calculation of the FSR.
The FSR of MyoPS, sarcoplasmic synthesis (SPS), collagen synthesis (CPS), and protein synthesis was determined from the incorporation of deuterium-labeled alanine into protein, using the enrichment of body water (corrected for the mean number of deuterium moieties incorporated per alanine, 3.7) as the surrogate precursor labeling between subsequent biopsies. In brief, the standard equation used was
where APEAla = deuterium enrichment of protein-bound alanine, APEP = mean precursor enrichment over the time period, and t is the time between biopsies.
Muscle architecture and strength assessment.
We also analyzed changes in muscle architecture, as these represent a technique with sufficient resolution to detect subtle changes in muscle architecture (albeit not muscle mass) after only short periods of exercise training (1). Exercised leg vastus lateralis ultrasonographic images were obtained at rest using B-mode ultrasonography (Mylab 70; Esaote Biomedica) with a 100 mm, 10- to 15-MHz, linear array probe. Ultrasound images were taken at a specific point at full knee extension when the participant was lying supine. The transducer was aligned in the fascicle plane to capture an optimal portion of fascicles. The muscle architecture parameters fascicle length and pennation angle were quantified by the same unblinded investigator (M. V. Franchi) from the ultrasound scans using Image J 1.42q (National Institutes of Health). The visible portion of the fascicle length was assessed directly using this software. Pennation angle was measured as the intersection between fascicles and the deep tendon aponeurosis. Isometric muscle strength was measured in a sitting position using an isokinetic dynamometer (Isocom; Isokinetic Technologies, Eurokinetics) throughout a range of six knee joint angles from 90 to 40°, with full extension corresponding to 0°. Subjects were seated in the dynamometer chair and secured into position using straps across the chest. Contractions lasted for 4 s, with a rest period of 45 s between contractions and a rest period of 90 s between knee joint angle changes. The maximum isometric torque value (MVC) was chosen for data analysis.
Statistical analyses.
Descriptive statistics were produced for all data sets to check for normal distribution (accepted if P > 0.05) using a Kolmogorov-Smirnov test. All data are presented as means ± SE. Differences within groups were detected by repeated-measures one-way (time) and two-way (ex type × time) ANOVA with a Bonferroni correction using GraphPad Prism (La Jolla, CA) Software Version 5. Differences for muscle strength and architectural measurements were analyzed by paired t-test. Correlations were assessed using Pearson Product Moment Correlation Coefficient. The α-level of significance was set at P < 0.05.
RESULTS
Body water and plasma alanine enrichment.
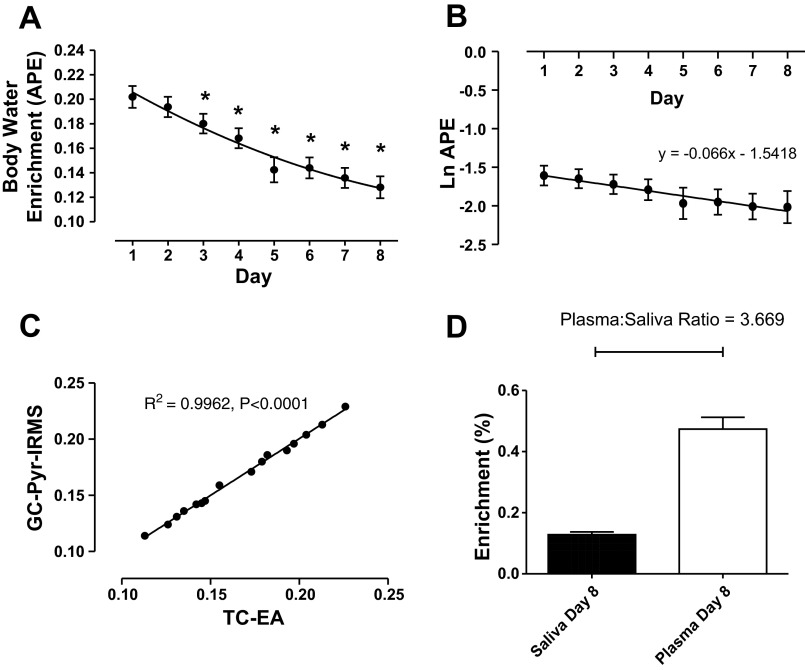
Mean body water enrichment over the 8-day period can be observed in Fig. 2A. A single bolus of 150 ml of 70% D2O led to a body water enrichment of 0.202 ± 0.009% 24 h postingestion (range: 0.162–0.237%). Body water enrichment followed an exponential decay pattern (Fig. 2A), decaying slowly and significantly over the 8-day period at a rate of ∼0.009/day. Raw APE values were natural logarithm converted to determine the mean decay constant (see Fig. 2B), and from this an estimation of mean half-life for body water elimination can be made; this was calculated as 11 ± 0.9 days.
Fig. 2.
A: exponential time course of body water enrichment over 8 days following oral bolus of 150 ml of deuterium oxide (D2O; 70 atom%). B: natural logarithm transformed body water enrichment for calculation of decay constant and elimination half-life. C: correlation of 2 different body water analytical techniques using high-temperature conversion elemental analyzer (TC-EA) direct liquid injection and gas chromatography-pyrolysis-isotope ratio mass spectrometry (GC-Pyr-IRMS). D: comparison of plasma alanine enrichment and saliva enrichment on day 8. *Significantly different from initial day 1 time point, P < 0.01. APE, atom% excess.
Saliva samples from two subjects were prepared via a modification of a method described by Mahsut et al. (34), where deuterium from water is transferred onto the hydrogen positions of acetone under high pH incubation. These samples were then analyzed via GC-pyrolysis-IRMS. This was performed to validate that there was no dilution in enrichment with saliva due to contaminants that might have been present. Table 1 and Fig. 2C provide a comparison of the APE for the two participants, as measured using both the direct liquid injection method for high-temperature conversion elemental analyzer (TC-EA) and the GC acetone exchange methods, and there was no difference between the two methods, showing highly significant correlation between the two techniques (P < 0.0001, r2 = 0.9962; Fig. 2C) underlining the validity of TC-EA method. Furthermore, water extracted from day 8 plasma samples provided further validity for the method (Table 2).
Table 1.
Comparison between MS techniques for measuring BW enrichment following D2O ingestion and the use of saliva as a surrogate for plasma
|
Participant 1 BW (APE) |
Participant 2 BW (APE) |
||
|---|---|---|---|
| TC/EA | GC-Pyr-IRMS | TC/EA | GC-Pyr-IRMS |
| 0.229 | 0.226 | 0.204 | 0.204 |
| 0.213 | 0.213 | 0.197 | 0.196 |
| 0.190 | 0.193 | 0.182 | 0.186 |
| 0.180 | 0.179 | 0.173 | 0.171 |
| 0.159 | 0.155 | 0.113 | 0.114 |
| 0.145 | 0.147 | 0.135 | 0.136 |
| 0.131 | 0.131 | 0.142 | 0.142 |
| 0.124 | 0.126 | 0.145 | 0.143 |
MS, mass spectrometry; D2O, deuterium oxide; BW, body water; APE, atom %excess; TC/EA, high-temperature conversion elemental analyzer; GC-Pyr-IRMS, gas chromatography-pyrolysis-isotope ratio mass spectrometry.
Table 2.
Comparison of saliva and plasma water among participants
| Saliva Water Day 8 (APE) | Plasma Water Day 8 (APE) | |
|---|---|---|
| Participant 1 | 0.124 | 0.124 |
| Participant 2 | 0.145 | 0.144 |
| Participant 3 | 0.128 | 0.126 |
| Participant 4 | 0.090 | 0.090 |
| Participant 5 | 0.154 | 0.157 |
| Participant 6 | 0.106 | 0.106 |
| Participant 7 | 0.165 | 0.166 |
| Participant 8 | 0.114 | 0.112 |
It has been demonstrated in the literature, based on GC-MS mass isotopomer distribution analysis calculations, that an average of 3.7 deuteriums are incorporated onto alanine from D2O out of a possible four carbon-hydrogen bonds, following a bolus of D2O in mammals (14, 43). Hence, this correction factor is used in the precursor-product equation to calculate FSR when body water enrichment is being used as the surrogate precursor. To validate this for our own calculations, the alanine enrichment in 8-day plasma samples was determined by GC-MS, and the mean plasma alanine/saliva body water ratio was calculated as 3.67 (Fig. 2D), therefore confirming the utility of 3.7 as a correction factor for use in the calculation of FSR in this study.
FSRs of MyoPS, SPS, and CPS.
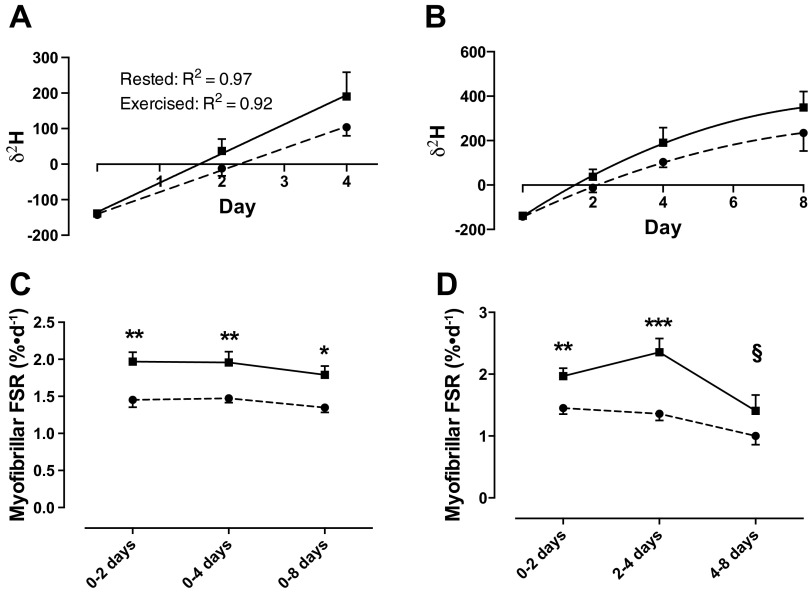
Figure 3, A and B, shows the mean δ2H in protein-bound alanine over the course of the 8-day training period. An exponential nonlinear pattern of incorporation was observed. Breaking this down into sections of time, we identify linear kinetics as being maintained for ≤4 days (Fig. 3A), after which it switches to nonlinear from 4 to 8 days (Fig. 3B). Therefore, the standard precursor-product calculation (as detailed in materials and methods) was only valid for FSR ≤4 days, after which a nonlinear model was applied to the data whereby
Fig. 3.
A: linear incorporation of deuterium into protein bound alanine over the 1st 4 days. B: illustration of nonlinear incorporation of deuterium into protein-bound alanine beyond 4 days. C: myofibrillar protein synthesis rates in exercised (solid line) and nonexercised (dashed line) legs over 8-day training period. D: temporal pattern of myofibrillar protein synthesis in exercised and nonexercised legs. ***Significantly different from rested leg at the same time point, P < 0.001; **P < 0.01; *P < 0.05; §significantly different from previous time point in same leg P < 0.05. FSR, fractional synthetic rate.
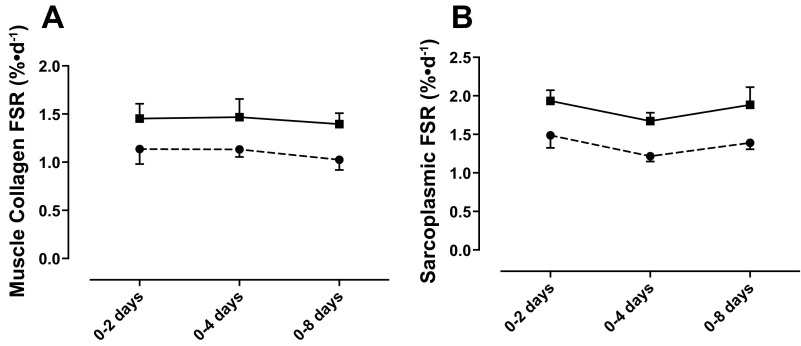
Using this equation, in the rested leg, MyoPS was 1.45 ± 0.10, 1.47 ± 0.06, and 1.35 ± 0.07%/day at 0–2, 0–4, and 0–8 days, respectively, which when calculated as percent/hour represented between 0.052 and 0.061%/h; this rate is identical to the rates that are observed using traditional AA stable isotope tracers (53). MyoPS was increased significantly in the exercised leg at 0–2 (1.97 ± 0.13%/day), 0–4 (1.96 ± 0.15%/day, P < 0.01; Fig. 3A), and 0–8 days (1.79 ± 0.12%/day, P < 0.05). Figure 3D illustrates the temporal pattern for stimulation of MyoPS over the 8-day training period. FSR in the exercised leg is significantly increased above that of the rested leg at 2–4 days (P < 0.001), after which it rapidly falls by ∼40% from 4 to 8 days (P < 0.01). CPS was, as expected, slower overall than MyoPS but followed a similar pattern over the 8 days, with the exercised leg showing a trend for greater FSR (2 days: 1.14 ± 0.13 vs. 1.45 ± 0.15%/day; 4 days: 1.13 ± 0.07 vs. 1.47 ± 0.18%/day; 8 days: 1.03 ± 0.09 vs. 1.40 ± 0.11%/day; Fig. 4A). However, due to a problem with sample recovery during collagen sample extraction, there were a number of missing data points; therefore, it was not possible to run statistical analyses on this data set. SPS, as with CPS, was found to be increased in the exercised leg at 0–2, 0–4, and 0–8 days (1.93 ± 0.15 vs. 1.48 ± 0.17, 1.67 ± 0.11 vs. 1.22 ± 0.07, and 1.88 ± 0.23 vs. 1.39 ± 0.23%/day, respectively; Fig. 4B); however, this was not statistically significant.
Fig. 4.
Muscle collagen (A) and sarcoplasmic protein synthesis (B) in exercised (solid line) and nonexercised (dashed line) legs over 8-day training period.
Strength and muscle architecture.
At 8 days we observed significant increases in fascicle length (1.08 ± 0.16%, P < 0.001) and pennation angle (1.83 ± 0.16%, P < 0.001), as measured using ultrasonography. There was a small increase in MVC of 3.4 ± 3.5%, but this was not statistically significant.
DISCUSSION
The purpose of the present study was to determine the efficacy of using the D2O tracer to determine the temporal synthesis of muscle protein subfractions at “rest” and in response to short-term RET in the form of successive exercise bouts. Additionally, we hoped to demonstrate the utility of this method for providing short-term resolution of the regulation of muscle mass under conditions where traditional AA tracer approaches are untenable and where changes in mass due to RET are not yet quantifiable with standard image measurement techniques (ultrasound, MRI, DEXA). Indeed, although we observed increases in fascicle length and pennation angle at the end of 8 days, which is suggestive of increased hypertrophic response, this change was very small, increasing by 1.08 and 1.8%, respectively. Furthermore, we aimed to validate the use of IRMS for quantifying these responses rapidly and robustly. Here, we show for the first time that, using a single bolus of D2O, we are able to detect synthesis rates of multiple protein fractions (MyoPS, CPS, and SPS) at rest and also increases in synthesis in response to an anabolic stimulus (exercise) vs. an internal nonexercised contralateral leg. This difference was detectable within 48 h of the first exercise bout, gradually declining from 2 to 8 days. This highlights the applicability of D2O for quantifying cumulative MPS over periods when detection of an increase in muscle mass (via imaging techniques) is not feasible.
RET remains the most feasible tool for increasing or maintaining muscle mass in both healthy young and old individuals (20, 44) in addition to frail elderly and clinical populations (10, 32, 55). Hypertrophy associated with resistance exercise is caused primarily by cumulative increases in MPS, leading to net protein accretion (4). Using traditional AA tracer techniques, resistance exercise has been shown to induce increases in MPS following a single bout (22, 30, 42), which can be augmented by the addition of a protein feed postexercise (57) and with adequate nutrition can be sustained for up to and beyond 24 h (12, 36, 42). It was our aim to measure participants in a “free-living” normal environment such that these studies were not controlled for feeding, with the exception of the protein taken postexercise, to provide a “maximal stimulus” to ensure that the study was not confounded by individuals consuming insufficient protein with which to sustain MPS. Indeed, following the first bout of RET we observed that MyoPS was increased by ∼36% compared with the nonexercised leg after 48 h (Fig. 3C). These data support the findings of the acute AA tracer work (acute infusions on different study days), which substantiates that with adequate nutrition MPS can be maintained for greater than 24 h postexercise (12, 36).
In the present data, we further validate the use of D2O in measuring turnover in skeletal muscle (16, 18, 33, 50) by highlighting for the first time the efficacy of the D2O tracer for monitoring the cumulative and temporal responses of protein synthesis to an anabolic stimulus (RET). Over 8 days of RET, MyoPS peaked between 2 and 4 days. Following this initial stimulation there was a drop in FSR of ∼40% from 4 to 8 days, which could be suggestive of a “muscle full” effect (3, 4, 8, 38) where the muscle has achieved a new set point of accommodation, requiring greater stimulation to sustain growth. Finally, although not significant, there was a slight drop in MyoPS in the nonexercised leg over the 8 days (Fig. 3D), and this may have been influenced by the free-living nature of the design; for example, no strict controls on diet and exercise were imposed on the participants. We conclude that future studies of this kind would thus benefit from diet and activity monitoring.
The importance of studying distinct muscle protein subfractions can be seen via the preferential acute increase in myofibrillar MPS in response to resistance vs. mitochondrial MPS after endurance exercise, both of which are prophetic of ensuing adaptations to each exercise mode (58). As with MyoPS, both CPS and SPS have been shown to be stimulated in a similar fashion immediately post-resistance exercise, highlighting the coordinated response of muscle proteins to stimulation by exercise (36). In the present study, we have seen similar coordinated effects. SPS was greater, albeit not quite significantly (P = 0.14, 0.07, and 0.16 for 0–2, 0–4, and 0–8 days respectively), over the 8-day training period in the exercised compared with the nonexercised leg. This lack of significant findings may be influenced by the diverse nature of the sarcoplasmic protein pool, and dramatic variance in turnover rates within this pool will also reflect the variance observed within the present data. Our present data on CPS support other published data (5, 36) demonstrating that muscle collagen turns over at a slower rate than the myofibrillar and sarcoplasmic fractions (∼25% lower than myofibrillar in the nonexercised state). As expected, CPS rates showed a pattern of change over the 8-day period that was similar to the myofibrillar fraction; however, because of sample processing problems, we were unable to produce complete data sets for all subjects, such that statistical representation of the data is limited. Despite this, we have shown that this approach can be used for the quantification of multiple muscle fractions in a single study, and further refinements to the protocol, sample processing, and the use of tandem MS will permit measurement of the turnover of additional protein pools, e.g., mitochondrial protein fractions.
It is also necessary to compare our FSRs to those captured using traditional 13C methodologies to qualify their quantitative utility. We obtained “rested” FSR for MyoPS of ∼1.25–1.47%/day, which equates to ∼0.05–0.06%/h, representative of that usually achieved with the use of 13C-AA tracers (53). This was also observed with rested SPS rates that were ∼0.051–0.062%/h in the present study, similar to rates quoted within the literature using 13C tracers (3, 36). Although CPS rates were slightly higher than some values quoted in previous literature [∼0.042–0.047%/h here compared with ∼0.018–0.025%/h (35, 36)], CPS rates of ≤0.06%/h have been reported (22). Therefore, using the present approaches, we arrive at values approximating those expected.
Herein, we have validated a highly sensitive stable isotope tracer approach for measuring human MPS using D2O. In one of the only other human studies of its kind, Gasier et al. (16) showed that, by providing a 300-ml bolus of 70% D2O, they were able to measure increased rates of MyoPS following a single bout of resistance exercise 24 h postexercise using the same unilateral model as the present study. To do this, these authors utilized GC-MS for measuring both bound protein and body water deuterium enrichment, reporting that because of low levels of enrichment in the body water (<0.5%), accurate data quantification was difficult due to limitations of the GC-MS approach they used. Here, we show that by giving a single 150-ml bolus of D2O and using a combination of TC/EA-IRMS and GC-pyrolysis-IRMS, we are able to sensitively and accurately measure body water and bound protein alanine enrichment to a precision of 1–3 δ2H. The substantial increase in measurement sensitivity provided by IRMS allows us to uncover subtle temporal differences in response to exercise not possible with standard GC-MS. Indeed, δ2H changes over 2 days in the present study ranged from 116 to 176 δ2H such large changes suggest that measurements of MyoPS may be possible over even shorter periods (e.g., several hours, given the appropriate D2O dosing protocol), with similar levels of accuracy using IRMS. Per hour, current δ2H changes would be equivalent to 2–3 δ2H; however, by increasing the D2O dosing threefold, with appropriate timing of doses to avoid side effects, we believe it is likely that rates of MyoPS could be measured over periods of hours, enhancing the utility of this approach and potentially replacing the need for intravenous AA tracers for measurement of protein turnover in certain situations.
To ease the burden on the participants within the present study, we chose to provide only a single small bolus of D2O (150 ml) at the beginning of the study based on the experimental design provided by MacDonald et al. (33), where a single 100-ml D2O (70 atom%) bolus was applied to measure MyoPS over a period of 4–14 days. Many published uses of D2O for monitoring metabolic turnover in both animals and humans usually provide regular daily doses of D2O following an initial large bolus, and this is implemented in an attempt to mimic that of AA tracers, whereby a large primed and continuous infusion is provided to maintain an isotopic steady state. However, it is possible to measure protein synthesis in the non-steady-state conditions (15, 46). Because of the slow turnover of water in the body, half-life is reported as ∼7–10 days (17, 33) and was estimated in the present study as a mean of 11 ± 0.9 days. MacDonald et al. (33) has shown that D2O elimination follows a slow exponential decay after bolus ingestion, as was observed here (Fig. 2A). This slow decay maintains body water enrichment within adequate levels for continuous incorporation into muscle protein and measurement of MPS over periods of 1 wk and potentially longer. The use of IRMS, with its high measurement sensitivity for isotopic abundance, ensures that body water enrichment needs to be raised by only a small amount (∼0.2%) compared with the 1–2% quoted by other researchers (50). Furthermore, this also minimizes the potential for the onset of the reported side effects from D2O, such as dizziness and nausea. This function of slow D2O elimination combined with high measurement precision makes this technique ideally suited for use within populations where regular D2O administration or high doses of D2O may be problematic (e.g., frail elderly) or contraindicated (e.g., children or critical care patient populations), highlighting the wide-ranging applicability of this tracer technique.
To summarize, using only a single 150-ml bolus of D2O, we show for the first time that it is possible to measure the temporal response of FSR within multiple muscle protein subfractions over a short period and also validate the approach in response to an anabolic stimuli (RET), from which we could determine the temporal and cumulative anabolic responses to exercise (compared with the contralateral nonexercise control leg). The increased sensitivity provided by GC-pyrolysis-IRMS allows accurate measurement of MyoPS, SPS, and CPS over as little as 2 days while also being able to robustly detect a stimulation of protein synthesis. Therefore, we reaffirm that D2O is a valid tracer approach for measuring MPS and muscle anabolism in extended free-living situations and will have wide application to assess the efficacy of clinical/nutritional/exercise interventions for maximizing mass/attenuating atrophy. Finally, despite holding much promise, the extent to which D2O can be utilized as a tool to “predict” chronic anabolic outcomes remains to be determined.
GRANTS
This work was supported by a grant from The Physiological Society awarded to P. J. Atherton and K. Smith, a project grant from the Dunhill Medical Trust to K. Smith, P. J. Atherton, D. J. Wilkinson, and M. V. Narici (R264/1112), and a Medical Research Council Confidence in Concept award (CIC12019) to P. J. Atherton, P. L. Greenhaff, N. J. Szewczyk, and K. Smith. D. J. Wilkinson is a Medical Research Council-Arthritis Research United Kingdom (MRC-ARUK) Centre-funded postdoctoral research fellow, and M. S. Brook is supported by a University of Nottingham Ph.D studentship. Equipment was funded through monies provided from an award by the MRC-ARUK Centre to the Universities of Nottingham and Birmingham.
DISCLOSURES
The authors declare no conflicts of interest, financial or otherwise.
AUTHOR CONTRIBUTIONS
D.J.W., M.V.N., P.L.G., P.J.A., and K.S. contributed to the conception and design of the research; D.J.W., M.V.F., M.S.B., P.J.A., and K.S. analyzed the data; D.J.W., N.J.S., P.J.A., and K.S. interpreted the results of the experiments; D.J.W. prepared the figures; D.J.W., M.V.N., J.P.W., W.K.M., N.J.S., P.L.G., P.J.A., and K.S. drafted the manuscript; D.J.W., M.V.F., M.S.B., M.V.N., J.P.W., W.K.M., N.J.S., P.L.G., P.J.A., and K.S. edited and revised the manuscript; D.J.W., M.V.F., M.S.B., M.V.N., J.P.W., W.K.M., N.J.S., P.L.G., P.J.A., and K.S. approved the final version of the manuscript; M.V.F., M.S.B., J.P.W., and W.K.M. performed the experiments.
ACKNOWLEDGMENTS
We acknowledge the technical and administrative support of Margaret Baker, Amanda Gates, and Tanya Fletcher.
REFERENCES
- 1.Alegre LM, Jiménez F, Gonzalo-Orden JM, Martín-Acero R, Aguado X. Effects of dynamic resistance training on fascicle length and isometric strength. J Sports Sci 24: 501–508, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Arango-Lopera VE, Arroyo P, Gutiérrez-Robledo LM, Pérez-Zepeda MU, Cesari M. Mortality as an adverse outcome of sarcopenia. J Nutr Health Aging 17: 259–262, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K, Rennie MJ. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr 92: 1080–1088, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Atherton PJ, Smith K. Muscle protein synthesis in response to nutrition and exercise. J Physiol 590: 1049–1057, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babraj JA, Cuthbertson DJ, Smith K, Langberg H, Miller B, Krogsgaard MR, Kjaer M, Rennie MJ. Collagen synthesis in human musculoskeletal tissues and skin. Am J Physiol Endocrinol Metab 289: E864–E869, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Barillaro C, Liperoti R, Martone AM, Onder G, Landi F. The new metabolic treatments for sarcopenia. Aging Clin Exp Res 25: 119–127, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Belloto E, Diraison F, Basset A, Allain G, Abdallah P, Beylot M. Determination of protein replacement rates by deuterated water: validation of underlying assumptions. Am J Physiol Endocrinol Metab 292: E1340–E1347, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Bohe J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol 532: 575–579, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busch R, Kim YK, Neese a R, Schade-Serin V, Collins M, Awada M, Gardner JL, Beysen C, Marino ME, Misell LM, Hellerstein MK. Measurement of protein turnover rates by heavy water labeling of nonessential amino acids. Biochim Biophys Acta 1760: 730–744, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Cadore EL, Casas-Herrero A, Zambom-Ferraresi F, Idoate F, Millor N, Gómez M, Rodriguez-Mañas L, Izquierdo M. Multicomponent exercises including muscle power training enhance muscle mass, power output, and functional outcomes in institutionalized frail nonagenarians. Age (Dordr). In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Churchward-Venne TA, Burd NA, Phillips SM. Nutritional regulation of muscle protein synthesis with resistance exercise: strategies to enhance anabolism. Nutr Metab (Lond) 9: 40, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuthbertson DJ, Babraj J, Smith K, Wilkes E, Fedele MJ, Esser K, Rennie M. Anabolic signaling and protein synthesis in human skeletal muscle after dynamic shortening or lengthening exercise. Am J Physiol Endocrinol Metab 290: E731–E738, 2006 [DOI] [PubMed] [Google Scholar]
- 12a.De Riva A, Deery MJ, McDonald S, Lund T, Busch R. Measurement of protein synthesis using heavy water labeling and peptide mass spectrometry: Discrimination between major histocompatibility complex allotypes. Anal Biochem 403: 1–12, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dietrichson P, Coakley J, Smith PE, Griffiths RD, Helliwell TR, Edwards RH. Conchotome and needle percutaneous biopsy of skeletal muscle. J Neurol Neurosurg Psychiatry 50: 1461–1467, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dufner DA, Bederman IR, Brunengraber DZ, Rachdaoui N, Ismail-Beigi F, Siegfried BA, Kimball SR, Previs SF. Using 2H2O to study the influence of feeding on protein synthesis: effect of isotope equilibration in vivo vs. in cell culture. Am J Physiol Endocrinol Metab 288: E1277–E1283, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Garlick PJ, McNurlan MA, Essén P, Wernerman J. Measurement of tissue protein synthesis rates in vivo: a critical analysis of contrasting methods. Am J Physiol Endocrinol Metab 266: E287–E297, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Gasier HG, Fluckey JD, Previs SF, Wiggs MP, Riechman SE. Acute resistance exercise augments integrative myofibrillar protein synthesis. Metabolism 61: 153–156, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Gasier HG, Fluckey JD, Previs SF. The application of 2H2O to measure skeletal muscle protein synthesis. Nutr Metab (Lond) 7: 31, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gasier HG, Riechman SE, Wiggs MP, Buentello A, Previs SF, Fluckey JD. Cumulative responses of muscle protein synthesis are augmented with chronic resistance exercise training. Acta Physiol (Oxf) 201: 381–389, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Häkkinen K, Kraemer WJ, Newton RU, Alen M. Changes in electromyographic activity, muscle fibre and force production characteristics during heavy resistance/power strength training in middle-aged and older men and women. Acta Physiol Scand 171: 51–62, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Häkkinen K, Newton RU, Gordon SE, McCormick M, Volek JS, Nindl BC, Gotshalk LA, Campbell WW, Evans WJ, Häkkinen A, Humphries BJ, Kraemer WJ. Changes in muscle morphology, electromyographic activity, and force production characteristics during progressive strength training in young and older men. J Gerontol A Biol Sci Med Sci 53: B415–B423, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Hilkert AW, Douthitt CB, Schlüter HJ, Brand WA. Isotope ratio monitoring gas chromatography/Mass spectrometry of D/H by high temperature conversion isotope ratio mass spectrometry. Rapid Commun Mass Spectrom 13: 1226–1230, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Holm L, van Hall G, Rose AJ, Miller BF, Doessing S, Richter EA, Kjaer M. Contraction intensity and feeding affect collagen and myofibrillar protein synthesis rates differently in human skeletal muscle. Am J Physiol Endocrinol Metab 298: E257–E269, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Husek P, Liebich HM. Organic acid profiling by direct treatment of deproteinized plasma with ethyl chloroformate. J Chromatogr B Biomed Appl 656: 37–43, 1994 [DOI] [PubMed] [Google Scholar]
- 24.International Atomic Energy Agency IAEA Human Health Series. Introduction to Body Composition Assessment Using the Deuterium Dilution Technique with Analysis of Saliva Samples by Fourier Transform Infrared Spectrometry. Vienna: IAEA Books, 2011 [Google Scholar]
- 25.Ivey FM, Roth SM, Ferrell RE, Tracy BL, Lemmer JT, Hurlbut DE, Martel GF, Siegel EL, Fozard JL, Jeffrey Metter E, Fleg JL, Hurley BF. Effects of age, gender, and myostatin genotype on the hypertrophic response to heavy resistance strength training. J Gerontol A Biol Sci Med Sci 55: M641–M648, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Jones TE, Stephenson KW, King JG, Knight KR, Marshall TL, Scott WB. Sarcopenia—mechanisms and treatments. J Geriatr Phys Ther 32: 83–89, 2009 [PubMed] [Google Scholar]
- 27.Kasumov T, Ilchenko S, Li L, Rachdaoui N, Sadygov RG, Willard B, McCullough AJ, Previs S. Measuring protein synthesis using metabolic 2H labeling, high-resolution mass spectrometry, and an algorithm. Anal Biochem 412: 47–55, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim PL, Staron RS, Phillips SM. Fasted-state skeletal muscle protein synthesis after resistance exercise is altered with training. J Physiol 568: 283–290, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar V, Atherton PJ, Selby A, Rankin D, Williams J, Smith K, Hiscock N, Rennie MJ. Muscle protein synthetic responses to exercise: effects of age, volume, and intensity. J Gerontol A Biol Sci Med Sci 67: 1170–1177, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 587: 211–217, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab 293: E453–E459, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Lønbro S, Dalgas U, Primdahl H, Johansen J, Nielsen JL, Aagaard P, Hermann AP, Overgaard J, Overgaard K. Progressive resistance training rebuilds lean body mass in head and neck cancer patients after radiotherapy—results from the randomized DAHANCA 25B trial. Radiother Oncol 108: 314–319, 2013 [DOI] [PubMed] [Google Scholar]
- 33.MacDonald AJ, Small AC, Greig CA, Husi H, Ross JA, Stephens NA, Fearon KCH, Preston T. A novel oral tracer procedure for measurement of habitual myofibrillar protein synthesis. Rapid Commun Mass Spectrom 27: 1769–1777, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Mahsut A, Wang SP, McLaren DG, Bhat G, Herath K, Miller PL, Hubbard BK, Johns DG, Previs SF, Roddy TP. Headspace analyses of 2H labeling of acetone: enabling studies of fatty acid oxidation in vivo. Anal Biochem 408: 351–353, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Miller BF, Hansen M, Olesen JL, Flyvbjerg A, Schwarz P, Babraj JA, Smith K, Rennie MJ, Kjaer M. No effect of menstrual cycle on myofibrillar and connective tissue protein synthesis in contracting skeletal muscle. Am J Physiol Endocrinol Metab 290: E163–E168, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Miller BF, Olesen JL, Hansen M, Døssing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K, Rennie MJ. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol 567: 1021–1033, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller BF, Robinson MM, Bruss MD, Hellerstein M, Hamilton KL. A comprehensive assessment of mitochondrial protein synthesis and cellular proliferation with age and caloric restriction. Aging Cell 11: 150–161, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Millward DJ, Bowtell JL, Pacy P, Rennie MJ. Physical activity, protein metabolism and protein requirements. Proc Nutr Soc 53: 223–240, 1994 [DOI] [PubMed] [Google Scholar]
- 39.Onder G, Della Vedova C, Landi F. Validated treatments and therapeutics prospectives regarding pharmacological products for sarcopenia. J Nutr Health Aging 13: 746–756, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Parise G, Yarasheski KE. The utility of resistance exercise training and amino acid supplementation for reversing age-associated decrements in muscle protein mass and function. Curr Opin Clin Nutr Metab Care 3: 489–495, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Phillips BE, Hill DS, Atherton PJ. Regulation of muscle protein synthesis in humans. Curr Opin Clin Nutr Metab Care 15: 58–63, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab 273: E99–E107, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Previs SF, Fatica R, Chandramouli V, Alexander JC, Brunengraber H, Landau BR. Quantifying rates of protein synthesis in humans by use of 2H2O: application to patients with end-stage renal disease. Am J Physiol Endocrinol Metab 286: E665–E672, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Raue U, Trappe TA, Estrem ST, Qian HR, Helvering LM, Smith RC, Trappe S. Transcriptome signature of resistance exercise adaptations: mixed muscle and fiber type specific profiles in young and old adults. J Appl Physiol 112: 1625–1636, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rennie MJ, Edwards RH, Halliday D, Matthews DE, Wolman SL, Millward DJ. Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin Sci (Lond) 63: 519–523, 1982 [DOI] [PubMed] [Google Scholar]
- 46.Rennie MJ, Smith K, Watt PW. Measurement of human tissue protein synthesis: an optimal approach. Am J Physiol Endocrinol Metab 266: E298–E307, 1994 [DOI] [PubMed] [Google Scholar]
- 47.Ripoche N, Ferchaud-Roucher V, Krempf M, Ritz P. D and 18O enrichment measurements in biological fluids in a continuous-flow elemental analyser with an isotope-ratio mass spectrometer using two configurations. J Mass Spectrom 41: 1212–1218, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Rittenberg D, Schoenheimer R. Deuterium as an indicator in the study of intermediary metabolism: XI. Further studies on the biological uptake of deuterium into organic substances, with special reference to fat and cholesterol formation. J Biol Chem 121: 235–253, 1937 [Google Scholar]
- 50.Robinson MM, Turner SM, Hellerstein MK, Hamilton KL, Miller BF. Long-term synthesis rates of skeletal muscle DNA and protein are higher during aerobic training in older humans than in sedentary young subjects but are not altered by protein supplementation. FASEB J 25: 3240–3249, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schoenheimer R, Rittenberg D. Deuterium as an indicator in the study of intermediary metabolism. Science 82: 156–157, 1935 [DOI] [PubMed] [Google Scholar]
- 52.Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr 82: 53–59, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Smith GI, Patterson BW, Mittendorfer B. Human muscle protein turnover—why is it so variable? J Appl Physiol 110: 480–491, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS One 5: e10805, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stene GB, Helbostad JL, Balstad TR, Riphagen II, Kaasa S, Oldervoll LM. Effect of physical exercise on muscle mass and strength in cancer patients during treatment-A systematic review. Crit Rev Oncol Hematol 88: 573–593, 2013 [DOI] [PubMed] [Google Scholar]
- 56.Toth MJ, LeWinter MM, Ades PA, Matthews DE. Impaired muscle protein anabolic response to insulin and amino acids in heart failure patients: relationship with markers of immune activation. Clin Sci (Lond) 119: 467–476, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.West DW, Burd NA, Coffey VG, Baker SK, Burke LM, Hawley JA, Moore DR, Stellingwerff T, Phillips SM. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. Am J Clin Nutr 94: 795–803, 2011 [DOI] [PubMed] [Google Scholar]
- 58.Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol 586: 3701–3717, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams JP, Phillips BE, Smith K, Atherton PJ, Rankin D, Selby AL, Liptrot S, Lund J, Larvin M, Rennie MJ. Effect of tumor burden and subsequent surgical resection on skeletal muscle mass and protein turnover in colorectal cancer patients. Am J Clin Nutr 96: 1064–1070, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr 84: 475–482, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Yarasheski KE, Smith K, Rennie MJ, Bier DM. Measurement of muscle protein fractional synthetic rate by capillary gas chromatography/combustion isotope ratio mass spectrometry. Biol Mass Spectrom 21: 486–490, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuan CL, Sharma N, Gilge DA, Stanley WC, Li Y, Hatzoglou M, Previs SF. Preserved protein synthesis in the heart in response to acute fasting and chronic food restriction despite reductions in liver and skeletal muscle. Am J Physiol Endocrinol Metab 295: E216–E222, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]